Abstract
PPARγ activators such as rosiglitazone (RSG) stimulate adipocyte differentiation and increase subcutaneous adipose tissue mass. However, in addition to preadipocyte differentiation, adipose tissue expansion requires neovascularization to support increased adipocyte numbers. Paradoxically, endothelial cell growth and differentiation is potently inhibited by RSG in vitro, raising the question of how this drug can induce an increase in adipose tissue mass while inhibiting angiogenesis. We find that adipose tissue from mice treated with RSG have increased capillary density. To determine whether adipose tissue angiogenesis was stimulated by RSG, we developed a novel assay to study angiogenic sprout formation ex vivo. Angiogenic sprout formation from equally sized adipose tissue fragments, but not from aorta rings, was greatly increased by obesity and by TZD treatment in vivo. To define the mechanism involved in RSG-stimulated angiogenesis in adipose tissue, the expression of proangiogenic factors by adipocytes was examined. Expression of VEGFA and VEGFB, as well as of the angiopoietin-like factor-4 (ANGPTL4), was stimulated by in vivo treatment with RSG. To define the potential role of these factors, we analyzed their effects on endothelial cell growth and differentiation in vitro. We found that ANGPTL4 stimulates endothelial cell growth and tubule formation, albeit more weakly than VEGF. However, ANGPTL4 mitigates the growth inhibitory actions of RSG on endothelial cells in the presence or absence of VEGF. Thus, the interplay between VEGF and ANGPTL4 could lead to a net expansion of the adipose tissue capillary network, required for adipose tissue growth, in response to PPARγ activators.
Keywords: neovascularization, endothelial cell, insulin resistance, diabetes, adipokines
a close relationship exists between ectopic fat accumulation in tissues such as muscle and liver and the development of insulin resistance. The primary defense against such ectopic lipid accumulation is a well-functioning adipose tissue capable of sequestering excess calories in the form of stored triglycerides (21). Individual adipocytes within adipose tissue can expand markedly and in this manner accommodate increased triglyceride storage. However, sustained adipocyte hypertrophy can lead to cell death, potentially causing inflammatory responses and insulin resistance (11). In the long term, sustained triglyceride sequestration requires tissue expansion, a process that is likely to depend on recruitment and differentiation of new adipocytes. In addition to preadipocyte differentiation, adipose tissue expansion absolutely requires the parallel growth of its capillary network. This requirement is evidenced by the finding that antiangiogenic agents mitigate genetic and high-fat diet-induced obesity and the observations of an increase in adipose tissue microcirculation during the onset of obesity (5, 7, 36, 43, 48).
One of the most important regulators of adipocyte differentiation is the transcription factor peroxisome proliferator activated receptor-γ (PPARγ), which is required for adipocyte differentiation both in vivo and in vitro (32, 46). Thiazolidinediones (TZDs) are a class of drugs that activate PPARγ and promote adipocyte differentiation (22, 29, 56). TZDs enhance insulin sensitivity in patients with type 2 diabetes, but the precise mechanism by which TZDs exert this therapeutic action is not clear. One hypothesis is that they lower ectopic fatty acid accumulation in muscle and liver by promoting free fatty acid deposition in subcutaneous adipose tissue formed de novo in response to PPARγ stimulation (2, 9, 13, 35, 40, 47). TZDs also enhance expression of mitochondrial genes in white adipocytes (4, 10, 50, 51) and adiponectin secretion (6), an adipokine that enhances fatty acid oxidation. Thus, the therapeutic mechanism of TZD action is thought to involve the expansion of adipose tissue with enhanced metabolic and endocrine activities. Adipocyte PPARγ also appears to be required for the formation of the capillary network of adipose tissue, because preadipocytes expressing PPARγ dominant negative constructs not only fail to differentiate into adipose cells but also fail to induce angiogenesis (18). The mechanisms by which adipocyte PPARγ targets induce adipose tissue angiogenesis are not well defined.
PPARγ is also present in endothelial cells, but paradoxically, multiple studies demonstrate that PPARγ activation suppresses the proliferation of endothelial cells in vitro and their differentiation into tube-like structures (24, 41, 52). Furthermore, the antiangiogenic effect of PPARγ ligands such as the TZDs occurs in vivo, as evidenced by cornea (34) and chick chorioallantoic membrane (41) angiogenesis assays. Indeed, the antiangiogenic actions of PPARγ activators have prompted the use of TZDs as potential anticancer therapies (16). These findings raise the question of how TZDs can promote the expansion of adipose tissue and at the same time inhibit angiogenesis.
In the current study, we have developed an ex vivo angiogenesis assay to determine whether the TZD rosiglitazone (RSG) enhances angiogenesis in adipose tissue. We find that both obesity and RSG treatment in vivo results in enhanced angiogenic potential of the adipose tissue capillary bed. Analysis of angiogenic factors in adipocytes of animals treated with RSG reveals enhanced expression of the essential proangiogenic factors VEGFA and VEGFB as well as of angiopoietin-like protein 4 (ANGPTL4, fasting-inducible adipocyte factor, PPARγ angiopoietin-related gene). We find that, in vitro, ANGPTL4 stimulates endothelial cell growth and differentiation, and, more importantly, it protects endothelial cells from the inhibitory actions of RSG. Thus, the combined production of VEGF and ANGPTL4 could lead to a net increase in angiogenesis in adipose tissue in response to systemic PPARγ stimulation.
