Abstract
Glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) are anti-diabetes/obesity hormones secreted from the gut after meal ingestion. We have shown that dietary-resistant starch (RS) increased GLP-1 and PYY secretion, but the mechanism remains unknown. RS is a fermentable fiber that lowers the glycemic index of the diet and liberates short-chain fatty acids (SCFAs) through fermentation in the gut. This study investigates the two possible mechanisms by which RS stimulates GLP-1 and PYY secretion: the effect of a meal or glycemic index, and the effect of fermentation. Because GLP-1 and PYY secretions are stimulated by nutrient availability in the gut, the timing of blood sample collections could influence the outcome when two diets with different glycemic indexes are compared. Thus we examined GLP-1 and PYY plasma levels at various time points over a 24-h period in RS-fed rats. In addition, we tested proglucagon (a precursor to GLP-1) and PYY gene expression patterns in specific areas of the gut of RS-fed rats and in an enteroendocrine cell line following exposure to SCFAs in vitro. Our findings are as follows. 1) RS stimulates GLP-1 and PYY secretion in a substantial day-long manner, independent of meal effect or changes in dietary glycemia. 2) Fermentation and the liberation of SCFAs in the lower gut are associated with increased proglucagon and PYY gene expression. 3) Glucose tolerance, an indicator of increased active forms of GLP-1 and PYY, was improved in RS-fed diabetic mice. We conclude that fermentation of RS is most likely the primary mechanism for increased endogenous secretions of total GLP-1 and PYY in rodents. Thus any factor that affects fermentation should be considered when dietary fermentable fiber is used to stimulate GLP-1 and PYY secretion.
Keywords: short-chain fatty acids, gene regulation, promoter, gut hormone, nutrition
glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) are gut-secreted peptides that have been proposed as potential anti-diabetes/obesity drugs. These two hormones are also naturally secreted in response to meal ingestion, but they degrade rapidly after endogenous secretion or exogenous injection (3, 5, 31, 38, 43). Thus pharmaceutical means to maintain substantial high plasma levels of GLP-1 and PYY are intensely targeted (6, 11, 16, 31, 38, 44).
Resistant starch (RS) is a dietary fermentable fiber that has previously been reported to increase plasma total GLP-1 and total PYY in animals. These increases have been confirmed in all animal models tested in our laboratory, including mice and rats of both sexes at different ages (23, 45). However, the mechanism is unclear.
Dietary RS resists digestion in the small intestine and, instead, is fermented in the large intestine (17, 22). It lowers the glycemic index of the diet and produces short-chain fatty acids (SCFAs) in the large intestine through fermentation (4, 24). Both actions could potentially influence GLP-1 and PYY secretion. In the present study, we tested these two possible mechanisms by which dietary RS regulates GLP-1 and PYY secretion. 1) Because GLP-1 and PYY secretions are stimulated by nutrient availability in the gut, the timing of blood sample collections could influence the outcome when two diets with different glycemic indexes are compared. Thus we examined GLP-1 and PYY plasma levels at various time points over a 24-h period in rats fed an RS diet. 2) We tested proglucagon (the gene that encodes GLP-1) and PYY gene expression patterns in specific areas of the gut in RS-fed rats and in an enteroendocrine (STC-1) cell line following exposure to SCFAs in vitro. 3) We showed that glucose tolerance was improved in diabetic mice fed RS, an indicator of improved GLP-1 and PYY activity.
Understanding how dietary RS modulates GLP-1/PYY secretion will provide an explanation for equivocal results reported in humans trials. It will also provide insight for development of pharmaceutical/nutritional alternatives for maintaining substantial high plasma levels of GLP-1 and PYY.
MATERIALS AND METHODS
Animals, housing conditions, and diets: studies 1, 2, and 5.
All animals were housed in a humidity- and temperature-controlled room (22 ± 2°C, 65–67% humidity) on a 12:12-h light-dark cycle with free access to food and water. For studies 1 and 2, Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) were individually housed in suspended wire-bottom cages. For study 5, C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were housed as a group in shoe-box cages. All animals were fed semipurified powder diets prepared in our laboratory. The control and RS diets (Table 1) were based on the AIN-93 diet formula for laboratory rodents (37). Animal protocols were approved by the Pennington Biomedical Research Center and the Louisiana State University Animal Care and Use Committees.
Table 1.
Composition of control and RS starch diets used in study 1
|
Diet |
||
|---|---|---|
| Control | RS | |
| Cornstarch, g | 424.5 | 0 |
| RS, g | 0 | 530.7 |
| Sucrose, g | 100 | 100 |
| Casein, g | 200 | 200 |
| Soybean oil, g | 70 | 70 |
| Cellulose, g | 156.2 | 50 |
| Mineral mix (AIN-93G), g | 35 | 35 |
| Vitamin mix (AIN-93G), g | 10 | 10 |
| Choline chloride, g | 1.3 | 1.3 |
| l-Cysteine, g | 3.0 | 3.0 |
| Total amount, g | 1,000 | 1,000 |
| Energy, kcal/g | 3.3 | 3.3 |
| RS content, % | 0 | 30 |
Amioca cornstarch (100% amylopectin) and Hi-Maize 260 were used for control and resistant starch (RS) diets, respectively; both starches were provided by National Chemical and Starch Company (Bridgewater, NJ). Metabolizable energy values were 3.5 kcal/g for Amioca cornstarch (data provided by National Starch and Chemical) and 2.8 kcal/g for Hi-Maize 260 (unpublished data based on bomb calorimetry). RS content in the diet was calculated on the basis of the amount of Hi-Maize (56% RS) used.
