Abstract
Purpose
To describe the prevalence of retinal vein occlusion (RVO) and its association with cardiovascular, inflammatory and hematological risk factors in a multi-ethnic cohort.
Methods
A population-based, cross-sectional study of 6,147 participants (whites, blacks, Hispanics, Chinese) from 6 U.S. communities. RVO was defined from retinal photographs taken from both eyes according to a standardized protocol. Risk factors were assessed from interviews, examinations, and laboratory and radiological investigations.
Results
The prevalence of RVO was 1.1% (0.9% for branch RVO and 0.2% for central RVO) and was similar across different ethnic groups: 0.9% in whites, 1.2% in blacks, 1.2% in Hispanics and 1.1% in Chinese (p=0.76). Independent risk factors associated with RVO were hypertension (odds ratio [OR] 2.06; 95% confidence interval [CI]: 1.18, 3.59), older age (OR 1.34, 95% CI: 1.00, 1.81, per decade increase), less education (OR 4.08, 95% CI: 2.20, 7.54), hypertriglyceridemia (OR 1.98; 95% CI: 1.10, 3.56), renal dysfunction (OR 1.85; 95% CI: 1.01, 3.39) and the presence of retinal arteriovenous nicking (OR 4.01; 95% CI: 2.06, 7.81) and focal arteriolar narrowing (OR 4.38; 95% CI: 1.44, 13.34). RVO was not significantly associated with direct measures of subclinical atherosclerosis (e.g., carotid intima media thickness and coronary artery calcium scores) or markers of inflammation (e.g., C reactive protein, interleukin-6), and endothelial dysfunction (e.g., soluble intercellular adhesion molecule-1) or coagulation (e.g., D-Dimer).
Conclusions
The prevalence of RVO is similar across different racial/ethnic groups. In the general population, RVO is associated with hypertension, dyslipidemia and renal dysfunction, but not with atherosclerotic disease, systemic inflammation and hematological abnormalities.
Retinal vein occlusion (RVO), including central and branch RVO, is a sight-threatening condition that most commonly affects middle-aged to older people. There are limited population-based studies on the epidemiology of RVO, with reported prevalence rates ranging from 0.3% to 1.6%.1-4 Reasons for the variability in prevalence rates may in part be due to racial/ethnic differences in study populations. Indeed, racial/ethnic variations in the prevalence of other major retinal diseases, such as age-related macular degeneration and diabetic retinopathy, are well recognized,5, 6 but ethnicity-specific prevalence data on RVO are lacking.
Although RVO has been associated with a variety of cardiovascular risk factors, its pathogenesis remains incompletely understood. Previous studies indicate that hypertension and related hypertensive retinal arteriolar changes (e.g., arteriovenous nicking) are the strongest and most consistent risk factors for RVO.1-3, 7 Studies have found less consistent relationships with other cardiovascular risk factors, such as smoking, diabetes, obesity, hyperhomocysteinemia and dyslipidemia.1, 2, 8 Furthermore, few studies have examined whether novel cardiovascular risk factors, such as biomarkers of inflammation (e.g., C-reactive protein), endothelial dysfunction (e.g., soluble intercellular adhesion molecule-1) and direct measures of subclinical systemic atherosclerosis (e.g., carotid artery disease), are also major risk factors for RVO in the general population.
The purposes of this study are to describe the prevalence of RVO in a multi-ethnic population-based cohort of whites, blacks, Hispanics, and Chinese, and to examine the associations of RVO with a range of traditional (e.g., blood pressure, diabetes, lipids, cigarette smoking) and novel (e.g., inflammation, hematological, endothelial dysfunction, subclinical atherosclerosis) cardiovascular risk factors.
METHODS
Study Population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study of men and women aged 45 to 84 years without a history of clinical cardiovascular disease, sampled from six United States communities.9 In brief, there were 6,814 participants at the first examination (July 2000 to August 2002). Of these, 6,176 returned for the second examination and had retinal photographs taken (August 2002 to January 2004).5, 10, 11 There were 29 participants with ungradable photographs for both eyes (mostly due to media opacities from cataract). These participants were excluded, leaving 6,147 for analysis.
The tenets of the Declaration of Helsinki were observed, and institutional review board approval was granted at each study site. Written informed consent was obtained from each participant. All procedures followed were in accordance with institutional guidelines.
