Abstract
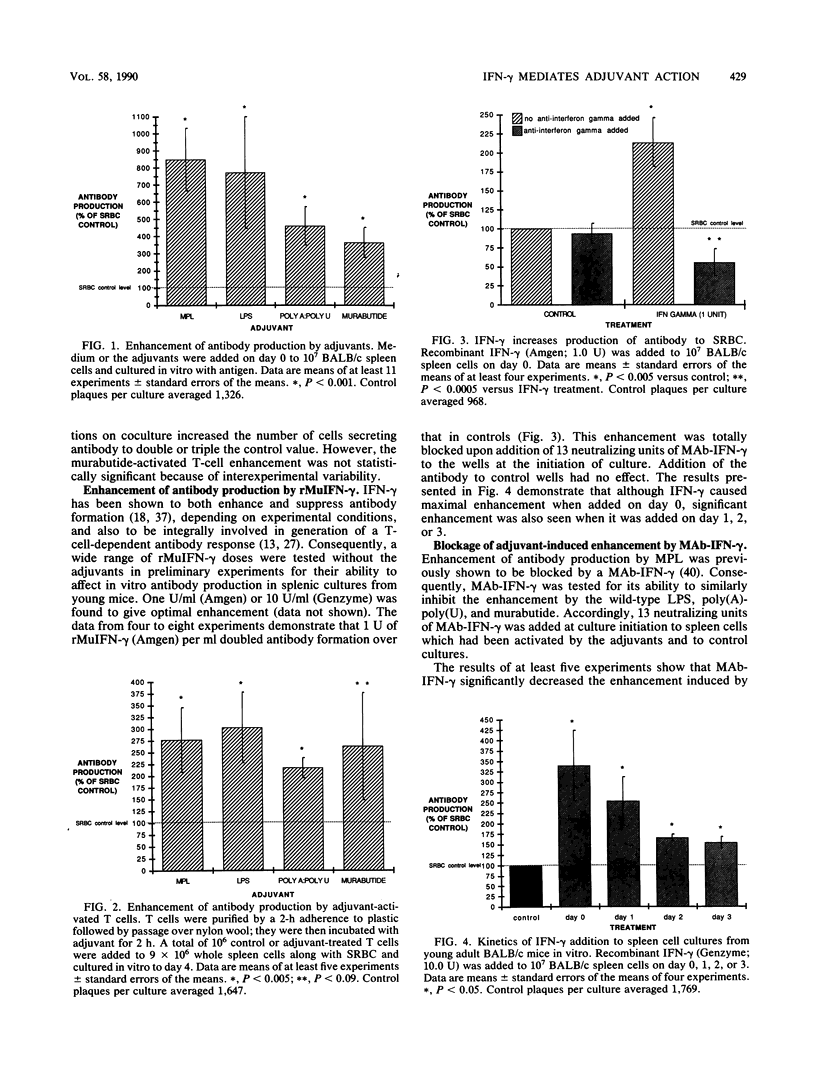
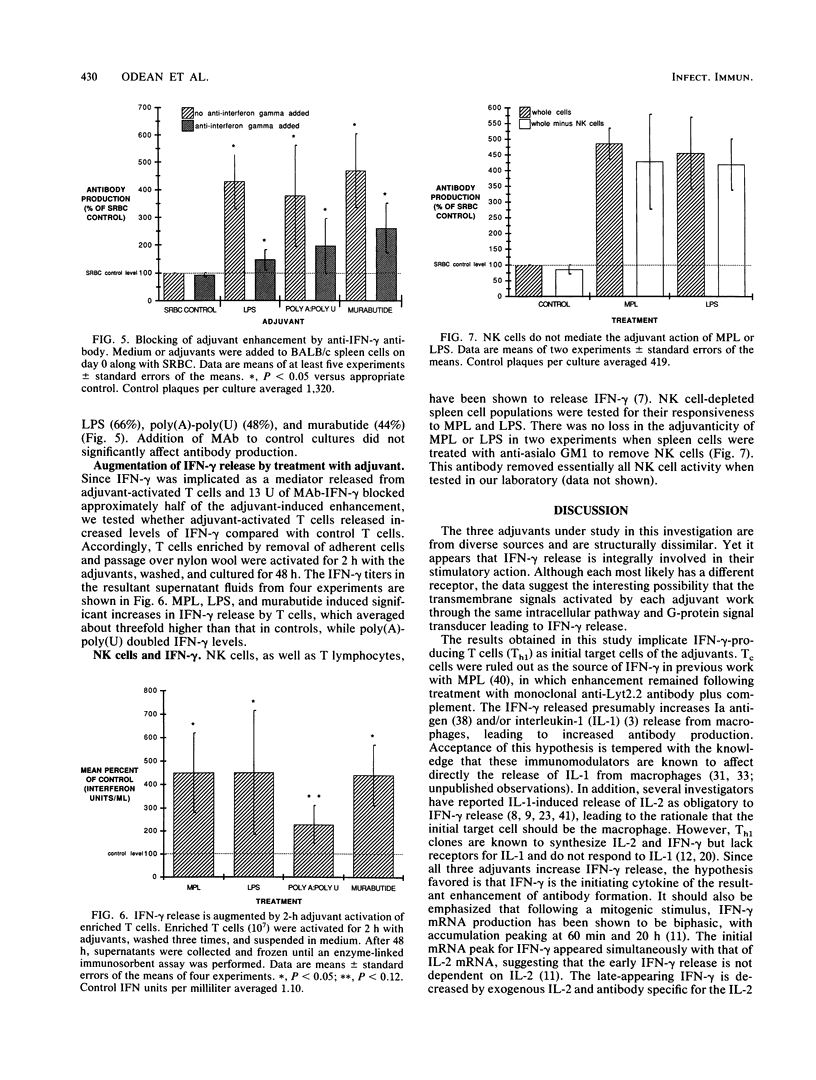
In a previous study the adjuvant action of a monophosphoryl lipid A, a nontoxic derivative of endotoxic lipopolysaccharide (LPS), was found to be negated by a monoclonal anti-gamma interferon (anti-IFN-gamma) antibody. The present investigation centered on three other adjuvants of diverse microbial origins, testing for their capacity to affect the release of IFN-gamma as an explanation for their antibody-enhancing action. The adjuvant action of each of the three, a wild-type LPS, synthetic poly(A)-poly(U) complexes, and a synthetic muramyl dipeptide, n-acetylmuramyl-L-alanyl-D-glutaminyl-n-butyl ester (murabutide), was transferable by adjuvant-stimulated T cells to normal spleen cells on coculture. Supernatant fluids from these T cells contained increased levels of IFN-gamma. Addition of a monoclonal anti-IFN-gamma antibody to adjuvant-stimulated spleen cell cultures reduced the adjuvant action by approximately one-half. Removal of natural killer cells from spleen cell populations prior to culture with antigen had no effect on the enhancement induced by LPS and monophosphoryl lipid A. It was concluded that the enhancement induced by the adjuvants LPS, poly(A)-poly(U), and murabutide is mediated in part by their action on T cells resulting in release of IFN-gamma suggesting activation of a common transmembrane signal.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abruzzo L. V., Rowley D. A. Homeostasis of the antibody response: immunoregulation by NK cells. Science. 1983 Nov 11;222(4624):581–585. doi: 10.1126/science.6685343. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Davies A. J. Requirement of thymus-dependent lymphocytes for potentiation by adjuvants of antibody formation. Nature. 1971 Oct 1;233(5318):330–332. doi: 10.1038/233330a0. [DOI] [PubMed] [Google Scholar]
- Arenzana-Seisdedos F., Virelizier J. L., Fiers W. Interferons as macrophage-activating factors. III. Preferential effects of interferon-gamma on the interleukin 1 secretory potential of fresh or aged human monocytes. J Immunol. 1985 Apr;134(4):2444–2448. [PubMed] [Google Scholar]
- Baker P. J., Hiernaux J. R., Fauntleroy M. B., Prescott B., Cantrell J. L., Rudbach J. A. Inactivation of suppressor T-cell activity by nontoxic monophosphoryl lipid A. Infect Immun. 1988 May;56(5):1076–1083. doi: 10.1128/iai.56.5.1076-1083.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick P. H., Johnson A. G. Poly A:U-induced secretion of T-lymphocyte helper factors. Scand J Immunol. 