Cell culture: studies 3 and 4.
STC-1 cells, an enteroendocrine intestinal cell line (kindly provided by Dr. D. Hanahan, University of California, San Francisco, CA), were grown in DMEM containing 15% horse serum, 2.5% fetal bovine serum, 100 U/ml penicillin, and 100 mg/l streptomycin (GIBCO-BRL, Grand Island, NY). HEK-293 cells were grown in DMEM containing 5% fetal bovine serum. STC-1 and HEK-293 cells were incubated at 37°C with 95% O2-5% CO2. Studies were performed 24–48 h (STC-1) or immediately (HEK-293) after the cells were plated. Cells were at 80–90% confluence when treatment began. Butyrate, propionate, and acetate (Sigma, St. Louis, MO) were diluted with PBS buffer, and the doses used in the cell culture were similar to their physiological concentrations determined in RS-fed animals (23, 25).
Hormone measurements.
Trunk blood from each rat was collected in chilled EDTA tubes. No inhibitors were added, and the blood was centrifuged at 2,000 g for 15 min at 4°C. Plasma total GLP-1, total PYY, and insulin were measured by RIA kits (Millipore, St. Charles, MO). Since the commercial RIA kits that measure the active form of GLP-1 were not available at the time of study, dipeptidylpeptidase-4 (DPP-4), the enzyme that rapidly degrades the active form of GLP-1, was measured. Plasma DPP-4 enzymatic activity was determined spectrophotometrically, with Gly-Pro p-nitroanilide p-toluene sulfonate used as the substrate (13, 28). Plasma glucose was measured using a QuantiChrom glucose assay kit (BioAssay Systems, Hayward, CA).
Effect of dietary RS on plasma total GLP-1 and total PYY during a 24-h period in rats: study 1.
The aim of study 1 was to determine whether the differences in glycemic index between the control and RS diets or the effect of a meal can influence the plasma levels of GLP-1 and PYY in RS-fed rats. One hundred adult male Sprague-Dawley rats were divided into two dietary groups and fed the control or the RS diet for 10 days. After 10 days, rats from each group were killed rapidly by decapitation at different times throughout the day (−1, 1, 3, 7, 11, 15, and 19 h after onset of the dark cycle, n = 7–8 for each dietary group at each time point). Total GLP-1, total PYY, glucose, insulin, and DPP-4 enzyme activity were measured. Food intake was recorded for 2 days before the end of study 1 by measurement of food jar weight and spillage for each rat. Body weight was recorded and fat pads were weighed at the end of study 1.
Effect of dietary RS on gut proglucagon and PYY gene expression in rats: study 2.
The aim of study 2 was to determine the location(s) in the gut where dietary RS stimulates proglucagon and PYY gene expression. For identification of the role of fiber in the RS diet, an additional fiber control group was added in study 2. The rats in the fiber control group were fed a diet with nonfermentable methylcellulose replacing the control cornstarch to reach fiber levels similar to those in the RS diet [38–40% fiber (wt/wt)]. Except for the differences in starch and fiber, the dietary components were the same for all diets (23). Therefore, although the fiber contents of the RS and the fiber control diet were similar, a fermentable fiber was used in the RS diet and a nonfermentable fiber was used in the fiber control diet.
Thirty adult female rats were assigned to one of three diet treatment groups (n = 10 each group), control, RS, and fiber control, for a 32-day study. At the end of the study, epithelial cells from the stomach, duodenum, jejunum, ileum, cecum, and colon of each rat were collected for total RNA extraction and mRNA expression by real-time RT-PCR (23, 45). Proglucagon and PYY gene expression was measured in the jejunum and distal gut (ileum, cecum, and colon). To compare the effects of RS on gene expression in the proximal and distal parts of the gut, we also measured ghrelin mRNA in the stomach and CCK mRNA in the duodenum.
The animals and methods used in study 2 are slightly different from those used in study 1, because we previously justified that such minor modifications do not change the interpretation of the study. We previously observed a consistent increase in GLP-1 and PYY in different animal models that were fed RS and subjected to different methods, including rats and mice of both sexes, compared with those fed two different control diets (regular AIN-93 rodent diet or a control diet with a diluted energy density equal to that of the RS diet) (23). Therefore, female rats were used, and diets were fed for a longer time to maximize the effect of RS on decreasing body fat accumulation. The body fat and food intake results from the present study were reported in our previous publication (23), which focused on body fat regulation, but not on proglucagon and PYY gene location in the gut.
Direct in vitro stimulating effects of SCFAs on proglucagon gene expression: study 3.
The aim of study 3 was to test the direct effects of fermentation products on proglucagon gene expression in vitro. STC-1 cells were incubated in normal-pH (pH 7.5) or low-pH (pH 6.0) medium with different concentrations of butyrate (0.016, 0.08, and 0.4 mM), propionate (0.1 and 1.0 mM), and acetate (3.0 and 30 mM) for 24 h. At the end of treatment, total RNA from STC-1 cells was extracted for measurement of proglucagon mRNA. PYY mRNA is undetectable in STC-1 cells (unpublished observation).
