Abstract
Metabolic flexibility is the capacity for the organism to adapt fuel oxidation to fuel availability. The inability to modify fuel oxidation in response to changes in nutrient availability has been implicated in the accumulation of intramyocellular lipid and insulin resistance. The metabolic flexibility assessed by the ability to switch from fat to carbohydrate oxidation is usually impaired during a hyperinsulinemic clamp in insulin-resistant subjects; however, this “metabolic inflexibility” is mostly the consequence of impaired cellular glucose uptake. Indeed, after controlling for insulin-stimulated glucose disposal rate (amount of glucose available for oxidation), metabolic flexibility is not altered in obesity regardless of the presence of type 2 diabetes. To understand how intramyocellular lipids accumulate and cause insulin resistance, the assessment of metabolic flexibility to high-fat diets is more relevant than metabolic flexibility during a hyperinsulinemic clamp. An impaired capacity to upregulate muscle lipid oxidation in the face of high lipid supply may lead to increased muscle fat accumulation and insulin resistance. Surprisingly, very few studies have investigated the response to high-fat diets. In this review, we discuss the role of glucose disposal rate, adipose tissue lipid storage, and mitochondrial function on metabolic flexibility. Additionally, we emphasize the bias of using the change in respiratory quotient to calculate metabolic flexibility and propose novel approaches to assess metabolic flexibility. On the basis of current evidence, one cannot conclude that impaired metabolic flexibility is responsible for the accumulation of intramyocellular lipid and insulin resistance. We propose to study metabolic flexibility in response to high-fat diets in individuals having contrasting degree of insulin sensitivity and/or mitochondrial characteristics.
Keywords: fuel selection, insulin sensitivity, mitochondria, lipid oxidation, skeletal muscle
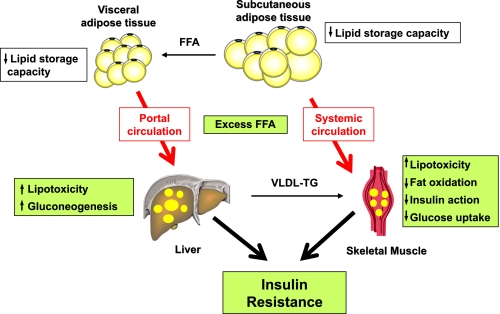
lipid accumulation in skeletal muscle of sedentary people is associated with impaired insulin-stimulated glucose metabolism (31). A reduced capacity of oxidative tissues and organs to adjust lipid oxidation to lipid availability can lead to tissue accumulation of lipids as triglycerides. Excess lipid accretion and/or lower triglyceride turnover can induce lipotoxicity, as reflected by the cellular accumulation of ceramides and diglycerides (39). These lipid species ultimately impair insulin signaling through different mechanisms, either increased serine phosphorylation of the insulin receptor and insulin receptor substrate 1 and/or reduced serine phosphorylation of PKB/Akt (38, 60) (Fig. 1). Therefore, the ability to increase lipid oxidation as a function of their availability eventually reduces the formation of ceramides and diglycerides leading to improved insulin sensitivity.
Fig. 1.
Model for fat-induced insulin resistance describing how a failure to appropriately store lipids into subcutaneous adipose tissue (quantitatively predominant) will lead to ectopic lipid deposition into visceral fat and insulin-sensitive tissues such as liver and skeletal muscle. These tissues will progressively develop a state of lipotoxicity, altering insulin signaling and action and contributing to whole body insulin resistance and deterioration of glucose tolerance.
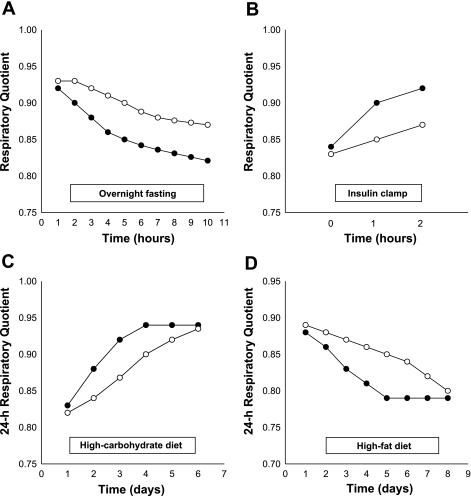
In general, the ability of a system (i.e., whole organism, organ, tissue, or cell) to adjust fuel oxidation to fuel availability is known as metabolic flexibility. This term was coined by Kelley and Mandarino as “the capacity to switch from predominantly lipid oxidation and high rates of fatty acid uptake during fasting conditions to the suppression of lipid oxidation and increased glucose uptake, oxidation, and storage under insulin-stimulated conditions” (26). In line with the above definition, the switch from carbohydrate to lipid oxidation [drop in respiratory quotient (RQ)] during an overnight fast or in response to high-fat diets should also be part of the assessment of metabolic flexibility (Fig. 2).
Fig. 2.
Different features of metabolic flexibility during overnight fasting (A), during a hyperinsulinemic clamp (B), in response to a high-carbohydrate diet (C), and in response to a high-fat diet (D). Metabolically flexible (•) and inflexible (○) subjects.
There is now a growing interest to assess the influence of metabolic flexibility, particularly to dietary fat as a mechanism to explain how lipids can accumulate in skeletal muscle. The switch in fuel oxidation will depend on the type and amount of nutrient available for oxidation at the cellular level. In tissues and organs, fuel availability (glucose, fatty acids, and amino acids) is integrated at the cellular level by fuel sensors that activate or inhibit specific metabolic pathways (47, 50). In response to fuel oversupply, anabolic pathways are activated, whereas the activity of hydrolytic and lipolytic pathways is increased when fuel availability is restricted. In addition, the ability to change substrate oxidation in response to nutritional status will depend on the genetically determined balance between cellular oxidation and storage capacities.
For example, in skeletal muscle, white (glycolytic) or red (oxidative) muscle homogenates respond differently to a supply of fatty acids or glucose. Glycolytic fibers have low rates of fat oxidation compared with oxidative fibers (30), which have high mitochondrial density and oxidative enzyme activities. As a consequence, the oxidative capacity of skeletal muscle may be of utmost importance to boost lipid oxidation to the level of lipid supply and therefore modulate insulin sensitivity. If skeletal muscle cannot match fat oxidation to lipid uptake, fat accumulation will ensue, which in turn will cause insulin resistance.
