Abstract
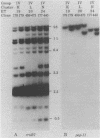
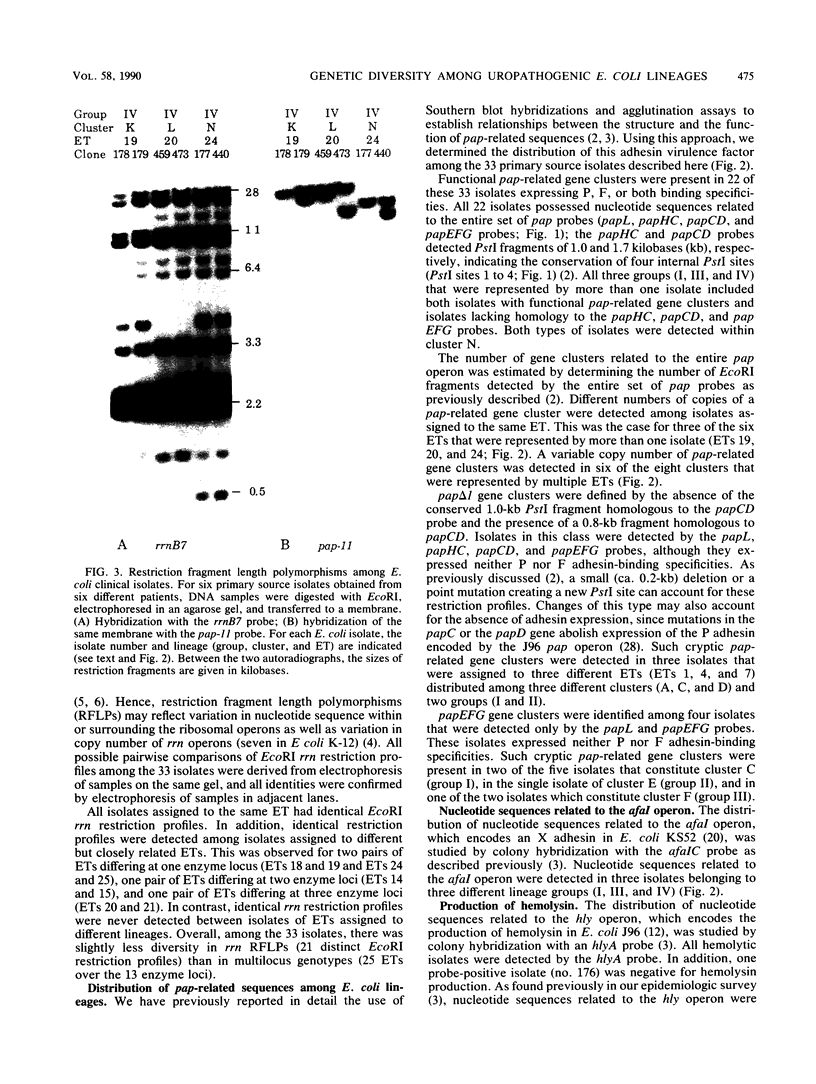
Among the adhesin-encoding virulence operons associated with uropathogenic Escherichia coli, only pap (pyelonephritis-associated pilus)-related gene clusters typically exhibit variation in their structure and chromosomal copy number. To access further such variability, we compared pap restriction fragment length polymorphisms (RFLPs) with those detected among rRNA (rrn) operons, which encode an essential host function unrelated to virulence. To place such findings in a phylogenetic perspective, the E. coli isolates were also characterized by using multilocus enzyme electrophoresis. Variation in the rrn RFLP profiles correlated with evolutionary divergence resolved by multilocus enzyme electrophoresis; isolates with identical rrn profiles represented the same or closely related electrophoretic types. In contrast, such isolates frequently had different pap-related RFLPs, indicating that these genetic variations have developed recently relative to the changes associated with essential rrn operons or metabolic enzymes. Despite such fluctuations, two lines of evidence indicate conditions under which the pap-related RFLPs can be stably maintained. First, for each of 20 patients with urosepsis, both the primary urinary tract isolate and the concurrent blood isolate were identical. Second, although obtained from different patients, some isolates representing the same electrophoretic type also had identical pap-related RFLPs. Thus, the genotypic diversity of this virulence adhesin operon was not generated during the course of acute infection or during laboratory manipulations. Since fecal E. coli isolates frequently carry chromosomally encoded pap-related gene clusters, these findings suggest that the intra- and interchromosomal recombination events generating the polymorphisms associated with the pap-related sequences likely occur among the E. coli of the commensal reservoir.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Pluschke G. Clonal analysis of descent and virulence among selected Escherichia coli. Annu Rev Microbiol. 1986;40:185–210. doi: 10.1146/annurev.mi.40.100186.001153. [DOI] [PubMed] [Google Scholar]
- Arthur M., Campanelli C., Arbeit R. D., Kim C., Steinbach S., Johnson C. E., Rubin R. H., Goldstein R. Structure and copy number of gene clusters related to the pap P-adhesin operon of uropathogenic Escherichia coli. Infect Immun. 1989 Feb;57(2):314–321. doi: 10.1128/iai.57.2.314-321.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur M., Johnson C. E., Rubin R. H., Arbeit R. D., Campanelli C., Kim C., Steinbach S., Agarwal M., Wilkinson R., Goldstein R. Molecular epidemiology of adhesin and hemolysin virulence factors among uropathogenic Escherichia coli. Infect Immun. 1989 Feb;57(2):303–313. doi: 10.1128/iai.57.2.303-313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg K. L., Squires C. L., Squires C. In vivo translation of a region within the rrnB 16S rRNA gene of Escherichia coli. J Bacteriol. 1987 Apr;169(4):1691–1701. doi: 10.1128/jb.169.4.1691-1701.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Ullrich A., Raker M. A., Gray A., Dull T. J., Gutell R. R., Noller H. F. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid. 1981 Jul;6(1):112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
