Abstract
Physical activity after spinal cord injury promotes improvements in motor function, but its effects following peripheral nerve injury are less clear. Although axons in peripheral nerves are known to regenerate better than those in the CNS, methods of accelerating regeneration are needed due to the slow overall rate of growth. Therefore we studied the effect of two weeks of treadmill locomotion on the growth of regenerating axons in peripheral nerves following injury. The common fibular nerves of thy-1-YFP-H mice, in which a subset of axons in peripheral nerves express yellow fluorescent protein (YFP), were cut and repaired with allografts from non-fluorescent littermates, and then harvested two weeks later. Mice were divided into groups of low-intensity continuous training (CT, 60 minutes), low-intensity interval training (IT; one group, 10 reps, 20 minutes total), and high-intensity IT (three groups, 2, 4, and 10 reps). One repetition consisted of two minutes of running and five minutes of rest. Sixty minutes of CT resulted in the highest exercise volume, whereas two reps of IT resulted in the lowest volume of exercise. The lengths of regenerating YFP+ axons were measured in images of longitudinal optical sections of nerves. Axon profiles were significantly longer than control in all exercise groups except the low-intensity IT group. In the CT group and the high-intensity IT groups that trained with four or 10 repetitions axons were more than twice as long as unexercised controls. The number of intervals did not impact axon elongation. Axon sprouting was enhanced in IT groups but not the CT group. Thus exercise, even in very small quantities, increases axon elongation in injured peripheral nerves whereas continuous exercise resulting in higher volume (total steps) may have no net impact on axon sprouting.
Keywords: Exercise, Recovery, Yellow fluorescent protein, Mouse, Nerve graft
Introduction
Exercise has been shown to improve motor function following spinal cord injury both in animal models and in clinical studies (Edgerton, et al., 1997, Hutchinson, et al., 2004, Skinner, et al., 1996). The effect of exercise on recovery from peripheral nerve injury has received less attention. Damaged axons in peripheral nerves are capable of significant regeneration but functional recovery in human patients with peripheral nerve injuries is poor (Brushart, 1998). The reason most often cited for poor recovery is that axon regeneration is slow (Fawcett and Keynes, 1990). Seven days of running-wheel training prior to peripheral nerve injury in rats has been found to increase afferent axonal outgrowth as measured from cultured DRG neurons (Molteni, et al., 2004). The extent of enhancement was related to the extent of running-wheel usage. Pre-injury exercise may “prime” adult dorsal root ganglion neurons for increased axon regeneration. In another recent study, treadmill training was found to enhance sensory functional recovery, as measured from nerve recordings (Marqueste, et al., 2004). However, there is little direct evidence of an effect of post-injury exercise on axon regeneration in injured peripheral nerves.
The data from studies of electrical stimulation, neurotrophins, and peripheral nerve injury are encouraging. If the proximal stump of a transected nerve is stimulated for as little as one hour at the time of surgical repair, axon regeneration is enhanced (Al-Majed, et al., 2000, English, et al., 2006). This enhancement is associated with an increase in BDNF and trkB in the regenerating neurons (Al-Majed, et al., 2000) and is dependent on neuronal neurotrophins (English, et al., 2006). Recent experiments have also shown that exercise affects an increase in neurotrophins (e.g., BDNF) (Berchtold, et al., 2005, Gomez-Pinilla, et al., 2002) and that neurotrophins are required for even control levels of axon regeneration after peripheral nerve injury (English, et al., 2005). Therefore, there is sufficient background to hypothesize that exercise should also enhance axon regeneration after peripheral nerve injury.
Treadmill training is readily applied and can be used by both laboratory animals and human subjects. In clinical studies of exercise with human subjects, continuous treadmill locomotion at a moderate to brisk pace is commonly used. When allowed to exercise voluntarily, laboratory mice utilize a markedly different pattern. This pattern is characterized by repeated short-duration runs (two minutes) at speeds that are close to maximum, with rest periods interspersed between runs (De Bono, et al., 2006), a form of interval training. In this study we provide direct evidence that treadmill training enhances the growth of regenerating axons in injured peripheral nerves. We also show that there are differences in the effects of these two forms of treadmill training on axon regeneration in the first two weeks following injury. A preliminary report of some of these findings has been made (English, et al., 2006).
