Abstract
Rich and ephemeral resources, such as carrion, are a source of intense interspecific competition among animal scavengers and microbial decomposers. Janzen [Janzen DH (1977) Am Nat 111:691–713] hypothesized that microbes should be selected to defend such resources by rendering them unpalatable or toxic to animals, and that animals should evolve counterstrategies of avoidance or detoxification. Despite the ubiquity of animal-microbe competition, there are few tests of Janzen's hypothesis, in particular with respect to antimicrobial strategies in animals. Here, we use the burying beetle Nicrophorus vespilloides, a species that obligately breeds on carcasses of small vertebrates, to investigate the role of parental care and avoidance as antimicrobial strategies. We manipulated competition between beetle larvae and microbes by providing beetles with either fresh carcasses or old ones that had reached advanced putrefaction. We found evidence for a strong detrimental effect of microbial competition on beetle reproductive success and larval growth. We also found that parental care can largely compensate for these negative effects, and that when given a choice between old and fresh carcasses, parents tended to choose to rear their broods on the latter. We conclude that parental care and carcass avoidance can function as antimicrobial strategies in this species. Our findings extend the range of behavioral counterstrategies used by animals during competition with microbes, and generalize the work of Janzen to include competition between microbes and insects that rely on carrion as an obligate resource for breeding and not just as an opportunistic meal.
Keywords: animal-microbe interactions, parental care, resource competition
Carrion is a rich and ephemeral resource that is used by a wide variety of animal scavengers and microbial decomposers (1–5). Competition among animal scavengers is often intense and may be manifest as aggression over the ownership of a carcass or rapid discovery and consumption of carrion (2–4). Of equal importance, if often less obvious, are contests between animal scavengers and microbial decomposers. To secure these resources for themselves and avoid being consumed, Janzen (1) proposed that bacterial and fungal decomposers should produce compounds that cause meat and other rich and ephemeral resources to rot, thereby rendering them unpalatable or toxic to animals. He further proposed that, in response to microbial activities, animals should evolve behavioral or physiological counterstrategies of avoidance or detoxification. Despite the ubiquity of animal-microbe competition in nature, experimental tests of Janzen's hypotheses are limited, in particular with respect to antimicrobial strategies in animals other than avoidance (5, 6) that would allow animals to use microbe-laden resources (7). Furthermore, there is little information on the adaptive consequences to animals of microbial competition (8). Such data are now needed to advance our understanding of the evolutionary and ecological implications of competition between animals and microbes.
When microbial decomposers compete with animal scavengers, the cost of defeat is typically strongly asymmetric among the participants, similar to the asymmetry between prey and predators described in the life-dinner principle (9). Whereas resource loss for the microbe usually results in death, resource loss for the animal is less dire because it can locate and consume another food source. For the vast majority of animals that feed on carrion, scavenging is a facultative strategy (5), suggesting that the costs associated with carcass loss are low and that the strength of selection for antimicrobial counterstrategies is weak (9). In contrast, the stakes are higher for animals that obligately rely on carrion as food for their offspring, such as in burying beetles of the genus Nicrophorus (10, 11). Although carcass loss for Nicrophorus species does not result in their immediate demise, it can result in total reproductive failure because breeding opportunities are highly limited due to the scarcity of suitable carrion (10, 11). Thus, burying beetles provide an excellent system for studying antimicrobial strategies because the costs of resource loss are likely to be symmetric among the participants, leading to strong selection for behavioral and physiological counterstrategies to contend with microbial competitors.
Burying beetle parents provide extensive care while the larvae develop on the carcass, including provisioning of predigested carrion and active defense against predators and conspecifics. Furthermore, parents prepare and clean the carcass by covering it with anal and oral secretions, which are assumed to provide behavioral and chemical defenses against microbial competitors (10–13). Thus, for these beetles, parental care may represent a neglected strategy for dealing with intense microbial competition over carrion. However, experimental evidence on how parental care influences competition with microbial decomposers is currently lacking. Likewise, there is no available information on the adaptive consequence to burying beetles of competition with microbes.
