Abstract
Background
Before coronary evaluation by modern imaging techniques was feasible, premorbid diagnoses of coronary artery anomalies (CAAs) were usually made fortuitously by invasive coronary angiography (ICA). However, this technique is limited by its invasive and projectional nature. Coronary magnetic resonance angiography (CMRA) and multi-slice computed tomography (MSCT) broadened clinical information by enabling visualisation of the coronary arteries in their anatomical environment.
Methods
This case series visualises and reviews anomalous coronary artery from the opposite sinus (ACAOS) and coronary artery fistulae. All CAAs were detected by means of 64-slice dual source computed tomography after 1000 cardiac scans at the Erasmus MC, Rotterdam, the Netherlands.
Results
Eight ACAOS cases, one anomalous left coronary artery from the pulmonary artery (ALCAPA) and one congenital aneurysm of an aortic sinus were found. Seven out often detected CAAs were considered malignant whereas three CAAs of the ACAOS type (retroaortic path) were considered benign. Significant coronary artery disease was found in three out of eight ACAOS cases. In one of the ACAOS cases complete evaluation of the anomalous coronary artery was limited by motion artifacts. All five cases of right ACAOS were referred for MSCT because the right coronary artery could not be located by invasive angiography.
Conclusion
All CAAs were easy to diagnose because of 3D imaging and high temporal and spatial resolution. High resolution made it possible to not only depict coronary artery abnormalities, but also to quantify luminal and vessel properties such as stenosis grade, aspects of plaque, anomalous vessel length, luminal area ratio and the asymmetry ratio. Because of its comprehensiveness, MSCT can be an effective imaging modality in patients suspected of coronary artery abnormalities caused by coronary artery disease, CAAs, or a combination of both. (Neth Heart J 2008;16:369-75.)
Keywords: coronary anomalies, anomalous coronary artery from the opposite sinus, coronary artery fistulae, computed tomography, invasive coronary angiography
MSCT-coronary angiography (MSCT-CA) is a minimally invasive, 3D imaging modality. It is able to evaluate the grade of stenosis of the coronary vessel lumen in patients suspected of coronary artery disease.1 In this way it is possible to estimate the haemo-dynamic consequences of atherosclerosis and to guide clinical decision-making. Furthermore, MSCT-CA enables accurate assessment of the vessel wall and coronary anatomy in a 3D fashion, thereby allowing optimal relative positioning of anatomical structures.2 3D visualisation enables determination of vessel origin and course but also helps to identify the dependant myo-cardial territory, a basic principle of CAA classification.3
Methods
This case series visualises and reviews main coronary artery anomalies as detected by 64-slice dual source computed tomography (Siemens, Forchheim, Germany) at the Erasmus MC, Rotterdam, the Netherlands.
A total of 1000 patients, 656 male (65.6%), mean age 63.8 (±11.8), age range 25 to 88, were evaluated between 1 April 2006 and 25 January 2008. The majority of scans were performed because of complaints of typical chest pain. Scan data were analysed using a dedicated workstation.
We will focus on anomalous coronary artery from the opposite sinus (ACAOS) and coronary artery fistulae because of their severe clinical implications.4-8 Coronary bridging was excluded from the analysis because of its high prevalence by MSCT-CA and its obscure clinical implications. MSCT images were evaluated using a modified 17-segment classification model of the American Heart Association.
Results
In this case series we focused on ACAOS and coronary artery fistulae because of their potential clinical implications. Eight ACAOS cases, one ALCAPA and one congenital aneurysm of an aortic sinus were found (table 1). Seven out often detected CAAs were considered malignant whereas three CAAs of the ACAOS type (retroaortic path) were considered benign. Significant coronary artery disease was found in three out of eight ACAOS cases. In one of the ACAOS cases, calculation of both the luminal area ratio and the asymmetry ratio of the anomalous coronary artery was limited by motion artifacts. All five cases of right ACAOS were referred for MSCT because the right coronary artery could not be visualised by invasive angiography.
Table 1 .