- Caugant D. A., Levin B. R., Lidin-Janson G., Whittam T. S., Svanborg Edén C., Selander R. K. Genetic diversity and relationships among strains of Escherichia coli in the intestine and those causing urinary tract infections. Prog Allergy. 1983;33:203–227. doi: 10.1159/000318331. [DOI] [PubMed] [Google Scholar]
- Caugant D. A., Levin B. R., Orskov I., Orskov F., Svanborg Eden C., Selander R. K. Genetic diversity in relation to serotype in Escherichia coli. Infect Immun. 1985 Aug;49(2):407–413. doi: 10.1128/iai.49.2.407-413.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Knapp S., Goebel W. Spontaneous deletions and flanking regions of the chromosomally inherited hemolysin determinant of an Escherichia coli O6 strain. J Bacteriol. 1983 Jun;154(3):1145–1152. doi: 10.1128/jb.154.3.1145-1152.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales B. A., Amyes S. G. The transfer of genes encoding production of mannose-resistant haemagglutinating fimbriae from uropathogenic enterobacteria. J Gen Microbiol. 1986 Aug;132(8):2243–2247. doi: 10.1099/00221287-132-8-2243. [DOI] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull S. I., Bieler S., Hull R. A. Restriction fragment length polymorphism and multiple copies of DNA sequences homologous with probes for P-fimbriae and hemolysin genes among uropathogenic Escherichia coli. Can J Microbiol. 1988 Mar;34(3):307–311. doi: 10.1139/m88-056. [DOI] [PubMed] [Google Scholar]
- Hull S., Clegg S., Sanborg Eden C., Hull R. Multiple forms of genes in pyelonephritogenic Escherichia coli encoding adhesins binding globoseries glycolipid receptors. Infect Immun. 1985 Jan;47(1):80–83. doi: 10.1128/iai.47.1.80-83.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R., Moseley S. L., Roberts P. L., Stamm W. E. Aerobactin and other virulence factor genes among strains of Escherichia coli causing urosepsis: association with patient characteristics. Infect Immun. 1988 Feb;56(2):405–412. doi: 10.1128/iai.56.2.405-412.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källenius G., Svenson S. B., Möllby R., Korhonen T., Winberg J., Cedergren B., Helin I., Hultberg H. Carbohydrate receptor structures recognized by uropathogenic E. coli. Scand J Infect Dis Suppl. 1982;33:52–60. [PubMed] [Google Scholar]
- Labigne-Roussel A., Schmidt M. A., Walz W., Falkow S. Genetic organization of the afimbrial adhesin operon and nucleotide sequence from a uropathogenic Escherichia coli gene encoding an afimbrial adhesin. J Bacteriol. 1985 Jun;162(3):1285–1292. doi: 10.1128/jb.162.3.1285-1292.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Lindberg F., Marklund B. I., Normark S. The PapG protein is the alpha-D-galactopyranosyl-(1----4)-beta-D-galactopyranose-binding adhesin of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5898–5902. doi: 10.1073/pnas.84.16.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Marklund B. I., Strömberg N., Lindberg F., Karlsson K. A., Normark S. Uropathogenic Escherichia coli can express serologically identical pili of different receptor binding specificities. Mol Microbiol. 