The aim of this study is to investigate the role of parental care and carcass choice as antimicrobial strategies in the burying beetle N. vespilloides. Because parental care in this species is facultative (14, 15), its form and level can be experimentally manipulated. Furthermore, the fitness consequences of competition with microbes can be quantified in terms of larval growth and survival throughout development. Larval growth, which occurs entirely while the larvae feed on the carcass, determines adult body size (16). Larval growth and body size are important fitness components that influence larval and pupal survival (16, 17) and success in competition for breeding opportunities among adults (18). Here, we first test for effects of competition with microbes on the reproductive success of breeding beetles. Finding overwhelming evidence for a strong detrimental effect on reproductive success and larval growth, we next examine the efficacy of parental care in mitigating the costs of this competition. We find that parental care partly compensates for the negative effects of microbial competition, and that when given the simultaneous choice between microbe-laden and fresh carcasses, parents tend to choose to breed on the latter. Our findings extend and generalize the work of Janzen (1) to include competition between microbes and insects that rely on carrion as an obligate resource for breeding and not just as an opportunistic meal.
Results
Number and Size of Offspring on Fresh and Old Carcasses.
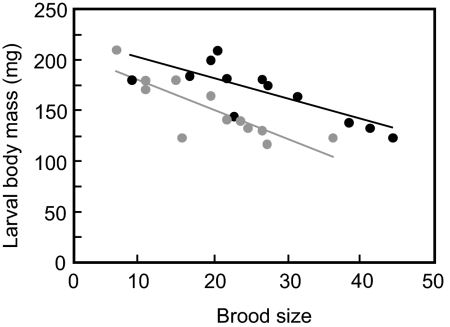
We first examined the effects of carcass age on the number and size of larvae to assess the impact of microbial competition on the parents' reproductive success. Here, as in later experiments, we provided single, mated females with either freshly thawed mouse carcasses (hereafter called fresh carcasses) or carcasses that had aged for 7 days (old carcasses) to enable progression through putrefaction due to microbial growth. Consistent with this expectation, old carcasses exhibited pronounced bloating and decomposition and generated highly unpleasant odors of decay. We find that average larval body mass decreased with increasing brood size (general linear model [GLM]: F1,21 = 35.64, P < 0.001), consistent with a tradeoff between the number and size of offspring (Fig. 1). However, carcass age had a significant effect on this tradeoff (GLM: F1,21 = 17.78, P < 0.001); larvae reared on old carcasses had a smaller body mass for a given brood size than larvae reared on fresh carcasses (Fig. 1), corresponding to a 17% decline in body mass. Given that larval body mass is strongly correlated with fitness, these data strongly support the idea that competition with microbes represents an important selective factor for N. vespilloides.
Fig. 1.
Effect of carcass age on the tradeoff between the number and size of offspring in Nicrophorus vespilloides broods reared on fresh carcasses (black circles and line) and old carcasses (gray circles and line).
Effects of Pre- and Posthatch Care on Microbial Competition.
We next examined the interaction between parental care and carcass age on the growth and survival of larvae until the time of dispersal. To eliminate potential artifacts due to differences in brood size across different treatments, we generated experimental broods comprised of 10 larvae, a size chosen because larvae at this density are not resource limited when raised on the carcasses used in our experiments (20–25 g). Larvae from old carcasses were ≈10% smaller at the time of dispersal than those from fresh carcasses (GLM: F1,61 = 10.37, P = 0.002; Fig. 2A), and parental care enhanced larval growth (GLM: F2,61 = 84.77, P < 0.001). In addition, we found a significant interaction between carcass age and the level of care on larval growth (GLM: F2,61 = 5.01, P = 0.010), implying that the effects of competition with microbes on larval growth are dependent upon the level of parental care (Fig. 2A). Specifically, both prehatch and posthatch care benefited larvae on old carcasses, whereas prehatch care did not benefit larvae on fresh carcasses (Fig. 2A). This interaction effect might reflect that prehatch preparation of the carcass by parents is more beneficial on old carcasses, or alternatively that parents improve their body condition by feeding off fresh carcasses, thereby enabling them to provide better quality posthatch care.
Fig. 2.