This review discusses the studies in which metabolic flexibility has been measured at the whole body level or specifically in skeletal muscle tissue or in muscle cells with particular emphasis on the comparison between insulin-resistant and insulin-sensitive individuals. In addition, we discuss the main determinants of metabolic flexibility, how metabolic flexibility should be measured, and which questions need to be answered to better understand the pathophysiology of insulin resistance.
Metabolic Flexibility and Macronutrient Oxidative Regulation
During long-term energy balance, macronutrient oxidation eventually has to match macronutrient intake such that no macronutrients are stored or lost (10). In other words, not only does 24-h energy expenditure have to be equal to 24-h energy intake, but 24-h RQ has to be equal to 24-h food quotient (FQ). The 24-h RQ corresponds to the mean proportion of macronutrient oxidized over a day, whereas 24-h FQ represents the proportion of daily dietary macronutrients available for oxidation (5). Many studies have shown that when people are in energy balance then 24-h RQ eventually matches 24-h FQ (6, 21, 44, 53, 56, 58). Increased availability of carbohydrate results in a rapid increase in carbohydrate oxidation associated with concomitant suppression of lipid oxidation. However, when dietary fat intake increases, one observes a much slower progressive increase in lipid oxidation paralleled by suppression of carbohydrate oxidation.
Both day-to-day variations in energy/macronutrient intake and day-to-day changes in energy expenditure lead to either slightly positive or negative energy balance. In response to these short-term variations in energy balance, carbohydrate and protein stores are closely regulated by an adjustment of oxidation to intake. Consequently, positive or negative energy balances are mostly buffered by changes in fat stores as evidenced by the tight correlation between fat storage and energy balance (1, 9). It is therefore only after short periods of time (one to a few days) that a difference in metabolic flexibility (switch from one type of fuel to another) can be observed. This situation can be particularly relevant in individuals exposed to an obesogenic environment (i.e., high-energy density diets and low physical activity). Whether the capacity to adapt fuel oxidation to fuel availability is preserved or impaired in states of insulin resistance is discussed below.
Metabolic Flexibility in Individuals with Insulin Resistance, Obesity or Family History of Type 2 Diabetes
Metabolic flexibility in response to fasting.
Fatty acids are the main readily available energy sources during the transition from the fed to the overnight-fasted condition, as indicated by the progressive fall in RQ. An impaired drop in RQ during an overnight fast (high fasting RQ) may be defined as metabolic inflexibility to lipid (Fig. 2A). Several studies suggest that fasting RQ is elevated in skeletal muscle from obese insulin-resistant (24) and type 2 diabetic adults (28). Similar findings have been reported at the whole body level in obese adolescents (41) and subjects with a family history of type 2 diabetes (7, 32). Furthermore, obese insulin-resistant individuals subjected to a 16-wk exercise and weight loss program enhanced their insulin sensitivity associated with a reduction in whole body fasting RQ (17). The relationship between fasting RQ and insulin sensitivity, however, has not always been observed. For instance, no association was found between fasting RQ and insulin-stimulated glucose disposal rate in healthy volunteers (61, 62), and Blaak et al. (4) reported lower fasting RQ in obese compared with lean subjects.
Differences in energy balance and macronutrient dietary composition are key determinant factors of the variability in fasting RQ or fuel oxidation. Accordingly, the response to a 1-day drastic increase in energy intake (∼4,500 kcal/day) preceded and followed by underfeeding (500 kcal/day) provided quite different 24-h RQ values (0.80, 0.88, and 0.85, respectively) (54). Additionally, consumption of a eucaloric high-fat or high-carbohydrate diet for 1 wk modified the fasting RQ according to the diet FQ (22, 34). Interestingly, when macronutrient intake and energy balance were carefully controlled, fasting RQ measured in a respiratory chamber for 48 h was similar between obese and lean subjects (64).
In addition to the influence of energy balance and diet composition, RQ is also affected by plasma substrate concentrations (e.g., glucose, FFA). Thus, as a result of hyperglycemia, glucose-dependent muscle glucose uptake can be increased, leading to a higher RQ. On the other hand, when ketone bodies are synthesized without further oxidation, a reduction in RQ is expected (55). These factors can eventually explain part of the differences in fasting RQ, particularly in people with diabetes. Another aspect to take into account when measuring metabolic flexibility in insulin-resistant vs. insulin-sensitive people is the difference between whole body and muscle RQ. Kelley et al. (23) observed that whole body RQ was directly correlated with leg RQ in nondiabetic individuals but not in type 2 diabetic subjects.
Future clinical studies must therefore be designed to ensure that participants are in energy balance and fed standardized diets of similar nutrient composition. Particular attention must be given to RQ data interpretation when differences in plasma glucose and/or FFA concentrations are observed. Finally, the assessment of whole body RQ as a marker of metabolic flexibility can lead to erroneous conclusions about skeletal muscle metabolism, at least in people with type 2 diabetes.
Acute metabolic flexibility in response to nutrients.
METABOLIC FLEXIBILITY TO HIGH-CARBOHYDRATE MEAL.
Metabolic flexibility in response to high-carbohydrate meals has not been frequently compared in subjects with different insulin sensitivity. Recently, the change in whole body RQ in subjects with and without a family history of type 2 diabetes was assessed (20). Individuals with a family history of diabetes usually have a lower insulin-stimulated glucose disposal rate during a hyperinsulinemic clamp, although it was not the case in that study. However, in response to a high-carbohydrate meal (1,000 kcal, 76% energy from carbohydrate), individuals with a family history of diabetes had a higher plasma insulin concentration and a similar increase in RQ compared with control subjects.
METABOLIC FLEXIBILITY DURING A HYPERINSULINEMIC CLAMP.
