Abstract
Neural stem/progenitor cells (NSCs/NPCs) give rise to neurons, astrocytes, and oligodendrocytes. It has become apparent that intracellular epigenetic modification including DNA methylation, in concert with extracellular cues such as cytokine signaling, is deeply involved in fate specification of NSCs/NPCs by defining cell-type specific gene expression. However, it is still unclear how differentiated neural cells retain their specific attributes by repressing cellular properties characteristic of other lineages. In previous work we have shown that methyl-CpG binding protein transcriptional repressors (MBDs), which are expressed predominantly in neurons in the central nervous system, inhibit astrocyte-specific gene expression by binding to highly methylated regions of their target genes. Here we report that oligodendrocytes, which do not express MBDs, can transdifferentiate into astrocytes both in vitro (cytokine stimulation) and in vivo (ischemic injury) through the activation of the JAK/STAT signaling pathway. These findings suggest that differentiation plasticity in neural cells is regulated by cell-intrinsic epigenetic mechanisms in collaboration with ambient cell-extrinsic cues.
Keywords: glia, JAK/STAT
The mammalian cerebral cortex originates from neural stem/neural progenitor cells (NSCs/NPCs), which self-renew and give rise to the three major brain cell types: neurons, astrocytes, and oligodendrocytes (1). The fate of NSCs/NPCs in the developing brain is believed to be determined by external cues that involve various types of cytokines and internal cellular programs (2, 3).
Among the three NSC/NPC progenies, astrocyte differentiation from NSCs/NPCs is largely dependent on the activation of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway (4, 5). Impairment of astrocyte differentiation in gene knockout mice lacking leukemia inhibitory factor (LIF) (6), LIF receptor β (7), gp130 (8), and STAT3 (9) strongly suggests that the JAK-STAT signaling pathway plays a critical role for astrogliogenesis in the developing central nervous system (CNS).
Cell-intrinsic programs regulating fate determination of NSCs/NPCs include epigenetic modifications such as chromatin remodeling and DNA methylation. The cytosine residue in CpG dinucleotides of vertebrate genomes is a well-known target for DNA methylation, leading to suppression of methylated genes. Establishment of the proper gene methylation patterns is essential for inactivation of the X-chromosome, genomic imprinting, and normal development. Consistently, abnormalities in DNA methylation are associated with tumorigenesis (10) and with several neurological disorders including Rett (RTT), immunodeficiency-centromeric instability-facial anomalies (ICF), fragile-X, and α-thalassemia mental retardation (ATRX) syndromes (11).
Mechanistically, DNA methylation is considered to elicit its effects by interfering with binding of transcriptional factors to their cognate recognition sequences (12) or by creating a binding site for members of a transcriptional repressor family, the methyl-CpG binding proteins (MBDs), that recognize methylated CpG sequences (13).
During development, NSCs/NPCs change their differentiation potential via alternation of epigenetic modification. In early neurogenic period, the fetal NSCs/NPCs are unable to generate astrocytes even when stimulated with known astrocyte-inducing cytokines such as LIF, which activate the JAK/STAT pathway, due to DNA hypermethylation in the promoter regions of astrocytic genes (14, 15). The promoter regions become demethylated as gestation proceeds, conferring astrocyte differentiation potential to NSCs/NPCs in response to astrocyte-inducing cytokines. After CNS development is complete, differentiated cells may still be exposed to a variety of stimuli including physiological and pathological stress: for example, it has been reported that focal cerebral ischemia triggers JAK/STAT activation (16). Meanwhile, it remains unclear how differentiated cells maintain their traits or how their differentiation plasticity is regulated under normal or pathological conditions. There should at least be a mechanism of escaping from pathologic astrogliogenic stimuli on non astrocytic lineages.
In this study, we demonstrate that expression of MBDs is highly relevant to differentiation plasticity against astrocytic stimuli in adult neural cells. Oligodendrocytes which are devoid of MBDs can respond to JAK-STAT pathway activators and differentiate into astrocytes in vitro. Ectopic expression of MeCP2, one of the MBDs, in oligodendrocytes can suppress astrocytic differentiation even in the presence of astrocyte-inducing cytokines. By means of oligodendrocyte-fate tracing system, we further show that oligodendrocytes can convert into astrocytic lineages after brain ischemic injury. We thus provide a model in which in vivo unexpected cellular differentiation plasticity could contribute to the pathogenesis of injuries in the CNS.
Results
Oligodendrocytes but Not Neurons Have the Capacity to Respond to Astrogliogenic Stimulation.