Prevalence of main coronary findings.
| Results | |
|---|---|
| Coronary findings | Patients % (n) |
| Right dominance | 851 (85.1) |
| Left dominance | 88 (8.8) |
| Balanced | 61 (6.1) |
| ACAOS | |
| RCA(interarterial) | 0.05 (5) |
| LCx (retroaortic) | 0.01 (1) |
| LM (retroaortic) | 0.02 (2) |
| Coronary artery fistulae | |
| ALCAPA | 0.01 (1) |
| Aorto-atrial fistula | 0.01 (1) |
ACAOS=anomalous origin of coronary artery from the opposite sinus of Valsalva, RCA=right coronary artery, LCx=left circumflex coronary artery, LM=left main coronary artery, ALCAPA=anomalous origin of the left coronary artery from the pulmonary arterys
Coronary artery anatomy
Current coronary evaluation by MSCT focuses on the right coronary artery (RCA), the left main coronary artery (LM), the left anterior descending coronary artery (LAD) and the left circumflex coronary artery (LCx). The RCA and LM have their ostium at the anterior right and left sinus of Valsalva respectively (figure 1). The LM divides into the LAD and LCx coronary arteries. Both the RCA (from the right) and LCx (from the left) encircle the atrioventricular groove. The coronary system is called right or left dominant if the RCA (85%) or the LCx (7 to 8%) crosses the cardiac crux and gives of the posterior descending coronary artery (PDA). If both the RCA and LCx perfuse the inferior interventricular septum (7 to 8%), the coronary system is called co-dominant or balanced.9 See table 1 for dominancy figures found in this study.
Figure 1 .



Volume-rendered images showing normal coronary anatomy: A) The LAD and PDA form a half-loop (arrows) and perfuse the interventricular septum in this right dominant system (superior view). B) The RCA and LCx form two half-loops forming the atrioventricular groove (arrowheads). They send out several branches in apical direction perfusing the right and leftside of the heart, respectively. The LAD has several diagonal branches (D) perfusing the anterior wall of the left ventricle. Encircling the atrioventricular groove from the left, we see the left circumflex coronary artery giving off two marginal obtuse branches (MO). C) Anterior view of main coronary arteries. The LM divides into the LAD and the LCx. In this patient, the LAD gives off three diagonal branches. The RCA gives off a large right ventricular branch (RVB). Ao=aorta, AR=antero-right coronary sinus, AL=antero-left coronary sinus, PA=pulmonary artery, LA=left atrium, RA=right atrium, A=apex of the heart, PDA=posterior descending coronary artery.
From its ostium, the RCA courses to the right between the pulmonary artery (anteriorly) and the right atrium (posteriorly). In this segment it usually gives off both the conus branch, perfusing the right ventricular outflow tract and the sinoatrial node artery. The RCA continues its course downwards into the atrioventricular groove and gives off one or more ventricular branches and/or acute marginal branch(es). At the end of its course, the RCA can give rise to the posterior descending coronary artery (PDA) directed towards the apex of the heart and/or the posterolateral artery (PL). The PL courses towards the left side of the heart ending in one or more posterolateral ventricular branches (PLV) that traverse in apical direction.
After the division of the LM (5 to 10 mm long), the LAD runs through the anterior interventricular groove towards the apex of the heart, giving off diagonal branches that perfuse the anterior wall of the left ventricle and several septal branches perforating the interventricular septum. The LCx runs through the atrioventricular groove on the left side of the heart with several side branches (marginal obtuse arteries) that perfuse the lateral wall of the left ventricle. The LCx ends in a left posterolateral branch (LPL) and if it travels up to the cardiac crux, it can (co)form the PDA.
Coronary artery anomalies
Classification criteria of CAAs have been intensely discussed, but there is still no absolute consensus about the most effective way of specifying CAAs. The anatomy of the coronary system is highly variable and correlation between anatomy and symptomatology is often absent or at least difficult to prove.
Although CAAs are rare with reported prevalences of 0.3 to 5.64% of patients undergoing invasive coronary angiography and 0.3 to 0.5% of autopsies, they can be responsible for cardiovascular morbidity and even mortality.10-12 Malignant anomalies may have serious implications such as angina pectoris, myocardial infarction, syncope, cardiac arrhythmias, congestive heart failure, or sudden death.13 Especially in the young, coronary anomalies are an important cause of sport-related sudden cardiac death. Coronary anomalies rank second as a cardiovascular cause of sudden death in the young behind hypertrophic cardiomyopathy.14 Because of the potential implications of coronary artery anomalies, they require accurate recognition, observation, medical treatment, stent placement and at times, surgical correction.