Methods
Animals and Surgical Procedures
All experimental procedures conformed to the Guidelines for the Use of Animals in Research of the Society for Neuroscience and were approved by the Institutional Animal Care and Use Committee of Emory University. All experiments were conducted using thy-1-YFP-H mice (Feng, et al., 2000) on a C57BL/6J background. The thy-1-YFP-H mice are maintained as heterozygotes (YFP+), so that half of all litters born to YFP+ mice will not contain the transgene. In these transgenic mice, yellow fluorescent protein is present in about 10% of all axons in peripheral nerves, enabling visualization of individual axons using fluorescence microscopy. Mice positive for the transgene (YFP+) were anesthetized with pentobarbital (90mg/kg; IP) and the common fibular (CF) nerve was exposed and cut with sharp scissors. The cut nerve was then repaired with a short (3 mm) segment of the CF nerve obtained from a WT littermate of the host mouse that was negative for the transgene. This nerve allograft and the cut ends of the CF nerve in the host mouse were carefully arranged on a short length of Gore-Tex tubing that had been cut in half longitudinally. The cut ends of the nerves were aligned as much as possible with those of the graft, placing the proximal stump of the host nerve with the proximal end of the graft from the donor, and similarly apposing the distal end of the graft with the original distal stump of the cut nerve. It was then secured with a 1:1 mixture of fibrin (3 μL, E.C. 2325986) and fibronectin (3 μL, E.C. 2891492), paired with an equal amount of thrombin (6 μL, E.C. 3.4.21.5) (English, et al., 2005). All of these reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA). The WT graft used to repair the cut nerve serves as a dark background against which regenerating YFP+ axons can be visualized
Exercise
Treadmill training began on the third post operative day. In the CT group (n=5), training consisted of one hour of continuous locomotion at a treadmill speed of 10 m/min, with no incline, applied five days per week for two weeks. All mice had been acclimated to the treadmill for five to ten minutes twice prior to surgery and none had been on the treadmill for at least 3 days before nerve transection. Mice ran on the treadmill at this speed with little or no persuasion, even on the third post operative day. When mice exercise voluntarily, they do not run continuously. They run at near-maximal speeds for short periods separated by rest periods (De Bono, et al., 2006). Therefore, mice in the IT group were run at a faster speed (20 m/min) for two minutes at a time, separated by 5-minute rest periods. Training for IT groups consisted of two (n=4 mice), four (n=3), or ten repetitions (n=4) per day. Although the intensity of exercise (steps per unit time) was higher in the high-intensity IT groups, the volume of exercise (total step number) was smaller for these animals due to less total time spent running on the belt. A treadmill speed of 10 m/min results in a step cycle frequency of 147 steps per minute (unpublished findings). Running continuously at this speed for 60 minutes results in a total volume of 8620 steps. A treadmill speed of 20 m/min results in a step cycle frequency of 295 steps per minute (unpublished findings). Even 10 repetitions at 20 m/min results in a total volume of only 5900 steps. Two repetitions at 20 m/min results in only approximately 1180 steps. Air jets were used judiciously as encouragement for IT mice to keep up with the belt. Mice in the control group remained caged for two weeks after nerve transection and did not receive treadmill training. Results from an additional group of mice that were exposed to 10 repetitions at 10 m/min (n=3) in preparing for the study are also reported because these results contrast those from both 10 m/min CT and 20 m/min IT.
Mice subjected to common fibular nerve transection were able to run at 10 m/min for 1 hour continuously and at 20 m/min intermittently beginning three days after surgery, despite loss of the ability to dorsiflex the ankle. Exercise capacity in mice varies with strain (Billat, et al., 2005, Lerman, et al., 2002), with C57BL/6J mice ranking poorly in aerobic performance amongst other strains (Lerman, et al., 2002, Lightfoot, et al., 2001). The intensity used in this study during high-intensity IT was close to that associated with maximal oxygen consumption for healthy C57BL/6J mice (i.e., 25 m/min, (Schefer and Talan, 1996)). Lerman et al. found that maximal treadmill speed for C57BL/6J mice was only 22.2 m/min (Lerman, et al., 2002). Mice evaluated in this study were able to maintain apparently close to maximal running speed for two-minute blocks of time and for multiple repetitions despite a significant physical limitation and with little encouragement. Although kinematic data were not collected, it was apparent that locomotor movements were compromised in these mice. Following transection of the common fibular nerve, function of ankle dorsiflexors muscles is lost.
Tissue Harvesting and Microscopy
At the end of the prescribed exercise period, mice were euthanized with an overdose of pentobarbital (150 mg/kg) and perfused through the heart with saline, followed by periodate-lysate-paraformaldehyde fixative (McLean and Nakane, 1974). The entire sciatic nerve was removed, placed on a microscope slide, and cover slipped using Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA). Edges of the cover slip were sealed with nail polish. Mounted nerves were viewed with a laser scanning confocal microscope (Zeiss LSM510). Horizontal optical sections 10 μm thick were obtained through the full extent of the graft. Stacks of images of adjacent microscope fields were stitched together using Adobe Photoshop. The result was a stack of stitched images containing the entire repaired CF nerve. All visible YFP+ axon profiles in these stacks of optical sections were measured from their distal tips to the surgical repair site using Image Pro Plus (Media Cybernetics, Silver Spring, MD USA).