The increase in RQ during a euglycemic-hyperinsulinemic clamp (ΔRQ) is the original and now common approach to evaluate the metabolic flexibility to carbohydrate (Fig. 2B). The advantage of the clamp procedure is that plasma glucose and insulin concentrations are carefully matched among subjects, even when they have different nutritional and/or metabolic conditions. Kelley and Mandarino (26) and others (14, 24, 65) described an impaired capacity to increase muscle and whole body glucose oxidation and storage during the clamp in insulin-resistant subjects. Additionally, when insulin sensitivity was improved after weight loss, a concomitant enhancement in metabolic flexibility and glucose disposal were observed (14, 35). These data indicate that the increase in ΔRQ is reflecting the amount of glucose entering the cells and being available for oxidation and storage (see below and Ref. 14).
Despite a number of studies that have shown impaired metabolic flexibility during a clamp in insulin-resistant vs. insulin-sensitive individuals, studies are lacking to identify the mechanisms linking metabolic inflexibility to glucose and insulin resistance.
METABOLIC FLEXIBILITY TO HIGH-FAT MEAL.
High-fat diets usually lead to positive energy balance, since fat-rich foods are more palatable and have higher energy density than other foods (49). Fat overload may represent a metabolic challenge for many individuals, even in energy balance conditions, who may indeed fail to appropriately upregulate skeletal muscle lipid oxidation, therefore causing intracellular lipid accumulation and insulin resistance. Although the rationale for metabolic inflexibility to lipid may appear obvious, few studies have so far investigated metabolic flexibility to lipid in individuals with contrasting degrees of insulin sensitivity.
Kelley and Simoneau (28) compared leg RQ in the postprandial state of nondiabetic vs. weight-matched diabetic subjects in response to a high-fat meal (737 kcal, 62% energy from fat). During postabsorptive conditions, diabetic individuals had higher RQ throughout the 6 h following the high-fat meal compared with nondiabetic subjects. Apparently, mitochondrial function was preserved, since key skeletal muscle mitochondrial markers such as citrate synthase (mitochondrial number), cytochrome c oxidase (electron transport chain activity), and 3-hydroxyacyl-CoA dehydrogenase activities were similar in both groups. On the other hand, the large difference in postprandial glycemia (∼2-fold) and lower skeletal muscle FFA uptake observed in diabetic vs. nondiabetic subjects likely drove the difference in leg RQ.
At the whole body level, the drop in RQ after a high-fat meal (1,000 kcal, 76% energy from fat) was examined in individuals with similar insulin sensitivity but with positive or negative family history of type 2 diabetes (20). In both groups, the changes in plasma glucose, FFA, and insulin concentrations were similar; however, individuals without a history of diabetes had larger decrease in RQ compared with offspring from diabetic parents. The lower reliance on fat oxidation for energy supply in offspring from diabetic parents suggests that impaired fat oxidation might precede insulin resistance.
In contrast, a study in 113 lean and 701 obese subjects who ate ∼50% of their daily energy requirement as fat, showed that insulin resistance measured by homeostasis model assessment of insulin resistance (HOMA-IR) was associated with increased postprandial fat oxidation (as percentage of energy expenditure) after controlling for confounding variables such as fat mass, fasting fat oxidation, sex, and physical activity (4).
Prolonged metabolic flexibility in response to diets.
METABOLIC FLEXIBILITY TO HIGH-CARBOHYDRATE DIETS.
Few studies have used the approach of prolonged dietary carbohydrate supplementation to evaluate the metabolic flexibility to carbohydrate, and none of them have evaluated muscle fuel metabolism (Fig. 2C). A carefully controlled study assessed the increase in 24-h RQ in lean and obese subjects who received a mixed diet for 3 days (50% energy from carbohydrate) containing twice the daily energy requirement (64). Surprisingly, both groups experienced a similar increase in RQ in response to the dietary challenge. Using a similar approach, Freymond et al. (12) compared children of obese and nonobese parents. Children of obese parents were heavier and tended to be fatter than children of nonobese parents. Both groups were evaluated under eucaloric conditions and after 3 days of progressive overfeeding with a mixed diet (2-fold energy excess on the 3rd day). There was no difference in the change in the 24-h RQ between groups after the 3-day overfeeding period. Both studies suggest similar metabolic flexibility to carbohydrate- enriched diets in obese and lean individuals.
METABOLIC FLEXIBILITY TO HIGH-FAT DIETS.
Metabolic flexibility to lipid has seldom been evaluated in the context of insulin resistance (Fig. 2D). Ukropcova et al. (62) assessed the body's ability to adjust 24-h fat oxidation to a 3-day eucaloric high-fat diet (50% energy from fat) in subjects with or without a family history of type 2 diabetes but similar insulin-stimulated glucose disposal rates. After 3 days of a high-fat diet, both groups had a similar decrease in 24-h RQ and therefore a similar increase in fat oxidation. However, a lesser decrease in RQ was observed during the sleeping period in the offspring of diabetic parents compared with controls. No association between sleep RQ and insulin sensitivity was observed, but a negative association with muscle mitochondrial content was found (62).
Metabolic flexibility in vitro.
Metabolic flexibility in vitro has been assessed in cultures of human myotubes. Unlike in vivo skeletal muscle, cultured myotubes are not influenced by the physiological milieu, which is kept constant. Therefore, the variability in the response to FFA, glucose, or insulin is determined mostly by genetic and/or epigenetic factors controlling metabolic pathways. Ukropcova et al. (61) investigated whether substrate switching is preserved in cultured myotubes and reflects the metabolic characteristics of the donors. Those authors investigated the suppression of palmitate oxidation by glucose in the absence of insulin, which was termed in vitro “suppressibility”. Contrary to expectations, in vitro suppressibility of fat oxidation was lower in subjects with higher whole body insulin sensitivity and higher metabolic flexibility to glucose. Since the in vivo measurement was done under insulin-stimulated conditions whereas the in vitro assay was performed without insulin, it may be inappropriate to compare these findings.
