Abstract
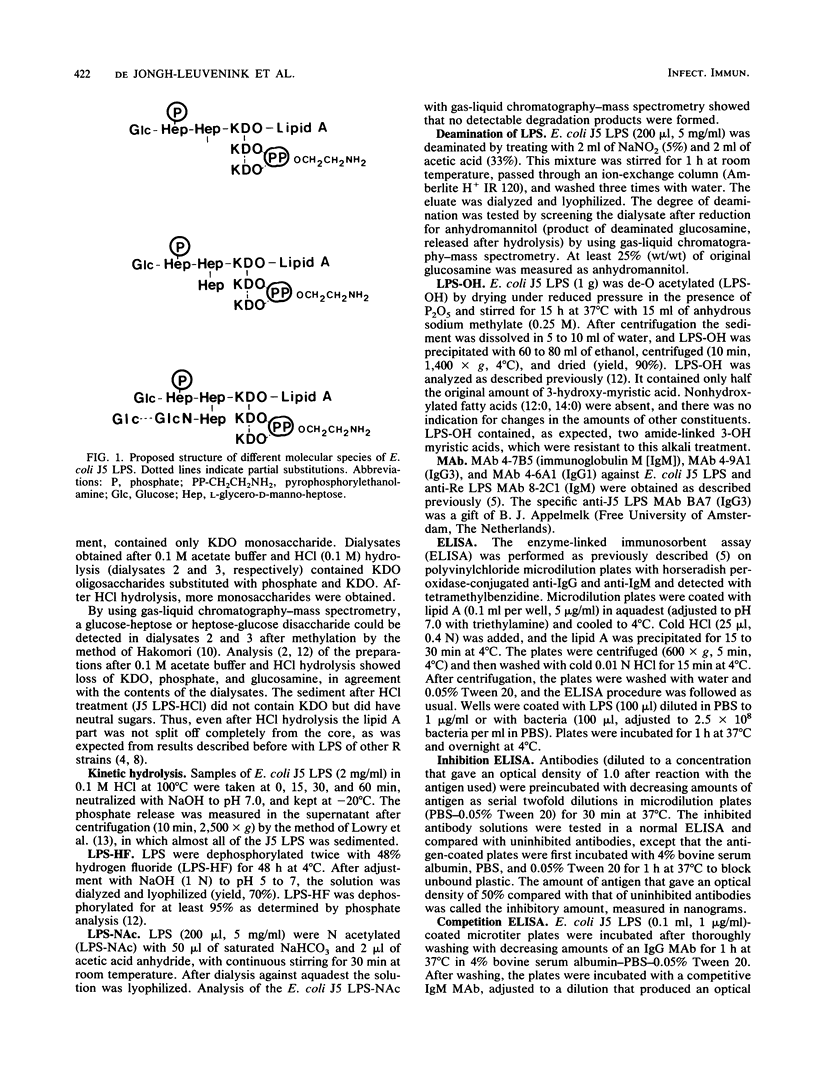
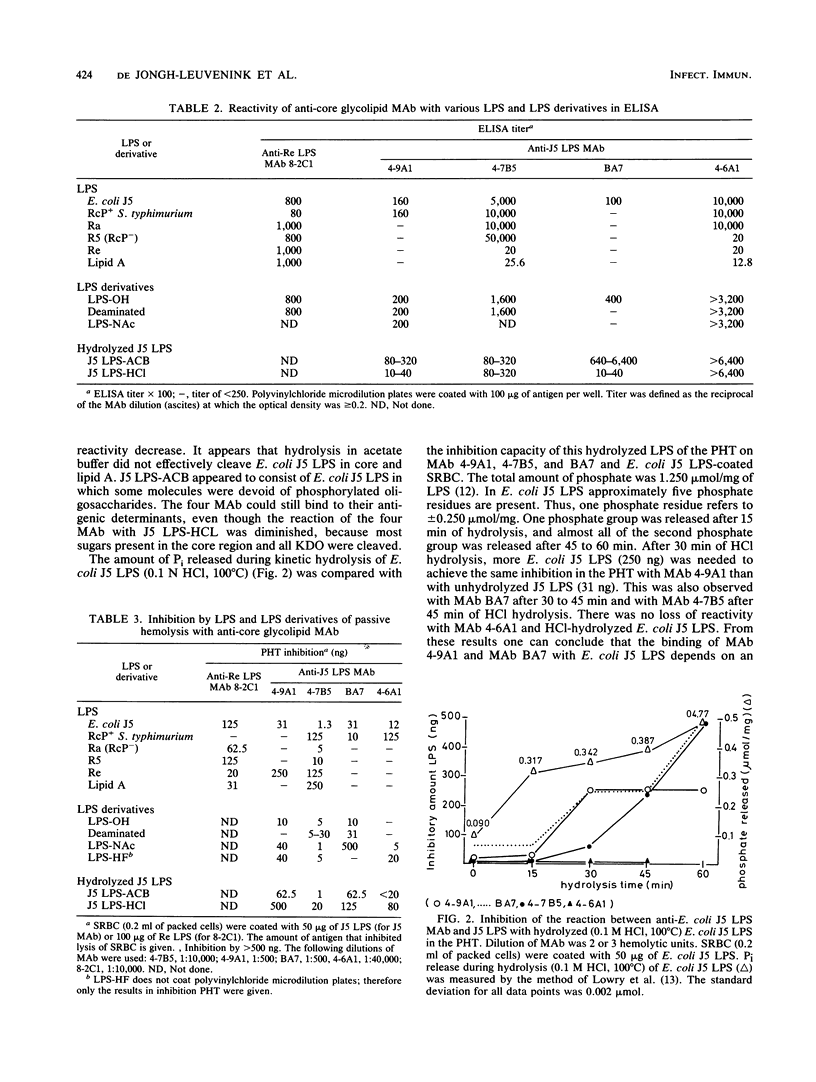
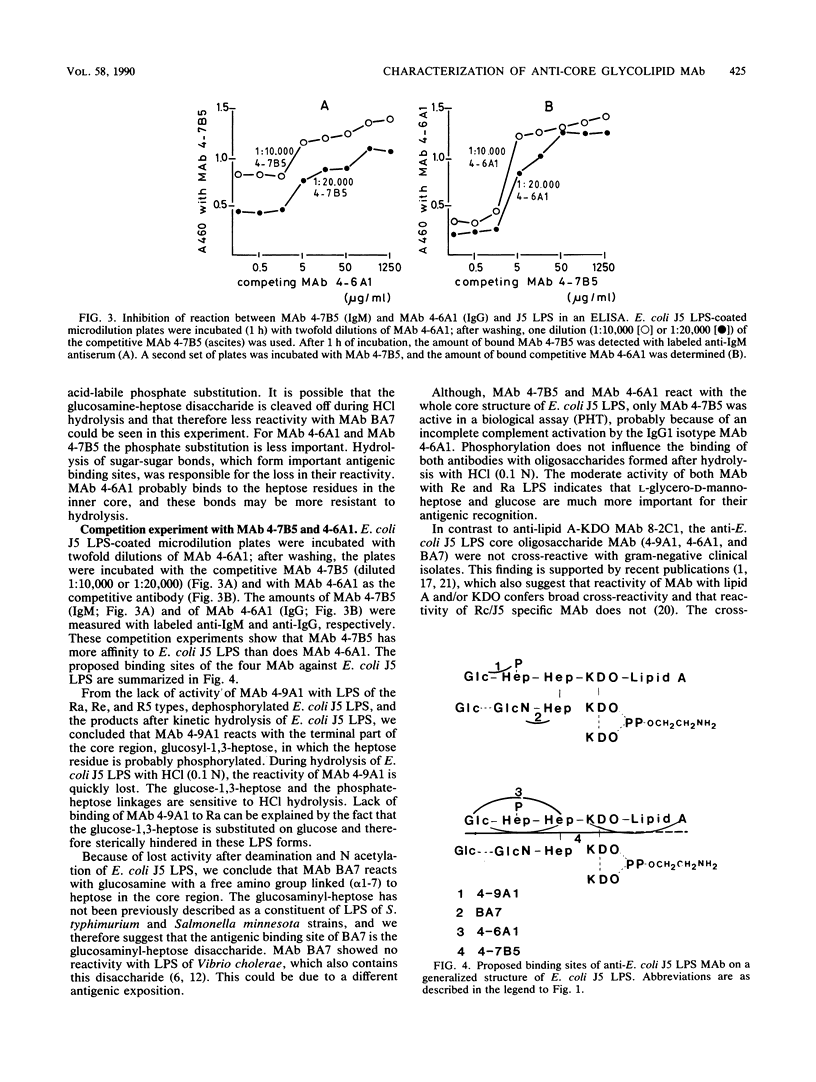
Five anti-core glycolipid monoclonal antibodies (MAb) (four against Escherichia coli J5 lipopolysaccharide [LPS] and one against the Re core glycolipid of Salmonella typhimurium) were characterized using LPS from several rough and smooth strains and derivatives of E. coli J5 LPS, obtained by N acetylation and hydrolysis. The MAb against E. coli J5 were not only weakly cross-reactive with clinical isolates, whereas the anti-Re MAb was highly cross-reactive. The MAb differed in their reaction pattern with E. coli J5 LPS. MAb 4-7B5 (immunoglobulin M) and MAb 4-6A1 (immunoglobulin G1) cross-reacted with LPS of Salmonella minnesota R5 and S. typhimurium Ra and Rc and little with Re and lipid A. The dominant binding site of these MAb was located in the glucose-heptose-heptose region and was independent of phosphate substitution. The MAb 4-9A1 reacted with the terminal part of the core region (glucose-heptose) and was dependent on phosphate substitution of the LPS. The MAb BA7 (immunoglobulin G3) was E. coli J5 LPS specific and reacted with the glucosaminyl-heptose disaccharide. Antibody 8-2C1 was directed against the common parts of LPS, 3-deoxy-D-manno-octulosonic acid, and lipid A, which are not (or only weakly) recognized by the four anti-J5 LPS MAb. Thus, MAb that are not cross-reactive can be directed against at least three different antigenic determinants present on the core oligosaccharide of E. coli J5 LPS.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogard W. C., Jr, Dunn D. L., Abernethy K., Kilgarriff C., Kung P. C. Isolation and characterization of murine monoclonal antibodies specific for gram-negative bacterial lipopolysaccharide: association of cross-genus reactivity with lipid A specificity. Infect Immun. 1987 Apr;55(4):899–908. doi: 10.1128/iai.55.4.899-908.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade H., Galanos C. A method to detect 2-keto-3-deoxyoctanat and related compounds on pherograms and chromatograms. Anal Biochem. 1983 Jul 1;132(1):158–159. doi: 10.1016/0003-2697(83)90440-2. [DOI] [PubMed] [Google Scholar]
