Abstract
Pheochromocytomas in patients with von Hippel-Lindau (VHL) syndrome and multiple endocrine neoplasia type 2 (MEN 2) differ in the types and amounts of catecholamines produced and the resulting signs and symptoms. We hypothesized the presence of different processes of catecholamine release reflecting differential expression of components of the regulated secretory pathway among the two types of hereditary tumors. Differences in catecholamine secretion from tumors in patients with VHL syndrome (n = 47) and MEN 2 (n = 32) were examined using measurements of catecholamines in tumor tissue, urine, and plasma, the last of which was under baseline conditions in all subjects and in a subgroup of patients who received intravenous glucagon to provoke catecholamine release. Microarray and proteomics analyses, quantitative PCR, and Western blotting were used to assess expression of tumor tissue secretory pathway components. The rate constant for baseline catecholamine secretion was 20-fold higher in VHL than in MEN 2 tumors (0.359 ± 0.094 vs. 0.018 ± 0.009 day−1), but catecholamine release was responsive only to glucagon in MEN 2 tumors. Compared with tumors from MEN 2 patients, those from VHL patients were characterized by reduced expression of numerous components of the regulated secretory pathway (e.g., SNAP25, syntaxin, rabphilin 3A, annexin A7, calcium-dependent secretion activator). The mutation-dependent differences in expression of secretory pathway components indicate a more mature regulated secretory pathway in MEN 2 than VHL tumors. These data provide a unique mechanistic link to explain how variations in the molecular machinery governing exocytosis may contribute to clinical differences in the secretion of neurotransmitters or hormones and the subsequent presentation of a disease.
Keywords: secretion, exocytosis, von Hippel-Lindau, multiple endocrine neoplasia type 2
the clinical manifestations of pheochromocytoma are diverse and highly variable (30, 35). The basis for this variability is only now becoming understood. In particular, with increasing recognition of the importance of hereditary influences on the development of pheochromocytomas it is now becoming apparent that differences in the underlying mutation can have a profound impact on the clinical presentation of tumors. Such influences include mutation-dependent effects on tumor location, propensity for malignancy, types of catecholamines produced, and resulting signs and symptoms (2, 10, 33). Patients with multiple endocrine neoplasia type 2 (MEN 2), a neoplastic syndrome due to mutations of the RET proto-oncogene, develop pheochromocytomas that produce epinephrine (20). In contrast, those due to mutations of the von Hippel-Lindau (VHL) tumor suppressor gene produce mainly norepinephrine and very little epinephrine, a consequence of lack of expression of phenylethanolamine N-methyltransferase, the enzyme that converts norepinephrine to epinephrine (21). The more symptomatic presentation and paroxysmal nature of the hypertension in patients with the former than the latter tumors has been linked to differences in the types of catecholamines produced but could also reflect differences in the nature of their secretion.
Exocytotic secretion of vesicular contents can occur by regulated and constitutive secretory pathways. Regulated secretion is a calcium-dependent exocytotic process responsive to neural input or secretogogues and the principle mechanism responsible for controlled release of neurotransmitters and hormones, including catecholamines (4, 11, 37). Constitutive secretion is an exocytotic process that is relatively insensitive to changes in calcium and is responsible for transport of proteins and other macromolecules to the plasma membrane and extracellular environment (11); it is a ubiquitous cellular pathway that in neurons and endocrine cells may also contribute to basal release of neurotransmitters and hormones (9, 32).
Although secretion of catecholamines from pheochromocytomas occurs autonomously without regulatory input from the central nervous system, the process nevertheless depends in part on calcium-dependent secretory pathways responsive to secretogogues such as histamine and glucagon (1, 12, 24, 31). Alterations in the modulatory components of the exocytotic machinery may contribute to indiscriminate calcium-dependent or calcium-independent secretion and resulting variations among tumors in the nature of catecholamine secretion and resulting signs and symptoms.
This study examined the hypothesis that pheochromocytomas in MEN 2 and VHL patients differ in the nature of catecholamine secretion, reflecting differences in expression of secretory pathway components. Catecholamine secretion was assessed from measurements of catecholamines in plasma and urine normalized to differences in tumor volume and catecholamine contents to provide estimates of rate constants for secretion into plasma and excretion into urine. Responses of plasma catecholamines to the secretogogue glucagon were examined to assess functional responsiveness of the catecholamine secretory process. Differential expression of components of the regulated secretory pathway was explored utilizing a database generated from previously published cDNA and oligonucleotide microarray studies of gene expression profiles in hereditary pheochromocytomas (6, 19). The database also included results from proteomics analyses employing capillary isoelectric focusing of enzyme-digested protein peptide fragments, nanoreverse-phase liquid chromatography, with identification of proteins from chromatographic eluants using electrospray ionization-tandem mass spectrometry (38). Relevant secretory pathway components identified from this database that showed differential expression between VHL and MEN 2 tumors were further validated using a combination of quantitative PCR and Western blotting.
METHODS
Clinical Investigations
Patients.
Subjects included 79 patients with histologically confirmed hereditary pheochromocytoma, 47 with tumors associated with VHL syndrome (20 females, 27 males), and 32 with MEN 2 (19 females, 13 males). VHL patients had a mean (±SD) age of 32 ± 14 yr (range 8–63 yr) at the time of biochemical testing for tumors that were resected on average 3.4 mo after blood samples were collected for biochemical diagnosis. MEN 2 patients were aged 38 ± 10 yr (range 17–57 yr) at the time of biochemical testing, which was carried out on average 2.2 mo before resection of tumors.
All MEN 2 patients and 44 of the 47 VHL patients had unilateral or bilateral adrenal tumors. Six VHL patients had extra-adrenal paragangliomas, including three who had both adrenal and extra-adrenal tumors. All patients had family and medical histories consistent with their syndromes, which in MEN 2 patients included medullary thyroid carcinoma, hyperparathyroidism in several patients, and multiple mucosal neuromas in two patients with MEN type 2B. No VHL patient among the series presented with pheochromocytoma as the sole manifestation of the syndrome (i.e., VHL type 2C). All had some additional clinical stigmata consistent with VHL syndrome, including the presence of hemiangioblastomas of the central nervous system and retina, renal carcinomas, pancreatic tumors, and cysts. Diagnosis of VHL syndrome or MEN 2 was confirmed by identification of germ line mutations of the VHL tumor suppressor gene or the RET proto-oncogene. Locations of mutations were available in 46 of the 47 VHL patients and 26 of the 32 MEN 2 patients. Among the VHL patients, partial deletions of the VHL gene were present in two patients and missense mutations in the others. Among the MEN 2 patients, 17 of the germline mutations were located at codon 634, three were located at codon 631, two each were at codons 611 and 618, and one each was at codons 791 and 918, the latter in a patient with MEN 2B. Patients provided written, informed consent for studies that were approved by the institutional review boards or ethics committees at the centers where they participated (institutional review boards of the National Institute of Child Health and Human Development and National Cancer Institute at the National Institutes of Health and ethics committees at the University of Florence, Florence, Italy, and Radboud University Nijmegen Medical Center, Nijmegen, The Netherlands).
Collection of blood and urine samples.
Blood samples were obtained from all patients, with an indwelling intravenous (iv) catheter inserted into a forearm vein, with patients supine for ≥20 min before blood collection. Samples of blood were transferred into tubes containing heparin as anticoagulant and immediately placed on ice until centrifuged (4°C) to separate the plasma. Plasma samples were stored at −80°C until assayed.
Twenty-four-hour urine samples were collected from 42 of the 47 VHL patients and 25 of the 32 MEN 2 patients. Samples were collected with 20 ml of 6 N hydrochloric acid as a preservative total urine volume was determined and aliquots kept at 4°C until assayed.