Similar studies have been designed to evaluate the capacity of myotubes to oxidize fat in response to lipid exposure (15). The increase in fat oxidation in response to palmitate called in vitro “adaptability” was positively related to in vivo insulin sensitivity and metabolic flexibility. In addition, in vitro adaptability was positively related to aerobic capacity, suggesting that myotubes established from lean, fit, insulin-sensitive subjects are more metabolically flexible in response to palmitate exposure in vitro. Similarly, palmitate oxidation has been shown to be lower in myotubes established from type 2 diabetic vs. matched nondiabetic controls (16). Together, these data suggest that intrinsic defects in fat oxidation are present in the skeletal muscle of insulin-resistant subjects. This is somehow consistent with studies showing reduced mitochondrial carnitine palmitoyltransferase I (CPT I) activity in the muscle of sedentary obese subjects with and without type 2 diabetes (29, 57). On the other hand, such impaired mitochondrial lipid oxidation could simply be the result of reduced energy demand and not necessarily due to impaired substrate switching. Indeed, the suppression of glucose oxidation in response to palmitate in the presence of insulin was similar in myotubes established from lean, obese, and type 2 diabetic donors (15).
Together, the above results are difficult to interpret in the context of mitochondrial dysfunction, metabolic flexibility, and insulin resistance. In fact, none of these studies have reported whether differences in mitochondrial density and/or activity may be the underlying mechanism explaining the variability in muscle lipid oxidation and/or lipid accumulation between insulin-resistant and insulin-sensitive individuals.
Determinant Factors of Metabolic Flexibility
Significant differences in mitochondrial number, structure and function have been described between insulin-resistant and insulin-sensitive subjects (3, 25, 36, 37, 40, 42, 43, 48, 59). The hypothesis that mitochondrial abnormalities may be a primary cause of metabolic inflexibility and insulin resistance has been raised but the causal link between the two still remains to be established (38). However, metabolic flexibility also depends on the rate at which nutrients are available to the cells, the ability of adipose tissue to handle fatty acids and even the method used for its calculation (i.e., ΔRQ). Below, we discuss the main variables to take into account when metabolic flexibility is assessed.
Baseline RQ.
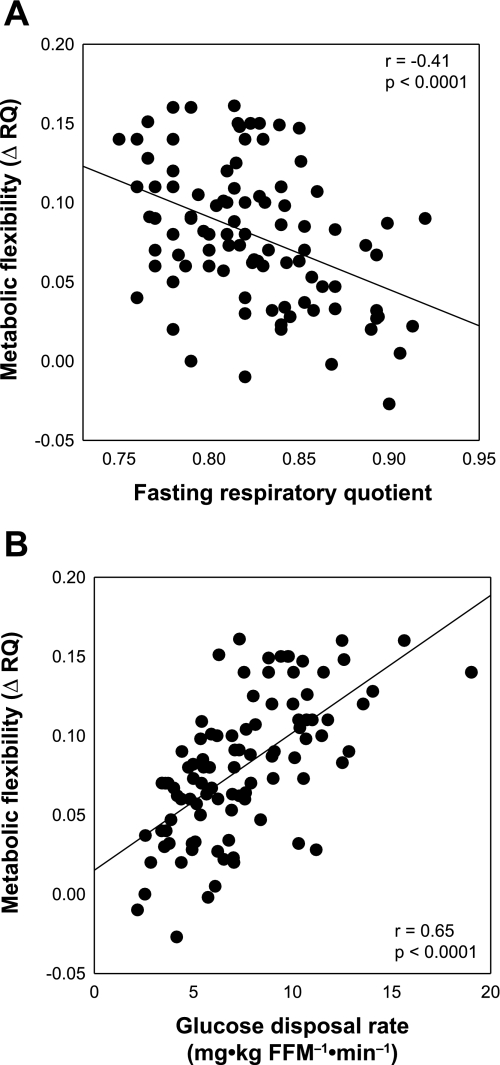
The difference between baseline and stimulated RQ (ΔRQ) is the usual way to estimate metabolic flexibility in response to meals, diets or euglycemic-hyperinsulinemic clamps. Depending on the testing paradigm, baseline RQ corresponds to the fasting RQ (hyperinsulinemic clamp or meal) or the initial 24-h RQ (diet). Accordingly, impaired metabolic flexibility may result from both a lower stimulated RQ and/or an elevated baseline RQ. In fact, fasting RQ is inversely related to metabolic flexibility to carbohydrate during a clamp (Fig. 3A). Fasting RQ is highly sensitive to differences in energy balance and diet composition within the few days preceding the measurement (22, 34, 54). Therefore, differences in metabolic flexibility may be explained by insufficient control of such variables. Subjects in negative energy balance or fed a high-fat diet have lower baseline RQ, increasing the potential to influence ΔRQ in response to energy macronutrients. The following example shows the influence of the baseline RQ on metabolic flexibility assessed by the ΔRQ (Table 1). Two well-matched individuals (A and B) are maintained under energy balance conditions in a metabolic chamber. They are fed for 1 wk with diets having different FQs (subject A = 0.92 and subject B = 0.82). Then, for another week, both receive a diet with an FQ of 0.75. Since both subjects are in energy balance, 24-h RQ is expected to match FQ at the end of each week. Using the ΔRQ as an index of metabolic flexibility, subject A will be characterized as flexible (ΔRQ = −0.17) and subject B as inflexible (ΔRQ = −0.07). Since both subjects were able to match the 24-h RQ to FQ at the end of each week, both subjects should be considered similarly flexible.
Fig. 3.
Correlation between metabolic flexibility [steady-state respiratory quotient (RQ) − fasting RQ = ΔRQ] and fasting RQ (A) and insulin-stimulated glucose disposal rate (B).
Table 1.
Influence of baseline RQ on metabolic flexibility assessed by the change in RQ (ΔRQ)
| Subject |
First Week |
Second Week
|
Δ24-h RQ
|
||
|---|---|---|---|---|---|
| FQ | 24-h RQ* | FQ | 24-h RQ* | (2nd–1st week) | |
| A | 0.92 | 0.92 | 0.75 | 0.75 | –0.17 |
| B | 0.82 | 0.82 | 0.75 | 0.75 | –0.07 |
Subjects A and B are two well-matched individuals maintained under energy balance conditions in a metabolic chamber. RQ, respiratory quotient. They were fed for 1 wk with diets having different food quotients (FQ). Then, for another week they received a diet with an FQ of 0.75.
Value at the end of the respective week.
This example clearly shows the relevance of having similar baseline RQ values when metabolic flexibility is assessed by the change in RQ. In addition, this example raises the concept of the time required to achieve a new equilibrium, since after 7–10 days it is generally expected that all individuals will be in equilibrium between 24-h RQ and FQ. However, subjects with higher metabolic flexibility will reach this equilibrium faster.