1988 Mar;2(2):255–263. doi: 10.1111/j.1365-2958.1988.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Maurer L., Orndorff P. E. Identification and characterization of genes determining receptor binding and pilus length of Escherichia coli type 1 pili. J Bacteriol. 1987 Feb;169(2):640–645. doi: 10.1128/jb.169.2.640-645.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milkman R., Stoltzfus A. Molecular evolution of the Escherichia coli chromosome. II. Clonal segments. Genetics. 1988 Oct;120(2):359–366. doi: 10.1093/genetics/120.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Kroll J. S., Moxon E. R., Selander R. K. Clonal population structure of encapsulated Haemophilus influenzae. Infect Immun. 1988 Aug;56(8):1837–1845. doi: 10.1128/iai.56.8.1837-1845.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics. 1978 Jul;89(3):583–590. doi: 10.1093/genetics/89.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren M., Normark S., Lark D., O'Hanley P., Schoolnik G., Falkow S., Svanborg-Edén C., Båga M., Uhlin B. E. Mutations in E coli cistrons affecting adhesion to human cells do not abolish Pap pili fiber formation. EMBO J. 1984 May;3(5):1159–1165. doi: 10.1002/j.1460-2075.1984.tb01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanley P., Lark D., Falkow S., Schoolnik G. Molecular basis of Escherichia coli colonization of the upper urinary tract in BALB/c mice. Gal-Gal pili immunization prevents Escherichia coli pyelonephritis in the BALB/c mouse model of human pyelonephritis. J Clin Invest. 1985 Feb;75(2):347–360. doi: 10.1172/JCI111707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov F., Orskov I. From the national institutes of health. Summary of a workshop on the clone concept in the epidemiology, taxonomy, and evolution of the enterobacteriaceae and other bacteria. J Infect Dis. 1983 Aug;148(2):346–357. doi: 10.1093/infdis/148.2.346. [DOI] [PubMed] [Google Scholar]
- Plos K., Hull S. I., Hull R. A., Levin B. R., Orskov I., Orskov F., Svanborg-Edén C. Distribution of the P-associated-pilus (pap) region among Escherichia coli from natural sources: evidence for horizontal gene transfer. Infect Immun. 1989 May;57(5):1604–1611. doi: 10.1128/iai.57.5.1604-1611.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Korhonen T. K., Väisänen-Rhen V., Williams P. H., Pattison P. E., Caugant D. A. Genetic relationships and clonal structure of strains of Escherichia coli causing neonatal septicemia and meningitis. Infect Immun. 1986 Apr;52(1):213–222. doi: 10.1128/iai.52.1.213-222.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stoltzfus A., Leslie J. F., Milkman R. Molecular evolution of the Escherichia coli chromosome. I. Analysis of structure and natural variation in a previously uncharacterized region between trp and tonB. Genetics. 1988 Oct;120(2):345–358. doi: 10.1093/genetics/120.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanborg Edén C., de Man P. Bacterial virulence in urinary tract infection. Infect Dis Clin North Am. 1987 Dec;1(4):731–750. [PubMed] [Google Scholar]