Glucagon provocation testing.
Glucagon was administered as a 1-mg iv bolus to 25 of the 47 VHL patients and 14 of the 32 MEN 2 patients. Blood samples were obtained immediately before and 2–3 min after injection of glucagon with the use of an iv catheter inserted into a forearm vein. Patients were in the supine position throughout the procedure.
Surgical specimen collection.
Samples of tumor tissue were obtained at surgery from 42 VHL patients and 32 MEN 2 patients. Extraneous tissue was removed, and the dimensions of tumors were recorded. The bulk of specimens were sent for formal pathological review, with smaller samples of each tumor divided into 10- to 50-mg pieces that were frozen on dry ice and stored at −80°C before further laboratory analyses, as described below.
Laboratory Analyses
Catecholamine determinations.
Plasma, urine, and tissue concentrations of catecholamines (norepinephrine, epinephrine, and dopamine) were quantified by liquid chromatography with electrochemical detection (18). Samples of tissue were weighed and homogenized in ≥5 vol of 0.4 M perchloric acid containing 0.5 mM EDTA. Homogenized samples were centrifuged (1,500 g for 15 min at 4°C) and supernatants collected and stored at −80°C until assayed. Concentrations of catecholamines were determined after extraction from plasma or perchloric acid tissue supernatants using alumina adsorption.
Microarray analyses.
Gene expression profiling of pheochromocytoma tumor tissue samples was carried out using cDNA and oligonucleotide microarrays as described in detail elsewhere (6, 19). The cDNA arrays utilized mRNA extracted from tumors of 12 patients with VHL syndrome and seven with MEN 2. Oligonucleotide arrays utilized tumor tissue samples from 11 VHL and 12 MEN 2 patients.
Proteomics.
The proteomics platform used in this study has been described in detail elsewhere (38). Protein extracts of tumor tissue samples from three MEN 2 and three VHL patients were pooled by group and digested with trypsin. Capillary isoelectric focusing was used to fractionate peptides. The fractions were further separated by nanoreverse-phase liquid chromatography. Chromatographic eluants were monitored using electrospray ionization-tandem mass spectrometry on a linear ion trap (ThermoFisher, Waltham, MA) to profile peptides. Protein identities were obtained by matching of peak list files to the human SwisProt sequence library with the NCBI Open Mass Spectrometry Search Algorithm database.
As described in our previous studies (5), the capillary isoelectric focusing-based proteome technology was combined with the spectral counting approach (29) for performing quantitative proteome analysis. In this work, a spectral count was defined as the identification of a single peptide tandem mass spectrum. Additional identifications of the same peptide, e.g., via fragmentation of another charge state, were each counted as additional spectral counts.
Quantitative PCR.
RNA was extracted from frozen samples of pheochromocytoma tissue after homogenization in TRIzol reagent (Invitrogen, Carlsbad, CA) followed by RNeasy Maxi (Qiagen, Valencia, CA). Total RNA (2 μg) was reverse transcribed to cDNA using random hexamers. Quantitative PCR (TaqMan PCR), carried out using a 7000 Sequence Detector (Applied Biosystems, Foster City, CA), was used for quantification of mRNA for secretory pathway genes, as described previously (23). The primers and TaqMan probes were provided as premade sets ordered through Applied Biosystems. 18S ribosomal RNA was used as a housekeeping gene. PCR amplifications of 18S ribosomal RNA and the genes of the secretory pathway were carried out in separated tubes. Reaction tubes contained 20 ng of cDNA product as template, 1× TaqMan Universal PCR Master Mix, 1× premade sets of primers, and TaqMan probes of the secretory pathway genes or 18S ribosomal RNA, all to a final volume of 50 μl with H2O. PCR involved 40 cycles at the following temperature parameters: 15 s at 95°C and 1 min at 60°C. Input RNA amounts were calculated manually using the comparative cycle threshold method for the target genes and 18S ribosomal RNA.
Western blot analysis.
Tissue proteins (20 μg) were separated by electrophoresis on polyacrylamide gels and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA). Membranes were incubated in blocking buffers for 1 h at room temperature and then with primary antibodies overnight at 4°C at optimized dilutions (Table 1). Membranes were washed three times for 10 min each with Tris-buffered saline (50 mM Tris, pH 7.4, 0.9% NaCl) containing 0.05% Tween-20 (TBS-T) and incubated with horseradish peroxidase-conjugated secondary antibodies. Membranes were then washed again three times for 10 min each with TBS-T. Target protein bands were visualized using the enhanced chemiluminescence method. Expression of pathway proteins in Western blots was assessed by densitometry using National Institutes of Health Image 1.62 software.
Table 1.
Sources and dilution conditions for primary antibodies of secretory pathway proteins
| Primary Antibodies | Sources | Dilution |
|---|---|---|
| Mouse anti-RPH3A | BD Biosciences, San Jose, CA | 1:500 |
| Mouse anti-CADPS | BD Biosciences | 1:500 |
| Rabbit anti-STX1* | Chemicon, Temecula, CA | 1:500 |
| Rabbit anti-ANXA7 | ProteinTech Group, Chicago, IL | 1:200 |
| Mouse anti-SNAP25 | Stressgen, Victoria, BC, Canada | 1:1,500 |
| Mouse anti-SNPH | BD Biosciences | 1:500 |
| Mouse anti-SYT5 | BD Biosciences | 1:1,000 |
| Mouse anti-CALM | Chemicon | 1:10,000 |
RPH3A, rabphilin 3A; CADPS, calcium-dependent secretion activator; STX1, syntaxin 1; ANXA7, annexin A7; SNAP25, synaptosomal-associated protein 25; SNPH, syntaphilin; SYT5, synaptotagmin 5; CALM, calmodulin.
Antibody recognizes both STX1A and STX1B.
Data Analyses
Catecholamine secretion.
Differences in plasma concentrations (nmol/l) or urinary outputs (μmol/day) of catecholamines in patients with pheochromocytoma compared with median values in a reference population of subjects without the tumor provided estimates for the amounts of catecholamines in plasma or urine that were derived from tumors. Rates of catecholamine secretion from tumors into plasma were estimated using the formula S = P × C × 1.44, where S is the rate of catecholamine secretion (μmol/day), P is the plasma concentration of catecholamines derived from tumors (nmol/l), C is the circulatory clearance of catecholamines from plasma (l/min), and the constant, 1.44, was used to convert secretion rates of nmol/min to μmol/day, the same units as for urine. The formula was based on that described elsewhere for the secretion of catecholamines into plasma from the sympathetic nerves or the adrenals (17). A circulatory clearance of 2 l/min was assumed, based on our data published elsewhere (22).
Rates of catecholamine secretion into plasma or excretion into urine (both in units of μmol/day) were divided by estimates of tumor volume to normalize for differences in tumor size and derive final rates in units of μmol·min−1·cc3 of tumor. Volumes of tumors (V) in cubic centimeters were estimated using the formula for the volume of a sphere, V = 4/3 πr3, where r, the radius in centimeters, was derived from estimated mean diameters (the latter calculated from the cubed roots of rectangular volumes).
Rate constants for catecholamine secretion into plasma or excretion into urine (day−1), representing the proportions of total catecholamines in a tumor secreted into plasma or excreted into urine over 1 day, were estimated by dividing rates of catecholamine secretion into plasma or excretion into urine (μmol/day) by total tumor catecholamine contents (μmol). Total tumor catecholamine contents were estimated from the product of tissue catecholamine concentrations and tumor mass (the latter derived from tumor volume, assuming a specific gravity of 1.0).