Anomalous origin of coronary artery from the opposite sinus of Valsalva (ACAOS)
ACAOS is a major subgroup of CAAs that can be life threatening.4-8 There are four types of ACAOS: the LM (figure 2), LCx (figure 3) or LAD originates from the right sinus of Valsalva, or the RCA (figures 4 and 5) originates from the left sinus of Valsalva. Because at least one coronary ostium in ACAOS is connected to the opposite sinus of Valsalva, this CAA always implicates an abnormal course, redirecting the coronary artery to its dependant myocardium.
Figure 2 .

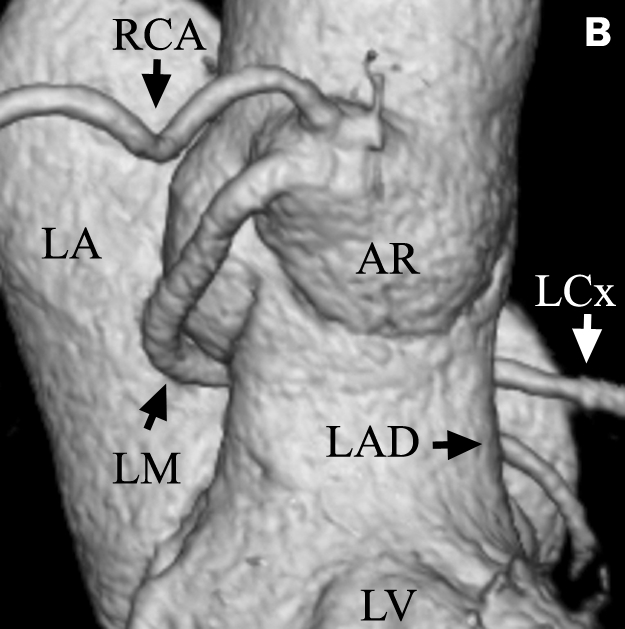

Left ACAOS (retroaortic course): A) Maximum intensity projection (MIP) of 4 mm thick slice. This image shows the LM originating from the right coronary sinus. The LM courses between the aorta and right atrium (dark surface) and between the aorta and leftatrium (highlighted surface) towards its dependent myocardial territory. Notethe bifurcation oftheLMintotheLCx andLAD coronary arteries (black arrowhead) and also the small conus branch with its separate ostium at the right sinus of Valsalva with a prevalence of about 50% (white arrowhead). B/C) Volume-rendered 3D images showing the contrast containing blood pool of the aorta, the coronary arteries and the left atrium. Only anatomy within the highest attenuation f actor range is visualised. Both the RCA and LM originate from the right sinus of Valsalva (anterior and posterolateral views). The LM curves backwards andfollows a retroaortic course. C) Image of the retroaortic course of the LM and its division in both the LAD and LCx coronary arteries. The upper part of the left atrium is made transparent. RA=right atrium, LA=left atrium, PA=pulmonary artery, LV=left ventricle, AR=anterior right coronary sinus, AL=anterior left coronary sinus, P=posterior coronary sinus, LCx=left circumflex coronary artery.
Figure 3 .
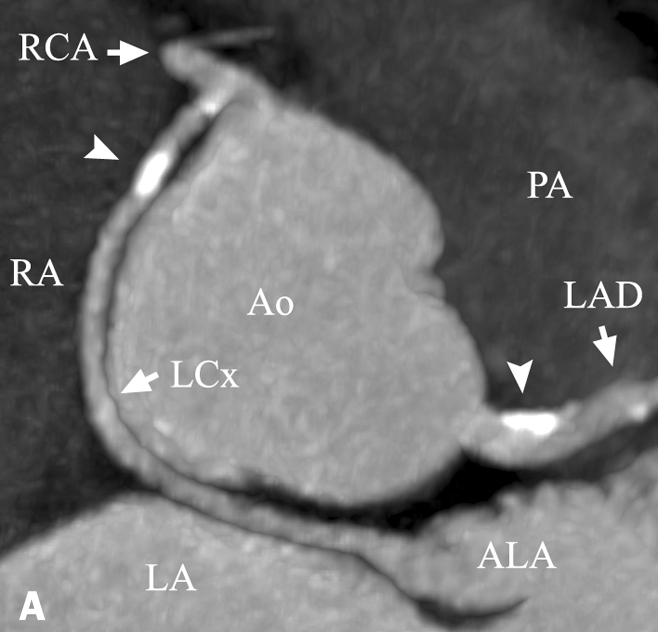


ACAOS of LCx (retroaortic course). The LCx has its ostium at the right coronary artery just after its origin at the anterior right coronary sinus: A) Maximum intensity projection (MIP) showing the anomalous course of the LCx between the aorta and the right and left atria. Note the calcified plaques in the LCx and the LM (arrowheads). Axial views of the LCX revealed significant stenosis (>50%) in this recently symptomatic patient. B) Volume-rendered image of coronary anatomy. The position of the PA was added for clarity. Note the LCx crossing thegreat cardiac vein (arrowheads). C) Invasive coronary angiography of the same patient. Note the stenosis in the LCx (arrowhead) at the location of the calcified lesion (figure A).