Retinal Photography
Fundus photography was performed at each site using a standardized protocol.5, 10-12 Participants were seated in a darkened room and both eyes were photographed using a 45-degree digital non-mydriatic camera. Two photographic fields (optic disc and macula centered) were taken from both eyes. All images were evaluated by trained graders masked to participants' characteristics at the Fundus Photograph Reading Center at the University of Wisconsin, Madison. The presence or absence of either central or branch RVO was defined according to a standardized protocol following the Beaver Dam Eye Study.1, 2 Recent central RVO was characterized by retinal edema, optic disc hyperemia or edema, scattered superficial and deep retinal hemorrhages, and venous dilation. Old central RVO were characterized by occluded and sheathed retinal veins, or vascular anastomosis at the optic disc. Branch RVO involved a more localized area of the retina in the sector of the obstructed venule and were characterized by scattered superficial and deep retinal hemorrhages, venous dilation, intraretinal microvascular abnormalities, and occluded and sheathed retinal venules. All cases of RVO were adjudicated by a retinal specialist (RK). The presence of any RVO was defined as presence of branch or central RVO in either right or left eye.
Retinal arteriovenous nicking and focal arteriolar narrowing were also graded from retinal photographs according to a standardized protocol.13 Retinal vascular caliber was measured using a computer-based program based on a detailed protocol described elsewhere.10-15 Reproducibility of retinal measurements has been previously reported, with intra- and inter-grader intraclass-correlation coefficients ranging from 0.78 to 0.99.10-15 Retinal vascular caliber was measured from one eye. Other qualitative retinal signs (e.g., arteriovenous nicking and focal arteriolar narrowing) were graded for both eyes.
Assessments and Definitions of Cardiovascular Risk Factors
Participants underwent a detailed and comprehensive interview, clinical examinations, and laboratory investigations for the assessment of cardiovascular risk factors, as described in detail in previous publications.9-11, 14 Variables for this analysis were based on data collected at the second examination, unless not available, in which case, data from the baseline examination were used. Resting blood pressure was measured using a standardized protocol and hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or current use of antihypertensive medications. Diabetes mellitus was defined as fasting glucose ≥7.0 mmol/L (126 mg/dL) or use of insulin or oral hypoglycemic medication. Body mass index was categorized as normal or overweight (grade 1, 2 or 3) according to the World Health Organization definitions. A detailed questionnaire was used to obtain information about education levels, medical history (e.g., hypertension, diabetes), cigarette smoking, alcohol consumption, and medication use (e.g., oral steroids, aspirin).
Fasting (>8 hours) blood samples were drawn from participants and analyzed for all the blood factors examined in this study following standardized protocols.14, 16 Hypertriglyceridemia was defined as triglyceride level of ≥200 mg/dL. Hypercholesterolemia was defined as total cholesterol >240 mg/dL. Hyperhomocysteinemia was defined as plasma homocysteine level of >12 micromol/L.17 Estimated glomerular filtration rate (eGFR, ml/min per 1.73m2) was calculated from serum creatinine using the refined Modification of Diet in Renal Disease equation.18 Renal dysfunction was defined as absent (eGFR≥90) or present (eGFR<90). When renal dysfunction was present, it was further categorized into mild (60≤eGFR<90) and moderate-severe (eGFR<60).19 Biomarkers of inflammation (e.g., C-reactive protein, interleukin-6), endothelial dysfunction (e.g., soluble intercellular adhesion molecule-1) and coagulation (e.g., D-dimer, Factor VIII, fibrinogen) and other hematological abnormalities (e.g., sphingomyelin, plasmin-α2-antiplasmin complex) were also measured as detailed elsewhere.14, 20
Detailed assessment of subclinical atherosclerosis was performed as one of the primary objectives of the MESA.16, 21 Carotid ultrasonography was used to determine carotid atherosclerosis and defined as present if ≥25% stenosis.21 Chest computed tomography was used to quantify coronary atherosclerosis and defined as present if Agatston calcium score ≥200.21 Ankle-brachial index was used to assess peripheral arterial atherosclerosis and was defined as present if ankle-brachial index <0.9.21
Statistical Analysis
RVO was analyzed as a binary outcome variable (present vs. absent). Cardiovascular risk factors were analyzed as present versus absent for binary traits (e.g., hypertension, renal disease, carotid artery disease) and categorized into quartiles or standard deviation difference for continuous traits (e.g., blood pressure, C-reactive protein, interleukin-6). More severe grading of the qualitative retinal signs (e.g., focal arteriolar narrowing, arteriovenous nicking) was sued in the person-specific analyses. Analysis of variance (ANOVA) or Chi-square tests were used to compare the prevalence rates of central, branch and any RVO by age, gender, ethnic groups. We used logistic regression models to estimate odds ratios (OR) for any RVO for each risk factor, adjusting for age, gender, ethnicity and study center. In multivariate analysis, we selected variables for simultaneous inclusion in the regression model if candidate variables were independently significant at p<0.05 in the model adjusting for age, gender, ethnicity and study center. As central and branch RVO may have differences in pathogenesis, we repeated the multivariate analysis after excluding central RVO in supplementary analysis. We also examined interactions with race by inclusion of cross-product terms of race and each risk factor in the models. All analyses were performed in SPSS version 12.0.1 (SPSS Inc, Chicago, Ill).