Effects of carcass age and parental care on larval growth, development, and survival in Nicrophorus vespilloides broods reared on fresh carcasses (black circles and lines) and old carcasses (gray circles and lines). (A) Larval growth until the time of dispersal from the carcass. (B) Larval growth at 24-h intervals from hatching until 120 h. Solid lines represent broods that received prehatch and posthatch care; dashed lines broods that received prehatch care only; and dotted lines broods that received no care. (C) Timing of larval dispersal from the carcass. (D) Larval survivorship until the time of dispersal from the carcass. The data are presented as mean ± 2 SE.
To explore further details on the effects of carcass age and parental care, we examined larval growth at 24-h intervals from hatching until the age of 120 h. As in the previous analysis, larvae grew significantly slower on old carcasses than on fresh ones (repeated-measures GLM, within subjects: F2.1,126.9 = 7.00, P = 0.001; Fig. 2B). Furthermore, decreasing levels of care had a negative impact on larval growth (repeated-measures GLM, within subjects: F4.2,126.9 = 73.71, P < 0.001), and shifted the time taken to reach the peak in larval body mass (Fig. 2B). Larval body mass peaked at the age of 96 h when parents provided either pre- or posthatch care, whereas it peaked at the age of 120 h when parents provided no care (Fig. 2B). In contrast to the previous analysis, there was no significant interaction between carcass age and parental care (repeated-measures GLM, within subjects: F4.2,126.9 = 1.42, P = 0.23; Fig. 2B), suggesting that the earlier finding was due to effects taking place after the larvae reached the age of 120 h.
Consistent with this suggestion, larvae dispersed later from old carcasses than from fresh ones (GLM: F1,61 = 5.84, P = 0.019; Fig. 2C). The timing of larval dispersal was also significantly affected by the level of parental care (GLM: F2,61 = 36.72, P < 0.001). Larvae dispersed earlier when females provided either pre- or posthatching care than when females provided no care (Fig. 2C). There was also a significant effect of the interaction between carcass age and parental care (GLM: F2,61 = 3.19, P = 0.048), indicating that the effect of the age of the carcass on the dispersal time of the larvae depended on the level of care (Fig. 2C).
Finally, though the level of parental care had a highly significant effect on larval survivorship (GLM: F2,61 = 17.20, P < 0.001; Fig. 2D), carcass age had no significant effect on larval survivorship (GLM: F1,61 = 0.14, P = 0.71).
Parental and Larval Behavior on Fresh and Old Carcasses.
The complex interactions identified herein suggest that females and/or larvae may alter their behavior according to the level of microbial competition they experience. To examine this possibility, we monitored the behavior of caring females and their dependent larvae over 2 days (24 h and 48 h after hatching). We found that carcass age had no significant effects on female behavior in terms of the overall time spent caring for larvae (repeated-measures GLM, between subjects: F1,36 = 1.22, P = 0.28; Fig. 3A), the time spent providing food to the larvae (repeated-measures GLM, within subjects: F1,36 = 1.13, P = 0.29; Fig. 3B), or time spent cleaning the carcass (repeated-measures GLM, within subjects: F1,36 = 0.35, P = 0.56; Fig. 3C). However, larvae reared on old carcasses spent significantly more time begging than those reared on a fresh carcass (repeated-measures GLM, between subjects: F1,36 = 4.63, P = 0.038; Fig. 3D). Given that begging reflects nutritional status and that larvae grew slower on old carcasses, this finding suggests that larvae were under increased nutritional stress when faced with high levels of microbial competition on old carcasses.
Fig. 3.
Effects of carcass age on parental and larval behavior in Nicrophorus vespilloides broods reared on fresh carcasses (black circles and lines) and old carcasses (gray circles and lines) over 2 days of observation. (A) Total time spent caring by female parents. (B) Time spent provisioning larvae with food by female parents. (C) Time spent cleaning the carcass by female parents. (D) Time spent begging by larvae. The data are presented as mean ± 2 SE.
Carcass Choice and Avoidance of Microbial Competition.
In light of our finding that competition with microbes on old carcasses had a detrimental effect on larval growth, we next sought to determine whether females showed a preference for fresh carcasses over old ones. When we provided mated females with a simultaneous choice between old and fresh carcasses, we found strongly contrasting results depending upon when choice was measured (Fig. 4 A and B). Initially, females showed a significant preference for associating with and feeding upon old carcasses (exact binomial test, P = 0.003). However, females were significantly more likely to rear their broods on fresh carcasses (exact binomial test, P = 0.014; Fig. 4B). Thus, despite the initial attraction to old carcasses, there was a strong maternal preference for breeding on fresh carcasses that were not previously colonized by microbial competitors.
