Abstract
Mimicking nature is both a key goal and a difficult challenge for the scientific enterprise. DNA, well known as the genetic-information carrier in nature, can be replicated efficiently in living cells. Today, despite the dramatic evolution of DNA nanotechnology, a versatile method that replicates artificial DNA nanostructures with complex secondary structures remains an appealing target. Previous success in replicating DNA nanostructures enzymatically in vitro suggests that a possible solution could be cloning these nanostructures by using viruses. Here, we report a system where a single-stranded DNA nanostructure (Holliday junction or paranemic cross-over DNA) is inserted into a phagemid, transformed into XL1-Blue cells and amplified in vivo in the presence of helper phages. High copy numbers of cloned nanostructures can be obtained readily by using standard molecular biology techniques. Correct replication is verified by a number of assays including nondenaturing PAGE, Ferguson analysis, endonuclease VII digestion, and hydroxyl radical autofootprinting. The simplicity, efficiency, and fidelity of nature are fully reflected in this system. UV-induced psoralen cross-linking is used to probe the secondary structure of the inserted junction in infected cells. Our data suggest the possible formation of the immobile four-arm junction in vivo.
Keywords: DNA nanotechnology, immobile DNA junction, self-replication, synthetic biology
The notion that DNA is merely the gene encoder of living systems has been eclipsed by the successful development of DNA nanotechnology (1–2). Using branched DNA as the main building block (also known as a “tile”) and cohesive single-stranded DNA (ssDNA) ends to designate the pairing strategy for tile-tile recognition, one can rationally design and assemble complicated nanoarchitectures from specifically designed DNA oligonucleotides. Objects in both two and three dimensions with a large variety of geometries and topologies have been built from DNA with excellent yield (3–9); this development enables the construction of DNA-based nanodevices (10–11) and DNA template directed organization of other molecular species (12–19). The construction of such nanoscale objects constitutes the basis of DNA nanotechnology. However, the synthetic scale of oligonucleotides limits the potential applications of DNA nanotechnology, especially when the nanostructure is designed to be made from long ssDNA (>100 bases). Replicable DNA nanostructures are thus a highly desirable goal to help overcome this barrier.
To this end, several possible solutions have been explored. For example, a three-point star motif has been replicated by chemical methods in which the parental nanomotif serves as the template to direct the chemical ligation of nonidentical DNA strands to form the next generation of the nanomotif (20). Another instance involves a 1.7-kb ssDNA that was used to assemble a DNA octahedron (together with five short strands); this molecule was cloned in a plasmid in bacteria (9). A nicking endonuclease was used to digest the amplified double-stranded plasmids to obtain the single-stranded heavy chain. This is an inspiring accomplishment, although the long strand did not form a complete nanostructure itself without the aid of the short strands. We recently reported the replication of a four-arm DNA nanojunction and a paranemic cross-over (PX) DNA molecule, using rolling-circle amplification (RCA)-based enzymatic methods (21–22). The rolling-circle mechanism is also involved in the replication of bacteriophage genomes and bacterial plasmids in vivo, so this result suggested that it might be possible to replicate DNA nanostructures by using viruses and bacteria. In fact, such a possibility was proposed previously (22–23). Moreover, DNA containing a designed hairpin structure has been incorporated into a vector as the substrate for a rolling-circle transcript to produce RNA ribozymes in Escherichia coli cells (24). However, to our knowledge, in vivo replication of artificial DNA nanostructures has not been achieved to date.
Results and Discussion
Design of the Replication Process.
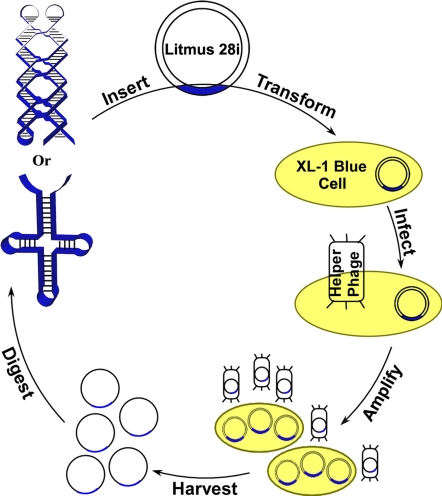
We present a biological method to replicate single-stranded DNA nanostructures. The design is illustrated schematically in Fig. 1. A single-stranded Holliday junction and PX DNA are chosen as model molecules, because they have been thoroughly characterized and successfully replicated by using RCA in vitro (21–22). The in vivo replication starts by inserting the desired nanostructure into double-stranded phagemid vector Litmus 28i (≈2.8-kb) with an ampicillin resistance gene and a single-stranded (M13) DNA replication origin. To do this, the ssDNA that folds into the designed nanostructure is extended at both the 5′ and 3′ ends, hybridized to its Watson-Crick complement with end extensions to form a double-stranded (DS) insert with proper sticky ends, and ligated with correspondingly restricted Litmus 28i. The vector with the DS insert is then transformed into E. coli cells (XL1-Blue), which are later incubated in an ampicillin-containing culture to ensure the selective amplification of Litmus 28i-transformed cells. At the next stage, the transformed cells in a single colony are infected by helper phage M13KO7 that carries a kanamycin resistance gene. M13KO7 has the special property that rather than self-replicating, it preferentially packages single-stranded phagemid bearing the M13 origin and secretes it into the culture medium (25). The infected cells are then amplified in a medium containing kanamycin. Finally, the phages are precipitated, and their single-stranded DNA molecules are extracted and restricted. A high copy number of the DNA nanostructure is thus obtained as a result of the exponential replication of phagemid vectors in bacteria cells.