Consequently, the assessment of metabolic flexibility should take into account the differences in baseline RQ and the timing required to match 24-h RQ to FQ. One alternative to control for the baseline RQ is to include this factor as a covariate in a regression analysis model. Such an approach requires large sample sizes, something often difficult to afford in clinical studies. Undoubtedly, the best option to reduce the variability in baseline RQ is to maintain individuals for a period of time on a given diet under energy balance conditions to match 24-h RQ to FQ. The next step is to evaluate the differences in the timing required to reach the equilibrium between fuel oxidation and availability. To do that, one can measure the day-to-day change in RQ in a respiratory chamber and calculate the number of days necessary to reach 50% of the expected change in RQ (ΔRQ50%). Such a ΔRQ50% may be in the range of 1–2 days in response to high-carbohydrate diets and in the range of 3–5 days in response to high-fat diets.
Glucose disposal rate.
Metabolic inflexibility to glucose and impaired glucose storage during a hyperinsulinemic clamp is consistently reported in insulin-resistant subjects. In fact, a direct relationship between metabolic flexibility to glucose and glucose disposal rate is described (14, 24, 61, 62) (Fig. 3B). We (14) recently described that insulin-stimulated glucose disposal rate is the main determinant of the change in RQ during a clamp, explaining ∼50% of its variance. The commonly reported metabolic inflexibility and impaired glucose storage in insulin-resistant individuals is expected, since cellular glucose uptake is decreased and cellular glucose available for oxidation and storage is low (8). As a consequence, data corrected for glucose disposal rate, a mechanism proximal to glucose oxidation, indicates no difference in metabolic flexibility and nonoxidative glucose disposal rate between subjects with or without type 2 diabetes and matched for body mass index, sex, and race. Furthermore, after controlling for glucose disposal rate, no improvement in metabolic flexibility is observed after weight loss in type 2 diabetic individuals subjected to a 1-yr intensive lifestyle intervention including energy restriction and increased physical activity (14). When subjects were divided into quartiles of insulin sensitivity, the insulin-sensitive group (upper quartile; n = 25) had about a fourfold higher glucose disposal rate, nonoxidative glucose disposal rate, and increased steady-state RQ compared with the insulin-resistant group (lower quartile; n = 25). However, after adjustment for differences in glucose disposal rates, there was no longer any difference in nonoxidative glucose disposal rate and steady-state RQ between groups (Ref. 14; Table 2). Further support for these findings comes from studies in which glucose disposal rates were matched by increasing the glucose or insulin infusion rates (27, 66). Together, the results indicate that the impaired metabolic flexibility and glucose storage so often observed during a clamp in insulin-resistant individuals are the consequence of impaired glucose transport rather than a defective cellular glucose oxidative and nonoxidative metabolism.
Table 2.
Steady-state RQ between subjects with high and low insulin-stimulated glucose disposal rate
| Highest Insulin Sensitivity | Lowest Insulin Sensitivity | |
|---|---|---|
| Female/male | 19/6 | 12/13* |
| DM/non-DM | 4/21 | 23/2* |
| White/black/other | 15/10/0 | 23/0/2* |
| Age, yr | 54.3±1.4 | 60.1±1.6* |
| BMI, kg/m2 | 32.9±0.5 | 33.0±0.5 |
| Body fat, % | 39.0±1.5 | 35.3±1.2 |
| GDR, mg·kg FFM–1·min–1 | 11.8±0.4 | 4.0±0.2 |
| Nonoxidative GDR, mg·kg FFM–1·min–1 | 8.5±0.5 | 2.3±0.2† |
| Steady-state RQ | 0.94±0.01 | 0.87±0.01† |
Values are means ± SE.
P < 0.05,
P > 0.44 after controlling for sex, race, presence of type 2 diabetes mellitus (DM), age, and insulin-stimulated glucose disposal rate (GDR; P < 0.02 when values are not controlled for GDR).
Adipose tissue lipid storage capacity and plasma FFA concentration.
Just as intracellular glucose availability influences metabolic flexibility, higher plasma lipid concentration also drives fuel oxidation by increasing fat oxidation (14, 33, 46, 52). Additionally, high plasma FFA concentration impairs insulin-stimulated glucose disposal rate and decreases intracellular glucose, which in turn reduces glucose oxidation and consequently enhances lipid oxidation (2, 8).
Since adipose tissue is the main source of plasma FFA, the capacity to store and release FFA from adipose tissue may influence metabolic flexibility (11). For instance, patients with lipodystrophy (partial or total absence of subcutaneous adipose tissue) have similar fat oxidation than control individuals when fed a eucaloric, mixed diet (51). However, in response to excess energy as fat, lipodystrophic subjects have higher fat oxidation compared with control individuals (51). Accordingly, a lesser decrease in RQ after a fat overload might be the result of enhanced adipose tissue lipid storage capacity rather than impaired lipid oxidative capacity.
Mitochondrial oxidative capacity.
It is known that mitochondrial content and activity determine fatty acid oxidation in response to lipid. This has been shown by comparing fatty acid oxidation rates between homogenates of red (oxidative) and white (glycolytic) skeletal muscles (30). These data and the evidence indicating multiple mitochondrial abnormalities in type 2 diabetic and insulin-resistant individuals (3, 25, 36, 37, 40, 42, 43, 48, 59) led to the hypothesis that lower mitochondrial capacity is associated with reduced resting lipid oxidation and therefore increased muscle lipid accumulation. Such muscle mitochondrial dysfunction was suggested to be an intrinsic characteristic of muscle cells, since fat oxidation in response to palmitate was lower in myotubes from insulin-resistant vs. insulin-sensitive individuals (16, 61). However, comparisons among obese, diabetic, and lean individuals have failed to appropriately match the groups for physical fitness, opening up the possibility that mitochondrial dysfunction may be related to lower physical activity in metabolically inflexible subjects (45).