RESULTS
Participants' characteristics in the MESA have been previously presented.5, 14 In our study population, there were 66 cases of RVO (57 branch RVO and 9 central RVO) identified (65 participants). One participant had bilateral RVO (central RVO on the left and branch RVO on the right eye). This participant did not have any retinal microvascular characteristics. Table 1 shows that the prevalence of RVO increased with age (p≤0.01), but did not vary by gender. The Figure shows that the prevalence rates of RVO and its subtypes were largely similar across different ethnic groups, ranging from 0.9% in whites to 1.2% in blacks and Hispanics.
Table 1.
Prevalence of Retinal Vein Occlusion by Age and Gender Groups, the Multi-Ethnic Study of Atherosclerosis
| Branch Retinal Vein Occlusion |
Central Retinal Vein Occlusion |
Any Retinal Vein Occlusion |
|||||
|---|---|---|---|---|---|---|---|
| N | n (%) | p* | n (%) | p* | n (%) | p* | |
| Overall | 6147 | 57 (0.9) | 9 (0.2) | 65 (1.1) | |||
| Age, years | |||||||
| 45−54 | 1437 | 4 (0.3) | 0.01 | 0 (0.0) | 0.01 | 4 (0.3) | 0.002 |
| 55−64 | 1805 | 19 (1.1) | 0 (0.0) | 19 (1.1) | |||
| 65−74 | 1896 | 22 (1.2) | 7 (0.4) | 29 (1.5) | |||
| 75 and above | 1009 | 12 (1.2) | 2 (0.2) | 13 (1.3) | |||
| Gender | |||||||
| Male | 2931 | 31 (1.1) | 0.31 | 4 (0.1) | 0.85 | 35 (1.2) | 0.31 |
| Female | 3216 | 26 (0.8) | 5 (0.2) | 30 (0.9) | |||
RVO = retinal vein occlusion; N = No. at risk; n = No. with event; % = prevalence
p-value for trend for age groups or based on chi-square test comparing ethnic and gender differences
Figure.
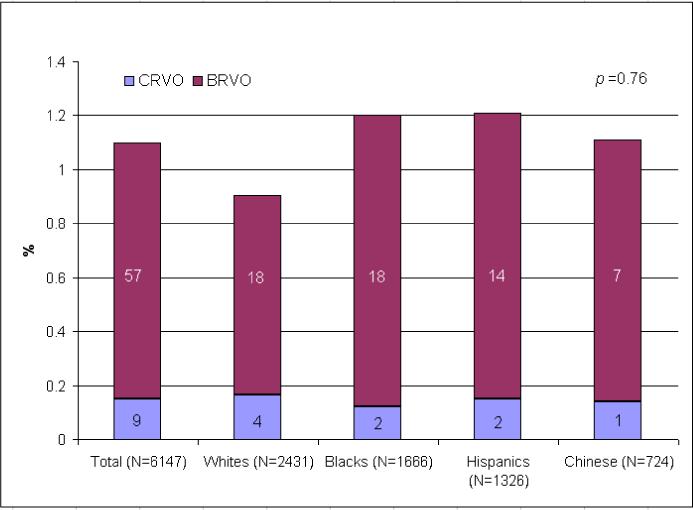
Prevalence of Retinal Vein Occlusion in the Multi-Ethnic Study of Atherosclerosis (CRVO, central retinal vein occlusion; BRVO, branch retinal vein occlusion)
Table 2 shows the associations of RVO with various traditional cardiovascular risk factors. After adjusting for age, gender, ethnicity and study center, each decade increase in age, lower education level, hypertension, higher blood pressure, and hypertriglyceridemia were associated with increased odds of RVO. Diabetes and smoking were not associated with RVO.
Table 2.