Fig. 1.
Schematic drawing showing the in vivo replication of a DNA nanostructure. A single-stranded DNA nanostructure (four-arm junction or paranemic cross-over DNA) is inserted into phagemid, transformed into XL1-Blue cells, and amplified in vivo in the presence of helper phages. High copy numbers of cloned nanostructures can be obtained readily by using standard molecular biology techniques.
Replication of a Four-Arm DNA Nanojunction.
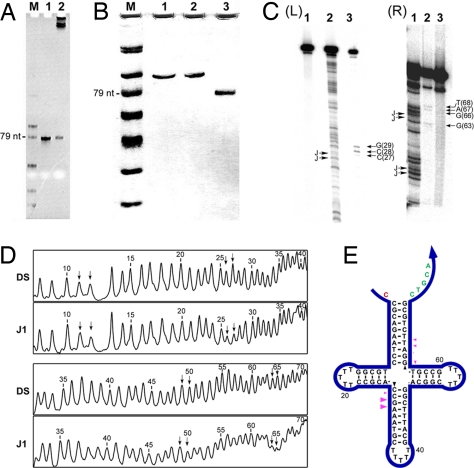
As a pilot experiment, we tested the in vivo replication of a four-arm DNA nanojunction (J1) that is 79 nt long. First, the correct DS insert was verified by assaying the restricted double-stranded phagemid extracted from the amplified single colony of transformed cells with denaturing PAGE [supporting information (SI) Fig. S1]. Among four randomly chosen colonies, three appeared to have the correct insert. The error was most likely due to self-ligation of the initially digested phagemid. Glycerol stock of cells containing the correctly inserted vector was then prepared for long-term storage and for later amplification use. The in vivo replication efficiency was evaluated by using a denaturing PAGE assay (Fig. 2A). It is clear that intact J1 strand is the only replication product visible from the gel. (The top bands represent digested and undigested phagemid vectors, along with helper phage genomic DNA.) Approximately 6 pmol of J1 was produced (estimated from the band intensities in the gel image) from 10 ml of saturated cell culture (OD600 = 1.0). It is very important to point out that this amplification is fully scalable. The final yield of nanostructure is proportional to the volume of the culture medium used. For example, 150 pmol of J1 is expected to be produced from 250 ml of culture medium; this estimate is consistent with our final yield, considering the material lost in the final PAGE purification step. The overall yield might be improved further by optimizing restriction conditions.
Fig. 2.
In vivo replication of a cloverleaf four-arm junction. (A) A 10% denaturing PAGE showing the final replication product. Lane M, 10-nt ssDNA ladder; lane 1, 10-pmol original J1 junction; lane 2, replication product yield from 10-ml saturated cell culture. (B) A 20% nondenaturing PAGE analysis of replicated J1. Lane M, 10-nt ssDNA ladder; lane 1, annealed original J1; lane 2, annealed replicated J1; lane 3, annealed random-sequence 79-mer ssDNA. (C) Endo VII cleavage of the J1 cloverleaf. The two images, Left (L) and Right (R) contain the results of the same experiment, but R has been run further than L to resolve cleavage positions nearer the 3′ end of the strand. Lanes L1 and R3 are untreated controls. Lanes L2 and R1 contain A–G sequencing ladders. The positions of possible cross-overs are indicated by “J” symbols. Lanes L3 and R2 contain the products of Endo VII cleavage experiments. The sites of bands are indicated. (D) Quantitative scans of hydroxyl radical autofootprinting gels. Two sets of traces are indicated: DS, when the molecule is paired with its Watson–Crick complement, and J1, when it is folded into its junction structure. Nucleotides are numbered from the 5′ end, which is labeled. The positions of expected junction-flanking nucleotides are indicated by arrows. In agreement with previous studies of this junction, protections of the J1 strand relative to the DS strand are seen at positions 26 and 27 and at positions 64 and 65 (see E for numbering). In addition, a small amount of protection is seen at position 50 as well, possibly indicating a small amount of cross-over isomerization. (E) Schematic summarizing the results of autofootprinting and Endo VII cleavage experiments. Autofootprinting protection positions are indicted by black triangles and endo VII cleavage positions are indicated by magenta triangles. The main sites of protection or cleavage are in agreement with previous studies of J1.