Independently of the origin of these mitochondrial abnormalities, it is not clear whether the extent to which muscle mitochondria are impaired is sufficient to influence metabolic flexibility to lipid, especially in resting conditions. Some suggestion comes from the comparison between subjects with and without a history of type 2 diabetes (62). The former group had on average a 22% less mitochondrial DNA copy number (marker of mitochondrial number) than control subjects. Increased mitochondrial number was related to a higher decrease in sleeping RQ in response to a 3-day isoenergetic high-fat diet. However, when mitochondrial number was related to 24-h RQ after the high-fat diet period, no association between mitochondrial number and the change in 24-h RQ was observed. Sleeping RQ may be relevant, because under this condition most of the energy comes from lipid. However, since energy expenditure is minimal in resting conditions, the absolute lipid oxidative demand is hardly a metabolic challenge for muscle mitochondria. As indicated in the previous section, a higher decrease in RQ after fat overload may well be a consequence of reduced adipose tissue lipid storage capacity.
Another interesting experimental paradigm is to assess the metabolic flexibility to lipids (e.g., meals or diets) between athletes and sedentary individuals. Clearly, one knows that athletes have better muscle oxidative capacity (i.e., mitochondrial number and activity); however, such a simple study has not been performed yet. Alternatively, elderly individuals matched for body mass and fat content to young volunteers showed on average 26% lower maximal aerobic consumption (vo2max) vs. young individuals (6). Since vo2max has been shown to be directly related to muscle mitochondrial density (62, 63), one could expect an improved metabolic flexibility in young vs. elderly individuals. However, after 4 days of intervention, both groups were similarly able to match whole body fuel oxidation to fuel intake.
On the other hand, fuel oxidation may not necessarily be reduced in presence of mitochondrial dysfunction. For instance, frank mitochondrial dysfunction in humans (e.g., myopathies with impaired electron transport chain activity) is accompanied by increased fuel oxidation to compensate for the impaired mitochondrial ATP synthesis (13). Similarly, mice with muscle-specific PGC1α (PPARγ coactivator-1α) deletion have defective mitochondrial function (e.g., lower staining for cytochrome c oxidase and succinate dehydrogenase); however, they have increased metabolic rate, lower RQ, and improved insulin-stimulated glucose uptake in skeletal muscle compared with wild-type animals (19). Therefore, mitochondrial dysfunction may well be associated with high fuel oxidation and enhanced insulin sensitivity.
Another hypothesis has recently been proposed by Koves et al. (30), stating that lipid oversupply can promote excessive lipid oxidation, which will disconnect the coupling between β-oxidation and the TCA cycle, generating excessive amounts of incompletely oxidized acyl-carnitine intermediates. The latter lipid species may interfere with insulin signaling and glucose transport through currently unknown mechanisms. This is in line with the finding that high-fat diets increase mitochondrial biogenesis and fat oxidation in parallel with a decrease in insulin sensitivity (18), possibly as a consequence of increased partially oxidized lipid intermediates.
Taken together, the literature does not support the hypothesis that impaired mitochondrial function leads to lower metabolic flexibility to lipid. In fact, it remains largely unknown whether muscle mitochondrial abnormalities described in insulin-resistant individuals are sufficient to affect metabolic flexibility to lipid.
Other determinant factors of metabolic flexibility.
In a recent study, we (14) identified other factors determining metabolic flexibility to glucose in humans. As expected, metabolic flexibility to glucose during a clamp was related to surrogate markers of insulin resistance such as fasting plasma glucose, FFA, and insulin concentrations. In addition, we observed that metabolic flexibility to glucose was directly related to plasma adiponectin concentration. Since most of these variables are interrelated, we performed a stepwise multiple regression analysis including these variables. Only glucose disposal rate and steady-state plasma FFA concentration (the latter explained 3% of the variance in metabolic flexibility) were independent determinants of the change in RQ (ΔRQ) during a euglycemic-hyperinsulinemic clamp accounting for half of its variance. It is now necessary to assess more deeply the cellular determinants of metabolic flexibility to lipid in order to improve our understanding of the underlying causes of impaired metabolic flexibility to lipid.
Conclusions
Most of the research on metabolic flexibility has focused on the capacity to metabolize glucose in response to an overload of carbohydrate during a euglycemic-hyperinsulinemic clamp. However, the difference in metabolic flexibility during the clamp in individuals with varying insulin sensitivity is mostly the consequence of variability in insulin-stimulated glucose disposal rate (14). Furthermore, the mitochondrial capacity to oxidize acetyl-CoA coming from glucose is conserved in insulin-resistant subjects even if mitochondrial defects have been reported in those individuals (3, 25, 36, 37, 40, 42, 43, 48, 59). These findings should not negate the potential role of mitochondrial defects in insulin resistance, since during a euglycemic-hyperinsulinemic clamp lipid demand as an energy source is mostly suppressed.
The assessment of metabolic flexibility to lipid will probably unravel defects in lipid oxidative capacity. Surprisingly, there are no studies evaluating metabolic flexibility to lipid in subjects with contrasting degree of insulin resistance. Furthermore, most studies have used fasting RQ as a marker of metabolic flexibility to lipid. However, fasting RQ is not a reliable indicator of lipid oxidation capacity, since RQ under fasting conditions is mostly influenced by energy balance and dietary macronutrient composition (22, 34, 54). In addition, under resting conditions, energy demand with concomitant fat oxidation requirement is hardly a metabolic challenge for muscle mitochondria. Consequently, an eventual defect in fat oxidation is unlikely to be evidenced in resting conditions.
The rate at which fat oxidation adjusts to high fat intake is variable among individuals (21, 53), but only scarce evidence exists about which factors determine this variability. However, this adaptation cannot take much more time than that required to deplete the glycogen stores, i.e., a few days to a week. The question then becomes: is there any importance in assessing the metabolic flexibility to lipid, since eventually fat oxidation will match fat intake in all individuals? The answer is yes, since the speed of the adaptation will probably impact the amount of lipid accumulation in the muscle and therefore impact insulin sensitivity. Individuals able to increase fat oxidation quickly in response to day-to-day changes in fat intake will eventually have lower muscle fat accumulation and be less prone to insulin resistance compared with slower adapters. On the basis of the RQ profile for metabolically flexible and inflexible subjects shown in Fig. 2D, and considering an energy intake of 2,500 kcal/day (protein intake 20% of total energy), the metabolically inflexible individuals would accumulate ∼200 g more fat than flexible subjects after 1 wk of a high-fat diet (60% of total energy).