Relationships of Traditional Cardiovascular Risk Factors and Retinal Vein Occlusion, the Multi-Ethnic Study of Atherosclerosis
| Any Retinal Vein Occlusion |
||
|---|---|---|
| Traditional Risk Factors | OR (95% CI)* | p |
| Age per 10 years increase | 1.44 (1.12, 1.85) | 0.005 |
| Education (<8 years vs. ≥8 years) | 3.87 (2.07, 7.23) | <0.001 |
| Smoking, current vs. never | 1.03 (0.44, 2.39) | 0.95 |
| Alcohol, current vs. never | 0.79 (0.44, 1.41) | 0.79 |
| Hypertension | 2.32 (1.34, 4.01) | 0.003 |
| Systolic blood pressure, per SD increase, mmHg | 1.53 (1.21, 1.92) | <0.001 |
| Diastolic blood pressure, per SD increase, mmHg | 1.71 (1.35, 2.17) | <0.001 |
| Pulse pressure, per SD increase, mmHg | 1.41 (1.17, 1.69) | <0.001 |
| Diabetes, present vs. absent | 1.18 (0.62, 2.23) | 0.62 |
| BMI (WHO categories) | ||
| Normal (<25 kg/m2) | Reference | |
| Grade 1 Overweight (≥25 but <30 kg/m2) | 0.94 (0.51, 1.72) | 0.83 |
| Grade 2 Overweight (≥30 but <40 kg/m2) | 1.06 (0.55, 2.04) | 0.56 |
| Grade 3 Overweight (≥40 kg/m2) | 1.00 (0.23, 4.41) | 0.99 |
| Hypercholesterolemia, >240mg/dL | 1.08 (0.48, 2.42) | 0.86 |
| LDL cholesterol, per SD increase, mg/dL | 0.91 (0.70, 1.17) | 0.46 |
| HDL cholesterol, per SD increase, mg/dL | 0.93 (0.70, 1.23) | 0.60 |
| Hypertriglyceridemia, ≥200 mg/dL | 2.07 (1.17, 3.69) | 0.01 |
Risk factors examined but not significant (p>0.05) include pack-years of smoking, fasting glucose, glycosylated hemoglobin, diabetes duration, medications (aspirin, oral steroids, non-steroidal anti-inflammatory drugs, hormone replacement therapy).
BMI = body mass index; WHO = World Health Organization; LDL = low density lipoprotein; HDL = high density lipoprotein; SD = standard deviation
Odds ratio (95% confidence interval) adjusted for age (except for increase in age), gender, ethnicity and study center
Table 3 shows the association of RVO with novel risk factors, including biomarkers of inflammation, coagulation/hematological abnormalities, renal dysfunction, retinal microvascular characteristics and subclinical atherosclerosis. Of the range of risk factors examined, only hyperhomocysteinemia and increased serum creatinine were significantly associated with RVO. Other markers of inflammation (e.g., C-reactive protein) and endothelial dysfunction (e.g., soluble intercellular adhesion molecule-1) were not associated with RVO. Increased odds of RVO were also seen in people with renal dysfunction, retinal arteriovenous nicking and focal arteriolar narrowing. The presence of atherosclerotic disease in the carotid, coronary, and peripheral arteries was not associated with RVO.
Table 3.
Relationships of Novel Inflammatory, Hematological and Atherosclerotic Risk Factors and Retinal Vein Occlusion, the Multi-Ethnic Study of Atherosclerosis
| Any Retinal Vein Occlusion |
||
|---|---|---|
| Novel Risk Factors | OR (95% CI)* | p |
| Inflammatory factors | ||
| C-reactive protein, per SD increase, mg/L | 0.94 (0.71, 1.25) | 0.66 |
| Fibrinogen, per SD increase, mg/dl | 0.93 (0.72, 1.21) | 0.60 |
| Hematological / Coagulation factors | ||
| Hyperhomocysteinemia, > 12 micromol/L | 1.88 (1.04, 3.41) | 0.04 |
| Creatinine, per SD increase, mg/dL | 1.21 (1.05, 1.39) | 0.008 |
| Factor VIII, per SD increase, % | 0.94 (0.73, 1.21) | 0.62 |
| D-dimer, per SD increase, μg/ml | 1.09 (0.84, 1.42) | 0.50 |
| Renal dysfunction | ||
| Absent | Reference | |
| Mild | 2.23 (1.28, 3.88) | 0.005 |
| Moderate-Severe | 3.73 (1.57, 8.88) | 0.003 |
| Retinal microvascular characteristics | ||
| Arteriovenous nicking | 5.71 (3.04, 10.70) | <0.001 |
| Focal arteriolar narrowing | 6.35 (2.17, 18.61) | 0.001 |
| Subclinical atherosclerosis | ||
| Carotid artery atherosclerosis, ≥25% stenosis | 1.34 (0.69, 2.59) | 0.39 |
| Coronary artery atherosclerosis, Agatston calcium score ≥200 | 1.22 (0.65, 2.28) | 0.54 |
| Peripheral artery atherosclerosis, ankle-brachial index <0.9 | 1.00 (0.30, 3.26) | 0.99 |
Risk factors examined but not significant (p>0.05) include interleukin-6, sphingomyelin, plasmin-α2-antiplasmin complex, soluble intercellular adhesion molecule-1, retinal arteriolar and venular calibers.