Denaturing PAGE provides information only on the length of DNA strands. Therefore, the purified J1 strand was subjected to a number of other assays to verify the correct replication of the junction structure. Fig. 2B shows the nondenaturing analysis of the replicated J1 strand. Original sense J1 and replicated J1 strand exhibited exactly the same mobility on a nondenaturing gel, whereas a 79-mer ssDNA with a random sequence migrated much faster, implying the proper folding of the replicated J1 strand. A further test of the structure of the J1 cloverleaf is provided by T4 endonuclease VII (endo VII). The cleavage pattern is shown in Fig. 2C, and it is summarized by the magenta triangles in Fig. 2E. The cleavage occurs at the expected site, 2 or 3 nt 3′ to the junction on the cross-over segment (26). The cleavage is somewhat stronger on the lower left segment than on the upper right one. Although consistent with a previous digestion study on the same molecule, the position of cleavage is not the strongest evidence for the proper cross-over structure, because endo VII has a requirement for minimum-length arms, a requirement that the short horizontal arms may not satisfy (27).
We have reported previously that enzymatically generated unusual DNA motifs can by characterized by hydroxyl radical autofootprinting experiments; RCA-generated PX molecules were characterized in this fashion (22). Whereas the technique was originally developed by Churchill et al. (28) to analyze DNA branched junctions, and J1 in particular, it is natural to characterize the cloned J1 cloverleaf structure by this method. In this technique, hydroxyl radicals are generated by Fenton chemistry involving Fe(II)EDTA2− (29–30). This technique gives single-nucleotide resolution of nucleotide susceptibility to attack and produces a quantitative pattern of backbone cleavage at all possible sites.
The strategy of autofootprinting experiments is to compare the chemical attack pattern of each strand when it is part of an unconventional species with the pattern obtained when the strand is hybridized with its normal Watson–Crick complement (31). Experiments with four-stranded four-arm junctions indicate that the patterns of two noncontiguous strands are the same in both environments, whereas the other two strands, crossing over at the branch point, exhibit protections at the site of the junction. From those experiments, it was concluded that strands with the same patterns in both pairing environments probably have continuous double-helical conformations near the junction, whereas the other strands (showing protection near the branch point) form cross-over structures (28).
The results of the hydroxyl radical autofootprinting experiments on the J1 cloverleaf molecule are shown in Fig. 2D, and they are shown schematically in Fig. 2E by black arrowheads. It is clear that the most salient points of protection are those flanking the branch point in the strand segments on the lower left and on the upper right, with no protection at the equivalent positions on the upper left or lower right segments. It is known that in the presence of Mg2+, the four-arm branched junction assorts pairs of adjacent arms into two stacking domains (28). It is possible for either pair of junction-flanking corners to form the cross-over strands, with the other two being virtually undistorted double-helix-like strands. It is known that the cross-over strands are determined by the junction-flanking nucleotides (32), so one would expect that replicated J1 cloverleaf should demonstrate the same pattern as J1. Indeed, that is the case: The nucleotides that are protected are the same ones seen in the original four-stranded J1 molecule. Combined with the endo VII digestion results, the data suggest that the replicated J1 cloverleaf has adopted the designed structure.
Replication of a PX DNA Molecule.
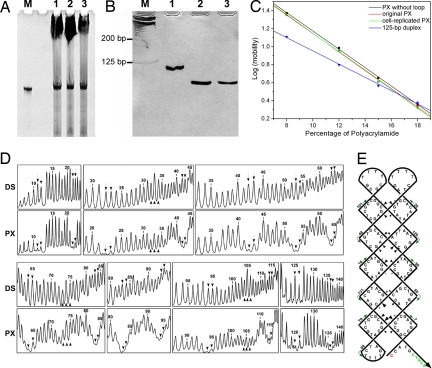
PX DNA, which has a cross-over at every possible position between its two parallel helical domains (33), is structurally more complicated than the J1 cloverleaf. Thus, we challenged our system by replicating a PX DNA molecule, which is folded from a 146-nt ssDNA. Following the same amplification protocol applied to J1, PX DNA was replicated in vivo with equally high efficiency (Fig. 3A). From denaturing PAGE analysis, it can be estimated that a ≈50-pmol PX strand was replicated from 120 ml of saturated cell culture. It is notable that DNA species shorter than the full-length PX strand were present in the restriction digestion product. These byproducts are truncated PX molecules that were also observed in the in vitro RCA replication of the same PX motif. However, the byproducts represent a much lower percentage here (≈20%) than that in the in vitro RCA replication products (≈50%). This is not unexpected, because the cellular machinery provides a series of enzymes and factors besides polymerase that facilitate the replication process. The in vivo replication efficiency is thus much higher than that seen in vitro (22).
Fig. 3.