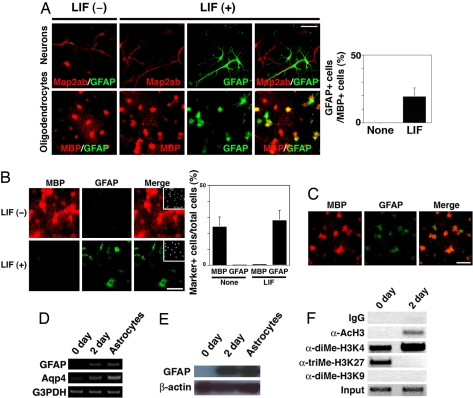
As a first step toward unraveling the mechanisms regulating the cellular identity of differentiated neural cells, we sought to examine whether neurons and oligodendrocytes express a typical astrocytic marker, glial fibrillary acidic protein (GFAP), in response to LIF stimulation. We used adult hippocampus-derived multipotent NPCs (AHPs) to obtain each differentiated cell type (17). When neurons were incubated with LIF for 2 days, no cells were found to be simultaneously positive for both the neuronal marker microtubule-associated protein 2ab (Map2ab) and GFAP (Fig. 1A, Upper). In this experiment, GFAP-positive cells were most likely to have differentiated from AHPs in response to LIF stimulation. In contrast, about 20% of cells that were positive for a mature oligodendrocyte marker, myelin basic protein (MBP), also became positive for GFAP in the LIF-stimulated condition, demonstrating their differentiation plasticity (Fig. 1A, Lower and Right graph). We also observed GFAP expression in a significant number of cells that were positive for another oligodendrocyte marker, CNPase [supporting information (SI) Fig. S1]. When we extended culturing with LIF to 4 days, MBP expression almost disappeared and the cells instead became exclusively GFAP-positive (Fig. 1B). We obtained similar results using a different astrocyte marker, S100β, with MBP (Fig. S2), and another oligodendrocyte marker, RIP, with GFAP (not shown). These results suggest that oligodendrocytes were converted into GFAP-positive astrocytes. GFAP expression in MBP-positive oligodendrocytes was observed, in the presence of LIF, even after treatment with the DNA polymerase inhibitor aphidicolin to arrest cell division (Fig. 1C). By measuring BrdU uptake, we confirmed that the cells were indeed growth-arrested (not shown), which suggests that cell division is dispensable for oligodendrocyte-astrocyte (O-A) conversion.
Fig. 1.
Differentiation plasticity of oligodendrocytes. (A) AHP-derived neurons and oligodendrocytes were cultured for 2 days with or without LIF (50 ng/ml). Cells were then stained with sets of antibodies for Map2ab (Upper, red)/GFAP (Upper, green) and MBP (Bottom, red)/GFAP (Lower, green). (Scale bar: 50 μm.) The percentage of GFAP-positive cells in MBP-positive cells was quantified (Right). Data are mean ± SD. (B) Oligodendrocytes were cultured with or without LIF (50 ng/ml) for 4 days and subsequently stained for MBP (red) and GFAP (green). Insets: Hoechst nuclear staining of each field. (Scale bar: 50 μm.) Percentages of MBP- and GFAP-positive cells in total cells were quantified (Right). Data are mean ± SD. (C) GFAP-positive astrocytes appeared with LIF treatment for 2 days even when the cells were growth-arrested by aphidicolin (10 μg/ml). MBP (red), GFAP (green). (Scale bar: 50 μm.) (D) AHP-derived oligodendrocytes (0 day), oligodendrocytes incubated with LIF (50 ng/ml) for 2 days (2 day), and AHP-derived astrocytes were analyzed by RT-PCR using specific sets of primers against gfap, Aqp4 and G3PDH. (E) AHP-derived oligodendrocytes (0 day), oligodendrocytes incubated with LIF (50 ng/ml) for 2 days (2 day), and AHP-derived astrocytes were analyzed by Western blot using an antibody against GFAP. A band corresponding to the molecular weight of GFAP protein (∼50 kDa) was detected. (F) Differentiated oligodendrocytes (0 day) were incubated with LIF (50 ng/ml) for 2 days and then subjected to ChIP assay using control IgG, anti-diMe-H3K4, -diMe-H3K9, -triMe-H3K27 and -AcH3 antibodies. Co-immunoprecipitated gfap gene fragment (−18bp to + 513bp) was amplified by PCR with a specific set of primers. Nucleotide positions are those of GenBank accession number Z48978.
Oligodendrocyte-Astrocyte Conversion Is Accompanied by Epigenetic Modification Change.