Microarray and proteomics.
Data from cDNA and oligonucleotide microarray analyses and from the proteomics analysis were merged into a single FileMaker Pro database (Santa Clara, CA). This database included Gene Ontology function annotations with links to online databases (e.g., PubMed, Ensembl) to facilitate searches of relevant genes and proteins. Secretory pathway components within the database were identified using the search terms “catecholamine,” “secretion,” “exocytosis,” “synapse,” “granule,” and “vesicle.” The database was additionally searched for individual components that had known functions in secretory pathways or were established to be constituents of the synaptic vesicle proteome. Relevant information about the genes and corresponding proteins so identified was then transferred to an Excel spreadsheet for further sorting according to differences in expression by microarray or proteomics analyses. Sorting was based on a significant (P < 0.01) difference in gene expression by cDNA or oligonucleotide array analysis or a larger than threefold difference in spectral count strength by proteomics analysis. Further selection of catecholamine secretory pathway components for followup validation studies and inclusion in this report was based on searches of PubMed for articles supporting the role of identified components in secretory pathways.
Statistics.
Differences in catecholamine secretion and expression of pathway components among the two groups of tumors were assessed by t-tests. Paired t-tests were used to assess changes in plasma concentrations of catecholamines after glucagon, with two-way repeated-measures analysis of variance used to assess differences in responses between groups. Relationships between variables were examined by linear regression analysis, with significance assessed using Pearson's correlation coefficient. Data were transformed to the base 10 logarithm before statistical analyses.
RESULTS
Catecholamine Secretion
Baseline secretion.
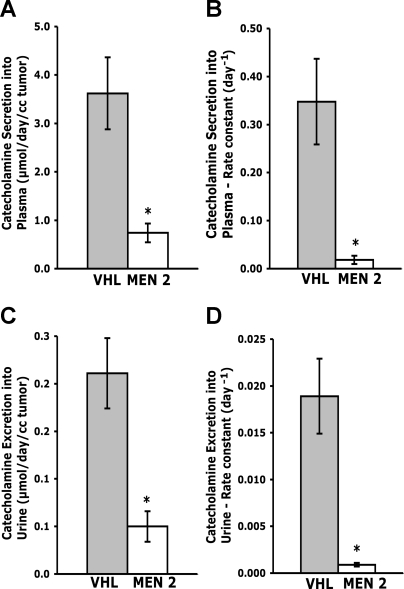
Rates of catecholamine secretion from pheochromocytomas were considerably higher for tumors from VHL patients than from MEN 2 patients, as assessed from both plasma concentrations and urinary outputs of catecholamines, and also when normalized for differences in tumor volume or total tumor contents of catecholamines (Fig. 1). Catecholamine secretion into plasma was 4.9-fold higher (P < 0.0001) for tumors from patients with VHL syndrome than in those from patients with MEN 2 (means ± SE, 3.62 ± 0.74 vs. 0.74 ± 0.19 μmol·day−1·ml−1). Urinary excretion of tumor-derived catecholamines was similarly 4.2-fold higher (P < 0.0001) in VHL than in MEN 2 patients (0.211 ± 0.037 vs. 0.050 ± 0.016 μmol·day−1·ml−1).
Fig. 1.
Rates (A and C) and rate constants (B and D) for catecholamine secretion into plasma (A and B) and excretion into urine (C and D) from von Hippel-Landau (VHL)- and multiple endocrine neoplasma type 2 (MEN 2)-associated pheochromocytomas. Data are shown as means ± SE for 42 VHL-associated tumors and 24–28 MEN 2-associated tumors. *P < 0.0001, lower rates or rate constants of catecholamine secretion into plasma or excretion into urine in MEN 2- than in VHL-associated pheochromocytomas.
Rate constants for catecholamine secretion were 19.3-fold higher (P < 0.0001) for tumors from VHL patients than in those from MEN 2 patients (0.348 ± 0.089 vs. 0.018 ± 0.008 day−1) and indicated that 35% of the catecholamine contents of VHL tumors were secreted into plasma each day compared with only 1.8% for MEN 2 tumors (Fig. 1). Rate constants for excretion of catecholamines into urine were similarly 21-fold higher (P < 0.0001) for tumors from VHL than from MEN 2 patients (0.0189 ± 0.0040 vs. 0.0009 ± 0.0002 day−1), indicating that 1.89% of the catecholamine contents of VHL tumors was excreted unchanged into urine each day compared with only 0.09% for MEN 2 tumors.
Rates of catecholamine excretion into urine were positively related (r = 0.832, P < 0.0001) to rates of catecholamine secretion into plasma; similarly, rate constants for catecholamine excretion were strongly positively related (r = 0.903, P < 0.0001) to rate constants of catecholamine secretion into plasma (data not shown). Rates of catecholamine secretion into plasma were much higher (P < 0.0001) than rates of catecholamine excretion into urine, indicating that only 5.8–6.8% of the catecholamines secreted into plasma were extracted by the kidneys and excreted unchanged into urine.
Secretogogue-stimulated secretion.
Intravenous administration of glucagon elicited distinctly divergent catecholamine secretory responses from pheochromocytomas in patients with MEN 2 compared with those with VHL syndrome (Fig. 2). Although baseline plasma concentrations of norepinephrine were higher (P = 0.018) in VHL patients than in MEN 2 patients, only MEN 2 patients showed a robust catecholamine secretory response to glucagon. Plasma concentrations of norepinephrine were increased threefold (P = 0.0002) and epinephrine 10-fold (P < 0.0001) in MEN 2 patients, whereas plasma concentrations of norepinephrine were unchanged and epinephrine increased only twofold (P < 0.0001) in VHL patients. Analysis of variance confirmed that patients with pheochromocytomas associated with MEN 2 had much more robust responses to glucagon of both plasma norepinephrine (F = 42.7, P < 0.0001) and epinephrine (F = 30.5, P < 0.0001) than those with the tumor due to VHL syndrome.
Fig. 2.
Plasma concentrations of norepinephrine (A and B) and epinephrine (C and D) before (BL) and after iv administration of glucagon (GLUC) in patients with VHL- (A and C) and MEN 2-associated (B and D) pheochromocytomas. Data are shown as means ± SE. *P < 0.0002, increases in plasma concentrations of norepinephrine or epinephrine after GLUC; †P < 0.0001, larger responses of plasma norepinephrine or epinephrine to GLUC in MEN 2 patients than in VHL patients.
Expression of Secretory Pathway Components
Microarray and proteomics.
Data from oligonucleotide microarrays are available at the Gene Expression Omnibus (GEO) on-line public database (http://www.ncbi.nlm.nih.gov/geo/) using the GEO accession identifier GSE12642. The overall results for cDNA and oligonucleotide microarray analyses have been published elsewhere (6, 19). Those previously reported results, however, do not include the details of the pathway components described here. The present analysis is restricted to components of secretory pathways selected according to the Gene Ontology search criteria and evidence for differential expression, as outlined in methods. This initial analysis yielded 1,400 components identified according to the search criteria, of which 356 showed evidence of differential expression, with these corresponding to 223 unique genes and corresponding proteins (this listing is available as Supplemental Table S1; Supplemental Material for this article is available at the AJP-Endocrinology and Metabolism web site). Among these unique components, 123 showed evidence of higher expression in MEN 2 tumors than in VHL tumors, 72 showed lower expression in VHL than in MEN 2 tumors, and in 28 the evidence was unclear (as assessed from either or both the microarray and proteomics data). Forty-six of these components were further selected on the basis of a PubMed search, indicating functional roles in secretory pathways (Table 2).