Fig. 4.
Preferences of mated Nicrophorus vespilloides females when given a choice between a fresh carcass (black bars) and an old carcass (gray bars). (A) Percent of females first visiting a fresh or an old carcass. (B) Percent of females breeding on a fresh or an old carcass.
Discussion
Janzen (1) hypothesized that microbial toxins and secondary metabolites that cause food to spoil provide microbes with a competitive edge against their larger and more mobile vertebrate competitors. In turn, he argued, animals should evolve countermeasures to avoid or compensate for the negative effects of competition with microbes. In this work we use a uniquely suited experimental system to study Janzen's hypotheses in more detail. Specifically, we show that though the burying beetle N. vespilloides is significantly burdened by competition with microbes for access to the carcasses they rely upon to raise their larvae, these beetles have evolved a series of behavioral countermeasures that mitigate these negative effects.
When breeding on old carcasses, burying beetles face a number of reproductive costs. We quantified these costs by measuring central fitness components in burying beetles: brood size, larval growth, and dispersal time. Larvae reared on old carcasses were 17% smaller than larvae reared on fresh carcasses. Most interestingly, this difference persisted even in very small broods that were not resource limited. Thus, the cost incurred by microbial competition is not caused by a reduction in the biomass available to the developing larvae, but rather through the deterioration of the resource quality. This suggestion is buttressed by the finding that larvae beg more when reared on old carcasses, which is indicative of their poor nutritional state (19). Furthermore, beetle larvae dispersed from the old carcass significantly later than their counterparts reared on fresh carcasses. The effects of competition from microbes on old carcasses have considerable fitness consequences for the beetles because larval mass influences larval and pupal survival (16, 17), and determines adult size, which in turn influences success at securing mates and carcasses for rearing broods (18). The effects of increasing dispersal time have not been directly quantified in the field but may increase the risk of total brood failure because at any point during larval development, the entire carcass may be found and consumed by other scavengers. Overall, larvae reared on old carcasses should survive less well and grow into smaller adults that are reproductively handicapped.
In the face of these costs, beetle parents displayed at least 2 behavioral strategies that play a role in dealing with microbial competition for carrion: (i) avoidance of old carcasses colonized by microbes and (ii) elaborate forms of parental care before and after hatching. When provided with a simultaneous choice between a fresh and an old mouse carcass, female beetles initially showed a preference for old carcasses either due to their strong smell or because they represent a better feeding opportunity. Nevertheless, this initial choice was later reversed, so that females showed a strong preference for breeding on fresh carcasses. This finding shows that females can assess the suitability and quality of resources available for breeding and make an adaptive choice by avoiding the old carcasses that provided the lowest reproductive output. Avoidance of microbe-laden resources, to avoid toxic compounds produced by microbes or infection (20, 21), is a general behavioral strategy adopted by many animals that face intense competition with microbes (5, 6, 22). In our study, breeding females suffered a lower reproductive output when forced to breed on old and microbe-laden carcasses. Thus, our findings suggest that a preference for fresh carcasses is adaptive because it allows females to avoid costs associated with breeding on microbe-laden resources. These findings also show that females assess the quality of available resources and do not necessarily rear their young on the first carcass that is located, which raises the intriguing possibility that beetles face similar decisions in nature and might occasionally reject lower-quality carcasses (23).
