Abstract
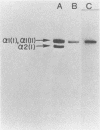
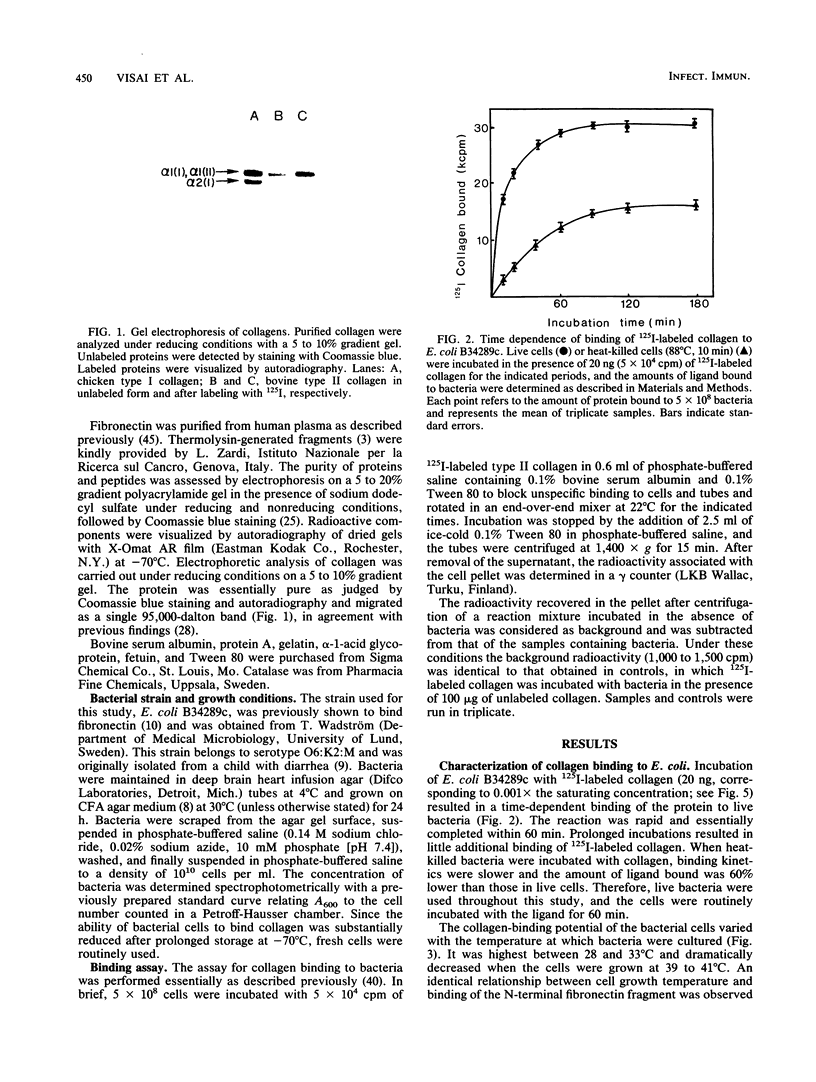
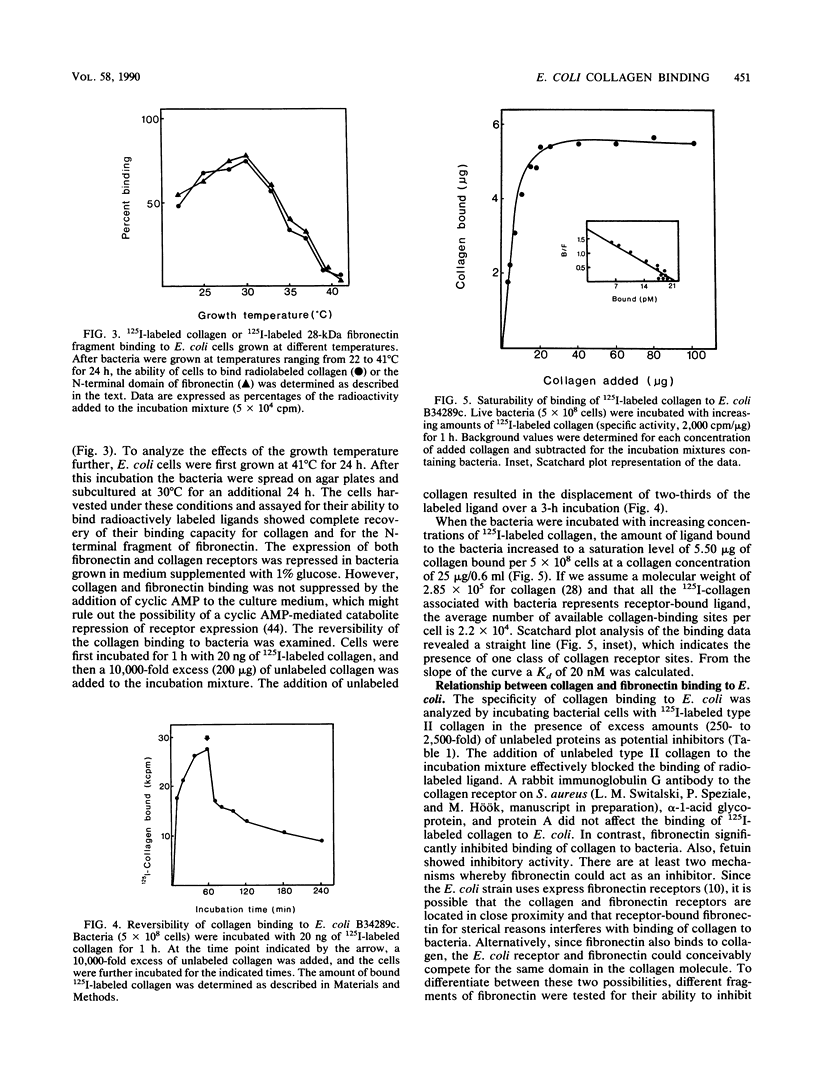
An enterotoxigenic strain of Escherichia coli, B34289c, has been shown to bind the N-terminal region of fibronectin with high affinity (G. Fröman, L. M. Switalski, A. Faris, T. Wadström, and M. Höök, J. Biol. Chem. 259:14899-14905, 1984). We now report that this strain also binds collagen. The binding of 125I-labeled type II collagen to bacteria was time dependent and reversible. Bacteria expressed a limited number of collagen receptors (2.2 x 10(4) per cell) and bound collagen with a Kd of 20 nM. All collagen types tested (I to V) as well as all tested cyanogen bromide-generated peptides [alpha 1(I)CB2, alpha 1(I)CB3, alpha 1(I)CB7, alpha 1(I)CB8, and alpha 2(I)CB4] were recognized by bacterial receptors, as demonstrated by the ability of these proteins to inhibit the binding of 125I-labeled collagen to bacteria. Of several unlabeled proteins tested in competition experiments, fibronectin and its N-terminal region strongly inhibited binding of the radiolabeled collagen to E. coli cells. Conversely, collagen competed with an 125I-labeled 28-kilodalton fibronectin fragment for bacterial binding. Collagen bound to bacteria could be displaced by excess amounts of either unlabeled fibronectin or its N-terminal fragment. Similarly, collagen could displace 125I-labeled N-terminal peptide of fibronectin bound to the bacterial cell surface. Bacteria grown at 41 degrees C or in the presence of glucose did not express collagen or fibronectin receptors. These results indicate the presence of specific binding sites for collagen on the surface of E. coli cells and furthermore that the collagen and fibronectin binding sites are located in close proximity, possibly on the same structure.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker U., Timpl R. Cyanogen bromide peptides of the rabbit collagen a1-chain. FEBS Lett. 1972 Oct 15;27(1):85–88. doi: 10.1016/0014-5793(72)80415-0. [DOI] [PubMed] [Google Scholar]
- Borsi L., Castellani P., Balza E., Siri A., Pellecchia C., De Scalzi F., Zardi L. Large-scale procedure for the purification of fibronectin domains. Anal Biochem. 1986 Jun;155(2):335–345. doi: 10.1016/0003-2697(86)90443-4. [DOI] [PubMed] [Google Scholar]
- Briles E. B., Haskew N. B. Isolation of cloned variants of a rat hepatoma cell line with altered attachment to collagen, but normal attachment to fibronectin. Exp Cell Res. 1982 Apr;138(2):436–441. doi: 10.1016/0014-4827(82)90194-x. [DOI] [PubMed] [Google Scholar]