- Brade H., Galanos C. Common lipopolysaccharide specificity: new type of antigen residing in the inner core region of S- and R-form lipopolysaccharides from different families of gram-negative bacteria. Infect Immun. 1983 Oct;42(1):250–256. doi: 10.1128/iai.42.1.250-256.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade H., Moll H., Rietschel E. T. Structural investigations on the inner core region of lipopolysaccharides from Salmonella minnesota rough mutants. Biomed Mass Spectrom. 1985 Oct;12(10):602–609. doi: 10.1002/bms.1200121007. [DOI] [PubMed] [Google Scholar]
- Fuller N. A., Wu M., Wilkinson R. G., Heath E. C. The biosynthesis of cell wall lipopolysaccharide in Escherichia coli. VII. Characterization of heterogeneous "core" oligosaccharide structures. J Biol Chem. 1973 Nov 25;248(22):7938–7950. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Gigliotti F., Shenep J. L. Failure of monoclonal antibodies to core glycolipid to bind intact smooth strains of Escherichia coli. J Infect Dis. 1985 Jun;151(6):1005–1011. doi: 10.1093/infdis/151.6.1005. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Kaca W., de Jongh-Leuvenink J., Zähringer U., Rietschel E. T., Brade H., Verhoef J., Sinnwell V. Isolation and chemical analysis of 7-O-(2-amino-2-deoxy-alpha-D-glucopyranosyl)-L-glycero-D-manno-heptose as a constituent of the lipopolysaccharides of the UDP-galactose epimerase-less mutant J-5 of Escherichia coli and Vibrio cholerae. Carbohydr Res. 1988 Aug 15;179:289–299. doi: 10.1016/0008-6215(88)84125-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Miner K. M., Manyak C. L., Williams E., Jackson J., Jewell M., Gammon M. T., Ehrenfreund C., Hayes E., Callahan L. T., 3rd, Zweerink H. Characterization of murine monoclonal antibodies to Escherichia coli J5. Infect Immun. 1986 Apr;52(1):56–62. doi: 10.1128/iai.52.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutharia L. M., Crockford G., Bogard W. C., Jr, Hancock R. E. Monoclonal antibodies specific for Escherichia coli J5 lipopolysaccharide: cross-reaction with other gram-negative bacterial species. Infect Immun. 