When avoidance of microbe-laden carcasses is not possible, adult burying beetles provide extensive parental care that largely, although not entirely, compensates for the harmful effects to larvae of microbial competition. We found that prehatch care enhanced larval growth compared with no care when parents bred on old carcasses, whereas prehatch care had no such effect on fresh carcasses (Fig. 2A). The adaptive value of prehatch care has been largely ignored in previous studies on burying beetles (24), although it is assumed to function at least partly to preserve the resource for the larvae (11, 12, 24). Our data show that parental care before hatching is particularly beneficial when faced with microbial competition on old carcasses. During the prehatch care period, parents might mitigate the negative effects of microbes by depositing antimicrobial peptides on the surface of the carcass (13), feeding off the carcass to enhance their own ability to provide care for the larvae, and/or consuming the gut, a primary source of microbes that decompose carrion. Meanwhile, posthatch care enhanced larval growth on old and fresh carcasses alike (Fig. 2A). Our behavioral data reveal that parents provided a similar level of posthatch parental care regardless of carcass age. Thus, there was no evidence that breeding parents adjusted their behavior to the level of competition from microbes. This contrasts with larvae, which begged more when reared on an old carcass than when reared on a fresh carcass, suggesting that larvae were hungrier and in poorer condition when faced with high levels of competition from microbes. This suggestion is consistent with the slower growth of larvae reared on old carcasses.
More generally, our findings on the role of parental care as an antimicrobial strategy are relevant for understanding of the evolution of parental care. Parental care is defined as any parental behavior that enhances offspring growth or survival by neutralizing environmental conditions that are harmful to offspring (25). In insects, parental care is often associated with breeding on bonanza resources, including carrion and dung (26, 27). Tallamy and Wood (28) suggested that the dispersion, quality, and persistence of resources influences the extent of resource competition that insects must contend with, and that parental care is an effective strategy for dealing with intense resource competition. Traditionally, the focus has been on resource competition with conspecifics or other animals (27, 28). However, as argued by Janzen (1), carrion, dung, and other bonanza resources are highly attractive to many microbes, and our finding that parental care plays a role in diminishing harmful effects from competition with microbes suggests that competition from microbes might be an overlooked component that has promoted the evolution of parental care.
Throughout this work we have discussed microbes as though they were a coherent group acting in concert to repel animal scavengers. The reality is undoubtedly more complex and warrants further research. Dead animals are quickly colonized by saprophytic microbes and bacteria that migrate through the gut (29, 30). Many such microbes, including the coliform enterics Clostridium botulinum, Yersinia pestis, and Bacillus sp., are either directly or indirectly toxic to a variety of consumers (31–33). However, many microbial toxins—antibiotics as a prime example—have lethal effects on other microbes. At present it is unclear if the effects we observe on beetle larvae are driven by microbial toxins that evolved due to competition with beetles and other animals or by toxins that evolved due to competitive interactions among microbes with incidental effects on beetles (34). Distinguishing between these possibilities is not trivial and is a major focus of our future work that will involve identification of the relevant microbes as well as the nature and targets of their toxic products. This work will also explore the chemical response of beetles to counteract microbes, a response that is critical to beetle fitness whether they are directly targeted by microbes or incidentally harmed by them.
In summary, we have shown that N. vespilloides burying beetles are significantly burdened by competition with microbes for the carcasses they rely on for breeding. When provided with a choice, female beetles tend to avoid carcasses colonized by microbes. However, when this is not possible, parental care before and after hatching can largely compensate for the deleterious effects of microbial competition. Thus, we conclude that parental care can function as an antimicrobial strategy in this species. Many insects and other invertebrates use rich and ephemeral resources as an obligatory resource for breeding, and not just a meal. Thus, animal countermeasures against microbial competition would be particularly important in these species. Our work both validates and greatly expands Janzen's (1) original conjectures, and suggests that the systems most suited to examining the interactions between microbial decomposers and animal scavengers are those where the costs associated with resource loss are symmetric.
Materials and Methods
Subjects and General Procedures.
For details on the origins and husbandry of the beetles, we refer to ref. 35.
For our experiments, we selected pairs of unrelated virgin female and male beetles, which were placed in small containers (12 × 8 × 2 cm) lined with moist paper for mating. Mated females were placed into larger containers (17 × 12 × 6 cm) filled with 1 cm of moist peat. Females were provided with a previously frozen mouse carcass (supplied from Livefoods Direct Ltd.). In all experiments, we provided females with either a fresh or an old mouse carcass. The fresh carcasses had been thawed on the same day they were provided to the beetles; the old carcasses had been thawed 7 days earlier and kept in the laboratory in containers filled with 1 cm of moist peat at 20 °C until provided to the beetles. The size of the mouse carcass was standardized (range 20–25 g) to control for potential effects of carcass size.