Figure 4 .



Right ARCAOS (interarterial course): A) Maximum intensity projection (MIP), superior view. The right coronary artery originates at the left sinus of Valsalva and courses between the aorta and the pulmonary artery. Note the significant calcified plaque (arrowhead). B) Volume-rendered image showing the anomalous path of the RCA (between arrowheads). C) Volume-rendered image showing the highly calcified plaque (white arrowhead). Note the high take-off of both the LM and RCA just above the aortic cusps. RA=right atrium, LA=left atrium, PA=pulmonary artery, Ao=aorta, AL/AR=anterior left and right aortic cusps, LV=left ventricle.
Figure 5 .



Right ACAOS (interarterial course): A) Volume-rendered image of left sinus of Valsalva (superior view) with B and C marking the intussuscepted and maximum vessel diameter respectively (pictures B and C). B) Luminal measurements at the ostium. '3D'software enables determination of the exact axial plane of a coronary artery. A 'pixel lens' was used to locate multiple 200 Houndsfield unit pixels for demarcation of the vessel border. The ovoid shape of the RCA was quantified by the asymmetry ratio as 0.33 (smallest/largest diagonal) (arrowheads). C) Cross section of RCA at point C. Luminal stenosis was quantified as 0.875 (luminal area ratio, see arrows).
The anomalous coronary artery can cross the aorta by four routes: retroaortic, interarterial, subpulmonary (beneath the right ventricular outflow tract) or pre-pulmonary. Because incidence figures are relatively low compared with the study populations used for research, global incidence figures vary considerably. Angelini found a global incidence of 0.92% for anomalous origination of the RCA from the left sinus of Valsalva (right ACAOS) and 0.15% for anomalous origination of the left coronary artery from the right sinus of Valsalva (left ACAOS).3
ACAOS can result in sudden cardiac death but symptoms can also be milder, such as palpitations, syncope, angina pectoris or dyspnoea.15 These symptoms are often the reason for coronary imaging. Haemo-dynamic significance and resulting symptomatology in ACAOS is directly related to the anomalous path of the vessel. Especially the interarterial course has a severe prognosis and is therefore referred to as malignant. However, the exact pathophysiological mechanism remains elusive.16,17 Traditionally, symptomatology was thought to be caused by the rising pressure within both the aorta and pulmonary artery during exercise, thereby compressing the anomalous coronary artery by a scissor-like mechanism. Recently, the theory of intussusception was added by Angelini and co-workers claiming that most interarterial coronary arteries are not in between the aorta and pulmonary artery at the point where the aorta and pulmonary artery are in closest proximity but are in fact incorporated in the aortic vessel wall.4 Angelini defines three possible mechanisms of stenosis. First, hypoplasia of the intussuscepted part of the vessel can cause coronary artery stenosis. Distally to the hypoplastic part of the vessel, luminal circumference increases. Obviously the intussuscepted part of the vessel cannot develop normally. A second mechanism is based on the ovoid shape of the intussuscepted coronary artery making it more susceptible to lateral forces thereby decreasing total luminal area during systole. The intussuscepted part of the vessel could, therefore, become haemodynamically significant during exercise if not at rest. A third mechanism correlates the stenosed length of the vessel to haemodynamic consequences. Because of its improved temporal and spatial resolution, MSCT technology enables us to measure the grade of stenosis, luminal shape and length of the anomalous path in ACAOS patients (figure 5). Main aspects of detected ACAOS cases are summarised in table 2.
Table 2 .