1977;6(11):1133–1144. doi: 10.1111/j.1365-3083.1977.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Chedid L. A., Parant M. A., Audibert F. M., Riveau G. J., Parant F. J., Lederer E., Choay J. P., Lefrancier P. L. Biological activity of a new synthetic muramyl peptide adjuvant devoid of pyrogenicity. Infect Immun. 1982 Feb;35(2):417–424. doi: 10.1128/iai.35.2.417-424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. B., Cline M. J., Merigan T. C. PPD-stimulated interferon: in vitro macrophage-lymphocyte interaction in the production of a mediator of cellular immunity. Cell Immunol. 1971 Dec;2(6):602–613. doi: 10.1016/0008-8749(71)90008-6. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Johnson H. M., Farrar J. J. Regulation of the production of immune interferon and cytotoxic T lymphocytes by interleukin 2. J Immunol. 1981 Mar;126(3):1120–1125. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Grabstein K., Dower S., Gillis S., Urdal D., Larsen A. Expression of interleukin 2, interferon-gamma, and the IL 2 receptor by human peripheral blood lymphocytes. J Immunol. 1986 Jun 15;136(12):4503–4508. [PubMed] [Google Scholar]
- Greenbaum L. A., Horowitz J. B., Woods A., Pasqualini T., Reich E. P., Bottomly K. Autocrine growth of CD4+ T cells. Differential effects of IL-1 on helper and inflammatory T cells. J Immunol. 1988 Mar 1;140(5):1555–1560. [PubMed] [Google Scholar]
- Helman S. W., Wallace J. H. Differential regulation of the immune response to SRBC by monoclonal antibodies to interferon-gamma. Proc Soc Exp Biol Med. 1989 May;191(1):55–59. doi: 10.3181/00379727-191-42889. [DOI] [PubMed] [Google Scholar]
- Holda J. H., Maier T., Claman H. N. Evidence that IFN-gamma is responsible for natural suppressor activity in GVHD spleen and normal bone marrow. Transplantation. 1988 Apr;45(4):772–777. doi: 10.1097/00007890-198804000-00021. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Rivière Y., Montagnier L., Michelson M., Lacour J., Lacour F. Enhancement of interferon-mediated protein kinase in mouse and human plasma in response to treatment with polyadenylic-polyuridylic acid (poly A:poly U). J Interferon Res. 1982;2(2):209–215. doi: 10.1089/jir.1982.2.209. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Baron S. The nature of the suppressive effect of interferon and interferon inducers on the in vitro immune response. Cell Immunol. 1976 Jul;25(1):106–115. doi: 10.1016/0008-8749(76)90100-3. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Torres B. A. Recombinant mouse interferon-gamma regulation of antibody production. Infect Immun. 1983 Aug;41(2):546–548. doi: 10.1128/iai.41.2.546-548.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kaye J., Gillis S., Mizel S. B., Shevach E. M., Malek T. R., Dinarello C. A., Lachman L. B., Janeway C. A., Jr Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol. 1984 Sep;133(3):1339–1345. [PubMed] [Google Scholar]