In vivo replication of a PX DNA molecule. (A) A 10% denaturing PAGE showing the final replication product. Lane M, 10-pmol original sense PX; lanes 1–3, each lane contains final replication product yield from ≈40-ml saturated cell culture. (B) A 12% nondenaturing PAGE analysis of replicated PX. Lane M, 25-bp dsDNA ladder; lane 1, annealed 146-mer ssDNA with random sequence; lane 2, annealed original sense PX; lane 3, annealed replicated PX. (C) Ferguson analysis of PX molecules and a 125-bp dsDNA (duplex). PX molecules show very similar Ferguson slopes that are significantly different from that of the duplex. (D) Densitometer scans of autofootprinting gels. The DS lanes correspond to the cloned molecule paired with its Watson–Crick complement, and the PX lanes are the PX molecule itself. Downward pointing arrows indicate cross-over positions, and upward pointing arrows are present at loop positions. Protection is seen to flank each of the cross-overs. In addition, a small amount of protection is seen at sites 33 and 105, which might occlude each other. The single loop at position 72 does not seem to be protected significantly. (E) Schematic summarizing the autofootprinting results. Nucleotide positions are numbered, and positions of protection are shown as triangles in rough proportion to the extent of protection for the junction regions. Colored nucleotides indicate the sites of restriction.
Three experiments were performed to confirm that the in vivo-replicated PX strand can still form the designed nanostructure. First, nondenaturing PAGE (Fig. 3B) demonstrated that the annealed in vivo-replicated PX molecule migrated at the same rate as the original PX molecule. As a negative control, a 146-mer ssDNA with random sequence ran much slower than the PX molecules because of its relatively loose secondary structure. Second, Ferguson analysis (Fig. 3C) was used to characterize the friction constant exhibited by the DNA molecules within the nondenaturing polyacrylamide gel; the friction constant is directly related to the surface area and shape of DNA nanostructures. The data points of the PX molecule replicated in vivo and the original PX molecule overlap each other. Furthermore, they both display a very similar slope to that of a previously reported PX motif composed of the same core structure but lacking dT4 loops or ssDNA extensions at the ends of its helical domains (PX without loops) (33). In contrast, the plot for a 125-bp dsDNA exhibits a significantly different slope. This observation strongly suggests the correct folding of the replicated PX structure. Finally, the strongest evidence comes from the hydroxyl radical autofootprinting experiment. The results of autofootprinting experiments are shown in Fig. 3D, and they are summarized in a schematic in Fig. 3E. The results are identical to the previous studies on this same PX nanostructure (22, 33), indicating cross-overs exactly where predicted by the design of the PX molecule. There is some minor protection in the two loops at the top of the diagram, possibly indicating occlusion of each other's structures. Thus, all three assays confirm that we have replicated the PX nanostructure correctly.
Searching for the Immobile Nanojunction in Vivo.
Now that we have confirmed that the in vivo-replicated DNA strands can fold into the designed nanostructures in vitro, it is interesting to ask whether these nanostructures also retain their native conformation in the cellular environment. The mobile Holliday junction is a central intermediate in DNA recombination (34–36), but the unnatural immobile DNA nanojunction has not been found in vivo. Here, we intended to use psoralen cross-linking (37) to probe the in vivo secondary structure of the single-stranded J1 insert. It is known that 4,5′,8-trimethylpsoralen (TMP) intercalates between DNA base pairs and preferentially cross-links two adjacent thymine bases (38) on opposite strands of a DNA double helix (i.e., 5′-TA or AT, which is also considered as a “hot-spot” for TMP to cross-link) when excited by 365-nm UV irradiation. Cross-linking between neighboring thymine and cytosine (e.g., 5′-TG) is also possible but is much less likely (38). This technique has been used widely to study the secondary structure of DNA and RNA in vitro and in vivo. It is especially useful for in vivo nucleic acid structural studies, because psoralen, once cross-linked with thymine (or uracil) bases, “freezes” the in vivo secondary structure of DNA (or RNA) by altering their topologies, which can be readily detected by subsequent in vitro assays, such as PAGE and electron microscopy.
To validate this method for our purpose, we first performed in vitro cross-linking on the J1 cloverleaf and a series of its derivatives and characterized them using denaturing PAGE (Fig. S2). The J1 derivatives were designed so that they all keep the same bases flanking the junction point, but each has only zero or one hot-spot on the long arms. On another derivative molecule, dT5 loops were substituted by dA5 loops to rule out their influence on the cross-linking products. It is clear that two common products were generated from all J1 derivatives after psoralen and UV treatment, regardless of their different arm, loop, and end extension sequences. Those two products are therefore directly related to the pyrimidines flanking the junction site. This is not surprising, given the fact that the junction structure has been shown to be a preferred substrate for intercalating drugs (39). Among various derivative molecules, J1-O, which has no regular hot-spot on any arm, was selected for an in vivo cross-linking study, because we expected TMP to photoreact preferentially with bases at its junction point and thus directly indicate the formation of a junction structure. Indeed, when J1-O was denatured by 40% (vol/vol) formamide, it became immune to TMP (Fig. S3), suggesting that the success of cross-linking depends strongly on its native conformation. Moreover, because J1-O lacks “5′-TA” or “5′-AT” sequences, double-stranded vectors in the cell are unlikely to be cross-linked within the J1-O DS insert domain (Fig. S4), strengthening the interpretation of the in vivo cross-linking result.