We next asked whether a change in chromatin modifications around the gfap transcription initiation site in oligodendrocytes occurred during the O-A transition. As shown in Fig. 1D, transcription of gfap and of another astrocytic marker, aquaporin4 (Aqp4) (18), was upregulated, and immunoblot analysis (Fig. 1E) revealed that GFAP levels were also strikingly higher. Histone H3-lysine 4 (H3K4) methylation and H3 acetylation are consistent markers of transcriptionally active genes, whereas silent genes are marked by increased levels of H3K9 and H3K27 methylation (19). Chromatin immunoprecipitation (ChIP) experiments showed that H3K4 methylation and H3 acetylation increased during the O-A transition (Fig. 1F), in agreement with the immunocytochemical data, revealing that the chromatin status of gfap was altered toward the active state following LIF stimulation. We could not detect a distinct signal corresponding to H3K9 methylation, which may indicate that gfap transcription is poised but not actively suppressed in oligodendrocytes by H3K27 methylation (20). In support of this idea, H3K27 trimethylation on gfap was observed before LIF treatment (Fig. 1F, 0 day).
Oligodendrocytes Convert into Astrocytes in Vivo After Injury.
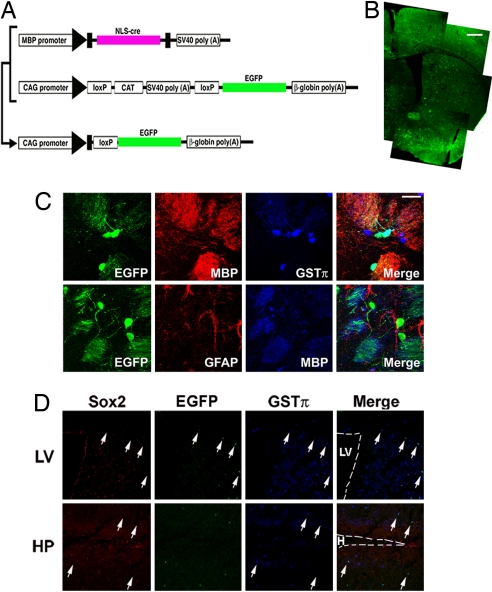
Given that O-A transition was observed in vitro when oligodendrocytes were stimulated by astrocyte-inducing cytokine, we then asked whether O-A conversion also occurs in vivo. To this end, we specifically labeled oligodendrocytes in vivo and traced their fates with the Cre-loxP recombination system (Fig. 2 A and B). We intercrossed two transgenic mouse lines: 1) MBP-Cre-tg (21), in which Cre recombinase is expressed under the control of the mbp promoter, and 2) CAG-CAT-EGFP-tg (22), in which EGFP expression is induced in Cre-expressing cells and their progeny under the control of the ubiquitous CAG promoter. Accordingly, in double-transgenic mice, once cells differentiate into MBP-expressing mature oligodendrocytes, they should sustain EGFP expression irrespective of what cell type they later become. We confirmed the previously determined specificity of the MBP promoter (21), and found that EGFP expression occurred exclusively in MBP-positive oligodendrocytes, but not in other cell types such as GFAP-positive astrocytes, in the adult brain of double-transgenic mice under normal conditions (Fig. 2C). The EGFP expression was not observed in a NSC/NPC marker Sox2-positive cells localized in the subventricular zone of the lateral ventricle or the subgranular zone of the hippocampus (Fig. 2D). EGFP was not expressed in cells positive for NG2, a marker for immature oligodendrocytes and/or glial progenitors (23) (data not shown). These results suggest that activation of the mbp promoter is confined to mature oligodendrocytes.
Fig. 2.
Oligodendrocyte-specific labeling with EGFP. (A) Schematic of transgenic constructs used to trace the lineage of oligodendrocytes. In double-transgenic mice harboring the upper two transgenes, Cre recombinase is expressed in MBP-expressing oligodendrocytes and excises the floxed CAT-SV40 poly(A) fragment, resulting in constitutive expression of EGFP under the control of the ubiquitous CAG promoter. (B) Distribution of EGFP-positive cells in the adult brain. (Scale bar: 500 mm.) (C) EGFP expression was confined to MBP- and GST-π-positive mature oligodendrocytes in the adult brain under normal conditions (Upper). Expression of EGFP and GFAP was mutually exclusive (Lower). (Scale bar: 20 μm.) (D) Expression of EGFP in neurogenic regions of the adult brain. Arrows indicate representative cells expressing EGFP and the oligodendrocyte marker GST-π. Sox2 expression was not observed in the EGFP-positive cells. LV, lateral ventricle; HP, hippocampus; H, hilus. (Scale bar: 100 mm.)