Main aspects of detected ACAOS.
| ACAOS type (n=8) | Right (1) | Right (2) | Right (3) | Right (4) | Right (5) | LCx (6) | Left (7) | Left (8) |
|---|---|---|---|---|---|---|---|---|
| Anomalous path | IA | IA | IA | IA | IA | Retroaortic | Retroaortic | Retroaortic |
| Ostium above coronary cusp | Yes | No | No | Yes | Yes | No | No | No |
| Prox. ovoid shape (yes/no) | Yes | Yes | Yes | Yes | Yes | No | No | No |
| Luminal area ratio | 1 | 0.5 | 0.875 | CTO after 1.8 cm | 1 | 1 | 1 | NM |
| Asymmetry ratio | 0.69 | 0.39 | 0.33 | 0.21 | 0.62 | 1 | 1 | NM |
| Max. vessel diameter after (cm) | 1.39 | 1.58 | 2.15 | CTO after 1.8 cm | NA | NA | NA | NA |
| Non-decisive CAG | Yes | Yes | Yes | Yes | Yes | No | No | No |
| Significant CAD (yes/no) | Yes | No | No | Yes | No | Yes | No | No |
| Treatment | Meds | CABG/ meds | Meds | PCI(LCx)/ meds | CABG/ Meds | Meds | None | Meds |
| Age (years) | 66 | 47 | 43 | 58 | 49 | 73 | 61 | 68 |
The luminal area ratio is defined as the ratio of the area of the vessel at its ostium with respect to the area of the vessel after its anomalous path (maximum vessel diameter). This ratio measures the grade of stenosis caused by the anomalous path of the coronary artery. The asymmetry ratio is defined as the ratio of the smallest to the largest diameter of the ovoid vessel It measures vulnerability for lateral compression of the vessel. The luminal area ratio and asymmetry ratio were not calculated for different cardiac phases. Definitions amended from Angelini et al.5,6 CAD=coronary artery disease, IA=interarterial, NA=not applicable, NM=non measurable because of motion artifacts, PCI=percutaneous coronary intervention, CABG=coronary artery bypass graft, CTO=chronic total obstruction, LCx=left circumflex.
Aortic root to right heart shunts
Three main categories of shunts involving the aortic root can be distinguished: congenital aneurysm of an aortic sinus of Valsalva with fistula, anomalous origin of the left coronary artery from the pulmonary artery also known as ALCAPA or Bland-White-Garland syndrome and coronary arterial-venous fistula (CAF). A congenital aneurysm of an aortic sinus of Valsalva is caused by separation or lack of fusion between the media of the aorta and the annulus fibrosis of the aortic valve. Usually, communication exists between the aorta and the right ventricle but the fistula can also drain into the right atrium (figure 6).
Figure 6 .


Aorto-atrial fistula (longitudinal and atrioventricular planes): A) Image of fistula between the aorta (supravalvular) and the right atrium. Calcification of the aortic valve is visible (black arrowhead). Note the tricuspid valve (black arrow). B) Image of atrioventricular plane (course of the RCA). Note the jet into the right atrium (arrowhead) showing a left to right shunt. RV=right ventricle, AR=anterior right sinus of Valsalva. Note the sinus coronarius (arrow).
ALCAPA, also known as the Bland-White-Garland syndrome, is seen in one in 300,000 live births.18 Prevalence of ARCAPA or anomalous right coronary artery arising from the pulmonary artery is estimated to be one in 50,000 of the general population.19 Because symptoms in ARCAPA are less pronounced as compared with ALCAPA and can even remain unnoticed, prevalence figures are not very robust and are based on a limited number of case reports.20 ARCAPA is generally not considered to be a lethal defect in infancy or childhood and often presents later in life.
ALCAPA (figure 7) is often noticed in early infancy or childhood by failure to thrive, irritability, pallor, cardiomegaly on chest radiography and ECG abnormalities suggestive of myocardial injury. If left untreated, approximately 90% of infants die before they are 12 months old and only a few survive into adulthood.21 After the second month of life, the vascular resistance of the pulmonary bed starts to decrease and therefore the pressure in the pulmonary artery drops. This causes decreased perfusing pressure of the anomalous LAD and diminished coronary flow to the dependant myocardium. Only if there is a substantial collateral flow can both the perfusing pressure and blood oxygenation suffice and ALCAPA remain unnoticed until adulthood or even later. Adults can be asymptomatic or more commonly have diverse symptoms such as syncope, angina pectoris, dyspnoea or sudden cardiac death.22,23
Figure 7 .