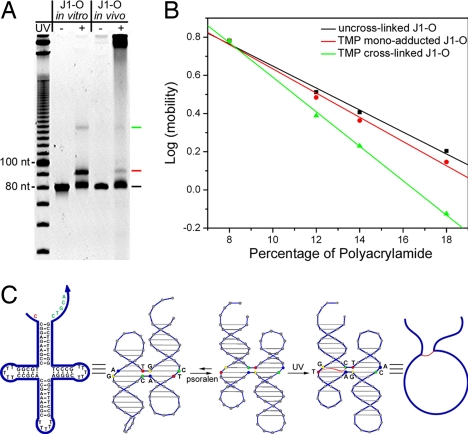
We cloned J1-O in vivo in the same way that we cloned J1 and PX. The transformed cells were infected by helper phage, allowed to grow until saturation, and harvested by gentle centrifugation. The harvested cells were permeabilized by a solution containing EDTA, followed by UV-induced TMP cross-linking treatment. The phagemid vectors extracted from the UV-treated cells were then restricted and run side by side on a 14% denaturing polyacrylamide gel with the in vitro cross-linking products of J1-O (Fig. 4A). It is clear that the product generated by in vivo cross-linking matches that produced by in vitro cross-linking. Note that on the far right lane, the smearing at the starting position of electrophoresis represents digested or undigested vectors. Another observation here is that the in vivo cross-linking products are relatively fewer than those seen in vitro. This is because most single-stranded vectors that contain J1-O as cross-linking substrates are packed and released into the culture medium, leaving the infected cells rich in replicative form (double-stranded) phagemids, whose DS insert cannot be cross-linked. Nevertheless, the same in vivo and in vitro cross-linking products from J1-O can be observed here with no ambiguity.
Fig. 4.
Psoralen cross-linking of the replicated junction (J1-O) structure in vivo and in vitro. (A) A 14% denaturing PAGE analysis of cross-linking products from J1-O. In vivo and in vitro cross-linking products are compared side by side and can be seen to be exactly the same. Black, red, and green lines were labeled beside bands representing un-cross-linked, mono-adducted, and cross-linked J1-O, respectively (see B). A 10-base DNA ladder is loaded in the far left lane as reference. (B) Ferguson analysis of the cross-linking products using denaturing PAGE. Colors of datasets correspond to the labels on the side of the cross-linking product bands in A. The product represented by the upper band shows a significantly different friction constant from the other two DNA species, implying its unique topology. (C) Schematic drawing of the mechanism in which psoralen reacts with the J1-O molecule. As illustrated, the psoralen molecule first induces the balance shift between the two cross-over isomers, and then cross-links (represented by a red curve) the two pyrimidines at the junction site in the presence of UV, altering the topology of the J1-O molecule.
To understand the topology of the cross-linked products, Ferguson analysis was performed by using denaturing PAGE (Fig. 4B). The un-cross-linked J1-O and the product represented by the lower band (marked by a red line) on Fig. 4A exhibited similar friction constants. In contrast, the friction constant of the other product (marked by a green line on Fig. 4A) was significantly different, suggesting a different topology. It is well known that besides covalently bridging two pyrimidines, psoralen can also react with a single pyrimidine at the susceptible point, forming a monoadduct (37–38). Therefore, the product with similar topology to the bare J1-O is considered likely to be the mono-TMP adduct and the other product is considered to be the cross-linked J1-O, which resembles a circle bearing two antennae under denaturing conditions (see molecular models in Fig. 4C).
A conundrum arises when one examines the details of the cross-linking pattern: The pyrimidines disposed to be cross-linked appear to be on opposite helices if the junction adopts the usual cross-over isomer (stacking pairs) seen for J1 (28). However, Kallenbach and his colleagues (40) have shown that the presence of intercalating drugs can reverse the favored cross-over isomer. We have ascertained that this occurs when J1-O is in the presence of psoralen (Fig. S5). The cross-over reversal is shown in the schematic of Fig. 4C. Thus, the cross-linking is not aberrant, and the pattern is consistent with the data. Therefore, the same cross-linking pattern found in vivo as in vitro can be attributed to the proper of folding of J1-O in the context of a vector within the cellular environment.
Conclusions
We have demonstrated a system that utilizes viruses and bacterial cells to replicate complete artificial DNA nanostructures. All steps involved in the replication process are based on well established molecular cloning techniques (41), making the method easy to master and reproduce. However, applying those techniques to DNA bearing complicated secondary structures is not necessarily trivial. Instead, the work reported here is significant, both for applications and on a fundamental basis. Compared with the in vitro enzymatic amplification method, in vivo replication provides higher efficiency by taking advantage of the naturally existing cellular machinery. Such improvement becomes obvious when more complicated nanostructures (e.g., PX DNA) are encountered. It is also worth pointing out that the replication is fully scalable. Only a small amount of DNA (subpicomole quantities) is needed to start the replication. Once successfully transformed by the correctly inserted vector, the cells can be stored for later amplification. The amplification can be scaled up readily by increasing the volume of the culture medium. Moreover, by replicating two different nanostructures (Holliday junction and PX) and the same nanostructure formed by different DNA sequences (J1 and J1-O), we expect this strategy to be universally useful for replicating nanostructures formed by a single DNA strand.