Direct in vitro stimulating effects of SCFAs on PYY promoter activity: study 4.
The aim of study 4 was to test the direct effect of SCFAs on PYY gene expression in vitro. Because, on the basis of our search, no cell lines express the PYY gene naturally, HEK-293 cells transfected with PYY promoter were used in study 4. Because HEK-293 cells grow poorly in low-pH (pH 6.0) medium, they were incubated in normal-pH (pH 7.5) medium. PYY-luciferase gene reporter constructs −770/+37 and −127/+37 were a gift from Prof. Leiter (Tufts University, Boston, MA). Preliminary results showed that PYY promoter activation by butyrate (10 mM), propionate (10 mM), and acetate (30 mM) was the same in HEK cells transfected with construct −770/+37 and HEK cells transfected with construct −127/+37. Thus the construct −127/+37 was used for subsequent studies. HEK-293 cells were transfected with 0.4 μg of DNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). The dose-response study was conducted 24 h after transfection. Cells were treated with different levels of each SCFA (0.08, 0.4, 2, 10, and 20 mM butyrate; 0.01, 0.1, 1, and 10 mM propionate; 0.3, 3, and 30 mM acetate) for 20 h. For the time-response study, transfected cells were treated with one of three SCFAs, butyrate (2 mM), propionate (10 mM), or acetate (30 mM), for 1, 3, 8, 12, 24, or 48 h. At the end of treatment, cells were harvested in lysis buffer (luciferase assay system; Promega, Madison, WI), and luciferase activity was determined with a luminometer (Microlumat Plus, Berthold Technologies) and normalized to the protein content of the cells.
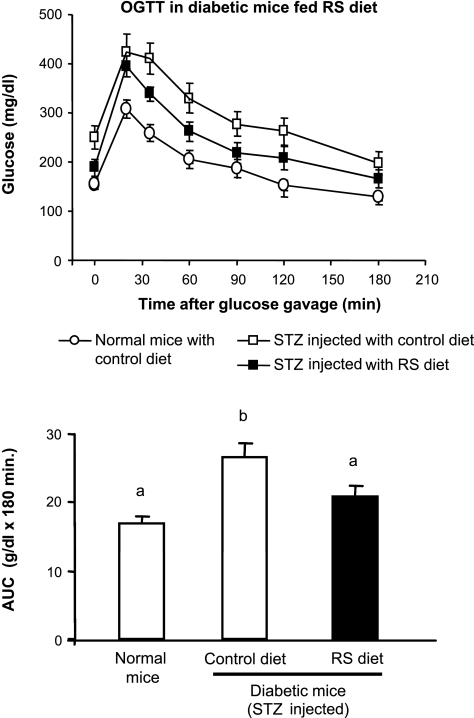
Oral glucose tolerance test in streptozocin-induced diabetic mice fed RS: study 5.
The aim of study 5 was to determine whether dietary RS can improve glucose tolerance in a diabetic animal model. A well-established diabetic mouse model induced by multiple low-dose streptozocin (STZ) injections (18, 27) was used. Adult male C57BL/6J mice were fed the control or the RS diet for 10 days. Then the mice were injected intraperitoneally with 0.5 M citrate buffer (pH 4.5) or STZ (40 mg/kg in citrate buffer, 0.1 ml/10 g body wt) for 5 consecutive days while continuing to consume the diets. Blood glucose was monitored daily by tail bleeding during the injection to ensure that hyperglycemia was established for STZ-injected mice. At 4 days after the last STZ injection, all mice were fasted for 5 h during the light period and gavaged with a glucose solution (50 mg glucose/mouse). The blood glucose levels were measured at 0, 15, 30, 45, 60, 90, and 120 min after the glucose gavage, and the area under the curve (AUC) during the oral glucose tolerance test (OGTT) was examined.
Statistical analysis.
Values are means ± SE. One-way ANOVAs with post hoc adjusted Tukey's tests were used to determine statistical significance among the groups. For the OGTT, AUC was determined by calculation of the entire AUC [“total-area” AUC method (35)].