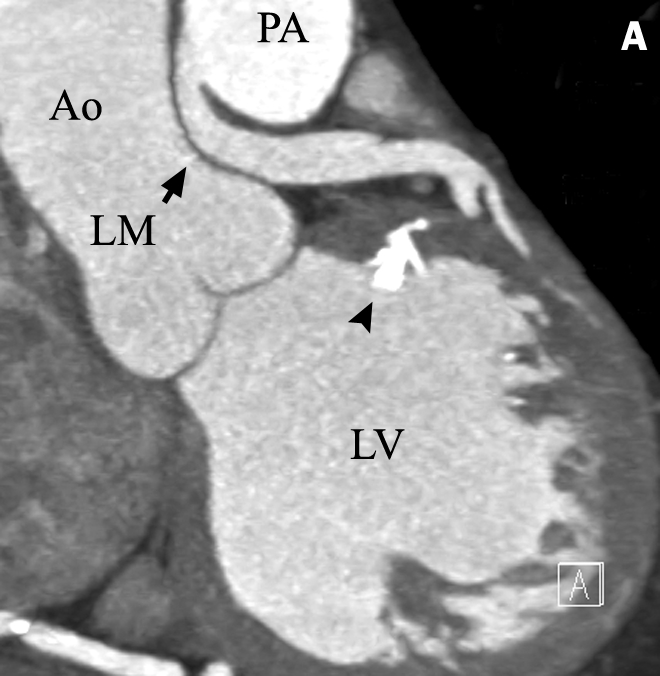


ALCAPA or Bland- White-Garland syndrome in a 61 -year-old female. After diagnosis, the anomalous LM was re-implanted in the aorta: A) Longitudinal view showing the connection between the pulmonary artery and the left main coronary artery. Note the difference in contrast intensity between the LM and the PA suggestive of retrograde flow in the left main coronary artery. In this patient extensive calcifications were visible in the RCA and left ventricle (arrowhead). B) Volume-rendered image (posterior view) showing the ostium of the LM at the right pulmonary artery. Note the large RCA with its impressive dependant myocardium. C) Anterior view of RCA with multiple collaterals directed towards the leftside of the heart. Note the corkscrew-like appearance of the large ventricular branch (VB) of the right coronary artery.
Because of the substantial radiation exposure and the use of potentially nephrotoxic contrast agents, MSCT is usually avoided in infants. Clinical history, plain chest radiography, electrocardiography, MRI and echocardiography are important for diagnosis. At older age, the risk of any adverse event caused by radiation diminishes and the risk/benefit ratio is shifted.24 3D capabilities of MSCT facilitate an accurate description of coronary artery anatomy including collateral flow, even in the hands of operators not specifically trained in diagnosing CAAs.25 This may facilitate the diagnosis of ALCAPA, especially if symptoms are less pronounced and/or variable, as in adults.
Discussion
We found a prevalence of 1% for ACAOS or coronary artery fistulae. The majority of detected CAAs (80%) were of the ACAOS type with a prevalence of 0.8%. Angelini and co-workers found a prevalence of 1.07% by invasive coronary angiography.25 Recently Cademartiri and colleagues reported a prevalence for ACAOS of 1.5% as detected by MSCT-CA in a series of 543 patients.26 According to the authors, the number of detected CAAs in this study was positively influenced by selection bias. More than 10% of their study population were referred for MSCT-CA after suspicion of a CAA by means of invasive coronary angiography. Because baseline characteristics differ between studies and the general population, generalisations should be taken with caution.