We have used commercialized kits (competent cells, phagemid vectors, restriction enzymes, etc.) throughout the study. In the future, the conditions can be optimized (e.g., engineering the vector) to improve the replication yield further. The cloning of DNA nanostructures with knotted topologies has yet to be tested. Nevertheless, the method reported here could potentially become a routine laboratory method to prepare moderate amounts of long ssDNA with designated sequences. An exciting possibility is that this technique could find application in the evolution and in vitro selection of DNA nanostructures. In this case, it can serve to amplify enriched DNA nanostructures after each selection cycle.
The tolerance of natural systems for artificial objects is of great importance in synthetic biology. An interesting yet open question is how the cellular replication machinery is affected by a foreign DNA insert. Although it is beyond the scope of this work to address this question, several clues can be obtained here. First, we found that J1 and an unstructured 79-mer ssDNA were replicated equally well by using the same in vivo cloning procedure (Fig. S6).Second, both J1 and PX retained sequence (Figs. S7 and S8) and structure integrity after replication. These findings suggest that the efficiency and fidelity of the natural replication system is not significantly hindered by the secondary structure of the inserted DNA. Third, another viral vector (M13mp18) is also capable of replicating J1 but with much lower efficiency and reproducibility (Fig. S9), possibly owing to the conflicts between the native M13 structure (44) and the inserted artificial nanostructure. Thus, the construction of the vector is crucial to achieve efficient replication. Finally, the psoralen cross-linking data suggest not only that nanostructures can be replicated by viruses and bacteria cells, but also that some artificial nanostructures might possibly survive in vivo. Although extreme care needs to be taken when interpreting the cross-linking data, the existence of immobile Holliday junctions in E. coli cells has been strongly implied by our data. More concrete evidence may be obtained from in vivo autofootprinting (42–43). In conclusion, we expect this work to open more opportunities to scale up structural DNA nanotechnology and to provide helpful insight on the possible existence and impact of artificial nanostructures in living systems.
Materials and Methods
Materials.
Detailed information for materials used in this study, including DNA oligoonucleotides, restriction enzymes, ligase, phagemid vectors, competent cell, helper phage, and chemicals, can be found in SI Text.
In Vivo Replication of DNA Nanostructures.
Equal-molar sense and antisense nanostructure (J1, J1-O, or PX; see SI Text for sequences) strands (90 nM) were annealed from 94°C to room temperature over 45 min in 1× TAE-Mg buffer [to mM Tris-acetic acid (pH 8.0), 12.5 mM magnesium acetate, and 1 mM EDTA] to yield a DS insert. Two micrograms of Litmus 28i (500 μg/ml) were double restriction digested by 20 units of PstI and 15 units of SacI in 50 μl of 1× NE Buffer 1 [10 mM Bis-Tris-Propane-HCl, 10 mM MgCl2, 1 mM DTT (pH 7.0)] at 37°C for 3 h. The reaction was stopped by the addition of denaturing buffer, and the digested vector was purified via agarose gel electrophoresis. Digested vector (100 ng) was ligated with 0.16 pmol of preannealed DS insert (≈3-fold excess) in 20 μl o f1× T4 ligase buffer [50 mM Tris·HCl, 10 mM MgCl2, 1 mM ATP, and 10 mM DTT (pH 7.5)] at 4°C overnight. Ligated vector (50 ng) was transformed into competent XL1-Blue cells, plated on LB-ampicillin (LB-Amp) agar plates, and incubated at 37°C overnight. Double-stranded phagemid was extracted from cells in 5 ml of saturated cultures that were amplified from a single colony by using the plasmid miniprep kit. The correct insertion was verified by restriction digestion, followed by denaturing PAGE. One milliliter of glycerol stock of XL1-Blue cells (OD600 = 0.5) with correctly inserted phagemid were then infected by 50 μl of 1 × 1011 M13KO7 helper phage and incubated overnight at 37°C in 250 ml of LB-Amp culture containing 25 μg/ml kanamycin A. The cells were harvested for later cross-linking studies. The bacteriophage particles that contain single-stranded vector were precipitated from the supernatant by addition of 10 g of PEG and 7.5 g of NaCl, followed by centrifugation at 10,000 × g. Protein shells were removed from the single-stranded vector by phenol/chloroform extraction. DNA was recovered by ethanol precipitation, redissolved in 0.9 ml of water, and restricted by 500 units of PstI and 360 units of SacI in the presence of 1 nmol of restriction helpers in 1 ml of 1× NE Buffer 1. The digested single-stranded vector was resolved on a 10% denaturing polyacrylamide gel, and the correctly replicated insert (J1, J1-O, or PX) was excised from the gel and eluted. Typically, 50–100 pmol of ssDNA can be recovered.
Characterization of Replicated Nanostructure.
Nondenaturing PAGE, Ferguson analysis, hydroxyl radical autofootprinting, and endonuclease VII cleavage experiments were used to characterize the replicated DNA nanostructures. Detailed protocols can be found in SI Text.