Data Analysis
Cumulative histograms of the distributions of axon profile lengths in these nerves were constructed with a bin size of 100 μm. Averages of these histograms were computed for each group. Statistical groups consisted of four (2-rep group), three (4-rep group), and four (4-rep group) nerve grafts in which axons were measured. The distribution of axon profile lengths measured in nerve allografts is not statistically normal (English, et al., 2005, Groves, et al., 2005), a requirement for the use of parametric tests such as t-tests or analysis of variance (ANOVA). Thus, statistical significance of differences between treatment groups in these experiments was evaluated using two different approaches. First, a nonparametric statistical test for independent samples, the Mann-Whitney U test, was used to determine whether the distributions of axon profile lengths measured in different treatment groups are from the same population. This method makes no assumption about the nature of these distributions. Second, the median axon profile length was determined in each nerve studied in each mouse. These values were found to be distributed normally, but the variances of different samples were not homogeneous (Levene’s test, p>0.05), also a requirement for use of parametric statistics. Therefore, they were log-transformed before evaluating the significance of differences between the experimental groups using ANOVA and post hoc (Fisher Least Significant Differences (LSD)) testing. The numbers of axon profiles proximal and distal to the surgical repair site were counted in each nerve graph studied. The ratio of those counts, the number of distal profiles per proximal profile, was calculated as a sprouting index. This index is used as a global measure of the amount of regenerative sprouting that had occurred in the 2-week survival period (Groves, et al., 2005). The significance of differences in sprouting index in the different treatment groups was evaluated using analysis of variance (ANOVA) and the Fisher LSD post hoc test. Probabilities of less than or equal to 0.05 were considered statistically significant.
Results
Typical results from a treadmill trained and an unexercised mouse are shown in Figure 1. The two panels in this figure are each a montage of images from several microscope fields taken at the same optical plane through a surgically repaired CF nerve, two weeks after nerve repair. The proximal stump of the nerve is toward the top in each panel, and the proximal surgical repair site is indicated by the upper arrows. The distal extent of the nerve allograft used to repair the cut nerve is indicated by the lower arrows. An allograft from a non-fluorescent mouse was used to provide a dark background against which YFP+ regenerating axons could be visualized. In some mice fluorescent debris from anterograde (Wallerian) degeneration is found in the original distal stump of the cut nerve. If no grafts were used, it would be impossible in these instances to identify fine processes of regenerating axons among this debris. The YFP+ axons in Figure 1 extend for considerable distances from the surgical repair site during this two week period, some coursing completely through the graft and into the original distal stump. In optical sections of the nerve from the exercised animal (Figure 1, Right panel), more of these long axons are present and fewer short axons are found near the surgical repair site than in the image from the unexercised mouse (Figure 1, Left panel).