- Kennedy J. C., Axelrad M. A. An improved assay for haemolytic plaque-forming cells. Immunology. 1971 Feb;20(2):253–257. [PMC free article] [PubMed] [Google Scholar]
- Langford M. P., Weigent D. A., Chan T. S., Johnson H. M., Stanton G. J. Antibodies to the carboxyl terminus of mouse interferon-gamma neutralize its immunoregulatory and antiviral activities. J Interferon Res. 1987 Feb;7(1):95–101. doi: 10.1089/jir.1987.7.95. [DOI] [PubMed] [Google Scholar]
- Le J., Lin J. X., Henriksen-DeStefano D., Vilcek J. Bacterial lipopolysaccharide-induced interferon-gamma production: roles of interleukin 1 and interleukin 2. J Immunol. 1986 Jun 15;136(12):4525–4530. [PubMed] [Google Scholar]
- Leclerc C., Vogel F. R. Synthetic immunomodulators and synthetic vaccines. Crit Rev Ther Drug Carrier Syst. 1986;2(4):353–406. [PubMed] [Google Scholar]
- Lefrancier P., Derrien M., Jamet X., Choay J., Lederer E., Audibert F., Parant M., Parant F., Chedid L. Apyrogenic, adjuvant-active N-acetylmuramyl-dipeptides. J Med Chem. 1982 Jan;25(1):87–90. doi: 10.1021/jm00343a018. [DOI] [PubMed] [Google Scholar]
- Lei M. G., Morrison D. C. Specific endotoxic lipopolysaccharide-binding proteins on murine splenocytes. I. Detection of lipopolysaccharide-binding sites on splenocytes and splenocyte subpopulations. J Immunol. 1988 Aug 1;141(3):996–1005. [PubMed] [Google Scholar]
- Leibson H. J., Gefter M., Zlotnik A., Marrack P., Kappler J. W. Role of gamma-interferon in antibody-producing responses. 1984 Jun 28-Jul 4Nature. 309(5971):799–801. doi: 10.1038/309799a0. [DOI] [PubMed] [Google Scholar]
- Liebson H. J., Marrack P., Kappler J. B cell helper factors. II. Synergy among three helper factors in the response of T cell- and macrophage-depleted B cells. J Immunol. 1982 Oct;129(4):1398–1402. [PubMed] [Google Scholar]
- McGhee J. R., Farrar J. J., Michalek S. M., Mergenhagen S. E., Rosenstreich D. L. Cellular requirements for lipopolysaccharide adjuvanticity. A role for both T lymphocytes and macrophages for in vitro responses to particulate antigens. J Exp Med. 1979 Apr 1;149(4):793–807. doi: 10.1084/jem.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Nakano M., Uchiyama T., Saito K. Adjuvant effect of endotoxin; antibody response to sheep erythrocytes in mice after transfer of syngeneic lymphoid cells treated with bacterial lipopolysaccharide in vitro. J Immunol. 1973 Feb;110(2):408–413. [PubMed] [Google Scholar]