Odds ratio (95% confidence interval) adjusted for age, gender, ethnicity and study center
The final multivariable model is presented in Table 4. RVO was significantly related to older age, less education, hypertension, hypertriglyceridemia, renal dysfunction, retinal arteriovenous nicking and focal arteriolar narrowing. The association between less education and RVO was present in all ethnic groups and was significant even after further adjustments for income and cigarette smoking (both are not significant risk factors in our study). In supplementary analysis after excluding central RVO, the associations of branch RVO with these risk factors remained (data not shown).
Table 4.
Relationships of Risk Factors and Retinal Vein Occlusion in Multivariate Analysis, the Multi-Ethnic Study of Atherosclerosis
| Any Retinal Vein Occlusion |
||
|---|---|---|
| Risk Factors | OR (95% CI)* | p |
| Age, per 10 years increase | 1.34 (1.00, 1.81) | 0.05 |
| Education (<8 years vs. ≥8 years) | 4.08 (2.20, 7.54) | <0.001 |
| Hypertension | 2.06 (1.18, 3.59) | 0.01 |
| Hypertriglyceridemia | 1.98 (1.10, 3.56) | 0.02 |
| Renal dysfunction (present vs. absent) | 1.85 (1.01, 3.39) | 0.04 |
| Creatinine, per SD increase, mg/dL | 1.20 (1.06, 1.37) | 0.004 |
| Arteriovenous nicking | 4.01 (2.06, 7.81) | <0.001 |
| Focal arteriolar narrowing | 4.38 (1.44, 13.34) | 0.01 |
| Hyperhomocysteinemia | 1.40 (0.74, 2.63) | 0.30 |
Odds ratio (95% confidence interval) adjusted for age, gender, ethnicity, study center, systolic and diastolic blood pressure (except for hypertension), education status (except for education), and serum levels of creatinine (except for renal dysfunction), renal dysfunction (except for creatinine), homocysteine (except for hyperhomocysteinemia), and triglyceride (except for hypertriglyceridemia)
We also performed several additional supplementary analyses (data not shown). First, to exclude non-linear associations of RVO with the variables that were analyzed as continuous variables (e.g., per standard deviation difference in factor VIII, D-Dimer), we categorized these variables into binary traits (above versus below 90th percentile) and repeated the logistic regression analyses to assess their associations with RVO. The results were largely similar, with no additional significant associations found. Second, we have compared means and prevalence of the risk factors examined between the small number of people with CRVO and people with BRVO. There were no significant differences. Finally, we also examined potential interactions with ethnicity for the significant risk factors and found no statistically significant interactions (all p>0.05, Table 4).
DISCUSSION
Our study provides new population-based data on the prevalence of RVO in a multi-ethnic sample of people in the general population without a history of clinical cardiovascular disease. We found an overall prevalence of RVO of 1.1% with rates similar across the four different ethnic groups in the U.S., namely the whites, blacks, Hispanic and Chinese Americans. We also examined the associations of RVO with a wide spectrum of traditional and novel cardiovascular risk factors. Besides hypertension, we found that hypertriglyceridemia, renal dysfunction, and focal retinal arteriolar abnormalities were associated with RVO. The association between homocysteine and RVO might be a result of confounding from other vascular risk factors. Importantly, we did not find any association of RVO with many markers of systemic inflammation, endothelial dysfunction and hematological/coagulation diseases, and with direct measures of subclinical atherosclerosis in the carotid, coronary and peripheral leg circulation.