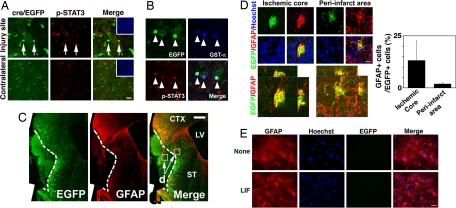
Because GFAP expression is dramatically induced by CNS injury (24), we subjected the double-transgenic mice to middle cerebral artery occlusion (MCAO) and traced the lineage of EGFP-positive cells. MCAO has been reported to activate the JAK/STAT pathway, as is the case with LIF stimulation (16). Consistent with the previous finding, we indeed detected STAT3 activation in oligodendrocytes after MCAO injury (Fig. 3A). Cells positive for both EGFP and phosphorylated STAT3 seemed to retain oligodendrocytic characteristics as judged by oligodendrocyte-specific marker expression 3 days after MCAO (Fig. 3B). Two weeks after surgery, we found that significant numbers of EGFP-positive cells became GFAP-positive astrocytes in and around the infarct area (Fig. 3 C and D), but not in unlesioned areas. Almost all other EGFP-positive/GFAP-negative cells still expressed MBP and appeared to retain oligodendrocytic characteristics (Fig. S3). We found no evidence for BrdU uptake in EGFP-positive cells after MCAO, probably because they did not divide during the O-A transition (Fig. S4). EGFP and GFAP double-positive cells were negative for cleaved caspase 3, indicating that they were not apoptotic (Fig. S5). Although one might argue that the mbp promoter was ectopically trans-activated in these GFAP-expressing astrocytes after injury, we could not detect EGFP expression in cultured astrocytes derived from adult MBP-cre/EGFP mice even when the cells were stimulated with LIF (Fig. 3E). Furthermore, the possibility that EGFP-positive cells de-differentiated into NSCs/NPCs is unlikely, because we could not detect ectopic or re-expression of the NSC/NPC neural marker Sox2 in these cells after injury (Fig. S6). Moreover, EGFP-positive cells also became GFAP-positive when the transgenic mice were subjected to another injury model, cold injury (Fig. S7), implying that the O-A transition occurs after diverse types of brain injury.
Fig. 3.
STAT activation and O-A conversion after ischemic injury. (A) Activation of the JAK/STAT pathway in EGFP-positive cells was evaluated by immunostaining using an antibody against phospo-STAT3 (p-STAT3) in the injured and contralateral sides of the striata 72 h after ischemic injury. Arrows indicate representative cells positive for both EGFP and p-STAT3. (Scale bar: 20 μm.) Insets: Hoechst nuclear staining of each field. (B) p-STAT3 was also detected in GSTπ-expressing EGFP-positive cells 72 h after injury (arrowheads). (Scale bar: 20 μm.) (C) A representative brain section stained with anti-GFP and -GFAP antibodies 2 weeks after MCAO surgery. Scale bar = 500 μm. CTX, cortex; CC, corpus callosum; LV, lateral ventricle; ST, striatum. (D) Higher magnification views of square areas in C. GFAP expression appeared in EGFP-expressing cells in the ischemic core (Left square arrowed in C, Upper Left) and peri-infarct (Right square arrowed in C, Upper Right) areas. Representative cells positive for both GFAP and EGFP are shown; Lower Right image in each group is a superimposition of the other three images. (Scale bar: 20 μm.) Three-dimensional digital images of each area are also shown (ischemic core area, Bottom Left; peri-infarct area, Bottom Right). Percentage of GFAP-positive cells in EGFP-positive cells was quantified for each area (graph). Data are mean ± SD. (E) Astrocytes prepared from adult MBP-Cre/EGFP transgenic mice were left untreated or treated with LIF for 4 days, and then stained for EGFP (green) and GFAP (red). (Scale bar: 50 μm.) EGFP expression was not induced even when the cells were stimulated with LIF.
Neuron-Specific Expression of Methyl CpG Binding Proteins (MBDs) in CNS.