Larval Growth and Survival.
In experiments with unmanipulated broods, females were allowed to breed on either a fresh (n = 12) or an old (n = 12) carcass. When the brood dispersed from the carcass, we recorded the brood size and total brood mass. For all subsequent experiments, we generated standardized broods with 10 larvae (mean ± SD natural brood size: 21 ± 10 offspring, range 2–47 offspring) (36) after the protocol of refs. 14, 15, and 37. These broods always contained offspring of mixed maternity. We provided females with broods only after their own eggs had started hatching because females exhibit temporal kin recognition, killing larvae that arrive before their own eggs have started to hatch (38).
The standardized broods were randomly assigned to 1 of the 6 treatment groups: (i) fresh carcass and no care (n = 10), (ii) old carcass and no care (n = 11), (iii) fresh carcass and prehatch care (n = 11), (iv) old carcass and prehatch care (n = 12), (v) fresh carcass and prehatch and posthatch care (n = 11), and (vi) old carcass and prehatch and posthatch care (n = 12). Broods that received no care were placed on a carcass that had not been prepared by a female. We opened these carcasses with a sterile razor blade to allow the larvae access to the carcass. Broods that received prehatch care were placed on a carcass prepared by a female, but where she was removed immediately before we introduced the larvae. Broods that received prehatch and posthatch care were placed on a carcass prepared by a female, and where she was allowed to remain with the larvae until they dispersed from the carcass. We monitored effects of these treatments on growth by weighing all larvae at 24-h (±15 min) intervals starting at the time of hatching and ending when the larvae dispersed from the carcass, and on larval survivorship by recording the number of larvae that were alive at dispersal.
Parental and Larval Behavior.
We observed parental and larval behaviors for standardized broods of 10 larvae that were raised on either fresh (n = 19) or old (n = 19) carcasses. In all broods, the female remained with the larvae until they dispersed from the carcass. Observations were conducted under photographic red light using instantaneous scan sampling every 1 min for 30 min in accordance with our established protocol (14,15–16, 19, 36, 37). We did observations 24 and 48 h after the larvae were placed on the carcass. At each scan, we recorded the behavior of the female. A female was defined as providing care when she engaged in provision of food for the larvae, cleaning the carcass, processing carrion, guarding, and interacting with the larvae; we excluded grooming, walking, and standing still (36). Food provisioning was defined as mouth-to-mouth contact between the female and at least 1 larva. Cleaning the carcass was defined as adding secretions to or manipulating the surface of the carcass. We calculated the percentage time the female spent at each activity at each day of observation.
At each scan, we also counted the number of begging larvae in that brood. A larva was scored as begging when raising its head toward the female while waving the legs or touching the female (36). Larvae beg only when parents are in near proximity. Thus, we also recorded the number of scans that the female was near the larvae, defined as a distance corresponding to less than the width of her pronotum from the nearest offspring. We calculated the average percentage time spent begging by each larva in the brood as b = (Σ b/L) · (100/p), where Σb is the total number of begging events in a brood during an observation session, L is the number of larvae in a given brood, and p is the number of scans during which the female was near the larvae (37).
Parental Choice.
We provided females with a choice to breed on an old or a fresh carcass (n = 30). Each female was first mated in a separate box before she was released in the middle of a large experimental box (27 × 20 × 8 cm) filled with 1 cm of peat. Fresh and old carcasses were attached with dental floss to either side of the box to prevent the female from pulling them together. We recorded the position of the female if visible and photographed the carcasses to record activity at the 2 carcasses twice a day (at 9:00 a.m. and 4:30 pm). We considered the female to have found both carcasses if the female was visibly present at a carcass or if there had been a change in the position of the carcass. We recorded the location and number of eggs at either half of the box (i.e., near the fresh or old carcass). We also noted whether the female was providing care for the larvae at the fresh or the old carcass. In the analyses on female choice, we excluded females who failed to lay eggs (n = 6), whose eggs failed to hatch (n = 7), or who provided care on both carcasses (n = 2).
Acknowledgments.