Despite the importance of RVO as a major cause of visual loss, there are only a small number of population-based studies that have previously examined the prevalence of RVO. The Blue Mountains Eye Study (BMES) reported, in white Australians, the highest rate of RVO (1.6%). The pooled data report on white and black participants in the Atherosclerosis Risk in Communities Study and Cardiovascular Health Study (ARIC & CHS) reported the lowest rate (0.3%) of RVO.1, 3 In the predominantly white population in the Beaver Dam Eye Study (BDES), a prevalence estimate of 0.6% was reported.2 It has been suggested that these variations may be related to racial/ethnic differences. Our study, however, shows clearly that there are no major racial/ethnic differences in the prevalence of RVO. Thus, previously observed variations in prevalence estimates are likely related to other differences between these studies, including study design, participant characteristics (e.g., older age corresponding to the higher prevalence rate in the BMES, cultural or environmental differences) and study methodology (e.g., retinal photography from only one eye with two fields corresponding to the lower prevalence in the ARIC & CHS, contrasting to the BMES with retinal photographs from both eyes, each with six fields).
Our data confirm the strong and known association of hypertension and related focal retinal arteriolar abnormalities with RVO, consistent with clinical knowledge and other population studies.1-3 Retinal arteriolar signs, such as arteriovenous nicking and focal narrowing, represent established microvascular damage caused by long-standing elevated blood pressure. It has been suggested that sclerotic retinal arteriolar walls may compress underlying venules, especially at arteriovenous crossings. This in turn can transform the normal laminar venous blood flow into turbulent flow, facilitating the formation of venous thrombus and downstream venular occlusion.22 In addition, arteriolar insufficiency resulting from focal arteriosclerosis has been suggested to play a pathogenic role in RVO.2, 23 These hypotheses are supported by prospective data from the BMES and the BDES, demonstrating that arteriovenous nicking and focal arteriolar narrowing are strong predictors of incident RVO.2, 7, 23
We also found that people with hypertriglyceridemia and hyperhomocysteinemia were more likely to have RVO. These associations are important as both of these risk factors are modifiable and known to be associated with venous thrombosis. Several case-control studies have documented higher levels of triglyceride in patients with RVO.24, 25 Our study extends these observations to an ethnically diverse population without overt cardiovascular disease. Alterations in platelet function, enhanced procoagulation, and plasma viscosity may contribute to the link between hypertriglyceridemia and RVO.25-27 Similarly, elevated homocysteine level has been associated with RVO in several studies, including the BMES.8, 28-30 It has been proposed that the oxygen free radicals generated from oxidation of homocysteine can cause venous endothelial cell damage, initiating coagulation processes that ultimately lead to formation of venular thrombosis.29 Nevertheless, as shown in our multivariate analysis, the association between hyperhomocysteinemia and RVO attenuated and lost its statistical significance, indicating that it could be a result of shared pathways from related cardiovascular risk factors, such as renal function.
Persons with higher creatinine levels and with renal dysfunction, even those with only mild impairment, were more likely to have RVO in our study. These findings agree with recent prospective data from the BDES, in which persons with elevated creatinine level (≥1.4 mg/dl) were shown to have 60% higher risk of RVO over 15 years of follow-up.23 However, the underlying pathophysiological mechanisms are unclear. Since RVO and nephropathy are both closely related to hypertension, one may expect this association to be due to concomitant damage in the retinal and renal vasculature by hypertension. Although we have adjusted for blood pressure in our multivariate models, residual confounding from long-term exposure to high blood pressure cannot be totally excluded. Additional studies are clearly needed to further elucidate the mechanistic pathways that may underlie the association between renal dysfunction and RVO risk.
There has been much controversy regarding the relationship between RVO and systemic atherosclerosis.28 While some investigators believe that atherosclerosis plays an important role in the pathogenesis of RVO, previous studies have provided inconsistent results.1, 31-35 The MESA provides a unique opportunity to address this, as participants had an extensive and detailed assessment of atherosclerosis in three major arterial beds (carotid artery via ultrasonography, coronary artery via chest computer tomography and lower-limb peripheral arteries via ankle-brachial index). Similarly, we examined a range of markers of systemic inflammation, endothelial dysfunction and hematological/coagulation diseases. We found no association with these measures, suggesting that in the general population, atherosclerosis, inflammation and hematological disease are not major risk factors for RVO.
Of note, we found a strong association between lower education and RVO, even after adjusting for potential socioeconomic (income) and lifestyle (e.g., cigarette smoking) factors. The reason underlying this association remains to be determined, but could be related to poorer control of unaccounted cardiovascular risk factors that may be important in the development of RVO (e.g., chronic or uncontrolled hypertension and dyslipidemia).