Psoralen Cross-Linking Study.
Detailed information for protocols used in psoralen cross-linking experiments can be found in SI Text.
Supplementary Material
Acknowledgments.
We thank Profs. Jiunn-Liang Chen and Thomas D. Tullius for helpful discussions. This work was supported by grants from the National Science Foundation (NSF), the Office of Naval Research, the Air Force Office of Scientific Research, the Army Research Office (ARO), and the National Institutes of Health and by funding from Arizona State University (to H.Y.), by grants from the NSF, ARO, and the U.S. Department of Energy (subcontract from the Research Foundation of State University of New York) (to N.C.S.), and by grants from the W. M. Keck Foundation and the National Center for Research Resources to New York University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 17593.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805416105/DCSupplemental.
References
- 1.Seeman NC. An overview of structural DNA nanotechnology. Mol Biotech. 2007;37:246–257. doi: 10.1007/s12033-007-0059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seeman NC. DNA in a material world. Nature. 2003;421:427–431. doi: 10.1038/nature01406. [DOI] [PubMed] [Google Scholar]
- 3.Lin C, Liu Y, Rinker S, Yan H. DNA tile based self-assembly: Building complex nanoarchitectures. ChemPhysChem. 2006;7:1641–1647. doi: 10.1002/cphc.200600260. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Seeman NC. The synthesis from DNA of a molecule with the connectivity of a cube. Nature. 1991;350:631–633. doi: 10.1038/350631a0. [DOI] [PubMed] [Google Scholar]
- 5.Rothemund PWK. Folding DNA to create nanoscale shapes and patterns. Nature. 2006;440:297–302. doi: 10.1038/nature04586. [DOI] [PubMed] [Google Scholar]
- 6.Douglas SM, Chou JJ, Shih WM. DNA-nanotube-induced alignment of membrane proteins for NMR structure determination; Proc Natl Acad Sci USA; 2007. pp. 6644–6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He Y, et al. Hierarchical self-assembly of DNA into symmetric supermolecular polyhedra. Nature. 2008;452:198–201. doi: 10.1038/nature06597. [DOI] [PubMed] [Google Scholar]
- 8.Goodman RP, et al. Rapid chiral assembly of rigid building blocks for molecular nanofabrication. Science. 2005;310:1661–1665. doi: 10.1126/science.1120367. [DOI] [PubMed] [Google Scholar]
- 9.Shih WM, Quispe JD, Joyce GF. A 1.7-kilobase single-stranded DNA that folds into a nanoscale octahedron. Nature. 2004;427:618–621. doi: 10.1038/nature02307. [DOI] [PubMed] [Google Scholar]
- 10.Liedl T, Sobey TL, Simmel FC. DNA based nano-devices. Nanotoday. 2007;2:36–41. [Google Scholar]
- 11.Seeman NC. From genes to machines: DNA nanomechanical devices. Trends Biochem Sci. 2005;30:119–125. doi: 10.1016/j.tibs.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le JD, et al. DNA-templated self-assembly of metallic nanocomponent arrays on a surface. Nano Lett. 2004;4:2343–2347. [Google Scholar]
- 13.Zhang J, Liu Y, Ke Y, Yan H. Periodic square-like gold nanoparticle arrays templated by self-assembled 2D DNA nanogrids on a surface. Nano Lett. 2006;6:248–251. doi: 10.1021/nl052210l. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, et al. Two-dimensional nanoparticle arrays show the organizational power of robust DNA motifs. Nano Lett. 2006;6:1502–1504. doi: 10.1021/nl060994c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma J, Chhabra R, Liu Y, Ke Y, Yan H. DNA-templated self-assembly of two-dimensional and periodical gold nanoparticle arrays. Angew Chem Int Ed. 2006;45:730–735. doi: 10.1002/anie.200503208. [DOI] [PubMed] [Google Scholar]
- 16.He Y, Tian Y, Ribbe AE, Mao C. Antibody nanoarrays with a pitch of ≈20 nanometers. J Am Chem Soc. 2006;128:12664–12665. doi: 10.1021/ja065467+. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Lin C, Li H, Yan H. Aptamer-directed self-assembly of protein arrays on a DNA nanostructure. Angew Chem Int Ed. 2005;44:4333–4338. doi: 10.1002/anie.200501089. [DOI] [PubMed] [Google Scholar]
- 18.Malo J, et al. Engineering a 2D protein-DNA crystal. Angew Chem Int Ed. 2005;44:3057–3061. doi: 10.1002/anie.200463027. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Liu H, Ye T, Kim J, Mao C. DNA-directed assembly of single-wall carbon nanotubes. J Am Chem Soc. 2007;129:8696–8697. doi: 10.1021/ja072838t. [DOI] [PubMed] [Google Scholar]
- 20.Eckardt LH, et al. DNA nanotechnology: Chemical copying of connectivity. Nature. 2002;420:286. doi: 10.1038/420286a. [DOI] [PubMed] [Google Scholar]
