Abstract
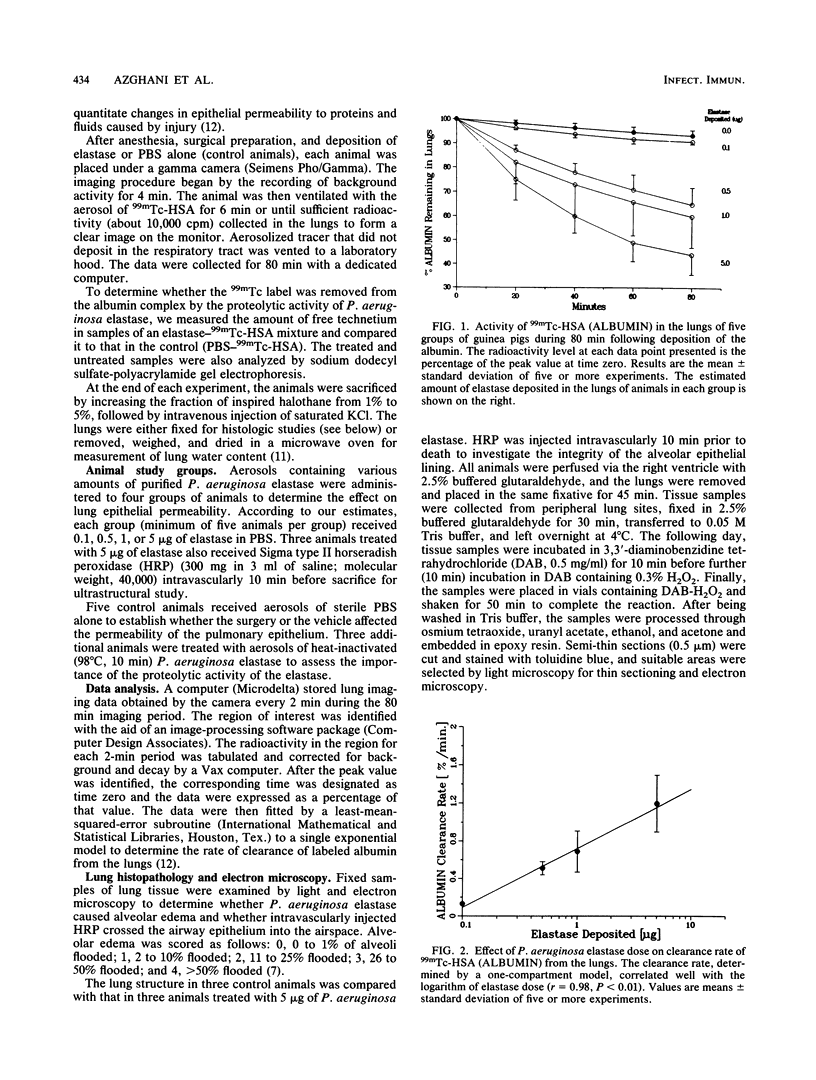
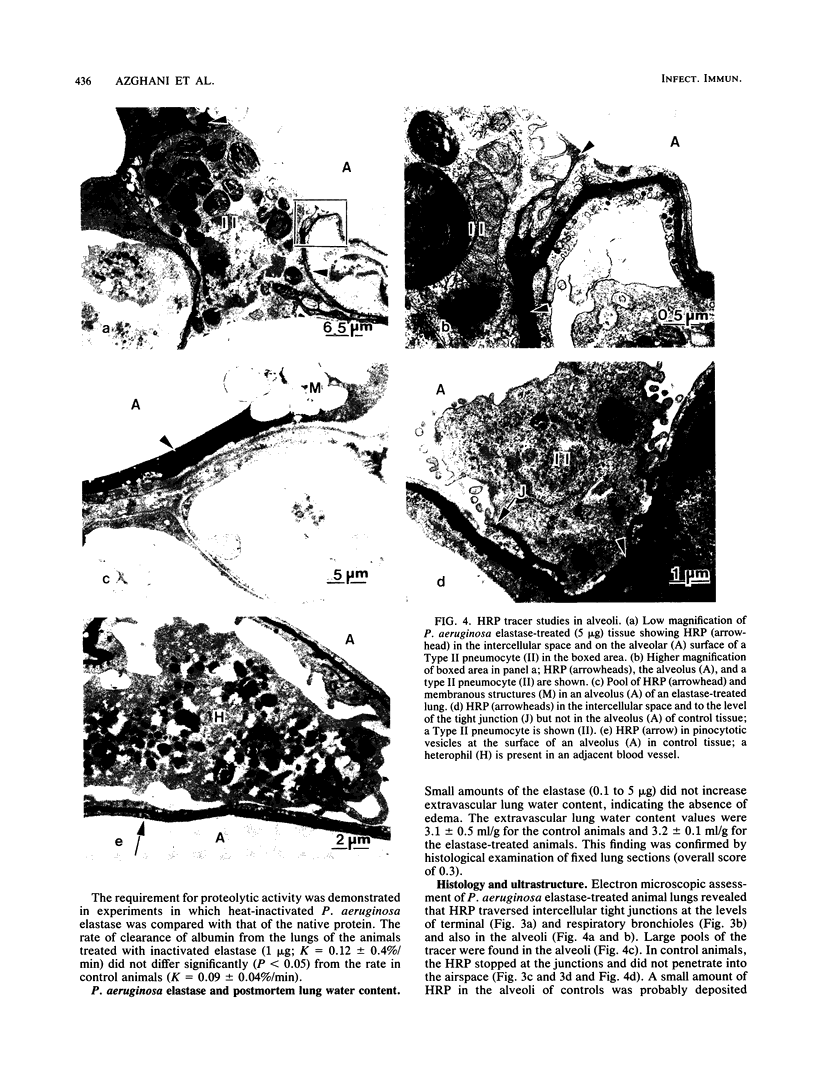
Elastase-deficient mutants of Pseudomonas aeruginosa are less virulent than the wild type and are easily cleared from the lungs of guinea pigs. The effect of P. aeruginosa elastase on lung epithelium, however, is not yet understood. We addressed the hypothesis that breach of the epithelial barrier by elastase from P. aeruginosa allows invading organisms and toxic substances to penetrate the interstitium. We measured the clearance of aerosolized technetium-labeled albumin (molecular weight, 69,000) from the lungs of anesthetized guinea pigs with the aid of a gamma camera and a dedicated computer. Aerosols of the elastase (0.1 to 5 micrograms) increased the rate of clearance of labeled albumin from the lungs in proportion to the elastase dose. Electron microscopic studies using horseradish peroxidase as a tracer revealed that elastase interrupts intercellular tight junctions of the epithelial lining, thereby increasing the permeability to macromolecules. The amounts of elastase used in this report did not cause interstitial or alveolar edema, as determined by both postmortem extravascular lung water volume measurement and morphological examination. The data indicate that the elastase is a potentially important virulence factor in acute lung infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachofen M., Weibel E. R. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med. 1982 Jan;3(1):35–56. [PubMed] [Google Scholar]
- Blackwood L. L., Stone R. M., Iglewski B. H., Pennington J. E. Evaluation of Pseudomonas aeruginosa exotoxin A and elastase as virulence factors in acute lung infection. Infect Immun. 1983 Jan;39(1):198–201. doi: 10.1128/iai.39.1.198-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck L. W., Morihara K., Abrahamson D. R. Degradation of soluble laminin and depletion of tissue-associated basement membrane laminin by Pseudomonas aeruginosa elastase and alkaline protease. Infect Immun. 1986 Oct;54(1):149–153. doi: 10.1128/iai.54.1.149-153.