Figure 1.
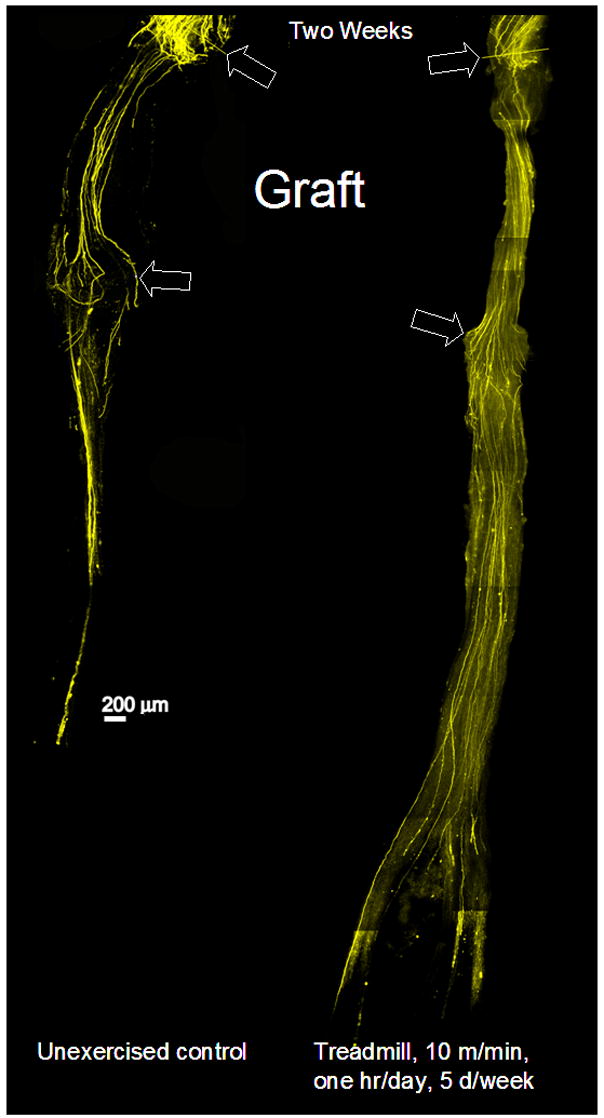
Low magnification images of two common fibular nerves are displayed to show the effect of continuous training on the regeneration of cut axons. Each nerve had been cut and surgically repaired 2 weeks earlier using a ca. 3 mm graft from a wild type donor mouse. The boundaries of each graft are indicated by arrows. The overall image of each nerve is a montage constructed from images of several microscope fields, each taken at the same depth through whole mounts of the nerve. By two weeks after nerve repair, any residual fluorescence in the original distal stump (distal to the graft) due to the degeneration of the host axons has disappeared. Fluorescence found in this region thus represents profiles of regenerating axons that have grown entirely through the grafts. The nerve on the right came from a mouse that had received treadmill training after the nerve repair. The nerve on the left was from a mouse that was unexercised.
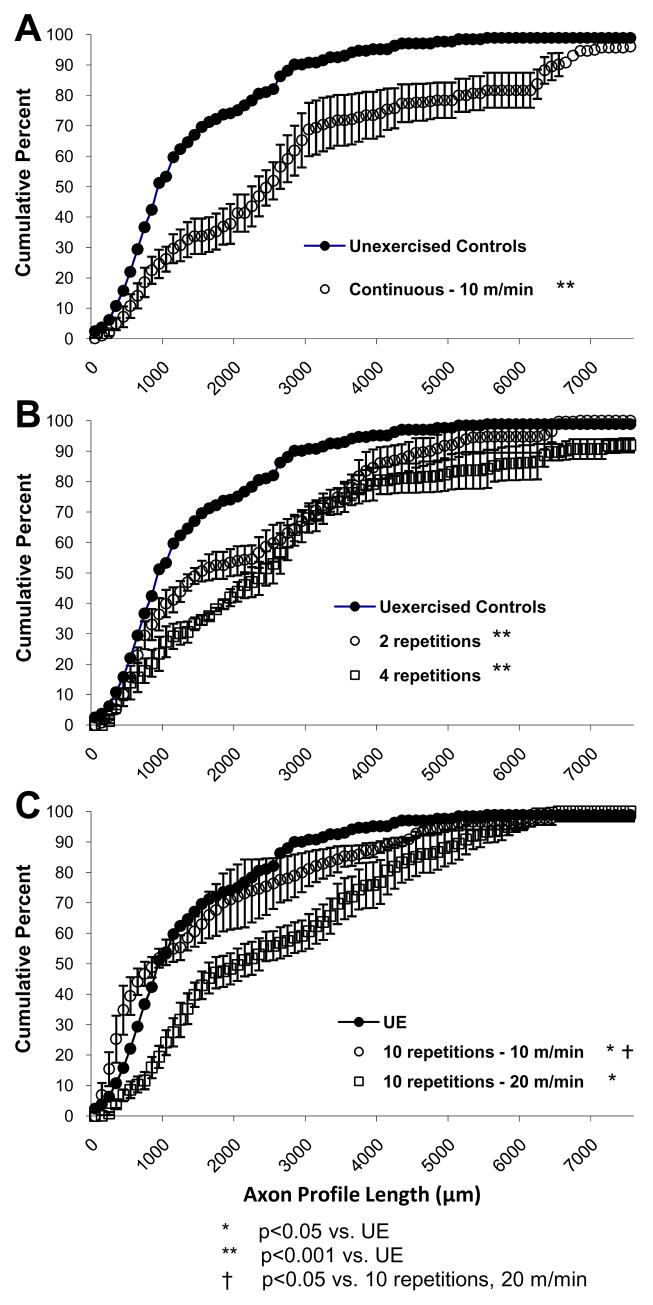
Data from mice that had been exercised with low-intensity continuous training or high-intensity intermittent training are shown in Figure 2. Measurements of axon profile lengths were made from stacks of optical sections through nerves and grafts, from the proximal surgical repair site to the distal tips of growth cones. Most measured axons that were visualized terminated distally, or in the direction of the distal stump, although some measured axons had reached the distal stump, turned around, and grown proximally. Using stacks of images through the nerves acquired with the confocal microscope helped enable tracing of these axons from their terminations to the repair site. Cumulative histograms of the distributions of these axon profile lengths were constructed for nerves from control and experimental mice. Each point in the cumulative histograms in these panels represents a mean. Error bars represent standard errors. Five mice were unexercised (UE) and served as a control group. Treadmill training at 10 m/min for 1 hour (CT); or at 20 m/min for four minutes (IT, 2 reps), eight minutes (IT, 4 reps), and 20 minutes (IT, 10 reps) results in a significant shift in axon profile length distributions as compared to unexercised mice (p<0.001 for CT, and 2 reps, 4 reps, and 10 reps at 20 m/min, Figure 2). The distributions of axon profile lengths from these exercised groups are shifted downward and rightward. This means that for any given axon profile length, significantly more axons were encountered at that length or longer in the exercised group than in the sedentary control group.