We thank James H. Brown, Rees Kassen, Michelle Scott, Tom Sherratt, David Wilkinson, and anonymous reviewers for comments on the manuscript. The work was supported financially by Natural Environment Research Council Grant NE/G000131/1 and the University of Manchester.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Janzen DH. Why fruits rot, seeds mold, and meat spoils. Am Nat. 1977;111:691–713. [Google Scholar]
- 2.Kruuk H. Competition for food between vultures in East Africa. Ardea. 1967;55:171–192. [Google Scholar]
- 3.Kruuk H. The Spotted Hyena: A Study of Predation and Social Behavior. Chicago: Univ of Chicago Press; 1972. [Google Scholar]
- 4.Houston DC. Scavenging efficiency of turkey vultures in tropical forest. Condor. 1986;88:318–323. [Google Scholar]
- 5.DeVault TL, Rhodes OE, Jr, Shivik JA. Scavenging by vertebrates: Behavioural, ecological, and evolutionary perspectives on an important energy transfer pathway in terrestrial ecosystems. Oikos. 2003;102:225–234. [Google Scholar]
- 6.Burkepile DE, et al. Chemically mediated competition between microbes and animals: Microbes as consumers in food webs. Ecology. 2006;87:2821–2831. doi: 10.1890/0012-9658(2006)87[2821:cmcbma]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Shivik JA. Are vultures birds, and do snakes have venom, because of macro- and microscavenger conflict? BioScience. 2006;56:819–823. [Google Scholar]
- 8.Rohlfs M, Obmann B, Petersen R. Competition with filamentous fungi and its implication for a gregarious lifestyle in insects living on ephemeral resources. Ecol Entomol. 2005;30:556–563. [Google Scholar]
- 9.Dawkins R, Krebs JR. Arms races between and within species. Proc R Soc Lond B. 1979;205:489–511. doi: 10.1098/rspb.1979.0081. [DOI] [PubMed] [Google Scholar]
- 10.Eggert A-K, Müller JK. In: The Evolution of Social Behavior in Insects and Arachnids. Choe JC, Crespi BJ, editors. Cambridge, UK: Cambridge Univ Press; 1997. pp. 216–236. [Google Scholar]
- 11.Scott MP. The ecology and behavior of burying beetles. Ann Rev Entomol. 1998;43:595–618. doi: 10.1146/annurev.ento.43.1.595. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki S. Suppression of fungal development on carcasses by the burying beetle Nicrophorus quadripunctatus (Coleoptera: Silphidae) Entomol Sci. 2001;4:403–405. [Google Scholar]
- 13.Hoback WW, Bishop AA, Kroemer J, Scalzitti J, Shaffer JJ. Differences among antimicrobial properties of carrion beetle secretions reflect phylogeny and ecology. J Chem Ecol. 2004;30:719–729. doi: 10.1023/b:joec.0000028427.53141.41. [DOI] [PubMed] [Google Scholar]
- 14.Smiseth PT, Lennox L, Moore AJ. Interaction between parental care and sibling competition: Parents enhance offspring growth and exacerbate sibling competition. Evolution. 2007;61:2331–2339. doi: 10.1111/j.1558-5646.2007.00192.x. [DOI] [PubMed] [Google Scholar]
- 15.Smiseth PT, Ward RSJ, Moore AJ. Parents influence asymmetric sibling competition: Experimental evidence with partially dependent young. Ecology. 2007;88:3174–3182. doi: 10.1890/06-1992.1. [DOI] [PubMed] [Google Scholar]
- 16.Lock JE, Smiseth PT, Moore AJ. Selection, inheritance, and the evolution of parent-offspring interactions. Am Nat. 2004;164:13–24. doi: 10.1086/421444. [DOI] [PubMed] [Google Scholar]
- 17.Smith RJ. Effect of larval body size on overwinter survival and emerging adult size in the burying beetle, Nicrophorus investigator. Can J Zool. 2002;80:1588–1593. [Google Scholar]
- 18.Müller JK, Eggert A-K, Dressell J. Intraspecific brood parasitism in the burying beetle Necrophorus vespilloides (Coleoptera: Silphidae) Anim Behav. 1990;40:491–499. [Google Scholar]