RESULTS
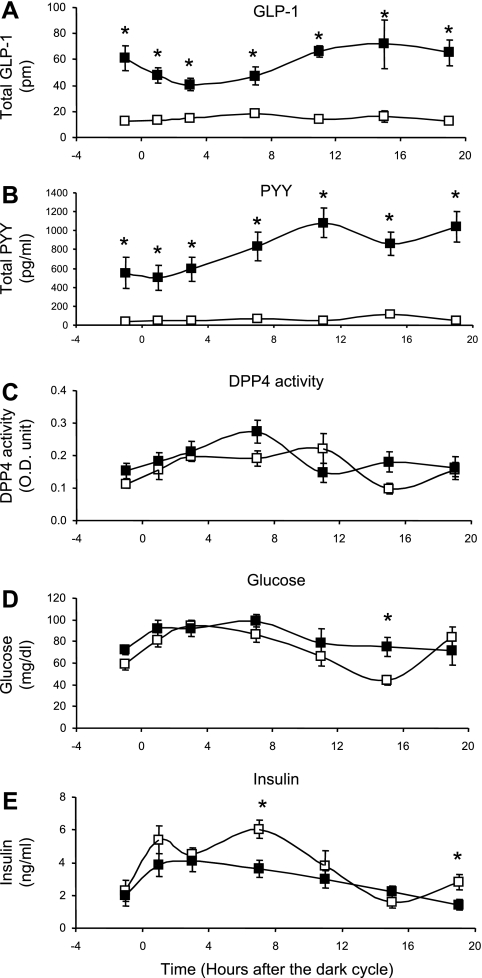
Plasma total GLP-1 and total PYY were increased over a 24-h period at every sample collection time point in RS-fed rats (Fig. 1). After the rats from study 1 were fed RS diets for 10 days, their body fat was significantly lower than that of the control-fed rats and their food intake was similar to that of the control-fed rats (Table 2). Plasma total GLP-1 (Fig. 1A) and total PYY (Fig. 1B) in RS-fed rats were significantly elevated at every sample collection time point over a 24-h period compared with the control rats that were fed a diet with the same energy density (P < 0.01). DPP-4 activity (Fig. 1C) was not different between the two dietary treatment groups. As we expected, there was less fluctuation in glucose (Fig. 1D) and insulin (Fig. 1E) over the 24-h period in the RS-fed rats than in the control-fed rats because of the lower glycemic index of the RS diet. However, these differences did not change the dietary RS-induced sustained elevation of GLP-1 and PYY. Also, the significantly higher levels of GLP-1 and PYY were observed in dark and light periods. Thus the day-long increases in GLP-1 and PYY in RS-fed rats are not due to meal effects, the differences in postprandial dietary glycemic indexes of the two diets, or timing of sampling; rather, the increases are more likely the result of a factor that persisted throughout the day. We next examined proglucagon and PYY gene expression in different parts of the gut.
Fig. 1.
Total glucagon-like peptide-1 (GLP-1), total peptide YY (PYY), dipeptidylpeptidase-4 (DPP-4) activity, blood glucose, and insulin in rats fed control diet (□) or resistant starch (RS) diet (▪) ad libitum for 10 days in study 1. Total GLP-1 and total PYY in RS-fed rats were significantly increased compared with controls (P < 0.01) at every sample collection time point over a 24-h period. DPP-4 activity was not significantly different between the groups at all time points. Blood glucose was similar between the groups, except at 1 time point. Insulin in RS-fed rats was lower at 2 time points than in controls. OD, optical density. Values are means ± SE (n = 6–8 for each group at each time point). *P < 0.05 vs. control at the same time collection point.
Table 2.
Body fat and food intake for rats fed RS diet for 10 days: study 1
|
Diet |
||
|---|---|---|
| Control | RS | |
| Body fat, g | 4.39±0.10 | 3.55±0.11* |
| Body fat/body wt, % | 1.61±0.03 | 1.35±0.04* |
| 48-h cumulative food intake, g | 40.5±0.5 | 39.5±0.9 |
Values are means ± SE. Body fat was calculated as the sum of epididymal fat, perirenal fat, and retroperitoneal fat; 48-h cumulative food intake was measured at the end of study 1.
P < 0.01 vs. control (by Student's t-test).
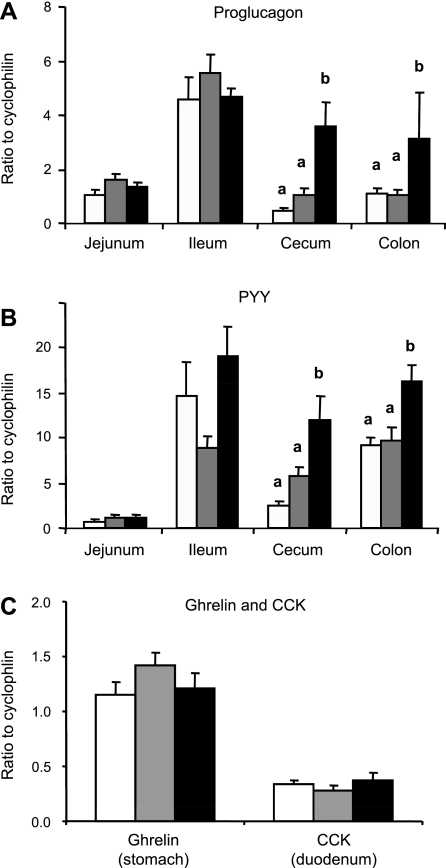
Proglucagon and PYY mRNA expression was higher in the cecum and colon, where RS fermentation occurs in rats (Fig. 2). Along with increased plasma total GLP-1 and PYY (23), proglucagon (Fig. 2A) and PYY (Fig. 2B) gene expression was significantly increased in the cecum and colon in RS-fed group compared with the control and nonfermentable fiber control groups. Interestingly, dietary RS did not affect proglucagon and PYY gene expression in the ileum, although the highest noninduced expressions for both genes were detected in the ileum. In contrast, ghrelin mRNA expression in the stomach and CCK mRNA expression in the duodenum were not different between the three groups (Fig. 2C), which suggests that RS has no effect on the upper gut. Thus the stimulating factor that specifically responds to RS might only exist in the lower gut, where RS ferments to produce SCFAs.
Fig. 2.
Proglucagon, PYY, and ghrelin and CCK gene expression in epithelial cells collected from different parts of the gut of rats fed control (open bars), nonfermentable fiber control (gray bars), and RS (filled bars) diets in study 2. Values are means ± SE for groups of 8–11 rats. Values that do not share a common letter (a, b) are significantly different (P < 0.05).