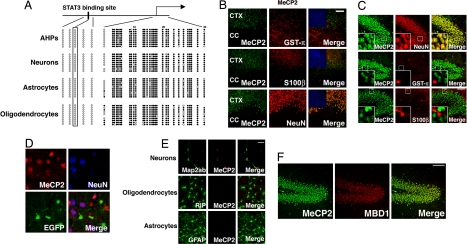
Because epigenetic modification is known to be critically involved in defining cell-typespecific gene expression, we hypothesized that an epigenetic mechanism could be responsible for the differentiation plasticity of these neural cells. We therefore examined the DNA methylation status of gfap in each neural cell type derived from adult AHPs by the bisulfite sequencing method (Fig. 4A) and found that the gfap promoter region around the STAT3 binding site was completely unmethylated, whereas the region around the transcriptional start site was extensively methylated, in all of the neural cell types. Next, we examined the expression of MeCP2, a member of the MBD family, because we have recently reported that MBDs play an important role in restricting astrocyte-specific gene expression in neurons that have differentiated from fetal NPCs (25). Representative brain sections stained with antibodies against MeCP2 and against markers of neurons (NeuN), oligodendrocytes (GST-π) and astrocytes (S100β) are shown in Fig. 4 B and C. In oligodendrocyte fate-tracing mice, as mentioned above, MeCP2 expression was not observed in oligodendrocytes that were labeled by EGFP (Fig. 4D). These results are consistent with previous reports that MBDs are expressed predominantly in neurons, and not in glial cells, in the CNS (26). Neurons, but not oligodendrocytes or astrocytes, that have differentiated from AHPs in vitro also express MeCP2 (Fig. 4E). As has been observed for fetal NSCs/NPCs and cells differentiated from them (25), we found that in all neural cell types derived from AHPs the region around the gfap transcription initiation site was extensively methylated (Fig. 4A), suggesting that this region is a target for MBD binding in cells expressing MBDs (26) (Fig. 4D) to suppress gfap expression. Furthermore, we also found that another MBD, MBD1, shows a quite similar distribution to MeCP2 in adult brain (Fig. 4F).
Fig. 4.
MeCP2 is predominantly expressed in neurons and suppresses astrocytic gene expression in oligodendrocytes when ectopically expressed. (A) Schematic representation of gfap locus (Upper). Methylation status of five CpG sites around the STAT3 binding site (black box), which is critical for LIF-induced activation of the gfap promoter, and of 30 CpG sites around the transcription initiation site (arrow) were examined for each cell type by bisulfite sequencing. Open and closed circles indicate unmethylated and methylated CpG sites, respectively. (B) MeCP2 expression (green) was examined in adult mouse brain sections. Neurons, oligodendrocytes, and astrocytes are identified with specific antibodies for NeuN (red, Upper middle), GST-π (red, center), and S100β (red, Lower middle), respectively. Staining of the hippocampal dentate gyrus region is shown. Insets: higher magnification views of square areas in each field. (Scale bar: 50 μm.) (C) MeCP2 is predominantly expressed in neurons. MeCP2 expression (green) was examined in the adult mouse cortex and corpus callosum. Oligodendrocytes, astrocytes, and neurons were identified with specific antibodies for GST-π (red, Upper rows), S100β (red, middle rows), and NeuN (red, Bottom rows), respectively. Insets: Hoechst nuclear images of each field. (Scale bar: 100 μm.) CTX, cortex; CC, corpus callosum. (D) EGFP-positive cells do not express MeCP2. MeCP2 expression (Upper Left) was observed in NeuN-positive (Upper Right) but not in EGFP-positive (Lower Left) cells. (E) MeCP2 (red) is expressed in Map2ab-positive neurons (Top, green), but not in RIP-positive oligodendrocytes (middle, green) or GFAP-positive astrocytes (Bottom, green) generated from AHPs in vitro. (Scale bar: 50 μm.) (F) Redundant expression of MBDs in vivo. Expression of MeCP2 (green, Left) and MBD1 (red, middle), another member of the MBD family, was examined in the adult hippocampal region. These two MBDs showed the same expression pattern (Right). (Scale bar: 100 μm.)
MeCP2 Is Sufficient to Restrict Astrocytic Gene Expression.
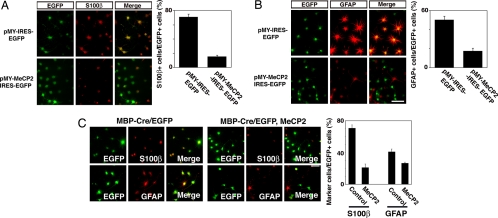
In light of the above findings, we examined the function of MeCP2 in terms of astrocytic differentiation of cells that are competent for LIF stimulation, i.e., NSCs/NPCs and oligodendrocytes. AHPs, which normally express GFAP in response to LIF, were transduced with recombinant retroviruses engineered to express EGFP (control) or both EGFP and MeCP2. In AHPs, the ectopic expression of MeCP2 led to suppression of astrocytic fate, consistent with our previous observation in fetal NSCs/NPCs (Fig. 5 A and B). Next, to examine the possibility that the O-A conversion potential of oligodendrocytes might be attributable to the lack of MBD expression, we transfected AHPs with MBP-Cre vector and control floxed neo or floxed neo-MeCP2-expressing vector together with floxed CAT-EGFP-expressing vector, and subsequently differentiated them into oligodendrocytes, then stimulated with LIF for 4 days. As shown in Fig. 5C, GFAP and S100β expression was clearly inhibited by the expression of MeCP2 in oligodendrocytes.