Figure 2.
Cumulative frequency distributions of YFP+ axon profile lengths measured in grafts from mice that experienced continuous and intermittent treadmill training. Panel A shows results from unexercised control mice and mice that trained at 10 m/min continuously for 1 hour. Panel B shows results from unexercised control mice and mice that trained with 2 and 4 repetitions at 20 m/min. Panel C shows results from unexercised control mice and mice that trained with 10 repetitions at 10 m/min and 10 repetitions at 20 m/min. Each 100 μm bin in these distributions represents the mean (±SEM) of 3 (CT), 4 (IT for 2 and 4 reps), 5 (IT for 10 reps), or 3 (IT for 10 reps at 10 m/min) mice.
Treadmill training at 10 m/min intermittently for 10 repetitions also resulted in a shift of axon profile lengths (p=0.02, Fig 2C). The p-value associated with this statistical comparison is larger by an order of magnitude as compared to the other pair-wise comparisons of cumulative frequency histograms. Although the probability value associated with a statistical test is not technically a measure of effect size, in this study it is associated with the smallest difference in cumulative frequency profiles. In addition, there was no statistically significant difference between the median axon profile lengths for this group and the unexercised control group (Figure 3). The Mann-Whitney U test is a very sensitive non-parametric alternative to the t-test which was used in this study because the axon profile lengths were not normally distributed. However, by definition, use of the Mann-Whitney U test increases the chance of Type I error (i.e., rejection of the null hypothesis when it is true). We believe it is likely that the discrepancy in the findings of the cumulative frequency distributions and the median axon lengths for this group is due to the sensitivity of the Mann-Whitney U test. As a result, the following interpretations focus on the much larger effect size and consistency of results for the other exercise trained groups rather than the much smaller, but statistically significant, effect based on the cumulative frequency plots.
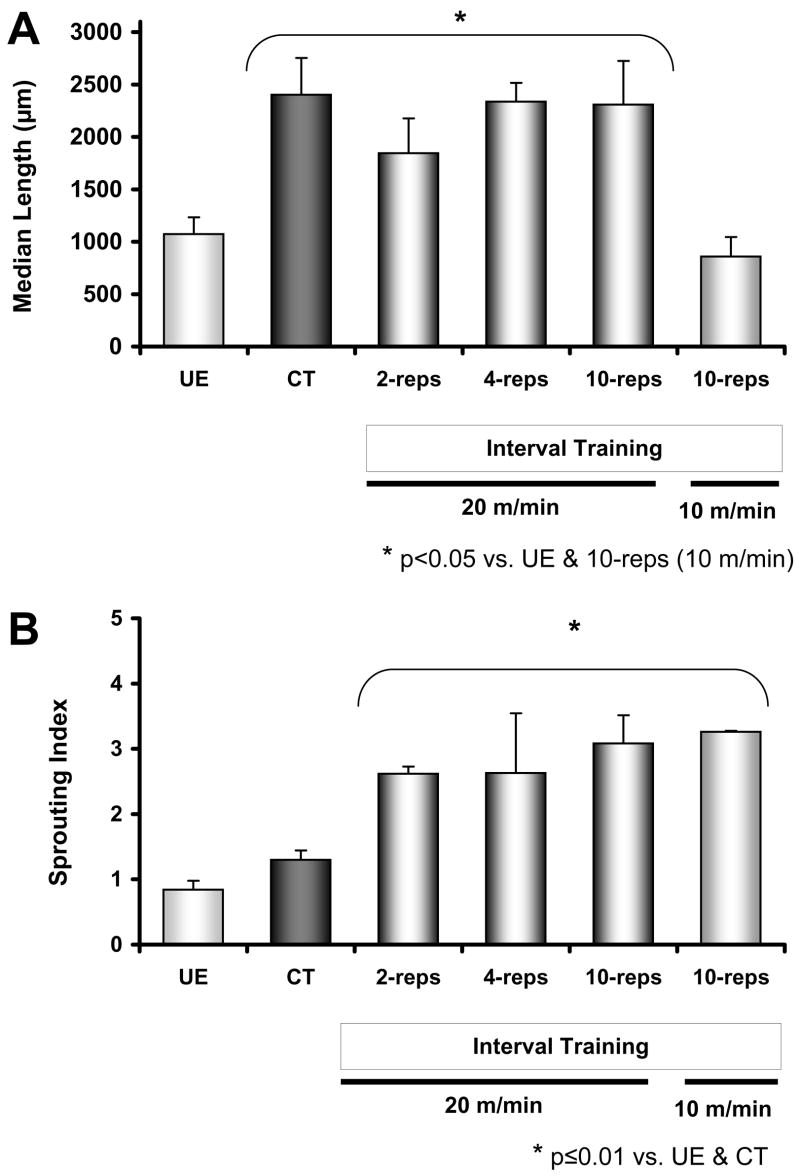
Figure 3.