- Chiang T. M., Kang A. H. Isolation and purification of collagen alpha 1(I) receptor from human platelet membrane. J Biol Chem. 1982 Jul 10;257(13):7581–7586. [PubMed] [Google Scholar]
- Couchman J. R., Rees D. A., Green M. R., Smith C. G. Fibronectin has a dual role in locomotion and anchorage of primary chick fibroblasts and can promote entry into the division cycle. J Cell Biol. 1982 May;93(2):402–410. doi: 10.1083/jcb.93.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedhar S., Ruoslahti E., Pierschbacher M. D. A cell surface receptor complex for collagen type I recognizes the Arg-Gly-Asp sequence. J Cell Biol. 1987 Mar;104(3):585–593. doi: 10.1083/jcb.104.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect Immun. 1977 Nov;18(2):330–337. doi: 10.1128/iai.18.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Faris A., Wadström T., Hök M. Binding of Escherichia coli to fibronectin. A mechanism of tissue adherence. J Biol Chem. 1984 Dec 10;259(23):14899–14905. [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Speziale P., Hök M. Isolation and characterization of a fibronectin receptor from Staphylococcus aureus. J Biol Chem. 1987 May 15;262(14):6564–6571. [PubMed] [Google Scholar]
- Gross R. J., Scotland S. M., Rowe B. Enterotoxin testing of Escherichia coli causing epidemic infantile enteritis in the U.K. Lancet. 1976 Mar 20;1(7960):629–631. doi: 10.1016/s0140-6736(76)90429-3. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L. Microbial toxins and diarrhoeal diseases: introduction and overview. Ciba Found Symp. 1985;112:1–13. doi: 10.1002/9780470720936.ch1. [DOI] [PubMed] [Google Scholar]
- Holderbaum D., Hall G. S., Ehrhart L. A. Collagen binding to Staphylococcus aureus. Infect Immun. 1986 Nov;54(2):359–364. doi: 10.1128/iai.54.2.359-364.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderbaum D., Spech T., Ehrhart L. A., Keys T., Hall G. S. Collagen binding in clinical isolates of Staphylococcus aureus. J Clin Microbiol. 1987 Dec;25(12):2258–2261. doi: 10.1128/jcm.25.12.2258-2261.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenaka T., Ishiguro M., Emura J., Kaufmann H., Isemura S., Bauer W., Schmid K. Isolation and partial characterization of the cyanogen bromide fragments of 1 -acid glycoprotein and the elucidation of the amino acid sequence of the carboxyl-terminal cyanogen bromide fragment. Biochemistry. 1972 Sep 26;11(20):3817–3829. doi: 10.1021/bi00770a022. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., McGoodwin E. B., Martin G. R., Klebe R. J., Fietzek P. P., Woolley D. E. Localization of the binding site for cell attachment in the alpha1(I) chain of collagen. J Biol Chem. 1978 Aug 25;253(16):5642–5646. [PubMed] [Google Scholar]