Fig. 5.
MeCP2 expression is sufficient for inhibition of astrocyte differentiation of NSCs/NPCs and oligodendrocytes. (A and B) AHPs were infected with recombinant retrovirus engineered to express only EGFP (pMY), or MeCP2 together with EGFP (pMY-MeCP2-IRES-EGFP), cultured with LIF for 4 days and subjected to immunostaining. GFP (A and B, green), S100β (A, red), GFAP (B, red). (Scale bar: 50 μm.) Percentages of S100β- and GFAP-positive cells in EGFP-positive cells were quantified (Upper and Lower graphs, respectively). Data are mean ± SD. (C) AHPs were transfected with MBP-Cre together with CAG-CAT-EGFP and CAG floxed neo, or with CAG-CAT-EGFP and CAG floxed neo-MeCP2, and were allowed to differentiate into oligodendrocytes for 4 days. The cells were then cultured with LIF for an additional 4 days, followed by immunocytochemical staining using antibodies against GFAP and S100β. (Scale bar: 50 μm.) Percentages of GFAP- and S100β-positive cells in EGFP-expressing cells were quantified (Right). Data are mean ± SD.
Discussion
It has become increasingly evident that a dynamic interplay between cellular extrinsic cues and cell intrinsic programs plays important roles in the fate determination of NSCs/NPCs. Among cell internal programs, epigenetic modification, especially DNA methylation, is important for acquisition of astrocyte-differentiation potential in NCSs/NPCs. In this work, we have shown that DNA methylation plays a critical role in restricting astrocytic property in neural cells in concert with MBDs. Notably, the astrocyte differentiation potential of oligodendrocytes may be attributable to loss of DNA methylation in the promoter regions of astrocytic genes and undetectable expression of MBDs. We have shown that oligodendrocytes derived from adult AHPs can respond to an astrocyte-inducing cytokine to become GFAP-positive astrocytes (Fig. 1). We further demonstrate that oligodendrocytes can also convert to GFAP-positive astrocytes after ischemic injury (Fig. 2), although because we have not examined functional maturation of the oligodendrocyte-derived astrocytes, this point will need to be addressed further in a future study.
Regarding cell plasticity, we have previously shown that even adult NSCs/NPCs retain substantial differentiation plasticity depending on the surrounding milieu (27). Ectopic expression of the proneural gene ascl (also known as mash1) induces oligodendrocyte differentiation of NSCs/NPCs located in one of the major neurogenic regions in the adult brain (the granular layer of the hippocampus), whereas it induces neuronal differentiation of NSCs/NPCs in another neurogenic region (the subventricular zone) and in in vitro culture conditions. Thus, it is becoming apparent that cells are more plastic than had previously been thought.
Our present study provides a molecular mechanism whereby cells can regulate their identity by repressing genes that are expressed in other lineages. Such an expression selectivity or exclusiveness of cell-type specific gene has been extensively examined in a series of studies of transcriptional repressor element1-silencing transcription factor (REST)/neuron-restrictive silencer factor (NRSF) (28). REST/NRSF suppresses neuronal gene expression in non-neural tissues to establish non-neuronal identity. Consistently REST/NRSF is expressed in non-neural tissues and dysfunction of REST/NRSF leads to ectopic neuronal gene expression in non-neural tissues. In contrast to the function of REST/NRSF, MBDs are expressed selectively in neurons in the CNS and repress glial genes (Figs. 4 and 5). We have previously shown by ChIP assay that MeCP2 binds to the highly methylated region of gfap in neurons that are expressing MeCP2 (25). MeCP2 deficiency in neurons may therefore lead to expression of genes that are normally astrocyte-specific. Indeed, many glial gene transcripts, including gfap, were found to be up-regulated in the brains of Rett syndrome patients with a mutation in mecp2 (29). However, no major phenotype related to cellular differentiation in MeCP2-deficient mice is known, probably due to functional redundancy and overlapping expression among MBDs (25) (Fig. 4F). To address this point more precisely, we must await future studies in mice with compound disruption of several MBD genes.