Effects of continuous training (CT) and interval training (IT; 2-reps, 4-reps, 10-reps at 20 m/min, and 10-reps at 10 m/min) on average median axon profile length (A) and sprouting index (B). Results from unexercised (UE) mice appear first in both panes. Except for the 10-rep IT group that trained at 10 m/min, exercise resulted in a significant enhancement of axon regeneration as compared to the UE group. However, only interval training paradigms significantly increased sprouting. Data are presented as mean (±SEM). The apparent lack of error bars for the 10-rep, 10m/min, group is due to very tight dispersion of sprouting index results for individual cases (i.e., individual results are 3.3, 3.3, and 3.2).
If median axon profile lengths of these groups are compared, all but the low-intensity IT (p=0.34) group were found to be significantly longer than unexercised controls (Fisher LSD, p≤0.01, Figure 3A). To evaluate whether the enhancement of axon regeneration observed in exercise trained animals might be the result of an increase in axon branching we calculated a sprouting index. This is simply a ratio of the number of fluorescent axon profiles measured in each mouse to the number of fluorescent axon profiles one mm proximal to the original surgical repair site. The index is a global measure of the average number of branches made by each cut axon (Groves, et al., 2005). These data are shown in Figure 3B. The sprouting index was significantly increased in all IT groups (Fisher LSD, p≤0.05). The mean sprouting index of the CT group was not significantly different from the UE group (p=0.39).
Discussion
The main finding of this study is that treadmill exercise, even in very small doses, enhances axon regeneration in the peripheral nervous system. As little as one hour daily of continuous treadmill locomotion at a slow speed, or two two-minute bouts of more intense treadmill locomotion at near maximal treadmill running speed (Lerman, et al., 2002) performed during the first two weeks after peripheral nerve transection and repair enhances growth of regenerating axons. Exercise, and in particular treadmill training, has been advocated for this purpose for patients with spinal cord injuries for some time (Edgerton, et al., 2004), but only recently has attention been devoted to its potential for treating peripheral nerve injuries. Marqueste et al. (2004) found that treadmill training after common fibular nerve transection and repair results in better mechano- and metabosensitive afferent recovery in rats (Marqueste, et al., 2004). A more recent report showed a positive effect of treadmill training on axon regeneration in rats after sciatic nerve crush (Seo, et al., 2006). However, this is the first study to provide direct evidence of a positive effect of post-injury exercise training on axon regeneration after peripheral nerve transection.
Volume and intensity are the two quintessential defining components of an endurance exercise program (i.e., one which is characterized by repetitive and prolonged physical activity involving most of the musculature and little or no added resistance) that determine the ensuing physiological adaptations in normal animals. In this study volume was manipulated primarily by altering the amount of time the mouse spent running on the treadmill. Intensity was manipulated by altering treadmill speed. Continuous training has been used more extensively in animal exercise studies and has been applied in previous studies of exercise after peripheral nerve injury (Marqueste, et al., 2004, Seo, et al., 2006). However, the preferred approach to patterned running for rodents is brief intervals of high-intensity running interspersed with periods of rest (De Bono, et al., 2006). We show here that training for large amounts of time at slower speeds and training for short amounts of time (even as little as 4 minutes) at higher speeds increased axon profile lengths at two weeks after nerve transection and repair. Continuous training and 4-rep IT especially increased the proportions of long axons (i.e., those ≥ 4000 μm, Figure 2). The lack of improvement in axon elongation with 10 repetitions of 10 m/min may mean that it is the application of higher intensity with lower volume that makes low-volume intermittent exercise effective. We employed a small number of volume-intensity combinations and therefore are limited in our interpretations as to the optimal balance. However, our results may be interpreted to mean that there are also differential effects of volume and intensity that determine how exercise impacts peripheral nerve regeneration.