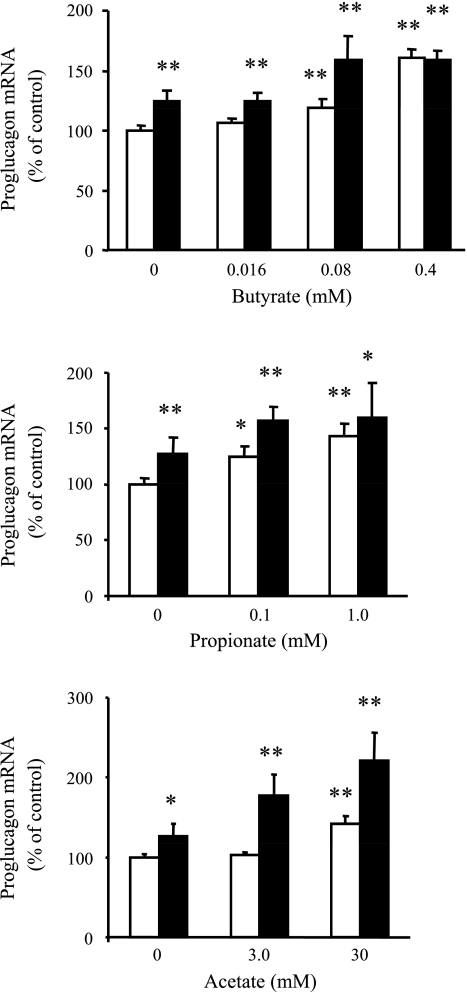
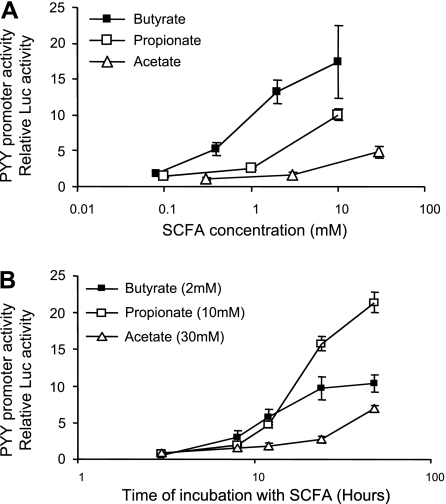
SCFAs directly increased proglucagon gene expression (Fig. 3) and PYY promoter activity (Fig. 4) in vitro. Fermentation of RS alters the large intestine environment by increasing the amounts of SCFA and lowering the pH in the large intestine. Three major SCFAs produced by fermentation are butyrate, propionate, and acetate. At concentrations similar to those obtained from cecal contents in RS-fed rats (23), butyrate, propionate, and acetate directly stimulated proglucagon gene expression in STC-1 cells individually in a dose-dependent manner (Fig. 3). Notably, when STC-1 cells were cultured in the same medium but with the pH of the medium adjusted from 7.5 to 6.0, the dose-response effects for all three SCFAs were generally increased (Fig. 3). In HEK-293 cells, the PYY promoter activity was dramatically increased by butyrate, propionate, or acetate in a dose- and time-response manner (Fig. 4).
Fig. 3.
Direct in vitro stimulating effects of short-chain fatty acids (SCFAs) on proglucagon gene expression in STC-1 cells in study 3. STC-1 cells were incubated in normal-pH (open bars) or low-pH (filled bars) medium with different concentrations of butyrate, propionate, and acetate for 24 h. Data are from 3–5 independent experiments with 3–9 replicates for each experiment. *P < 0.05; **P < 0.01 vs. control (0 mM in normal medium).
Fig. 4.
HEK-293 cells in study 4 were transiently transfected with rat PYY promoter fused to the luciferase reporter gene and incubated with different concentrations of SCFA for 20 h (A) or SCFA at different time periods (B). Values are expressed relative to luciferase (Luc) activity in cells transfected with control solution without promoter and incubated for the same period. Values are means ± SE for 2–5 independent experiments with 2–5 replicates in each experiment.
Glucose tolerance was improved in RS-fed diabetic mice (Fig. 5). STZ-treated mice exhibited glucose intolerance compared with vehicle-treated mice after 5 h of fasting in the light phase. Dietary RS significantly improved glucose tolerance in the STZ-injected mice (Fig. 5).
Fig. 5.
Glucose tolerance was improved in streptozocin (STZ)-induced diabetic mice fed RS in study 5. A: oral glucose tolerance test (OGTT) in normal mice and diabetic mice fed control or RS diet. Glucose concentration at time 0 was obtained after a 5-h fast. B: area under the curve (AUC) of OGTT for mice fed control (open bar) and RS (solid bar) diets. Values that do not share a common letter are significantly different (P < 0.05). Values are means ± SE (n = 8–9 for each group).