It has long been thought that, after CNS injury, oligodendrocytes can only die, which could explain subsequent demyelination (30). However, we have shown here that significant numbers of oligodendrocytes actually become GFAP-positive cells following injury, suggesting that O-A conversion may be in part responsible for postinjury demyelination; oligodendrocytes may instead provide a source of newly generated GFAP-positive astrocytes in damaged nervous systems in vivo. Although astrocytes form a glial scar and are considered to be detrimental for axonal regeneration in the injured CNS, it has been recently demonstrated that astrocytes also play a crucial role in wound healing and functional recovery in the subacute phase of spinal cord injury (31). During this phase, astrocytes migrate to compact the lesion, presumably excluding the inflammatory cells to prevent them from spreading in the parenchyma of the spinal cord. Assuming that astrocytes in the brain function as do those in the spinal cord, the oligodendrocyte-to-astrocyte conversion may contribute to an increase in the number of astrocytes after injury in the brain as well. We have also proposed an epigenetic mechanism to explain this differentiation plasticity of oligodendrocytes. To better understand how the injured brain might recover by shifting its cellular profile (e.g., from oligodendrocytes to astrocytes), this study emphasizes the need to consider both cell-intrinsic processes and cell-extrinsic cues. We suggest that epigenetic factors such as MBD proteins play important roles in the maintenance of cellular homeostasis after injury, providing a built-in system to relay signals from an ever-changing environment to the neural cell genome.
Materials and Methods
Cell Culture and in Vitro Differentiation.
Neural progenitor cells (AHPs) isolated from hippocampus of adult female Fischer 344 rats, used in this study, have been characterized previously (32). The methods for maintaining AHPs and inducing their differentiation into specific lineages have been reported (17). For a detailed description, see SI Text.
Reverse TranscriptionPolymerase Chain Reaction.
RNA isolation and reverse transcriptionpolymerase chain reaction (RT-PCR) were performed by an established method.
For a detailed description, see SI Text.
Western Blot Analysis.
Western blot analysis was performed by an established method.
For a detailed description, see SI Text.
Immunostaining.
Cells were fixed with 4% paraformaldehyde (PFA) and stained immunocytochemically, as described previously (25). For a detailed description, see SI Text.
Recombinant Retrovirus.
Retrovirus was produced as previously described (33). For a detailed description, see SI Text.
Bisulfite Sequencing.
Sodium bisulfite treatment of genomic DNA was performed essentially as described previously (34). For a detailed description, see SI Text.
Chromatin Immunoprecipitation Assay.
Chromatin immunoprecipitation (ChIP) was performed according to a protocol published by Upstate Biotechnologies. AHP-derived oligodendrocytes and the cells cultured with LIF for 2 days were exposed to formaldehyde, at a final concentration of 1%, added directly to the tissue culture medium. Co-immunoprecipitated DNA was used as a template for PCR with the following set of primers: Gfex1S (5′-TGACATCCCAGGAGCCAG-3′) and Gfex1AS (5′-CAGTCTCTCTGCTCACTAGCC-3′).
Animals.
MBP-Cre Tg mice (21) were provided by M. Miura (University of Tokyo, Japan). CAG-CAT-EGFP Tg mice (22) were a gift from J. Miyazaki (Osaka University, Japan). All experimental procedures and protocols were approved by the Animal Care and Use Committee of Keio University.
Focal Cerebral Ischemia.
Mice were anesthetized by nitrous oxide/oxygen/isoflurane (69/30/1%) administered through an inhalation mask during surgery. Details of surgical procedures are provided in SI Text.
Supplementary Material
Acknowledgments.
We thank Dr. M. Miura (University of Tokyo) for MBP-Cre transgenic mice, Dr. J. Miyazaki (Osaka University) for CAG-CAT-EGFP transgenic mice, Dr. T. Kitamura (University of Tokyo) for pMY vector and Plat-E cells, Dr. I. Saito (University of Tokyo) for pCALNL5 vector. We appreciate Drs. Y. Bessho and T. Matsui for valuable discussions. We also thank Drs. A. R. Muotri and I. Smith for helpful comments and critical reading of the manuscript. We are very grateful to N. Ueda for excellent secretarial assistance. Many thanks to N. Namihira, Y. Kuromi and K. Tsujimura for technical help. pCALNL5 was provided by RIKEN Brain Research Center, which is participating in the National Bio-Research Project of the Ministry of Education, Culture, Sports, Science and Technology, Japan. This work has been supported by a Grant-in-Aid for Young Scientists; a Grant-in-Aid for Science Research on Priority Areas; the Nara Institute of Science and Technology Global Centers of Excellence Program (Frontier Biosciences: Strategies for Survival and Adaptation in a Changing Global Environment) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and the Uehara Memorial Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808417105/DCSupplemental.