In addition to MSCT-CA, echocardiography and MRI form alternative noninvasive imaging modalities for diagnosing CAAs. In newborns, echocardiography supplemented with Doppler interrogation, both trans-oesophageal and transthoracic, is used for diagnosing large coronary fistulae and ALPACA. Echocardiog-raphy is highly accurate in detecting these CAAs without the use of ionising radiation or potentially nephrotoxic contrast agents.25 Unfortunately, at older age, when the BMI increases, transthoracic echo-cardiography becomes progressively less accurate and more operator dependant. Furthermore, evaluation by echocardiography is limited to the proximal part of the coronary arteries.27
MRI is the gold standard for functional assessment of the heart, it is noninvasive and uses no ionising radiation nor any nephrotoxic contrast agent.28 Furthermore coronary magnetic resonance angiography (CMRA) allows 3D visualisation of the main coronary arteries in their topographic environment, thereby circumventing an important drawback of invasive coronary angiography.25 However, its use is more time consuming as compared with MSCT-CA, repetitive (for some patients strenuous) breath-holds are required that are crucial for image quality and visualisation of the coronary arteries is incomplete, especially for the distal segments.29,30 With the use of CMRA, the intramural course and degree of compression inside the aortic wall in case of ACAOS can only be guessed at.25
Conclusion
The sensitivity of invasive angiography is limited by its projectional nature. In these cases CAAs can be detected by MSCT. All anomalies were easy to diagnose because of 3D imaging and high temporal and spatial resolution. High resolution has made it possible, not only to depict coronary artery abnormalities, but also to quantify luminal and vessel properties such as stenosis grade, aspects of plaque, anomalous vessel length, luminal area ratio and the asymmetry ratio.
Because of its comprehensiveness MSCT can be an effective imaging modality in patients suspected of coronary artery abnormalities caused by coronary artery disease, CAAs, or a combination of both. Familiarity with clinically significant CAAs and their appearance on MSCT images is essential for both effective diagnosis and patient treatment.
References
- 1.De Feyter PJ, Meijboom WB, Weustink A, Van Mieghem C, Mollet NR, Vourvouri E, et al. Spiral multislice computed tomography coronary angiography: a current status report. Clin Cardiol 2007;30:437-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi H Aschoff AJ, Brambs HJ, Hoffman MH. Multislice CT Imaging of anomalous coronary arteries. Eur Radiol 2004;14:2172-81. [DOI] [PubMed] [Google Scholar]
- 3.Angelini P. Coronary Artery Anomalies; An Entity in search of an Identity. Circulation 2007;115:1296-305. [DOI] [PubMed] [Google Scholar]
- 4.Angelini P, Trivellato M, Donis J, Leachman RD. Myocardial bridges: a review. Prog Cardiovasc Dis 1983;26:75-88. [DOI] [PubMed] [Google Scholar]
- 5.Angelini P, Velasco JA, Ott D, Khoshnevis GR. Anomalous coronary artery arising from the opposite sinus: descriptive features and pathophysiologic mechanisms, as documented by intravascular ultrasonography. J Invasive Cardiol 2003;15:507-14. [PubMed] [Google Scholar]
- 6.Angelini P, Walmsley RP, Libreros A, Ott DA. Symptomatic anomalous origination of left coronary artery from the opposite sinus of valsalva: clinical presentations, diagnosis, and surgical repair. Tex Heart Inst J 2006;33:171-9. [PMC free article] [PubMed] [Google Scholar]
- 7.Coronary artery surgery study (CASS): a randomized trial of coronary artery bypass surgery: survival data. Circulation 1983;68:939-50. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell JH, Haskell W, Snell P, Van Camp SP. Task Force 8: classification of sports. J Am Coll Cardiol 2005;45:1364-7. [DOI] [PubMed] [Google Scholar]
- 9.Reagan K, Boxt LM, Katz J. Introduction to coronary arterio-graphy. Radiol Clin North Am 1994;32:419-33. [PubMed] [Google Scholar]
- 10.Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation 2002;105:2449-54. [DOI] [PubMed] [Google Scholar]