Table 2.
Selected pathway components showing differential expression between VHL and MEN 2 tumors by microarray and/or proteomics analysis
| Gene Symbol | Component | Gene ID |
Microarray |
Proteomics (Δ) | PMID | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P Value | Δ | |||||||||||
| Positive control pathway components | ||||||||||||
| PNMT | Phenylethanolamine N-methyltransferase | 5409 | 0.000* | 11.8 | 300.1 | 11344198 | ||||||
| NPY | Neuropeptide Y | 4852 | 0.001* | 5.3 | 184.1 | 17470513 | ||||||
| CHGB | Chromogranin B | 1114 | 0.002 | 22.9 | 11.5 | 17639059 | ||||||
| SLC6A2 | Sodium-dependent norepinephrine transporter | 6530 | 0.006* | 4.3 | 8.2 | 16189177 | ||||||
| CHGA | Chromogranin A | 1113 | 0.010 | 7.0 | 3.9 | 18046660 | ||||||
| TH | Tyrosine hydroxylase | 7054 | 0.004 | 5.7 | 3.0 | 11344198 | ||||||
| Other selected pathway components | ||||||||||||
| SNAP25 | Synaptosomal-associated protein, 25 kDa† | 6616 | 0.000 | 0.4 | 22.5 | 18064413 | ||||||
| ANXA7 | Annexin A7† | 310 | 0.000 | 3.4 | 12438137 | |||||||
| RPH3A | Rabphilin 3A† | 22895 | 0.000 | 5.6 | 33.0 | 10364266 | ||||||
| CADPS | Ca2+-dependent secretion activator† | 8618 | 0.006* | 5.8 | 10460244 | |||||||
| CALM1 | Calmodulin 1† | 801 | 0.000 | 11.8 | 12145198 | |||||||
| CALM2 | Calmodulin 2† | 805 | 0.000* | 15.8 | 17601619 | |||||||
| SNPH | Syntaphilin† | 9751 | 0.001 | 2.3 | 14985338 | |||||||
| SYT1 | Synaptotagmin I† | 6857 | 0.059 | 0.4 | 43.1 | 18275379 | ||||||
| RAB27B | RAB27B, member RAS oncogene family† | 5874 | 0.006 | 3.1 | 21.4 | 17664848 | ||||||
| SYTL2 | Synaptotagmin-like 2† | 54843 | 0.001 | 5.3 | 11773082 | |||||||
| RAB8B | RAB8B, member RAS oncogene family | 51762 | 0.007 | 2.5 | 3.3 | 11278749 | ||||||
| RAB27A | RAB27A, member RAS oncogene family | 5873 | 0.007 | 1.6 | 61.3 | 17664848 | ||||||
| STX16 | Syntaxin 16 | 8675 | 0.004* | 1.5 | 9.5 | 18337752 | ||||||
| VAT1 | Vesicle amine transport protein 1 homolog | 10493 | 0.000* | 1.7 | 1.8 | 7905859 | ||||||
| ERC2 | ELKS/RAB6-interacting/CAST family member 2 | 26059 | 0.045 | 2.1 | 5.7 | 16421316 | ||||||
| PCSK2 | Proprotein convertase subtilisin/kexin type 2 | 5126 | 0.001 | 5.7 | 56.7 | 15196022 | ||||||
| PCSK1 | Proprotein convertase subtilisin/kexin type 1 | 5122 | 0.038 | 24.6 | 17556096 | |||||||
| SNAPAP | SNAP-associated protein | 23557 | 0.030* | 1.9 | 33.6 | 16280592 | ||||||
| NCAM1 | Neural cell adhesion molecule 1 | 4684 | 0.000 | 2.7 | 47.7 | 15800072 | ||||||
| NAPB | N-ethylmaleimide-sensitive factor attachment protein-β | 63908 | 0.002 | 3.1 | 38.2 | 16795052 | ||||||
| NSF | N-ethylmaleimide-sensitive factor | 4905 | 18.9 | 17080482 | ||||||||
| SCAMP1 | Secretory carrier membrane protein 1 | 9522 | 0.001* | 2.2 | 17355250 | |||||||
| SCAMP3 | Secretory carrier membrane protein 3 | 10067 | 0.003* | 1.7 | 17355250 | |||||||
| EXOC3 | Exocyst complex component 3 | 11336 | 0.001 | 5.0 | 12763070 | |||||||
| SYNGR1 | Synaptogyrin 1 | 9145 | 0.000 | 2.7 | 17355250 | |||||||
| SYTL1 | Synaptotagmin-like 1 | 84958 | 0.001 | 2.1 | 11243866 | |||||||
| RAB3B | RAB3B, member RAS oncogene family | 5865 | 305.0 | 7834495 | ||||||||
| VGF | VGF nerve growth factor inducible | 7425 | 0.868 | 1.1 | 192.0 | 16221685 | ||||||
| VAPA | VAMP-associated protein A | 9218 | 0.376 | 1.2 | 92.7 | 11511104 | ||||||
| VAPB | VAMP-associated protein B | 9217 | 0.353 | 1.3 | 74.4 | 11511104 | ||||||
| VAMP1 | Vesicle-associated membrane protein 1 | 6843 | 0.041 | 1.5 | 76.2 | 17355250 | ||||||
| STX1A | Syntaxin 1A | 6804 | 0.753 | 1.2 | 16.4 | 10369670 | ||||||
| STX1B2 | Syntaxin 1B2† | 112755 | 0.001 | 0.5 | 9200718 | |||||||
| UNC13B | Unc-13 homolog B (C. elegans)† | 10497 | 0.001 | 0.1 | 12893529 | |||||||
| ARHGDIB | Rho GDP dissociation inhibitor (GDI)-β | 397 | 0.000 | 0.3 | 0.01 | 8978674 | ||||||
| SV2A | Synaptic vesicle glycoprotein 2A | 9900 | 0.000 | 0.1 | 16436618 | |||||||
| SCGB3A1 | Secretoglobin, family 3A, member 1 | 92304 | 0.000 | 0.2 | 18194566 | |||||||
| GSN | Gelsolin (amyloidosis, Finnish type) | 2934 | 0.001 | 0.2 | 1.0 | 8388266 | ||||||
| SYT6 | Synaptotagmin VI | 148281 | 0.002 | 0.1 | 7791877 | |||||||
| SNIP | SNAP25-interacting protein | 80725 | 0.000 | 0.1 | 10625663 | |||||||
Values for microarrays indicate levels of significance (P values) and relative directional signals (Δ) of components in multiple endocrine neoplasma type 2 (MEN 2) vs. von Hippel-Lindau (VHL) tumors (for either oligonucleotide or cDNA arrays). PMID, PubMed unique identifiers; VAMP, vesicle-associated membrane protein. Values for the proteomics analysis indicate Δ of components in MEN 2 vs. to VHL tumors. Signals >1 indicate higher expression in MEN 2 than in VHL tumors and <1 lower expression in MEN 2 than VHL tumors. PMID provide confirmatory references either for components with previously established higher expression in MEN 2 than VHL tumors (positive control pathway components) or that support inclusion in the selection as components of secretory pathways.
Component showing significant differences by both cDNA and oligonuclotide arrays;
components selected for followup quantitative PCR.