DISCUSSION
The day-long increased patterns of GLP-1 and PYY in study 1 indicate that dietary RS increases GLP-1 and PYY independently from feeding status of the rats or glycemic status of the diet. Study 2 shows that the higher proglucagon and PYY mRNA expressions in RS-fed rats are specifically detected in the cecum and colon, but not in the ileum, although the highest noninduced expressions for both genes are in the ileum. Therefore, the stimulating factors for GLP-1 and PYY might exist only in the lower gut, where RS ferments. Furthermore, the nonfermentable fiber control group had an expression profile similar to that of the controls, suggesting that a simple bulking effect of RS is unlikely to be the cause. Rather, the fermentation of RS in the lower gut is likely a stimulator for the increased expressions of proglucagon and PYY in RS-fed rats. The fermentation of RS changed the environment of the large intestine by increasing SCFAs and decreasing pH. Studies 3 and 4 provide direct evidence that fermentation products increase GLP-1 and PYY expression in vitro.
These results provide a possible explanation for the equivocal effects of fiber on regulating gut hormone and glucose homeostasis (19, 42): “Not every kind of fiber is created equal.” Only fermentable fiber increases GLP-1 and PYY. Besides dietary RS, several other types of fermentable fibers also increase plasma GLP-1 and PYY in animals and humans (20, 21, 29). For example, oligofructose, another fermentable carbohydrate, stimulates GLP-1 secretion and improves glucose tolerance in a GLP-1-dependent manner (7–10, 14, 15).
Raben et al. (36) reported that dietary RS decreases plasma GLP-1 in human subjects. However, their test meal of RS was given to the subjects only once, and plasma GLP-1 was measured within the 5 h following the test meal. In such a short experimental period, RS has not been fermented to produce SCFAs, since the production of SCFAs usually requires 2–4 days in animals and humans. Additionally, they used a different control diet, which made immediate postprandial glycemia differences much more obvious between the control and the RS diet. Thus the decreased plasma GLP-1 is likely due to the immediate postprandial glycemic effect of an RS meal. Under our conditions, the effects of the overlap of fermentation from one meal to the next on GLP-1 production might overwhelm individual meal effects.
Furthermore, our data suggest that the continuous addition of fermentable fiber in the diet is another important factor for regulation of GLP-1 and PYY secretion. Because the microflora environment in the gut must have time to adapt to a new food source before the full effects of fermentation can take effect (12), RS has to be consumed for a long enough time to allow fermentation to occur and to stimulate GLP-1 and PYY production. Additionally, any factors that affect fermentation of RS might also alter the stimulating effect of RS on GLP-1 and PYY production. For example, caution should be given for certain dietary and pharmaceutical modulations, diseases, or medical procedures that will change gut microflora. These modulations and procedures might influence the effectiveness of RS through interference with gut microflora and fermentation.
One limitation of our study is that total GLP-1 and PYY, rather than their active forms, were measured. Nevertheless, we expect that the active forms of GLP-1 and PYY should also be increased in RS-fed animals proportional to the total GLP-1 and PYY. 1) As long as the fermentation continues, SCFAs in the lower gut should be continuously liberated (30) and serve as stimulators for GLP-1 and PYY expression. 2) Despite an increase in production of GLP-1 and PYY, their degradations should be the same, inasmuch as DPP-4 activity is the same in control and RS-fed rats. This implies that the difference in GLP-1 between control and RS feeding is due to increases in production of the active form of GLP-1. 3) Dietary RS improved glucose tolerance in diabetic mice, which indicates an increase in the biological function of GLP-1 and PYY in these mice.
Levels of elevated GLP-1 and PYY are elevated to a lesser extent by dietary RS than by pharmaceutical means. Because GLP-1 and PYY have pleiotropic functions, the full scope of biological functions requires further investigations for the sustained elevated, subpharmaceutical levels of GLP-1 and PYY in RS-fed animals. In the present studies, glucose control was improved in RS-fed diabetic mice (Fig. 5). However, dietary RS does not significantly change overall glucose and insulin levels in nondiabetic animals (Fig. 1) (unpublished observations). Furthermore, although body fat accumulation decreased in all our RS-fed animals, anorexia was not observed in RS-fed animals (Table 1), as described in our previous studies. The reduced food intake induced by exogenous injection of GLP-1 and PYY was only detected transiently (within hours) after injection and required high doses of GLP-1 and PYY (3, 34): usually >40 nmol/kg for GLP-1 and 10 nmol/kg for PYY3–36 (1, 2, 33). Such high doses can transiently generate much higher plasma levels of GLP-1 and PYY than those associated with dietary RS. Thus the levels of GLP-1 and PYY in RS-fed animals might be below the effective dose for decreasing food intake. It has been reported that insulin action was improved by PYY at a dose lower than the dose that decreases food intake (41). Glucose tolerance was also improved by endogenously released GLP-1 at a concentration lower than that achieved by exogenous injection at the dose that can produce a satiety effect. Thus the sustained elevated, subpharmaceutical level of GLP-1 might interact with the GLP-1 receptor located near the hepatic portal region to improve glucose tolerance in RS-fed animals (40).
The unpleasant effects of fermentation are a concern when dietary fermentable fiber is used. In our studies, we used 30% (wt/wt) RS in rodent diets. RS-fed animals initially had soft feces, but the symptoms were generally ameliorated after a few days of adaptation. No other side effects were observed in RS-fed animals. Because human subjects have varying tolerances for fermentation, understanding the mechanism of increased GLP-1 and PYY by RS can provide insight for development of alternatives for patients who are less tolerant to dietary fermentable fiber.