We propose that using the resting condition to test metabolic flexibility, particularly at the skeletal muscle level, is not appropriate, since the absolute lipid oxidation rate is minimal. Energy metabolism under exercise conditions provides a paradigm requiring highly coordinated regulation between fuel supply and the oxidative machinery. This approach in combination with muscle lipid and glycogen content determination may be useful to assess the role of mitochondrial density/function on metabolic flexibility to lipid.
In conclusion, evidence indicating mitochondrial defects as a driving factor of metabolic inflexibility and insulin resistance are far from conclusive or even unavailable. It will be important to test whether whole body or skeletal muscle metabolic flexibility to lipid is affected by muscle mitochondrial characteristics such as density, morphology, and activity. In addition, the role of metabolic flexibility in muscle lipid accumulation and the development of insulin resistance requires further studies. Only this kind of data will allow us to establish a causal link among impaired capacity to metabolize fat, muscle lipotoxicity, and insulin resistance.
GRANTS
J. Galgani is supported by a fellowship from The International Nutrition Foundation/Ellison Medical Foundation. E. Ravussin is supported by National Institutes of Health grants U01-AG-020478 and RO1-DK-60412.
Acknowledgments
We acknowledge Dr. Darcy Johannsen and Stacy Carling for their valuable help editing the manuscript.
REFERENCES
- 1.Abbott WG, Howard BV, Christin L, Freymond D, Lillioja S, Boyce VL, Anderson TE, Bogardus C, Ravussin E. Short-term energy balance: relationship with protein, carbohydrate, and fat balances. Am J Physiol Endocrinol Metab 255: E332–E337, 1988. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann OP, Dahl DB, Brechtel K, Machann J, Haap M, Maier T, Loviscach M, Stumvoll M, Claussen CD, Schick F, Haring HU, Jacob S. Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes 50: 2579–2584, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, Shulman GI. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 56: 1376–1381, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaak EE, Hul G, Verdich C, Stich V, Martinez A, Petersen M, Feskens EF, Patel K, Oppert JM, Barbe P, Toubro S, Anderson I, Polak J, Astrup A, Macdonald IA, Langin D, Holst C, Sorensen TI, Saris WH. Fat oxidation before and after a high fat load in the obese insulin-resistant state. J Clin Endocrinol Metab 91: 1462–1469, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Black AE, Prentice AM, Coward WA. Use of food quotients to predict respiratory quotients for the doubly-labelled water method of measuring energy expenditure. Hum Nutr Clin Nutr 40: 381–391, 1986. [PubMed] [Google Scholar]
- 6.Davy KP, Horton T, Davy BM, Bessessen D, Hill JO. Regulation of macronutrient balance in healthy young and older men. Int J Obes Relat Metab Disord 25: 1497–1502, 2001. [DOI] [PubMed] [Google Scholar]
- 7.De Pergola G, Pannacciulli N, Minenna A, Martina RA, Cannito F, Giorgino R. Fuel metabolism in adult individuals with a wide range of body mass index: effect of a family history of type 2 diabetes. Diabetes Nutr Metab 16: 41–47, 2003. [PubMed] [Google Scholar]
- 8.Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 103: 253–259, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flatt JP Body composition, respiratory quotient, and weight maintenance. Am J Clin Nutr 62: 1107S-1117S, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Flatt JP Dietary fat, carbohydrate balance, and weight maintenance. Ann NY Acad Sci 683: 122–140, 1993. [DOI] [PubMed] [Google Scholar]
- 11.Frayn KN Adipose tissue as a buffer for daily lipid flux. Diabetologia 45: 1201–1210, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Freymond D, Larson K, Bogardus C, Ravussin E. Energy expenditure during normo- and overfeeding in peripubertal children of lean and obese Pima Indians. Am J Physiol Endocrinol Metab 257: E647–E653, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Gadian D, Radda G, Ross B, Hockaday J, Bore P, Taylor D, Styles P. Examination of a myopathy by phosphorus nuclear magnetic resonance. Lancet 2: 774–775, 1981. [DOI] [PubMed] [Google Scholar]
- 14.Galgani JE, Heilbronn LK, Azuma K, Kelley DE, Albu JB, Pi-Sunyer X, Smith SR, Ravussin E. Metabolic flexibility in response to glucose is not impaired in people with type 2 diabetes after controlling for glucose disposal rate. Diabetes 57: 841–845, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaster M Metabolic flexibility is conserved in diabetic myotubes. J Lipid Res 48: 207–217, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Gaster M, Rustan AC, Aas V, Beck-Nielsen H. Reduced lipid oxidation in skeletal muscle from type 2 diabetic subjects may be of genetic origin: evidence from cultured myotubes. Diabetes 53: 542–548, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes 52: 2191–2197, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA 105: 7815–7820, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, Kulkarni RN, Shulman GI, Spiegelman BM. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest 117: 3463–3474, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heilbronn LK, Gregersen S, Shirkhedkar D, Hu D, Campbell LV. Impaired fat oxidation after a single high-fat meal in insulin-sensitive nondiabetic individuals with a family history of type 2 diabetes. Diabetes 56: 2046–2053, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Hill JO, Peters JC, Reed GW, Schlundt DG, Sharp T, Greene HL. Nutrient balance in humans: effects of diet composition. Am J Clin Nutr 54: 10–17, 1991. [DOI] [PubMed] [Google Scholar]
- 22.Hurni M, Burnand B, Pittet P, Jequier E. Metabolic effects of a mixed and a high-carbohydrate low-fat diet in man, measured over 24 h in a respiration chamber. Br J Nutr 47: 33–43, 1982. [DOI] [PubMed] [Google Scholar]