Among the identified components, there were six positive control pathway components previously established to show differential expression at both mRNA and protein levels (Table 2). Phenylethanolamine N-methyltransferase was identified to be differentially expressed at a high level of significance by both cDNA and oligonucleotide arrays. The enzyme was also identified by proteomics analysis to show a 300-fold higher expression in MEN 2 than in VHL tumors, a result consistent with a previous study (21). The 184-fold higher expression of neuropeptide Y protein and highly significant differences in neuropeptide Y gene expression by both cDNA and oligonucleotide arrays are also in agreement with previous data (13). The smaller differences in expression at the protein level, but still significant differences by at least one of the two microarray analyses, for tyrosine hydroxylase, the sodium-dependent norepinephrine transporter, and chromogranin A and B are also in agreement with previously published data showing higher expression of these components in MEN 2 than in VHL tumors (7, 14, 21, 25).
Among the other 40 selected components (Table 2), most were identified on the basis of PubMed searches as established components of the exocytotic secretory machinery (e.g., rabphilin 3A), some were identified as participating in the modulation of these components (e.g., calmodulin 1 and 2), and others were identified as secretory granule constituents or involved in the processing of such constituents (e.g., proprotein convertase). Among the secretory pathway components showing differential expression by either or both microarray and proteomics analyses, most showed higher expression in MEN 2 than in VHL tumors. Twelve components were selected for confirmatory quantitative PCR or Western blot analyses. These components included rabphilin 3A, RAB 27B, annexin A7, calcium-dependent secretion activator, calmodulin 1 and 2, syntaphilin, synaptotagmin-like 2, synaptotagmin 1, synaptosomal-associated protein-25 (SNAP25), syntaxin 1B2, and unc-13 homolog B. Three other secretory pathway components (rabphilin 3A-like, synaptotagmin 5, and synaptotagmin 13) that showed no differences by microarray or proteomic analyses were also included in followup quantitative PCR analyses.
Quantitative PCR.
Quantitative PCR confirmed the differences in expression indicated by microarray analyses in most but not every pathway component examined (Fig. 3). Syntaxin 1B2 in particular showed much higher expression by quantitative PCR in MEN 2 tumors than in VHL tumors, whereas the data from the oligonucleotide array analysis suggested that expression at the mRNA level should have been higher in VHL than in MEN 2 tumors. Similar results were observed for SNAP25.
Fig. 3.
Relative levels of mRNA expression in VHL- and MEN 2 associated pheochromocytomas for synaptosomal-associated protein 25 (SNAP25), syntaxin 1B2 (STX1B2), annexin A7 (ANXA7), rabphilin 3A (RPH3A), calcium-dependent secretion activator (CADPS), calmodulin 1 (CALM1), calmodulin 2 (CALM2), syntaphilin (SNPH), synaptotagmin 1 (SYT1), RAB27B, synaptotagmin 5 (SYT5), and rabphilin 3A-like (RPH3AL). Results are shown as means ± SE for data derived from 8 to 11 MEN 2 tumors and 9 to 17 VHL tumors. *P < 0.05, **P < 0.01, and ***P < 0.001, higher expression in tumors from MEN 2 than from VHL patients. † P < 0.05, lower expression in tumors from MEN 2 than from VHL patients.
Among the secretory pathway genes examined for differences in expression by quantitative PCR, the vast majority showed higher expression in pheochromocytomas from MEN 2 patients than in tumors from VHL patients (Fig. 3). Levels of SNAP25 mRNA were expressed in VHL tumors at only 26% (P = 0.016) of the levels in MEN 2 tumors, syntaxin 1B2 mRNA at 7% (P = 0.002), annexin A7 mRNA at 27% (P = 0.021), rabphilin 3A mRNA at 2% (P < 0.0001), calcium-dependent secretion activator mRNA at 6% (P = 0.0009), calmodulin 1 mRNA at 34% (P = 0.0014), calmodulin 2 mRNA at 18% (P = 0.019), syntaphilin mRNA at 13% (P = 0.0066), synaptotagmin 1 mRNA at 27% (P = 0.026), and RAB27B mRNA at 26% (P = 0.0025) of the levels in MEN 2 tumors. The mRNA for two other secretory pathway genes, synaptotagmin 5 and rabphilin 3A-like, showed 4.7- (P = 0.012) and 4.5-fold (P = 0.049) higher levels, respectively, in VHL than MEN 2 tumors. Several other secretory pathway genes examined by quantitative PCR, including unc-13 homolog B, synaptotagmin 13, and synaptotagmin-like 2, showed no differences in expression of mRNA between VHL and MEN 2 tumors (data not shown).
Levels of mRNA for SNAP25, annexin A7, rabphilin 3A, calcium-dependent secretion activator, calmodulin 1, synaptotagmin 1, and RAB27B showed significant (P < 0.05) negative relationships with rate constants for catecholamine secretion into plasma and excretion into urine (Table 3). Thus, the higher the level of expression of the above secretory pathway genes, the smaller the proportion of tumor catecholamine contents secreted into the circulation or excreted into urine per unit of time. In contrast, expression of the synaptotagmin 5 mRNA showed significant (P < 0.05) positive relationships with rate constants for catecholamine secretion into plasma and excretion into urine (Table 3) such that higher gene expression was associated with a larger proportion of tumor catecholamine contents secreted into the circulation or excreted into urine per unit of time.
Table 3.
Correlation matrix for relationships of mRNA expression with rate constants for catecholamine secretion and urinary excretion
| mRNA | Catecholamine Secretion, k−1 | Catecholamine Excretion, k−1 |
|---|---|---|
| SNAP25 | r=−0.51,P=0.021 | r=−0.55,P=0.019 |
| STX1B2 | r=−0.26,P=NS | r=−0.49,P=0.040 |
| ANXA7 | r=−0.51,P=0.010 | r=−0.50,P=0.016 |
| RPH3A | r=−0.70,P<0.001 | r=−0.81,P<0.001 |
| CADPS | r=−0.67,P<0.001 | r=−0.66,P<0.001 |
| CALM1 | r=−0.51,P=0.017 | r=−0.62,P=0.005 |
| CALM2 | r=−0.29,P=NS | r=−0.38,P=NS |
| SNPH | r=−0.46,P=0.043 | r=−0.56,P=0.017 |
| SYT1 | r=−0.70,P=0.002 | r=−0.69,P=0.004 |
| RAB27B | r=−0.52,P=0.018 | r=−0.60,P=0.008 |
| SYT5 | r=0.45,P=0.049 | r=0.59,P=0.009 |
| RPH3AL | r=0.02,P=NS | r=0.02,P=NS |
RPH3AL, rabphilin 3A-like; NS, not significant.
Western blots.
Western blots indicated higher tumor tissue levels of protein for SNAP25, syntaxin 1, annexin A7, rabphilin 3A, calcium-dependent secretion activator, and calmodulin in MEN 2 than in VHL tumors (Fig. 4).
Fig. 4.
Western blots showing expression of SNAP25, STX1, ANXA7, RPH3A, CADPS, and CALM in pheochromocytomas from VHL (left) and MEN 2 (right) patients. PC, mouse brain-positive control.
Differences in Western blots were particularly striking for rabphilin 3A and the calcium-dependent secretion activator, with both showing absent expression in VHL tumors and strong expression in nearly all MEN 2 tumors examined by Western blot. The findings at the protein level of particularly marked differences for these two secretory pathway components were in agreement with findings at the mRNA level (Fig. 3), where both components showed the most striking differences among all pathway components analyzed for differences in gene expression.
For the other proteins examined by Western blot (including SNAP25, syntaxin 1, annexin A7, and calmodulin 1), the differences in expression were more variable and less striking than for rabphilin 3A and the calcium-dependent secretion activator (Fig. 4). Nevertheless, densitometry analysis indicated that SNAP25 was expressed in VHL tumors at 29% (P = 0.029), annexin A7 at 30% (P = 0.017), and calmodulin at 21% (P = 0.004) of the levels in MEN 2 tumors. These differences were similar to those observed for expression of secretory pathway components at the mRNA level (Fig. 3).