- Koda J. E., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. Basal extracellular proteoglycan binds specifically to native type I collagen fibrils. J Biol Chem. 1984 Oct 10;259(19):11763–11770. [PubMed] [Google Scholar]
- Kurkinen M., Taylor A., Garrels J. I., Hogan B. L. Cell surface-associated proteins which bind native type IV collagen or gelatin. J Biol Chem. 1984 May 10;259(9):5915–5922. [PubMed] [Google Scholar]
- Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978 Dec 14;276(5689):718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- Löhler J., Timpl R., Jaenisch R. Embryonic lethal mutation in mouse collagen I gene causes rupture of blood vessels and is associated with erythropoietic and mesenchymal cell death. Cell. 1984 Sep;38(2):597–607. doi: 10.1016/0092-8674(84)90514-2. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Gay S. The collagens: an overview and update. Methods Enzymol. 1987;144:3–41. doi: 10.1016/0076-6879(87)44170-0. [DOI] [PubMed] [Google Scholar]
- Mollenhauer J., von der Mark K. Isolation and characterization of a collagen-binding glycoprotein from chondrocyte membranes. EMBO J. 1983;2(1):45–50. doi: 10.1002/j.1460-2075.1983.tb01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsén A., Jonsson A., Normark S. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature. 1989 Apr 20;338(6217):652–655. doi: 10.1038/338652a0. [DOI] [PubMed] [Google Scholar]
- Quaroni A., Isselbacher K. J., Ruoslahti E. Fibronectin synthesis by epithelial crypt cells of rat small intestine. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5548–5552. doi: 10.1073/pnas.75.11.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M. L., Koplan J. P., Wachsmuth I. K., Wells J. G., Gangarosa E. J., Guerrant R. L., Sack D. A. Epidemic diarrhea at Crater Lake from enterotoxigenic Escherichia coli. A large waterborne outbreak. Ann Intern Med. 1977 Jun;86(6):714–718. doi: 10.7326/0003-4819-86-6-714. [DOI] [PubMed] [Google Scholar]
- Rovasio R. A., Delouvee A., Yamada K. M., Timpl R., Thiery J. P. Neural crest cell migration: requirements for exogenous fibronectin and high cell density. J Cell Biol. 1983 Feb;96(2):462–473. doi: 10.1083/jcb.96.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin K., Hök M., Obrink B., Timpl R. Substrate adhesion of rat hepatocytes: mechanism of attachment to collagen substrates. Cell. 1981 May;24(2):463–470. doi: 10.1016/0092-8674(81)90337-8. [DOI] [PubMed] [Google Scholar]