- 23.Kelley D, Mokan M, Veneman T. Impaired postprandial glucose utilization in non-insulin-dependent diabetes mellitus. Metabolism 43: 1549–1557, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol Endocrinol Metab 277: E1130–E1141, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 49: 677–683, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Kelley DE, Mandarino LJ. Hyperglycemia normalizes insulin-stimulated skeletal muscle glucose oxidation and storage in noninsulin-dependent diabetes mellitus. J Clin Invest 86: 1999–2007, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest 94: 2349–2356, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab 279: E1039–E1044, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 42: 113–116, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Lattuada G, Costantino F, Caumo A, Scifo P, Ragogna F, De Cobelli F, Del Maschio A, Luzi L, Perseghin G. Reduced whole-body lipid oxidation is associated with insulin resistance, but not with intramyocellular lipid content in offspring of type 2 diabetic patients. Diabetologia 48: 741–747, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Lillioja S, Bogardus C, Mott DM, Kennedy AL, Knowler WC, Howard BV. Relationship between insulin-mediated glucose disposal and lipid metabolism in man. J Clin Invest 75: 1106–1115, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNeill G, Bruce AC, Ralph A, James WP. Inter-individual differences in fasting nutrient oxidation and the influence of diet composition. Int J Obes 12: 455–463, 1988. [PubMed] [Google Scholar]
- 35.Mingrone G, Manco M, Calvani M, Castagneto M, Naon D, Zorzano A. Could the low level of expression of the gene encoding skeletal muscle mitofusin-2 account for the metabolic inflexibility of obesity? Diabetologia 48: 2108–2114, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Mogensen M, Sahlin K, Fernstrom M, Glintborg D, Vind BF, Beck-Nielsen H, Hojlund K. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 56: 1592–1599, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267–273, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55, Suppl 2: S9–S15, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moro C, Bajpeyi S, Smith SR. Determinants of intramyocellular triglyceride turnover: implications for insulin sensitivity. Am J Physiol Endocrinol Metab 294: E203–E213, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100: 8466–8471, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perseghin G, Bonfanti R, Magni S, Lattuada G, De Cobelli F, Canu T, Esposito A, Scifo P, Ntali G, Costantino F, Bosio L, Ragogna F, Del Maschio A, Chiumello G, Luzi L. Insulin resistance and whole body energy homeostasis in obese adolescents with fatty liver disease. Am J Physiol Endocrinol Metab 291: E697–E703, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350: 664–671, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen KF, Dufour S, Shulman GI. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med 2: e233, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prentice AM Macronutrients as sources of food energy. Public Health Nutr 8: 932–939, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Rabol R, Boushel R, Dela F. Mitochondrial oxidative function and type 2 diabetes. Appl Physiol Nutr Metab 31: 675–683, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Randle PJ, Newsholme EA, Garland PB. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J 93: 652–665, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ravussin E Cellular sensors of feast and famine. J Clin Invest 109: 1537–1540, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54: 8–14, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Rolls BJ The role of energy density in the overconsumption of fat. J Nutr 130: 268S–271S, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov 3: 340–351, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Savage DB, Murgatroyd PR, Chatterjee VK, O'Rahilly S. Energy expenditure and adaptive responses to an acute hypercaloric fat load in humans with lipodystrophy. J Clin Endocrinol Metab 90: 1446–1452, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Schiffelers SL, Saris WH, van Baak MA. The effect of an increased free fatty acid concentration on thermogenesis and substrate oxidation in obese and lean men. Int J Obes Relat Metab Disord 25: 33–38, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Schrauwen P, van Marken Lichtenbelt WD, Saris WH, Westerterp KR. Changes in fat oxidation in response to a high-fat diet. Am J Clin Nutr 66: 276–282, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Schutz Y The adjustment of energy expenditure and oxidation to energy intake: the role of carbohydrate and fat balance. Int J Obes Relat Metab Disord 17, Suppl 3: S23–S27; discussion S41–S42, 1993. [PubMed] [Google Scholar]
- 55.Schutz Y, Ravussin E. Respiratory quotients lower than 0.70 in ketogenic diets. Am J Clin Nutr 33: 1317–1319, 1980. [DOI] [PubMed] [Google Scholar]
- 56.Shetty PS, Prentice AM, Goldberg GR, Murgatroyd PR, McKenna AP, Stubbs RJ, Volschenk PA. Alterations in fuel selection and voluntary food intake in response to isoenergetic manipulation of glycogen stores in humans. Am J Clin Nutr 60: 534–543, 1994. [DOI] [PubMed] [Google Scholar]
- 57.Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J 13: 2051–2060, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Smith SR, de Jonge L, Zachwieja JJ, Roy H, Nguyen T, Rood JC, Windhauser MM, Bray GA. Fat and carbohydrate balances during adaptation to a high-fat. Am J Clin Nutr 71: 450–457, 2000. [DOI] [PubMed] [Google Scholar]
- 59.Sreekumar R, Halvatsiotis P, Schimke JC, Nair KS. Gene expression profile in skeletal muscle of type 2 diabetes and the effect of insulin treatment. Diabetes 51: 1913–1920, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Summers SA, Nelson DH. A role for sphingolipids in producing the common features of type 2 diabetes, metabolic syndrome X, and Cushing's syndrome. Diabetes 54: 591–602, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Ukropcova B, McNeil M, Sereda O, de Jonge L, Xie H, Bray GA, Smith SR. Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. J Clin Invest 115: 1934–1941, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ukropcova B, Sereda O, de Jonge L, Bogacka I, Nguyen T, Xie H, Bray GA, Smith SR. Family history of diabetes links impaired substrate switching and reduced mitochondrial content in skeletal muscle. Diabetes 56: 720–727, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Welle S, Bhatt K, Shah B, Needler N, Delehanty JM, Thornton CA. Reduced amount of mitochondrial DNA in aged human muscle. J Appl Physiol 94: 1479–1484, 2003. [DOI] [PubMed] [Google Scholar]
- 64.Weyer C, Vozarova B, Ravussin E, Tataranni PA. Changes in energy metabolism in response to 48 h of overfeeding and fasting in Caucasians and Pima Indians. Int J Obes Relat Metab Disord 25: 593–600, 2001. [DOI] [PubMed] [Google Scholar]
- 65.Wohl P, Girman P, Pelikanova T. Inflexibility of energy substrate oxidation in type 1 diabetic patients. Metabolism 53: 655–659, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Yki-Jarvinen H, Sahlin K, Ren JM, Koivisto VA. Localization of rate-limiting defect for glucose disposal in skeletal muscle of insulin-resistant type I diabetic patients. Diabetes 39: 157–167, 1990. [DOI] [PubMed] [Google Scholar]