Densitometry analysis did not, however, reveal a significant difference in expression of syntaxin 1, which was largely due to a high level of protein expression in one particular VHL tumor (tumor 1 in Fig. 4). That particular tumor also showed a higher level of expression of SNAP25 and annexin A7 than the other VHL tumors and the corresponding lowest rate constants for catecholamine secretion into plasma (k = 0.079 vs. a mean of 0.247 day−1 for the other 6 VHL tumors). Similarly, one of the seven MEN 2 tumors (tumor 6) showed much lower expression of SNAP25 and syntaxin 1 than the other MEN 2 tumors. That tumor also had the correspondingly highest rate constant for catecholamine secretion into plasma compared with the other MEN 2 tumors (0.232 compared with a mean of 0.007 day−1 for the other MEN 2 tumors).
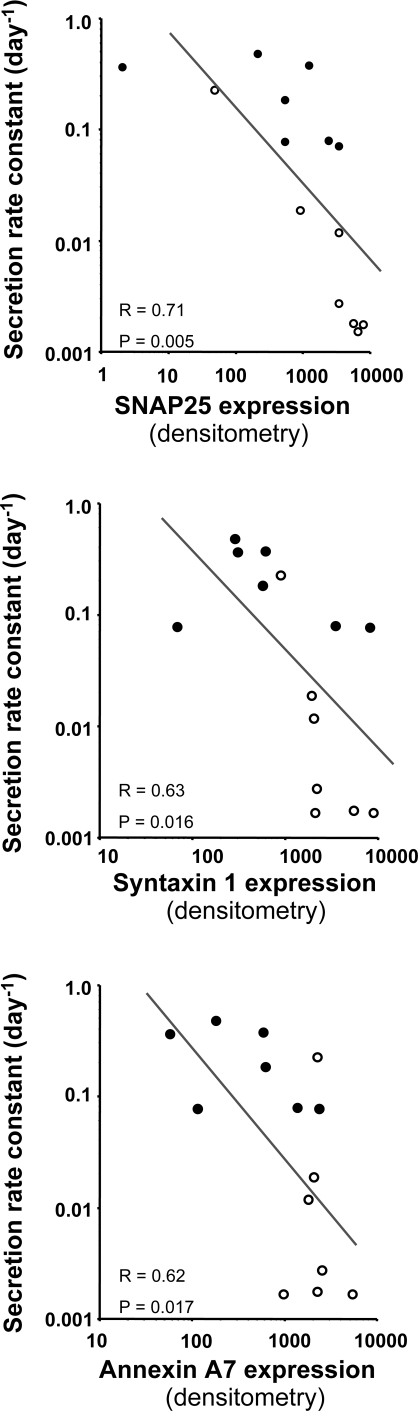
The above reciprocal relationships between catecholamine secretion and expression of secretory pathway components for tumors from individual patients with MEN 2 and VHL were mirrored by negative relationships between rate constants for catecholamine secretion into plasma and expression of SNAP25 (r = 0.71, P = 0.005), syntaxin 1 (r = 0.63, P = 0.016), annexin A7 (r = 0.62, P = 0.017), and calmodulin (r = 0.77, P = 0.002) for the data from all 14 tumors examined by densitometry analysis of Western blots (Fig. 5). There were also similarly significant negative relationships between rate constants for catecholamine excretion into urine and expression of SNAP25 (r = 0.57, P = 0.032), syntaxin 1 (r = 0.66, P = 0.011), annexin A7 (r = 0.71, P = 0.005), and calmodulin (r = 0.73, P = 0.004) examined by densitometry analysis of Western blots (data not shown).
Fig. 5.
Relationships between rate constants for catecholamine secretion into plasma and expression of SNAP25, STX1, and ANXA7 for pheochromocytomas associated with VHL syndrome (•) and MEN 2 (○). Expression of all 3 proteins was assessed from densitometry measurements of Western blots (Fig. 4).
DISCUSSION
Considerable progress has been made over the past decade in identifying various components of secretory pathways at the molecular level and elucidating their functions in exocytosis. The present report shows how this newfound knowledge can be utilized to understand pathological processes involving deregulated secretion of neurotransmitters and hormones and how these processes can contribute to the clinical presentation of a disease. More specifically, the data presented here establish mutation-dependent differences in expression of secretory pathway components in two types of neuroendocrine tumors characterized by differences in the nature of hormonal secretion and the subsequent presentation of related signs and symptoms.
We have previously established that pheochromocytomas in MEN 2 and in VHL syndrome exhibit highly distinct gene expression profiles (19). VHL tumors show activation of hypoxia-angiogenic signaling pathways, but compared with MEN 2, tumors show reduced expression of numerous components associated with catecholamine-related pathways. In particular, MEN 2 tumors express phenylethanolamine N-methyltransferase, the enzyme that converts norepinephrine to epinephrine, whereas VHL tumors do not (21). Subsequent differences in the relative amounts of norepinephrine and epinephrine produced by the two types of tumors and differing potencies of the two catecholamines on α- and β-adrenoceptors have been proposed to account for the more symptomatic nature of pheochromocytomas in MEN 2 than in VHL syndrome (21). This suggestion is in agreement with previous observations that certain signs and symptoms such as palpitations, anxiety, tremor, dyspnea, hyperglycemia, and paroxysmal hypertension are more common in patients with tumors producing epinephrine than in those that do not (3, 26, 27).
Here we establish additional differences in the nature of catecholamine secretion that likely also contribute to the more paroxysmal nature of hypertension in patients with tumors associated with MEN 2 than with VHL syndrome. More specifically, the low rates of basal catecholamine secretion in MEN 2 tumors and heightened responsiveness of these tumors to the secretogogue glucagon demonstrate the capacity of these tumors to secrete catecholamines in paroxysmal bursts compared with the more secretogogue-insensitive but sustained nature of secretion from tumors in VHL patients.
The smaller size of catecholamine stores and more rapid secretion-dependent turnover of these stores in VHL-related tumors than in MEN 2 tumors are findings consistent with the original observations by Crout and Sjoerdsma (15) many years ago that those tumors with larger stores of catecholamines produced smaller increases in urinary catecholamines than tumors with smaller stores. More importantly, the former tumors were more often characterized by increases in both epinephrine and norepinephrine, whereas the tumors with smaller stores but larger increases in urinary catecholamines invariably secreted only norepinephrine.
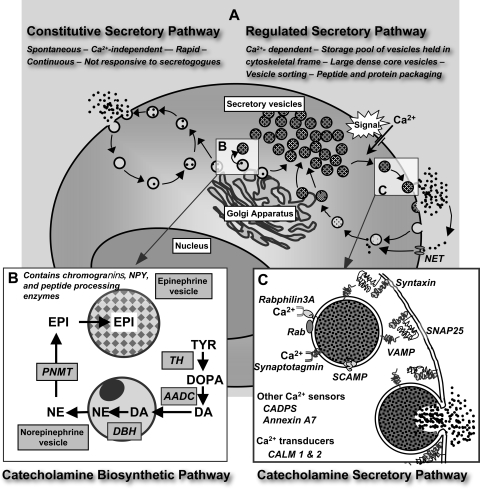
The generally lower expression of secretory pathway components but higher rate constants for catecholamine secretion in VHL than in MEN 2 tumors may seem counterintuitive but are in fact findings consistent with evolving concepts concerning the functions of constitutive and regulated secretory pathways (11, 28, 40). Regulated secretory pathways provide a means for selective sorting, processing, and storage of peptide and amine secretory products into synaptic vesicles or secretory granules available for calcium-dependent exocytotic release in response to specific triggering signals (Fig. 6). In order for these secretory products to be released strictly on demand, they must not enter the constitutive secretory pathway. The latter pathway is ubiquitous to all cells and functions to continuously replenish the cell membrane and provide constituents for the extracellular space. However, the constitutive pathway may also contribute to the spontaneous release of secretory products otherwise largely confined to the regulated pathway.