Strengths of our study include a large, multi-ethnic population-based sample of people, masked grading of two-field retinal photographs from both eyes with only few ungradable cases, and standardized and comprehensive assessment of cardiovascular risk factors. However, several limitations merit consideration. First, inferences to causation are limited by the study's cross-sectional design. Second, RVO were ascertained from two digital images per eye taken by a 45-degree non-stereoscopic, non-mydriatic fundus camera. Thus, our study results may not be directly comparable to some other population-based studies which used different methodologies (e.g., 30-degree stereoscopic photographs of six fields in the BMES and BDES).2, 3 Third, the rarity of RVO in our population might have limited our statistical power to detect associations for some risk factors and for sub-analysis of branch and central RVO. Additional studies with larger samples might be needed to verify our negative findings. Fourth, our study population was confined to persons without clinical cardiovascular disease. This may limit the generalizability of findings to people with cardiovascular disease and may also explain the lack of associations with some vascular risk factors, such as diabetes and smoking.2, 23 Nevertheless, our generally healthy study sample can also be considered a strength, as it minimizes the effects of cardiovascular medical treatments on the results. Finally, given the large number of risk factors examined, some findings could potentially be due to chance (e.g., education).
In summary, we showed that RVO occurs in 1% of the general adult population 45 years and older, and do not show substantial racial/ethnic differences in prevalence. In this respect, the epidemiology of RVO is different from age-related macular degeneration and diabetic retinopathy, which demonstrate clear racial/ethnic variations. RVO is strongly associated with hypertension and retinal arteriolar abnormalities, and may also be related to hypertriglyceridemia and renal dysfunction. However, in generally healthy people population, measures of atherosclerosis, systemic inflammation and hematological dysfunction are not major risk factors for RVO.
ACKNOWLEGEMENTS
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding:
This research was supported by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. Additional support was provided by NIH grant HL69979−03 (Klein R and Wong TY) and NEI Z01EY000403 (Cotch MF).
Footnotes
Conflicts of Interests/Disclosures: None.
REFERENCES
- 1.Wong TY, Larsen EK, Klein R, et al. Cardiovascular risk factors for retinal vein occlusion and arteriolar emboli: the Atherosclerosis Risk in Communities & Cardiovascular Health studies. Ophthalmology. 2005;112(4):540–547. doi: 10.1016/j.ophtha.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–141. [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol. 1996;114(10):1243–1247. doi: 10.1001/archopht.1996.01100140443012. [DOI] [PubMed] [Google Scholar]
- 4.Liu W, Xu L, Jonas JB. Vein occlusion in Chinese subjects. Ophthalmology. 2007;114(9):1795–1796. doi: 10.1016/j.ophtha.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Klein R, Klein BE, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology. 2006;113(3):373–380. doi: 10.1016/j.ophtha.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Wong TY, Klein R, Islam FM, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141(3):446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006;124(5):726–732. doi: 10.1001/archopht.124.5.726. [DOI] [PubMed] [Google Scholar]
- 8.Chua B, Kifley A, Wong TY, Mitchell P. Homocysteine and retinal vein occlusion: a population-based study. Am J Ophthalmol. 2005;139(1):181–182. doi: 10.1016/j.ajo.2004.06.084. [DOI] [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 10.Cheung N, Bluemke DA, Klein R, et al. Retinal arteriolar narrowing and left ventricular remodeling: the multi-ethnic study of atherosclerosis. J Am Coll Cardiol. 2007;50(1):48–55. doi: 10.1016/j.jacc.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung N, Sharret AR, Klein R, et al. Aortic distensibility and retinal arteriolar narrowing: the Multi-Ethnic Study of Atherosclerosis. Hypertension. 2007;50(4):617–622. doi: 10.1161/HYPERTENSIONAHA.107.091926. [DOI] [PubMed] [Google Scholar]
- 12.Cheung N, Islam AFM, Jacobs DR, et al. Arterial compliance and retinal vascular caliber in cerebrovascular disease. Annals of Neurology. 2007;62(6):618–624. doi: 10.1002/ana.21236. [DOI] [PubMed] [Google Scholar]