The most effective treatment for obesity has been gastric bypass surgery, which also cures type 2 diabetes in >80% of patients (32). Interestingly, after gastric bypass surgery, patients also have increased plasma GLP-1 and PYY levels (26, 32), leading to the theory that the long-term effectiveness of gastric bypass surgery on obesity and diabetes is in part due to the increased gut antiobesity hormones. Compared with pharmaceutical agents and surgery, the endogenous increase of GLP-1 and PYY by dietary RS is simple, noninvasive, inexpensive, and reliable.
Finally, although the data from the present study and our previous studies confirm that dietary RS consistently increases GLP-1 and PYY in rodents, the time frame of measured increases is 1–12 wk. It is uncertain how long the stimulation and the improved glucose tolerance could last. Another fermentable fiber (guar) reportedly improves carbohydrate tolerance within the first 18 wk of usage, but the beneficial effects disappeared after 24–64 wk (39). Thus a human study that lasts >1 yr is needed for full assessment of the effectiveness of RS on GLP-1 and PYY secretion.
In conclusion, we provide strong evidence that dietary RS upregulates total GLP-1 and PYY in a sustained day-long manner in rodents. Such increases are associated with the fermentation of RS in the lower gut. Although the conclusions need to be confirmed in humans, any factor that affects fermentation should be considered when dietary fermentable fiber is used to stimulate GLP-1 and PYY secretion.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R21 DK-073403-01A1, a Pennington Biomedical Research Center Functional Food Pilot Grant, and the LSU Ag Center Biotechnology Education for Students and Teachers Program.
Acknowledgments
We thank Dr. Zhanguo Gao and Elizabeth Lissy for technical assistance and Dr. Christopher Morrison for critically reading the manuscript.
This paper was approved for publication by the director of Louisiana Agricultural Experiment Station as manuscript no. 2008-239-1419.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 1044: 127–131, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Abbott CR, Small CJ, Sajedi A, Smith KL, Parkinson JR, Broadhead LL, Ghatei MA, Bloom SR. The importance of acclimatisation and habituation to experimental conditions when investigating the anorectic effects of gastrointestinal hormones in the rat. Int J Obes (Lond) 30: 288–292, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 132: 2131–2157, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Brennan CS Dietary fibre, glycaemic response, diabetes. Mol Nutr Food Res 49: 560–570, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Brubaker PL The glucagon-like peptides: pleiotropic regulators of nutrient homeostasis. Ann NY Acad Sci 1070: 10–26, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Brubaker PL Incretin-based therapies: mimetics versus protease inhibitors. Trends Endocrinol Metab 18: 240–245, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Cani PD, Daubioul CA, Reusens B, Remacle C, Catillon G, Delzenne NM. Involvement of endogenous glucagon-like peptide-1(7–36) amide on glycaemia-lowering effect of oligofructose in streptozotocin-treated rats. J Endocrinol 185: 457–465, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Cani PD, Dewever C, Delzenne NM. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr 92: 521–526, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes 55: 1484–1490, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Cani PD, Neyrinck AM, Maton N, Delzenne NM. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like peptide-1. Obes Res 13: 1000–1007, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Christopher R, Covington P, Davenport M, Fleck P, Mekki QA, Wann ER, Karim A. Pharmacokinetics, pharmacodynamics, and tolerability of single increasing doses of the dipeptidyl peptidase-4 inhibitor alogliptin in healthy male subjects. Clin Ther 30: 513–527, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Cummings JH, Macfarlane GT, Englyst HN. Prebiotic digestion and fermentation. Am J Clin Nutr 73: 415S–420S, 2001. [DOI] [PubMed] [Google Scholar]
- 13.David F, Bernard AM, Pierres M, Marguet D. Identification of serine 624, aspartic acid 702, and histidine 734 as the catalytic triad residues of mouse dipeptidyl-peptidase IV (CD26). A member of a novel family of nonclassical serine hydrolases. J Biol Chem 268: 17247–17252, 1993. [PubMed] [Google Scholar]
- 14.Delzenne NM, Cani PD, Daubioul C, Neyrinck AM. Impact of inulin and oligofructose on gastrointestinal peptides. Br J Nutr 93, Suppl 1: S157–S161, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Delzenne NM, Cani PD, Neyrinck AM. Modulation of glucagon-like peptide 1 and energy metabolism by inulin and oligofructose: experimental data. J Nutr 137: 2547S–2551S, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Drucker DJ The biology of incretin hormones. Cell Metab 3: 153–165, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr 46, Suppl 2: S33–S50, 1992. [PubMed] [Google Scholar]
- 18.Flodstrom M, Tyrberg B, Eizirik DL, Sandler S. Reduced sensitivity of inducible nitric oxide synthase-deficient mice to multiple low-dose streptozotocin-induced diabetes. Diabetes 48: 706–713, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Frost G, Brynes A, Leeds A. Effect of large bowel fermentation on insulin, glucose, free fatty acids, and glucagon-like peptide 1 (7-36) amide in patients with coronary heart disease. Nutrition 15: 183–188, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Gee JM, Johnson IT. Dietary lactitol fermentation increases circulating peptide YY and glucagon-like peptide-1 in rats and humans. Nutrition 21: 1036–1043, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Greenway F, O'Neil CE, Stewart L, Rood J, Keenan M, Martin R. Fourteen weeks of treatment with Viscofiber increased fasting levels of glucagon-like peptide-1 and peptide-YY. J Med Food 10: 720–724, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Haralampu S Resistant starch—a review of the physical properties and biological impact of RS. In: Carbohydrates and Polysaccharides. New York: Elsevier, 2000, p. 285–292.