Fig. 6.
Schematic model illustrating constitutive vs. regulated secretory pathways (A), with B and C indicating magnifications of secretory granules to illustrate the catecholamine biosynthetic pathway (B) and the catecholamine-regulated secretory pathway (C). For most definitions of abbreviations, see Table 2. TYR, tyrosine; DOPA, dihydroxyphenylalanine; DA, dopamine; NE, norepinephrine; EPI, epinephrine; AADC, aromatic amino acid decarboxylase; NET, norepinephrine transporter; VAMP, vesicle-associated membrane protein; SCAMP, secretory carrier membrane protein; PNMT, phenylethanolamine N-methyltransferase; DBH, dopamine β-hydroxylase.
In highly differentiated secretory cells, such as chromaffin cells of the adrenal medulla, the synthesis, processing, and sorting of secretory products into the appropriate regulated exocytotic secretory vesicles is achieved through tightly controlled and coordinated expression of numerous pathway components (Fig. 6). Secretory vesicles in this pathway undergo maturation through several steps, including fusion with other vesicles, removal of missorted material via budding, and sorting into different storage pools. Some of these pools are readily releasable, whereas others require a recruitment step before fusion at the cell membrane is possible. The generally lower expression of secretory pathway components in VHL than in MEN 2 tumors, including components necessary for synthesis of catecholamines, suggests a less mature chromaffin cell phenotype in VHL than in MEN 2 tumors. Presumably, the poorly differentiated secretory controls associated with the immature phenotype in VHL tumors result in less tightly regulated secretion of catecholamines as either a consequence of the channeling of these secretory products into the constitutive secretory pathway or their sorting into storage pools of catecholamines more readily released by spontaneous exocytosis than those in more fully differentiated chromaffin cells.
The above explanation of how lowered expression of regulated secretory pathway components in VHL tumors can lead to an increase in spontaneous secretion yet make the state of heightened secretion less responsive to secretogogues is in agreement with other observations in rat pheochromocytoma (PC12) cells infected with the repressor element 1-silencing transcription (REST) factor (9, 16, 36). This critical regulator of the neurosecretory phenotype reduces expression of numerous components of the regulated secretory pathway, including SNAP25, rabphilin 3A, chromogranin A, and chromogranin B (9). As we show here, expression of all four of these components is lower in VHL tumors than in MEN 2 tumors. Furthermore, there are numerous other genes with lower expression in VHL tumors than in MEN 2 tumors (e.g., annexin A7, calcium-dependent secretion activator, syntaphilin, N-ethylmaleimide-sensitive factor) that also harbor REST-binding sites (8, 34).
In addition to suppressed expression of numerous regulated secretory pathway components, overexpression of REST in PC12 cells increases spontaneous secretion of catecholamines but completely blocks stimulated secretion (9). REST-infected cells also have fewer and smaller secretory granules, with granules often surrounded by a clear halo (9, 16). These findings in PC12 cells overexpressing or infected with REST show remarkable similarities to the phenotypic features of VHL-associated pheochromocytomas. As shown here, tumors in VHL patients exhibit higher rates of spontaneous catecholamine secretion but are unresponsive to stimulated secretion. As we have shown elsewhere, VHL tumors are also characterized by lowered stores of catecholamines and fewer secretory vesicles, many of which include a clear halo surrounding a granule core and some of which appear to be empty without any granules (21, 25).
Further support for an overall restraining influence of regulated secretory pathway components on spontaneous catecholamine secretion in pheochromocytomas is provided by the negative relationships between rate constants of basal catecholamine secretion and the expression of individual secretory pathway components at both mRNA and protein levels. Together, these data and the considerations outlined above provide compelling evidence that differences in the nature of catecholamine secretion in hereditary pheochromocytomas reflect differences in expression of secretory pathway components.
It remains unclear how the underlying mutations lead to differences in expression of secretory pathway components, but it is tempting to speculate that the mechanism might involve REST. At the other functional end, the exact mechanism of how differences in secretory pathway components can have opposing influences on spontaneous and evoked secretion is also unclear. The difficulty here is compounded by involvement of many of the same exocytotic machinery components in different secretory pathways. Nevertheless, there are some components required for one pathway and not others. For example, ablation of SNAP25 abolishes evoked neurosecretion but has little influence on spontaneous secretion, whereas deletion of syntaxin results in a complete lethal block in both spontaneous and evoked secretion (39).
By translational links explaining how mutation-dependent differences in hormonal secretion and presentation of a disease may relate to differences in the expression of secretory pathway components, the present data lend clinical relevance to a large body of work directed at characterizing the molecular machinery responsible for exocytosis. Our findings in pheochromocytoma offer a unique clinical perspective for understanding the functions of secretory pathway components. The insight derived from these rare but highly dangerous tumors may have further relevance to other clinical conditions featuring disordered secretion of neurotransmitters and hormones, including more common disorders such as essential hypertension where abnormal release of norepinephrine from sympathetic nerves has long been recognized as a contributing factor but where the responsible mechanisms have yet to be clarified.
GRANTS
This work was supported by the intramural programs of the National Institute of Child Health and Human Development, the Center for Cancer Research, the National Cancer Institute, the National Human Genome Research Institute, and the National Institute of Neurological Disorders and Stroke at the National Institutes of Health, Bethesda, Maryland.