- 13.Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106(12):2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 14.Wong TY, Islam FM, Klein R, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA). Invest Ophthalmol Vis Sci. 2006;47(6):2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong TY, Knudtson MD, Klein R, Klein BE, Meuer SM, Hubbard LD. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 2004;111(6):1183–1190. doi: 10.1016/j.ophtha.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 16.Nelson JC, Jiang XC, Tabas I, Tall A, Shea S. Plasma sphingomyelin and subclinical atherosclerosis: findings from the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;163(10):903–912. doi: 10.1093/aje/kwj140. [DOI] [PubMed] [Google Scholar]
- 17.Castanon MM, Lauricella AM, Kordich L, Quintana I. Plasma homocysteine cutoff values for venous thrombosis. Clin Chem Lab Med. 2007;45(2):232–236. doi: 10.1515/CCLM.2007.038. [DOI] [PubMed] [Google Scholar]
- 18.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141(12):929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 19.Kottgen A, Russell SD, Loehr LR, et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2007;18(4):1307–1315. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 20.Ranjit N, Diez-Roux AV, Shea S, et al. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch Intern Med. 2007;167(2):174–181. doi: 10.1001/archinte.167.2.174. [DOI] [PubMed] [Google Scholar]
- 21.Holvoet P, Jenny NS, Schreiner PJ, Tracy RP, Jacobs DR. The relationship between oxidized LDL and other cardiovascular risk factors and subclinical CVD in different ethnic groups: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2006 doi: 10.1016/j.atherosclerosis.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Verougstraete C. Is change of the vessel wall a risk factor for venous thrombosis? Lancet. 1999;353(9170):2158. doi: 10.1016/S0140-6736(05)75596-3. [DOI] [PubMed] [Google Scholar]
- 23.Klein R, Moss SE, Meuer SM, Klein BEK. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol. 2007 doi: 10.1001/archopht.126.4.513. In Press. [DOI] [PubMed] [Google Scholar]
- 24.Brown GC. Central retinal vein obstruction with lipid exudate. Arch Ophthalmol. 1989;107(7):1001–1005. doi: 10.1001/archopht.1989.01070020063030. [DOI] [PubMed] [Google Scholar]
- 25.Dodson PM, Galton DJ, Hamilton AM, Blach RK. Retinal vein occlusion and the prevalence of lipoprotein abnormalities. Br J Ophthalmol. 1982;66(3):161–164. doi: 10.1136/bjo.66.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dodson PM, Westwick J, Marks G, Kakkar VV, Galton DJ. beta-thromboglobulin and platelet factor 4 levels in retinal vein occlusion. Br J Ophthalmol. 1983;67(3):143–146. doi: 10.1136/bjo.67.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moyer MP, Tracy RP, Tracy PB, van't Veer C, Sparks CE, Mann KG. Plasma lipoproteins support prothrombinase and other procoagulant enzymatic complexes. Arteriosclerosis, thrombosis, and vascular biology. 1998;18(3):458–465. doi: 10.1161/01.atv.18.3.458. [DOI] [PubMed] [Google Scholar]
- 28.Janssen MC, den Heijer M, Cruysberg JR, Wollersheim H, Bredie SJ. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93(6):1021–1026. doi: 10.1160/TH04-11-0768. [DOI] [PubMed] [Google Scholar]
- 29.Brown BA, Marx JL, Ward TP, et al. Homocysteine: a risk factor for retinal venous occlusive disease. Ophthalmology. 2002;109(2):287–290. doi: 10.1016/s0161-6420(01)00923-x. [DOI] [PubMed] [Google Scholar]
- 30.Yildirim C, Yaylali V, Tatlipinar S, Kaptanoglu B, Akpinar S. Hyperhomocysteinemia: a risk factor for retinal vein occlusion. Ophthalmologica. 2004;218(2):102–106. doi: 10.1159/000076144. [DOI] [PubMed] [Google Scholar]
- 31.Brown GC, Shah HG, Magargal LE, Savino PJ. Central retinal vein obstruction and carotid artery disease. Ophthalmology. 1984;91(12):1627–1633. doi: 10.1016/s0161-6420(84)34093-3. [DOI] [PubMed] [Google Scholar]
- 32.Rauh G, Fischereder M, Nasemann J, et al. Evaluation of atherosclerosis in patients with central retinal vein occlusion by carotid artery duplex scanning and echocardiography: a prospective case-control study. Eur J Med Res. 1996;1(9):429–432. [PubMed] [Google Scholar]
- 33.Sayag D, Gotzamanis A, Brugniart C, et al. [Retinal vein occlusion and carotid Doppler imaging]. J Fr Ophtalmol. 2002;25(8):826–830. [PubMed] [Google Scholar]
- 34.Nakazato K, Watanabe H, Kawana K, Hiraoka T, Kiuchi T, Oshika T. Evaluation of arterial stiffness in patients with branch retinal vein occlusion. Ophthalmologica. 2005;219(6):334–337. doi: 10.1159/000088374. [DOI] [PubMed] [Google Scholar]
- 35.Marin-Sanabria EA, Kondoh T, Yamanaka A, Kohmura E. Ultrasonographic screening of carotid artery in patients with vascular retinopathies. Kobe J Med Sci. 2005;51(1−2):7–16. [PubMed] [Google Scholar]