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma J. Y., Abe C., Tanamoto K., Hirao Y., Morihara K., Tsuzuki H., Yanagawa R., Honda E., Aoi Y., Fujimoto Y. Effectiveness of immunization with single and multi-component vaccines prepared from a common antigen (OEP), protease and elastase toxoids of Pseudomonas aeruginosa on protection against hemorrhagic pneumonia in mink due to P. aeruginosa. Jpn J Exp Med. 1978 Apr;48(2):111–133. [PubMed] [Google Scholar]
- Kawaharajo K., Homma J. Y., Aoyama Y., Morihara K. In vivo studies on protease and elastase from Pseudomonas aeruginosa. Jpn J Exp Med. 1975 Apr;45(2):89–100. [PubMed] [Google Scholar]
- MORIHARA K. PRODUCTION OF ELASTASE AND PROTEINASE BY PSEUDOMONAS AERUGINOSA. J Bacteriol. 1964 Sep;88:745–757. doi: 10.1128/jb.88.3.745-757.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel R. P., Hakim T. S., Smith T. T., Poulsen R. S. Quantitative morphology of permeability lung edema in dogs induced by alpha-naphthylthiourea. Lab Invest. 1983 Oct;49(4):412–419. [PubMed] [Google Scholar]
- Peterson B. T., Brooks J. A., Zack A. G. Use of microwave oven for determination of postmortem water volume of lungs. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jun;52(6):1661–1663. doi: 10.1152/jappl.1982.52.6.1661. [DOI] [PubMed] [Google Scholar]
- Peterson B. T., Dickerson K. D., James H. L., Miller E. J., McLarty J. W., Holiday D. B. Comparison of three tracers for detecting lung epithelial injury in anesthetized sheep. J Appl Physiol (1985) 1989 May;66(5):2374–2383. doi: 10.1152/jappl.1989.66.5.2374. [DOI] [PubMed] [Google Scholar]
- Schultz D. R., Miller K. D. Elastase of Pseudomonas aeruginosa: inactivation of complement components and complement-derived chemotactic and phagocytic factors. Infect Immun. 1974 Jul;10(1):128–135. doi: 10.1128/iai.10.1.128-135.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Cryz S. J., Friedman R. L., Iglewski B. H. Contribution of toxin A and elastase to virulence of Pseudomonas aeruginosa in chronic lung infections of rats. Infect Immun. 1982 Jun;36(3):1223–1228. doi: 10.1128/iai.36.3.1223-1228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]