Acknowledgments
Thanks are extended to Drs. Peter Munson and Jennifer Barb for assistance with statistical analyses.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Albertin G, Aragona F, Gottardo L, Malendowicz LK, Nussdorfer GG. Human pheochromocytomas, but not adrenal medulla, express glucagon-receptor gene and possess an in vitro secretory response to glucagon. Peptides 22: 597–600, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Amar L, Bertherat J, Baudin E, Ajzenberg C, Bressac-de Paillerets B, Chabre O, Chamontin B, Delemer B, Giraud S, Murat A, Niccoli-Sire P, Richard S, Rohmer V, Sadoul JL, Strompf L, Schlumberger M, Bertagna X, Plouin PF, Jeunemaitre X, Gimenez-Roqueplo AP. Genetic testing in pheochromocytoma or functional paraganglioma. J Clin Oncol 23: 8812–8818, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Aronoff SL, Passamani E, Borowsky BA, Weiss AN, Roberts R, Cryer PE. Norepinephrine and epinephrine secretion from a clinically epinephrine-secreting pheochromocytoma. Am J Med 69: 321–324, 1980. [DOI] [PubMed] [Google Scholar]
- 4.Aunis D, Langley K. Physiological aspects of exocytosis in chromaffin cells of the adrenal medulla. Acta Physiol Scand 167: 89–97, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Balgley BM, Wang W, Song T, Fang X, Yang L, Lee CS. Evaluation of confidence and reproducibility in quantitative proteomics performed by a capillary isoelectric focusing-based proteomic platform coupled with a spectral counting approach. Electrophoresis 29: 3047–3054, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Brouwers FM, Elkahloun AG, Munson PJ, Eisenhofer G, Barb J, Linehan WM, Lenders JW, De Krijger R, Mannelli M, Udelsman R, Ocal IT, Shulkin BL, Bornstein SR, Breza J, Ksinantova L, Pacak K. Gene expression profiling of benign and malignant pheochromocytoma. Ann NY Acad Sci 1073: 541–556, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brouwers FM, Glasker S, Nave AF, Vortmeyer AO, Lubensky I, Huang S, Abu-Asab MS, Eisenhofer G, Weil RJ, Park DM, Linehan WM, Pacak K, Zhuang Z. Proteomic profiling of von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2 pheochromocytomas reveals different expression of chromogranin B. Endocr Relat Cancer 14: 463–471, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Bruce AW, Donaldson IJ, Wood IC, Yerbury SA, Sadowski MI, Chapman M, Gottgens B, Buckley NJ. Genome-wide analysis of repressor element 1 silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) target genes. Proc Natl Acad Sci USA 101: 10458–10463, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruce AW, Krejci A, Ooi L, Deuchars J, Wood IC, Dolezal V, Buckley NJ. The transcriptional repressor REST is a critical regulator of the neurosecretory phenotype. J Neurochem 98: 1828–1840, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Bryant J, Farmer J, Kessler LJ, Townsend RR, Nathanson KL. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst 95: 1196–1204, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev 83: 581–632, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Chou YY, Lee YS. Ultrastructural and biochemical characterization of catecholamine release mechanisms in cultured human pheochromocytoma cells. Chin Med J (Engl) 111: 1018–1024, 1998. [PubMed] [Google Scholar]
- 13.Cleary S, Phillips JK, Huynh TT, Pacak K, Elkahloun AG, Barb J, Worrell RA, Goldstein DS, Eisenhofer G. Neuropeptide Y expression in phaeochromocytomas: relative absence in tumours from patients with von Hippel-Lindau syndrome. J Endocrinol 193: 225–233, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleary S, Phillips JK, Huynh TT, Pacak K, Fliedner S, Elkahloun AG, Munson P, Worrell RA, Eisenhofer G. Chromogranin A expression in phaeochromocytomas associated with von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2. Horm Metab Res 39: 876–883, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Crout JR, Sjoerdsma A. Turnover and metabolism of catecholamines in patients with pheochromocytoma. J Clin Invest 43: 94–102, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Alessandro R, Klajn A, Stucchi L, Podini P, Malosio ML, Meldolesi J. Expression of the neurosecretory process in pc12 cells is governed by rest. J Neurochem 105: 1369–1383, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhofer G Sympathetic nerve function—assessment by radioisotope dilution analysis. Clin Auton Res 15: 264–283, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Eisenhofer G, Goldstein DS, Stull R, Keiser HR, Sunderland T, Murphy DL, Kopin IJ. Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clin Chem 32: 2030–2033, 1986. [PubMed] [Google Scholar]
- 19.Eisenhofer G, Huynh TT, Pacak K, Brouwers FM, Walther MM, Linehan WM, Munson PJ, Mannelli M, Goldstein DS, Elkahloun AG. Distinct gene expression profiles in norepinephrine- and epinephrine-producing hereditary and sporadic pheochromocytomas: activation of hypoxia-driven angiogenic pathways in von Hippel-Lindau syndrome. Endocr Relat Cancer 11: 897–911, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhofer G, Lenders JW, Linehan WM, Walther MM, Goldstein DS, Keiser HR. Plasma normetanephrine and metanephrine for detecting pheochromocytoma in von Hippel-Lindau disease and multiple endocrine neoplasia type 2. N Engl J Med 340: 1872–1879, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Eisenhofer G, Walther MM, Huynh TT, Li ST, Bornstein SR, Vortmeyer A, Mannelli M, Goldstein DS, Linehan WM, Lenders JW, Pacak K. Pheochromocytomas in von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2 display distinct biochemical and clinical phenotypes. J Clin Endocrinol Metab 86: 1999–2008, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein DS, Eisenhofer G, Kopin IJ. Sources and significance of plasma levels of catechols and their metabolites in humans. J Pharmacol Exp Ther 305: 800–811, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res 6: 986–994, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Hernández-Guijo JM, Gandía L, Cuchillo-Ibáñez I, Albillos A, Novalbos J, Gilsanz F, Larrañaga E, de Pascual R, Abad F, García AG. Altered regulation of calcium channels and exocytosis in single human pheochromocytoma cells. Pflugers Arch 440: 253–263, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Huynh TT, Pacak K, Brouwers FM, Abu-Asab MS, Worrell RA, Walther MM, Elkahloun AG, Goldstein DS, Cleary S, Eisenhofer G. Different expression of catecholamine transporters in phaeochromocytomas from patients with von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2. Eur J Endocrinol 153: 551–563, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito Y, Fujimoto Y, Obara T. The role of epinephrine, norepinephrine, and dopamine in blood pressure disturbances in patients with pheochromocytoma. World J Surg 16: 759–763; discussion 763–764, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Lance JW, Hinterberger H. Symptoms of pheochromocytoma, with particular reference to headache, correlated with catecholamine production. Arch Neurol 33: 281–288, 1976. [DOI] [PubMed] [Google Scholar]
- 28.Lang T, Jahn R. Core proteins of the secretory machinery. In: Pharmacology of Neurotransmitter Release—Handbook of Experimental Pharmacology, edited by Südhof TC and Starke K. Berlin-Heidelberg, Germany: Springer-Verlag, 2008, vol. 184, p. 107–127. [DOI] [PubMed]
- 29.Liu H, Sadygov RG, Yates JR 3rd. A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem 76: 4193–4201, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Manger WM, Gifford RW. Clinical and Experimental Pheochromocytoma. Cambridge, MA: Blackwell Science, 1996.
- 31.Marley PD Mechanisms in histamine-mediated secretion from adrenal chromaffin cells. Pharmacol Ther 98: 1–34, 2003. [DOI] [PubMed] [Google Scholar]
- 32.McHugh EM, McGee R Jr, Fleming PJ. Sulfation and constitutive secretion of dopamine beta-hydroxylase from rat pheochromocytoma (PC12) cells. J Biol Chem 260: 4409–4417, 1985. [PubMed] [Google Scholar]
- 33.Neumann HP, Pawlu C, Peczkowska M, Bausch B, McWhinney SR, Muresan M, Buchta M, Franke G, Klisch J, Bley TA, Hoegerle S, Boedeker CC, Opocher G, Schipper J, Januszewicz A, Eng C. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA 292: 943–951, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Otto SJ, McCorkle SR, Hover J, Conaco C, Han JJ, Impey S, Yochum GS, Dunn JJ, Goodman RH, Mandel G. A new binding motif for the transcriptional repressor REST uncovers large gene networks devoted to neuronal functions. J Neurosci 27: 6729–6739, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pacak K, Eisenhofer G, Lenders JW. Pheochromocytoma: Diagnosis, Localization and Treatment. Oxford, UK: Wiley-Blackwell, 2007.
- 36.Pance A, Livesey FJ, Jackson AP. A role for the transcriptional repressor REST in maintaining the phenotype of neurosecretory-deficient PC12 cells. J Neurochem 99: 1435–1444, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Sudhof TC The synaptic vesicle cycle. Annu Rev Neurosci 27: 509–547, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Wang W, Guo T, Song T, Lee CS, Balgley BM. Comprehensive yeast proteome analysis using a capillary isoelectric focusing-based multidimensional separation platform coupled with ESI-MS/MS. Proteomics 7: 1178–1187, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Benditó G, Molnár Z, Becher MW, Valenzuela CF, Partridge LD, Wilson MC. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci 5: 19–26, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Wojcik SM, Brose N. Regulation of membrane fusion in synaptic excitation-secretion coupling: speed and accuracy matter. Neuron 55: 11–24, 2007. [DOI] [PubMed] [Google Scholar]