1984 Sep;45(3):631–636. doi: 10.1128/iai.45.3.631-636.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles M. J., Niswander C. A. Mouse monoclonal antibodies reactive with J5 lipopolysaccharide exhibit extensive serological cross-reactivity with a variety of gram-negative bacteria. Infect Immun. 1984 Dec;46(3):677–681. doi: 10.1128/iai.46.3.677-681.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M., Raubitschek A. A., Larrick J. W. Human monoclonal antibodies that recognize conserved epitopes in the core-lipid A region of lipopolysaccharides. J Clin Invest. 1987 May;79(5):1421–1430. doi: 10.1172/JCI112970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakulramrung R., Domingue G. J. Cross-reactive immunoprotective antibodies to Escherichia coli O111 rough mutant J5. J Infect Dis. 1985 Jun;151(6):995–1004. doi: 10.1093/infdis/151.6.995. [DOI] [PubMed] [Google Scholar]
- Schwartzer T. A., Alcid D. V., Numsuwan V., Gocke D. J. Immunochemical specificity of cross-reactive antibodies to lipopolysaccharide from Escherichia coli J5. J Infect Dis. 1987 May;155(5):1076–1076. doi: 10.1093/infdis/155.5.1076. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Teng N. N., Kaplan H. S., Hebert J. M., Moore C., Douglas H., Wunderlich A., Braude A. I. Protection against gram-negative bacteremia and endotoxemia with human monoclonal IgM antibodies. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1790–1794. doi: 10.1073/pnas.82.6.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E. J., Douglas H., Sherman J. E., Davis C. E., Braude A. I. Treatment of E. coli and klebsiella bacteremia in agranulocytic animals with antiserum to a UDP-gal epimerase-deficient mutant. J Immunol. 1973 Aug;111(2):433–438. [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]
- de Jongh-Leuvenink J., Bouter A. S., Marcelis J. H., Schellekens J., Verhoef J. Cross-reactivity of monoclonal antibodies against lipopolysaccharides of gram-negative bacteria. Eur J Clin Microbiol. 1986 Apr;5(2):148–151. doi: 10.1007/BF02013970. [DOI] [PubMed] [Google Scholar]