- 21.Lin C, Xie M, Chen JJ-L, Liu Y, Yan H. Rolling circle amplification of a DNA nanojunction. Angew Chem Int Ed. 2006;45:7537–7539. doi: 10.1002/anie.200602113. [DOI] [PubMed] [Google Scholar]
- 22.Lin C, Wang X, Liu Y, Seeman NC, Yan H. Rolling circle enzymatic replication of a complex multi-cross-over DNA nanostructure. J Am Chem Soc. 2007;129:14475–14481. doi: 10.1021/ja0760980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seeman NC. The construction of 3-D stick figures from branched DNA. DNA Cell Biol. 1991;10:475–486. doi: 10.1089/dna.1991.10.475. [DOI] [PubMed] [Google Scholar]
- 24.Ohmichi T, Maki A, Kool ET. Efficient bacterial transcription of DNA nanocircle vectors with optimized single-stranded promoters; Proc Natl Acad Sci USA; 2002. pp. 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vieira J, Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- 26.Mueller JE, Kemper B, Cunningham RP, Kallenbach NR, Seeman NC. T4 endonuclease VII cleaves the cross-over strands of Holliday junction analogs; Proc Natl Acad Sci USA; 1988. pp. 9441–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller JE, et al. Resolution of Holliday junction analogs by T4 endonuclease VII can be directed by substrate structure. J Biol Chem. 1990;265:13918–13924. [PubMed] [Google Scholar]
- 28.Churchill MEA, Tullius TD, Kallenbach NR, Seeman NC. A Holliday recombination intermediate is twofold symmetric; Proc Natl Acad Sci USA; 1988. pp. 4653–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tullius TD, Dombroski BA. Iron(II) EDTA used to measure the helical twist along any DNA molecule. Science. 1985;230:679–681. doi: 10.1126/science.2996145. [DOI] [PubMed] [Google Scholar]
- 30.Tullius TD, Dombroski BA. Hydroxyl radical “footprinting”: High-resolution information about DNA–protein contacts and application to lambda repressor and Cro protein; Proc Natl Acad Sci USA; 1986. pp. 5469–5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman NC. Current Protocols in Nucleic Acid Chemistry. New York: Wiley; 2002. Unit 12.1. [DOI] [PubMed] [Google Scholar]
- 32.Chen J-H, Churchill MEA, Tullius TD, Kallenbach NR, Seeman NC. Construction and analysis of mono-mobile DNA junctions. Biochemistry. 1988;27:6032–6038. doi: 10.1021/bi00416a031. [DOI] [PubMed] [Google Scholar]
- 33.Shen Z, Yan H, Wang T, Seeman NC. Paranemic cross-over DNA: A generalized Holliday structure with applications in nanotechnology. J Am Chem Soc. 2004;126:1666–1674. doi: 10.1021/ja038381e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoess R, Wierzbicki A, Abremski K. Isolation and characterization of intermediates in site-specific recombination; Proc Natl Acad Sci USA; 1987. pp. 6840–6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitts PA, Nash HA. Homology-dependent interactions in phage-lambda site specific recombination. Nature. 1987;329:346–348. doi: 10.1038/329346a0. [DOI] [PubMed] [Google Scholar]
- 36.Nunes-Duby SE, Matsumoto L, Landy A. Site-specific recombination intermediates trapped with suicide substrates. Cell. 1987;50:779–788. doi: 10.1016/0092-8674(87)90336-9. [DOI] [PubMed] [Google Scholar]
- 37.Cimino GD, Gamper HB, Isaacs ST, Hearst JE. Psoralens as photoactive probes of nucleic acid structure and function: Organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- 38.Esposito F, Brankamp RG, Sinden RR. DNA sequence specificity of 4,5′,8-trimethylpsoralen cross-linking. J Biol Chem. 1988;263:11466–11472. [PubMed] [Google Scholar]
- 39.Guo Q, Seeman NC, Kallenbach NR. Site-specific interaction of intercalating drugs with a branched DNA molecule. Biochemistry. 1989;28:2355–2359. doi: 10.1021/bi00432a001. [DOI] [PubMed] [Google Scholar]
- 40.Lu M, Guo Q, Kallenbach NR. The site-specific interaction of the anti-tumor antibiotic dynemicin with branched DNA molecules. J Biomol Struct Dyn. 1991;9:271–283. doi: 10.1080/07391102.1991.10507912. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russel DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2001. pp. 3.17–3.32. [Google Scholar]
- 42.Ottinger LM, Tullius TD. High-resolution in vivo footprinting of a protein–DNA complex using gamma-radiation. J Am Chem Soc. 2000;122:5901–5902. [Google Scholar]
- 43.Adilakshmi T, Lease RA, Woodson SA. Hydroxyl radical footprinting in vivo: Mapping macromolecular structures with synchrotron radiation. Nucleic Acids Res. 2006;34:e64. doi: 10.1093/nar/gkl291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zinder N, Horiuchi K. Multiregulatory element of filamentous bacteriophages. Microbiol Rev. 1985;49:101–106. doi: 10.1128/mr.49.2.101-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.