- Rydén C., Rubin K., Speziale P., Hök M., Lindberg M., Wadström T. Fibronectin receptors from Staphylococcus aureus. J Biol Chem. 1983 Mar 10;258(5):3396–3401. [PubMed] [Google Scholar]
- Simpson W. A., Beachey E. H. Adherence of group A streptococci to fibronectin on oral epithelial cells. Infect Immun. 1983 Jan;39(1):275–279. doi: 10.1128/iai.39.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speziale P., Hök M., Switalski L. M., Wadström T. Fibronectin binding to a Streptococcus pyogenes strain. J Bacteriol. 1984 Feb;157(2):420–427. doi: 10.1128/jb.157.2.420-427.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speziale P., Hök M., Wadström T., Timpl R. Binding of the basement membrane protein laminin to Escherichia coli. FEBS Lett. 1982 Sep 6;146(1):55–58. doi: 10.1016/0014-5793(82)80704-7. [DOI] [PubMed] [Google Scholar]
- Speziale P., Raucci G., Visai L., Switalski L. M., Timpl R., Hök M. Binding of collagen to Staphylococcus aureus Cowan 1. J Bacteriol. 1986 Jul;167(1):77–81. doi: 10.1128/jb.167.1.77-81.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawich E., Nimni M. E. Properties of a collagen molecule containing three identical components extracted from bovine articular cartilage. Biochemistry. 1971 Oct 12;10(21):3905–3911. doi: 10.1021/bi00797a017. [DOI] [PubMed] [Google Scholar]
- Sund H., Weber K., Mölbert E. Dissoziation der Rinderleber-Katalase in ihre Untereinheiten. Eur J Biochem. 1967 Jun;1(4):400–410. doi: 10.1111/j.1432-1033.1967.tb00088.x. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Speziale P., Hök M., Wadström T., Timpl R. Binding of Streptococcus pyogenes to laminin. J Biol Chem. 1984 Mar 25;259(6):3734–3738. [PubMed] [Google Scholar]
- Varmus H. E., Perlman R. L., Pastan I. Regulation of lac messenger ribonucleic acid synthesis by cyclic adenosine 3',5'-monophosphate and glucose. J Biol Chem. 1970 May 10;245(9):2259–2267. [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlund B., Merenmies J., Rauvala H., Miettinen A., Järvinen A. K., Virkola R., Holthöfer H., Korhonen T. K. The O75X adhesin of uropathogenic Escherichia coli: receptor-active domains in the canine urinary tract and in-vitro interaction with laminin. Microb Pathog. 1987 Aug;3(2):117–127. doi: 10.1016/0882-4010(87)90070-2. [DOI] [PubMed] [Google Scholar]
- Winkler J. R., John S. R., Kramer R. H., Hoover C. I., Murray P. A. Attachment of oral bacteria to a basement-membrane-like matrix and to purified matrix proteins. Infect Immun. 1987 Nov;55(11):2721–2726. doi: 10.1128/iai.55.11.2721-2726.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]