- Oppenheim J. J., Togawa A., Chedid L., Mizel S. Components of mycobacteria and muramyl dipeptide with adjuvant activity induce lymphocyte activating factor. Cell Immunol. 1980 Mar 1;50(1):71–81. doi: 10.1016/0008-8749(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J Biol Chem. 1982 Oct 10;257(19):11808–11815. [PubMed] [Google Scholar]
- Shalaby M. R., Weck P. K., Rinderknecht E., Harkins R. N., Frane J. W., Ross M. J. Effects of bacteria-produced human alpha, beta, and gamma interferons on in vitro immune functions. Cell Immunol. 1984 Apr 1;84(2):380–392. doi: 10.1016/0008-8749(84)90110-2. [DOI] [PubMed] [Google Scholar]
- Sidman C. L., Marshall J. D., Shultz L. D., Gray P. W., Johnson H. M. Gamma-interferon is one of several direct B cell-maturing lymphokines. 1984 Jun 28-Jul 4Nature. 309(5971):801–804. doi: 10.1038/309801a0. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld G., Mandel A. D., Merigan T. C. Time and dosage dependence of immunoenhancement by murine type II interferon preparations. Cell Immunol. 1978 Oct;40(2):285–293. doi: 10.1016/0008-8749(78)90336-2. [DOI] [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Johnson H. M., Oppenheim J. J. Regulation of murine macrophage Ia antigen expression by a lymphokine with immune interferon activity. J Exp Med. 1982 Dec 1;156(6):1780–1793. doi: 10.1084/jem.156.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Ribi E., Cantrell J. L. Isolation of a nontoxic lipid A fraction containing tumor regression activity. Cancer Res. 1981 Jul;41(7):2654–2657. [PubMed] [Google Scholar]
- Tomai M. A., Johnson A. G. T cell and interferon-gamma involvement in the adjuvant action of a detoxified endotoxin. J Biol Response Mod. 1989 Dec;8(6):625–643. [PubMed] [Google Scholar]
- Torres B. A., Farrar W. L., Johnson H. M. Interleukin 2 regulates immune interferon (IFN gamma) production by normal and suppressor cell cultures. J Immunol. 1982 May;128(5):2217–2219. [PubMed] [Google Scholar]
- Vilcek J., Henriksen-Destefano D., Siegel D., Klion A., Robb R. J., Le J. Regulation of IFN-gamma induction in human peripheral blood cells by exogenous and endogenously produced interleukin 2. J Immunol. 1985 Sep;135(3):1851–1856. [PubMed] [Google Scholar]
- Vogel S. N., Hilfiker M. L., Caulfield M. J. Endotoxin-induced T lymphocyte proliferation. J Immunol. 1983 Apr;130(4):1774–1779. [PubMed] [Google Scholar]