References
- 1.Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 2.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh J, Gage FH. Epigenetic control of neural stem cell fate. Curr Opin Genet Dev. 2004;14:461–469. doi: 10.1016/j.gde.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Bonni A, et al. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–483. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- 5.Nakashima K, et al. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999;284:479–482. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- 6.Bugga L, Gadient RA, Kwan K, Stewart CL, Patterson PH. Analysis of neuronal and glial phenotypes in brains of mice deficient in leukemia inhibitory factor. J Neurobiol. 1998;36:509–524. doi: 10.1002/(sici)1097-4695(19980915)36:4<509::aid-neu5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Koblar SA, et al. Neural precursor differentiation into astrocytes requires signaling through the leukemia inhibitory factor receptor. Proc Natl Acad Sci USA. 1998;95:3178–3181. doi: 10.1073/pnas.95.6.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakashima K, et al. Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J Neurosci. 1999;19:5429–5434. doi: 10.1523/JNEUROSCI.19-13-05429.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He F, et al. A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat Neurosci. 2005;8:616–625. doi: 10.1038/nn1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 11.Robertson KD, Wolffe AP. DNA methylation in health and disease. Nat Rev Genet. 2000;1:11–19. doi: 10.1038/35049533. [DOI] [PubMed] [Google Scholar]
- 12.Watt F, Molloy PL. Cytosine methylation prevents binding to DNA of a HeLa cell transcription factor required for optimal expression of the adenovirus major late promoter. Genes Dev. 1988;2:1136–1143. doi: 10.1101/gad.2.9.1136. [DOI] [PubMed] [Google Scholar]
- 13.Hendrich B, Bird A. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol Cell Biol. 1998;18:6538–6547. doi: 10.1128/mcb.18.11.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takizawa T, et al. DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev Cell. 2001;1:749–758. doi: 10.1016/s1534-5807(01)00101-0. [DOI] [PubMed] [Google Scholar]
- 15.Fan G, et al. DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development. 2005;132:3345–3356. doi: 10.1242/dev.01912. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki S, et al. Phosphorylation of signal transducer and activator of transcription-3 (Stat3) after focal cerebral ischemia in rats. Exp Neurol. 2001;170:63–71. doi: 10.1006/exnr.2001.7701. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh J, et al. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J Cell Biol. 2004;164:111–122. doi: 10.1083/jcb.200308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Y, et al. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- 19.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 20.Bernstein BE, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 21.Hisahara S, et al. Targeted expression of baculovirus p35 caspase inhibitor in oligodendrocytes protects mice against autoimmune-mediated demyelination. EMBO J. 2000;19:341–348. doi: 10.1093/emboj/19.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamoto S, et al. A novel reporter mouse strain that expresses enhanced green fluorescent protein upon Cre-mediated recombination. FEBS Lett. 2000;470:263–268. doi: 10.1016/s0014-5793(00)01338-7. [DOI] [PubMed] [Google Scholar]
- 23.Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- 24.Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- 25.Setoguchi H, et al. Methyl-CpG binding proteins are involved in restricting differentiation plasticity in neurons. J Neurosci Res. 2006;84:969–979. doi: 10.1002/jnr.21001. [DOI] [PubMed] [Google Scholar]
- 26.Jung BP, Zhang G, Ho W, Francis J, Eubanks JH. Transient forebrain ischemia alters the mRNA expression of methyl DNA-binding factors in the adult rat hippocampus. Neuroscience. 2002;115:515–524. doi: 10.1016/s0306-4522(02)00383-4. [DOI] [PubMed] [Google Scholar]
- 27.Jessberger S, Toni N, Clemenson GD, Jr, Ray J, Gage FH. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci. 2008;11:888–893. doi: 10.1038/nn.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballas N, Mandel G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr Opin Neurobiol. 2005;15:500–506. doi: 10.1016/j.conb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Colantuoni C, et al. Gene expression profiling in postmortem Rett Syndrome brain: Differential gene expression and patient classification. Neurobiol Dis. 2001;8:847–865. doi: 10.1006/nbdi.2001.0428. [DOI] [PubMed] [Google Scholar]
- 30.Lyons SA, Kettenmann H. Oligodendrocytes and microglia are selectively vulnerable to combined hypoxia and hypoglycemia injury in vitro. J Cereb Blood Flow Metab. 1998;18:521–530. doi: 10.1097/00004647-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Okada S, et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- 32.Gage FH, et al. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci USA. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morita S, Kojima T, Kitamura T. Plat-E: An efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- 34.Clark SJ, Harrison J, Paul CL, Frommer M. High sensitivity mapping of methylated cytosines. Nucleic Acids Res. 1994;22:2990–2997. doi: 10.1093/nar/22.15.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.