- 11.Angelini P, Villason S, Chan AV, Diez JG. Normal and anomalous coronary arteries in humans. In: Angelini P, editor. Coronary Artery Anomalies: A comprehensive Approach. Philadelphia: Lippincott Williams & Wilkins; 1999:27-150. [Google Scholar]
- 12.Alexander RW, Griffith GC. Anomalies of the coronary arteries and their clinical significance. Circulation 1956:14;800-5. [DOI] [PubMed] [Google Scholar]
- 13.Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn 1990;21:28-40. [DOI] [PubMed] [Google Scholar]
- 14.Taylor AJ, Virmani R. Coronary Artery Anomalies. In: Crawford MH, DiMarco JP, Paulus W (editors). Cardiology, 2nd ed. New York: Mosby; 2004:201-11. [Google Scholar]
- 15.Perloff JK. Congenital anomalies of the coronary circulation. In: Perloff JK, editor. The Clinical Recognition of Congenital Heart disease, 4th ed. Philadelphia: WB Saunders; 1994:738. [Google Scholar]
- 16.Geuns van RJ, Cadematiri F. Anatomy of the coronary arteries and vein in CT imaging. In: Schoepf UJ, editor. CT of the heart. Totowa, NJ: Humana, 2005; 219-28. [Google Scholar]
- 17.Cheitlin MD, De Castro CM, Mc Allister HA. Sudden death as a complication of anomalous left coronary origin of the anterior sinus ofValsalva: a not-so-minor congenital anomaly. Circulation 1974;50:780-7. [DOI] [PubMed] [Google Scholar]
- 18.Dodge-Khatami A, Mavroudis C, Backer CL. Anomalous origin of the left coronary artery from the pulmonary artery: collective review of surgical therapy. Ann Thorac Surg 2002;74:946-55. [DOI] [PubMed] [Google Scholar]
- 19.Williams IA, Gersony WM, Hellenbrand WE. Anomalous right coronary artery arising from the pulmonary artery: A report of 7 cases and a review of the literature. Am Heart J 2006;152:1004.e9-1004e17. [DOI] [PubMed] [Google Scholar]
- 20.Su JT, Krishnamurthy R, Chung T, G Wesley Vick, Kovalchin JP. Anomalous Right Coronary Artery from the Pulmonary Artery: Noninvasive Diagnosis and Serial Evaluation. J Cardiovasc Magn Res 2007;9:57-62. [DOI] [PubMed] [Google Scholar]
- 21.Wesselhoeft H, Fawcett JS, Johnson AL. Anomalous origin of the left coronary artery from the pulmonary trunk: its clinical spectrum, pathology and pathophysiology, based on 140 cases with seven further cases. Circulation 1968;38:403-25. [DOI] [PubMed] [Google Scholar]
- 22.Frescura C, Basso C, Thiene G, Corrado D, Pennelli T, Angelini A, et al. Anomalous origin of coronary arteries and risk of sudden death: a study based on an autopsy population of congenital heart disease. Hum Pathol 1998;29:689-95. [DOI] [PubMed] [Google Scholar]
- 23.Taylor AJ, Rogan KM,Virmani R. Sudden cardiac death associated with isolated congenital coronary artery anomalies. J Am Coll Cardiol 1992;20:640-7. [DOI] [PubMed] [Google Scholar]
- 24.Zanzonico P, Rothenberg LN, Strauss HW. Radiation exposure of computed tomography and direct intracoronary angiography: risk has its reward. J Am Coll Cardiol 2006;47:1846-9. [DOI] [PubMed] [Google Scholar]
- 25.Angelini P, Flamm SD. Newer Concepts for Imaging Anomalous Aortic Origin of the Coronary Arteries in Adults. Cath Cardiovasc Int 2007;69:942-54. [DOI] [PubMed] [Google Scholar]
- 26.Cademartiri F, La Grutta L, Malagò, Alberghina F, Meijboom WB, Pugliese F, et al. Prevalence of anatomical variants and coronary anomalies in 543 consecutive patients studied with 64-slice CT coronary angiography. Eur Radiol 2008;18:781-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandes F, Alam M, Smith S, Khaja F. The role of transeso-phageal echocardiography in identifying anomalous coronary arteries. Circulation 1993;88:2532-40. [DOI] [PubMed] [Google Scholar]
- 28.Bunce NH, Lorenz CH, John AS, Lesser JR, Mohiaddin RH, Pennell DJ. Coronary artery anomalies: Assessment with free-breathing three dimensional coronary MR angiography. Radiology 2003;227:201-8. [DOI] [PubMed] [Google Scholar]
- 29.Nemes A, Geleijnse ML, van Geuns RJ, Soliman OI, Vletter WB, Krenning BJ, et al. Dobutamine stress MRI versus threedimen-sional contrast echocardiography: It's all Black and White. Neth Heart J 2008;16:217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mc Connell MV, Ganz P, Selwyn AP, Li W, Edelman RR, Manning WJ. Identification of anomalous coronary arteries and their anatomic course by magnetic resonance coronary angiography. Circulation 1995;92:3158-62. [DOI] [PubMed] [Google Scholar]