- 19.Smiseth PT, Moore AJ. Signalling of hunger when offspring forage by both begging and self-feeding. Anim Behav. 2004;67:1083–1088. [Google Scholar]
- 20.Allcroft R. In: Mycotoxins in Foodstuffs. Wogan GN, editor. Cambridge, MA: MIT Press; 1965. pp. 153–162. [Google Scholar]
- 21.Rosen MN. In: Infectious and Parasitic Diseases of Wild Birds. Davis JW, et al., editors. Ames, IA: Iowa State Univ Press; 1971. pp. 100–117. [Google Scholar]
- 22.Selva N, Jedrzejewska B, Jedrzejewski W, Wajrak A. Factors affecting carcass use by a guild of scavengers in European temperate woodland. Can J Zool. 2005;83:1590–1601. [Google Scholar]
- 23.Wilson DS, Knollenberg WG. Food discrimination and ovarian development in burying beetles (Coleoptera: Silphidae: Nicrophorus) Ann Entomol Soc Am. 1984;77:165–170. [Google Scholar]
- 24.Eggert A-K, Reinking M, Müller JK. Parental care improves offspring survival and growth in burying beetles. Anim Behav. 1998;55:97–107. doi: 10.1006/anbe.1997.0588. [DOI] [PubMed] [Google Scholar]
- 25.Clutton-Brock TH. The Evolution of Parental Care. Princeton: Princeton Univ Press; 1991. [Google Scholar]
- 26.Wilson EO. Sociobiology: The New Synthesis. Cambridge, MA: Harvard Univ Press; 1975. [Google Scholar]
- 27.Tallamy DW. Insect parental care. BioScience. 1984;34:20–24. [Google Scholar]
- 28.Tallamy DW, Wood TK. Convergence patterns in subsocial insects. Ann Rev Entomol. 1984;31:369–390. [Google Scholar]
- 29.Kellerman GD, Waterman NG, Scharfenberger LF. Demonstration in vitro of post-mortem bacterial transmigration. Amer J Clin Pathol. 1976;66:911–915. doi: 10.1093/ajcp/66.5.911. [DOI] [PubMed] [Google Scholar]
- 30.Gill CO. A review: Intrinsic bacteria in meat. J Appl Bacteriol. 1979;47:367–378. doi: 10.1111/j.1365-2672.1979.tb01196.x. [DOI] [PubMed] [Google Scholar]
- 31.Castle KT, et al. Susceptibility of the Siberian polecat to subcutaneous and oral Yersinia pestis exposure. J Wildl Dis. 2001;37:746–754. doi: 10.7589/0090-3558-37.4.746. [DOI] [PubMed] [Google Scholar]
- 32.Harrison TM, Harrison SH, Rumbeiha WK, Sikarskie J, McClean M. Surveillance for selected bacterial and toxicologic contaminants in donated carcass meat fed to carnivores. J Zool Wildl Med. 2006;37:102–107. doi: 10.1638/05-022.1. [DOI] [PubMed] [Google Scholar]
- 33.Wild MA, Shenk TM, Spraker TR. Plague as a mortality factor in Canada lynx (Lynx canadensis) reintroduced to Colorado. J Wildl Dis. 2006;42:646–650. doi: 10.7589/0090-3558-42.3.646. [DOI] [PubMed] [Google Scholar]
- 34.Sherratt TN, Wilkinson DM, Bain RS. Why fruits rot, seeds mold, and meat spoils: A reappraisal. Ecol Model. 2006;192:619–626. [Google Scholar]
- 35.Smiseth PT, Ward RSJ, Moore AJ. Asynchronous hatching in Nicrophorus vespilloides, an insect in which parents provide food for their offspring. Funct Ecol. 2006;20:151–156. [Google Scholar]
- 36.Smiseth PT, Moore AJ. Does resource availability affect offspring begging and parental provisioning in a partially begging species? Anim Behav. 2002;63:577–585. [Google Scholar]
- 37.Smiseth PT, Darwell CT, Moore AJ. Partial begging: An empirical model for the early evolution of offspring signalling. Proc R Soc Lond B. 2003;270:1773–1777. doi: 10.1098/rspb.2003.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Müller JK, Eggert A-K. Time-dependent shifts between infanticidal and parental behavior in female burying beetles: A mechanism of indirect mother-offspring recognition. Behav Ecol Sociobiol. 1990;27:11–16. [Google Scholar]