The effects of exercise found in this study may be attributable to up regulation of neurotrophins resulting from increased neuronal activity. If the proximal stump of a cut nerve is stimulated for as little as one hour at the time of the surgical repair of the nerve, axon regeneration is enhanced (Al-Majed, et al., 2000, English, et al., 2006). This enhancement is associated with an increase in BDNF and trkB in the regenerating neurons (Al-Majed, et al., 2000) and is dependent upon neuronal neurotrophins (English, et al., 2006). Exercise increases BDNF in the brain (Berchtold, et al., 2005) and spinal cord (Gomez-Pinilla, et al., 2002, Zaheer, et al., 2006). Exercise also up regulates the BDNF receptor trkB (Macias, et al., 2007). Therefore, it is tempting to speculate that neurotrophins are involved in the enhanced axon elongation associated with exercise. In addition, large doses of BDNF have been found to be inhibitory to axon regeneration (Boyd and Gordon, 2002). If increased exercise volume and/or intensity are associated with increased neurotrophin upregulation, this may help explain the plateau we observed in axon elongation at 4 reps and/or with one hour of CT.
Enhanced axon regeneration could be produced by enhancing the growth of regenerating axons or by increasing the branching of those axons. Although the average median axon profile length in this study was increased with high-volume, low-intensity, and low-volume, high-intensity training, only IT increased sprouting. The sprouting index (i.e., ratio of distal to proximal axon counts) increased significantly in all IT mice, regardless of whether elongation of regenerating axons was enhanced. Continuous training enhanced axon elongation without increasing the sprouting index. Therefore, axon branching and elongation may be independently regulated in such a way that increasing volume actually negates the impact of exercise on branching.
This idea is supported by findings from a previous study where sprouting was inhibited in vivo with increased neuromuscular activity associated with wheel running in rats (Tam, et al., 2001). The volume of neural activity associated with CT in our study and the wheel running in the previous study may increase intracellular calcium to levels that interfere with axon sprouting (Kater and Mills, 1991). The effect may be that sprout terminals are overloaded with calcium and result in an overall reduction of sprouting. The end result may be to accelerate the normal process of pruning that occurs during the regeneration process (Mackinnon, et al., 1991).
This study provides strong evidence that exercise involving the affected limb enhances axon regeneration after peripheral nerve injury. It is also shown that volume and intensity modulate the effects of exercise. The negative correlation of exercise volume with sprouting, as well as the plateau in this enhancement with increasing volume are intriguing findings that await clarification. The results of this study provide a foundation for future investigation into the mechanisms of the effect of exercise on axon regeneration as well as to whether exercise also improves the specificity of reinnervation or functional outcomes after peripheral nerve injury.
Acknowledgments
Thanks are due to W. L. Gore & Associates, Inc., for the gift of the Gore-Tex tubing; and to Dr. Robert McKeon and Dr. Robert Gregor, both of whom read and commented on earlier versions of the manuscript. This work was supported with funding from grant HD43596. Manning J Sabatier was supported by the USPHS NIH Institutional Research and Academic Career Development grant, #K12 GM00680-05.