- 23.Keenan MJ, Zhou J, McCutcheon KL, Raggio AM, Bateman HG, Todd E, Jones CK, Tulley RT, Melton S, Martin RJ, Hegsted M. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity (Silver Spring) 14: 1523–1534, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Kendall CW, Emam A, Augustin LS, Jenkins DJ. Resistant starches and health. J AOAC Int 87: 769–774, 2004. [PubMed] [Google Scholar]
- 25.Kleessen B, Stoof G, Proll J, Schmiedl D, Noack J, Blaut M. Feeding resistant starch affects fecal and cecal microflora and short-chain fatty acids in rats. J Anim Sci 75: 2453–2462, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL, Wardlaw SL. Effects of roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab 90: 359–365, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Kunjathoor VV, Wilson DL, LeBoeuf RC. Increased atherosclerosis in streptozotocin-induced diabetic mice. J Clin Invest 97: 1767–1773, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marguet D, Baggio L, Kobayashi T, Bernard AM, Pierres M, Nielsen PF, Ribel U, Watanabe T, Drucker DJ, Wagtmann N. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci USA 97: 6874–6879, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massimino SP, McBurney MI, Field CJ, Thomson AB, Keelan M, Hayek MG, Sunvold GD. Fermentable dietary fiber increases GLP-1 secretion and improves glucose homeostasis despite increased intestinal glucose transport capacity in healthy dogs. J Nutr 128: 1786–1793, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Monsma DJ, Marlett JA. Rat cecal inocula produce different patterns of short-chain fatty acids than fecal inocula in in vitro fermentations. J Nutr 125: 2463–2470, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Murphy KG, Dhillo WS, Bloom SR. Gut peptides in the regulation of food intake and energy homeostasis. Endocr Rev 27: 719–727, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Naslund E, Kral JG. Impact of gastric bypass surgery on gut hormones and glucose homeostasis in type 2 diabetes. Diabetes 55, Suppl 2: S92–S97, 2006. [Google Scholar]
- 33.Parkinson JR, Dhillo WS, Small CJ, Chaudhri OB, Bewick GA, Pritchard I, Moore S, Ghatei MA, Bloom SR. PYY3-36 injection in mice produces an acute anorexigenic effect followed by a delayed orexigenic effect not observed with other anorexigenic gut hormones. Am J Physiol Endocrinol Metab 294: E698–E708, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Pittner RA, Moore CX, Bhavsar SP, Gedulin BR, Smith PA, Jodka CM, Parkes DG, Paterniti JR, Srivastava VP, Young AA. Effects of PYY[3-36] in rodent models of diabetes and obesity. Int J Obes Relat Metab Disord 28: 963–971, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Potteiger JA, Jacobsen DJ, Donnelly JE. A comparison of methods for analyzing glucose and insulin areas under the curve following nine months of exercise in overweight adults. Int J Obes Relat Metab Disord 26: 87–89, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Raben A, Tagliabue A, Christensen NJ, Madsen J, Holst JJ, Astrup A. Resistant starch: the effect on postprandial glycemia, hormonal response, and satiety. Am J Clin Nutr 60: 544–551, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J Nutr 123: 1939–1951, 1993. [DOI] [PubMed] [Google Scholar]
- 38.Small CJ, Bloom SR. The therapeutic potential of gut hormone peptide YY3-36 in the treatment of obesity. Expert Opin Investig Drugs 14: 647–653, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Track NS, Cawkwell ME, Chin BC, Chiu SS, Haberer SA, Honey CR. Guar gum consumption in adolescent and adult rats: short- and long-term metabolic effects. Can J Physiol Pharmacol 63: 1113–1121, 1985. [DOI] [PubMed] [Google Scholar]
- 40.Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D'Alessio DA. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 148: 4965–4973, 2007. [DOI] [PubMed] [Google Scholar]
- 41.van den Hoek AM, Heijboer AC, Voshol PJ, Havekes LM, Romijn JA, Corssmit EP, Pijl H. Chronic PYY3–36 treatment promotes fat oxidation and ameliorates insulin resistance in C57BL6 mice. Am J Physiol Endocrinol Metab 292: E238–E245, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Weickert MO, Spranger J, Holst JJ, Otto B, Koebnick C, Mohlig M, Pfeiffer AF. Wheat-fibre-induced changes of postprandial peptide YY and ghrelin responses are not associated with acute alterations of satiety. Br J Nutr 96: 795–798, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology 132: 2116–2130, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Xiao Q, Giguere J, Parisien M, Jeng W, St-Pierre SA, Brubaker PL, Wheeler MB. Biological activities of glucagon-like peptide-1 analogues in vitro and in vivo. Biochemistry 40: 2860–2869, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Zhou J, Hegsted M, McCutcheon KL, Keenan MJ, Xi X, Raggio AM, Martin RJ. Peptide YY and proglucagon mRNA expression patterns and regulation in the gut. Obesity (Silver Spring) 14: 683–689, 2006. [DOI] [PubMed] [Google Scholar]