References
- Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000;12:4381–4390. [PubMed] [Google Scholar]
- Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20:2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Billat VL, Mouisel E, Roblot N, Melki J. Inter- and intrastrain variation in mouse critical running speed. J Appl Physiol. 2005;98:1258–1263. doi: 10.1152/japplphysiol.00991.2004. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. A dose-dependent facilitation and inhibition of peripheral nerve regeneration by brain-derived neurotrophic factor. Eur J Neurosci. 2002;15:613–626. doi: 10.1046/j.1460-9568.2002.01891.x. [DOI] [PubMed] [Google Scholar]
- Brushart TM. Nerve repair and grafting. In: Green D, Hotchkiss R, Pederson W, editors. Green’s operative hand surgery. Churchill Livingstone; New York: 1998. pp. 1381–1403. [Google Scholar]
- De Bono JP, Adlam D, Paterson DJ, Channon KM. Novel quantitative phenotypes of exercise training in mouse models. Am J Physiol Regul Integr Comp Physiol. 2006;290:R926–934. doi: 10.1152/ajpregu.00694.2005. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, de Leon RD, Tillakaratne N, Recktenwald MR, Hodgson JA, Roy RR. Use-dependent plasticity in spinal stepping and standing. Adv Neurol. 1997;72:233–247. [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- English AW, Meador W, Carrasco DI. Neurotrophin-4/5 is required for the early growth of regenerating axons in peripheral nerves. Eur J Neurosci. 2005;21:2624–2634. doi: 10.1111/j.1460-9568.2005.04124.x. [DOI] [PubMed] [Google Scholar]
- English AW, Schwartz G, Meador W, Sabatier MJ, Mulligan A. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. J Neurobiol. 2006 doi: 10.1002/dneu.20339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Schwartz G, Mulligan AM, Sabatier MJ. Treadmill exercise promotes axon regeneration in peripheral nerves. Society for Neuroscience; Atlanta, GA: 2006. [Google Scholar]
- Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu Rev Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Groves ML, McKeon R, Werner E, Nagarsheth M, Meador W, English AW. Axon regeneration in peripheral nerves is enhanced by proteoglycan degradation. Exp Neurol. 2005;195:278–292. doi: 10.1016/j.expneurol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- Kater SB, Mills LR. Regulation of growth cone behavior by calcium. J Neurosci. 1991;11:891–899. doi: 10.1523/JNEUROSCI.11-04-00891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman I, Harrison BC, Freeman K, Hewett TE, Allen DL, Robbins J, Leinwand LA. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Appl Physiol. 2002;92:2245–2255. doi: 10.1152/japplphysiol.01045.2001. [DOI] [PubMed] [Google Scholar]
- Lightfoot JT, Turner MJ, Debate KA, Kleeberger SR. Interstrain variation in murine aerobic capacity. Med Sci Sports Exerc. 2001;33:2053–2057. doi: 10.1097/00005768-200112000-00012. [DOI] [PubMed] [Google Scholar]
- Macias M, Dwornik A, Ziemlinska E, Fehr S, Schachner M, Czarkowska-Bauch J, Skup M. Locomotor exercise alters expression of pro-brain-derived neurotrophic factor, brain-derived neurotrophic factor and its receptor TrkB in the spinal cord of adult rats. Eur J Neurosci. 2007;25:2425–2444. doi: 10.1111/j.1460-9568.2007.05498.x. [DOI] [PubMed] [Google Scholar]
- Mackinnon SE, Dellon AL, O’Brien JP. Changes in nerve fiber numbers distal to a nerve repair in the rat sciatic nerve model. Muscle Nerve. 1991;14:1116–1122. doi: 10.1002/mus.880141113. [DOI] [PubMed] [Google Scholar]
- Marqueste T, Alliez JR, Alluin O, Jammes Y, Decherchi P. Neuromuscular rehabilitation by treadmill running or electrical stimulation after peripheral nerve injury and repair. J Appl Physiol. 2004;96:1988–1995. doi: 10.1152/japplphysiol.00775.2003. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Molteni R, Zheng JQ, Ying Z, Gomez-Pinilla F, Twiss JL. Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci U S A. 2004;101:8473–8478. doi: 10.1073/pnas.0401443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schefer V, Talan MI. Oxygen consumption in adult and AGED C57BL/6J mice during acute treadmill exercise of different intensity. Exp Gerontol. 1996;31:387–392. doi: 10.1016/0531-5565(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Seo TB, Han IS, Yoon JH, Hong KE, Yoon SJ, Namgung U. Involvement of CDC2 in axonal regeneration enhanced by exercise training in rats. Med Sci Sports Exerc. 2006;38:1267–1276. doi: 10.1249/01.mss.0000227311.00976.68. [DOI] [PubMed] [Google Scholar]
- Skinner RD, Houle JD, Reese NB, Berry CL, Garcia-Rill E. Effects of exercise and fetal spinal cord implants on the H-reflex in chronically spinalized adult rats. Brain Res. 1996;729:127–131. [PubMed] [Google Scholar]
- Tam SL, Archibald V, Jassar B, Tyreman N, Gordon T. Increased neuromuscular activity reduces sprouting in partially denervated muscles. J Neurosci. 2001;21:654–667. doi: 10.1523/JNEUROSCI.21-02-00654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaheer A, Haas JT, Reyes C, Mathur SN, Yang B, Lim R. GMF-knockout mice are unable to induce brain-derived neurotrophic factor after exercise. Neurochem Res. 2006;31:579–584. doi: 10.1007/s11064-006-9049-3. [DOI] [PubMed] [Google Scholar]