MATERIALS AND METHODS
Reagents.
All chemicals, unless specified, were purchased from Sigma Chemical. Rabbit polyclonal anti-mouse/rat ANGPTL4 antibody was from Invitrogen. CD31 antibody used for immunomagnetic purification of microvascular endothelial cells (MEC) was from Serotec. Antibodies to CD31 (for immunohistochemistry), CD144 (VE-cadherin), and FITC-conjugated anti-BrdU antibody were from BD Pharmingen. Endothelial cell basal medium-2 supplemented with endothelial growth factors (EGM-2) was from Lonza. Matrigel was from BD Discovery Labware. Recombinant ANGPTL4 was from Abnova. SMARTpool siRNA against ANGPTL4 was purchased from Dharmacon along with nonspecific scrambled siRNA.
Animals.
Male 10-wk-old ob/ob and C57BL/6J mice (Jackson Laboratory) were housed (n = 2/cage) under 12:12-h light-dark cycles, with ad libitum access to food and water, and kept on standard pellet mouse chow. At 24–26 wk of age, the chow of one-half of the mice was supplemented with 5 mg·kg−1·day−1 of Avandia RSG maleate tablets (SmithKline Beecham Pharmaceuticals) or 10 mg·kg−1·day−1 of Actos tablets (Takeda Pharmaceuticals). Mice were killed at 26–28 wk after 2 wk of drug treatment, followed by 18 h of fasting. Thoracic aortas and epididymal fat pads were harvested. All procedures were approved by the University of Massachusetts Medical School Institutional Animal Care and Use Committee.
Preparation of adipose tissue explants.
Freshly harvested epididymal fat pads were placed in Krebs-Ringer solution buffered with HEPES (KRH), pH 7.4, supplemented with 2.5% BSA, and finely minced with scissors. Enzymatic digestion was performed in KRH supplemented with 1 mg/ml collagenase type I and 2.5% BSA (pH 7.4), using shaking orbital bath for 30 min at 37°C. The undigested tissue was separated from isolated adipocytes by filtration through chiffon material. The tissue remaining on the chiffon, comprising stromovascular fraction of adipose tissue with few remaining attached adipocytes, was collected, washed, cut into small (1 mm3) pieces, and embedded in Matrigel for the angiogenesis assay described below.
Ex vivo angiogenesis assay.
Freshly isolated thoracic aortas and epididymal fat pad pieces prepared as described above were washed under sterile conditions in several changes of ice-cold EGM-2. Aorta rings and epididymal fat pad explants were embedded in growth factor-depleted Matrigel as suggested by the manufacturer. Capillary sprouts from aorta rings and epididymal fat pad explants were counted daily for 2 wk along the perimeter of each sample under ×100 magnification.
Cell culture.
Human umbilical endothelial cells (HUVEC), 3T3-L1 preadipocytes, Cos-7 monkey kidney cells, C2C12 mouse myobalst cells, and HeLa human cervix carcinoma cells were purchased from American Type Culture Collection. HUVEC were cultured in EGM-2 in the presence of 5% CO2. All other cell types were cultured in DMEM supplemented with 10% FBS, 100 U penicillin/ml, and 100 μg streptomycin/ml (complete DMEM) in 10% CO2. Confluent 3T3-L1 cells were differentiated into adipocytes by incubation in complete DMEM supplemented with 0.5 mM 3-isobutyl-1-methylxanthine, 0.25 μM dexamethasone, and 1 μM insulin for 72 h, after which cells were cultured for an additional 5–6 days in complete DMEM. For siRNA experiments, 3T3-L1 adipocytes were transfected on day 5 after the beginning of differentiation, as described previously (26).
Isolation of primary MEC.
Epididymal fat pads from C57BL/6J mice (∼20–24 fat pads) were pooled and enzymatically digested as described above. Pieces of undigested tissue were layered on top of 45% Percoll solution in phosphate-buffered saline (PBS) and centrifuged at 400 g for 30 min at 4°C. The second layer containing MEC and microvascular fragments was transferred into EGM-2 for MEC and utilized for separation via differential cell attachment, as described previously (17). Rat anti-mouse CD31 antibody in combination with Dynabeads (Dynal Biotech) was used for immunomagnetic purification of MEC in accordance with the manufacturer's instructions. The identity of the purified cells as endothelial was confirmed by immunostaining and by the ability to form capillary tubes on Matrigel.
Capillary tube formation on matrigel.
HUVEC were plated in 24-well tissue culture plates (25,000 cells/well) precovered with growth factor-depleted Matrigel and incubated for 24 h as indicated in each experiment. Cells were fixed for 10 min in 4% paraformaldehyde in PBS. Differentiation of HUVEC into capillary-like tubes was assessed by two independent investigators by counting the number of capillary branches under ×100 magnification.
BrdU incorporation assay.
HUVEC were plated in 75 mm2 tissue culture flasks and incubated for 20 h as indicated in each experiment, followed by 4 h with 50 μM BrdU. Cells were harvested, resuspended in 1.5% BSA in PBS, and fixed in ice-cold 70% ethanol for 30 min. Cells were then incubated at room temperature for 30 min in 2 N HCl and 0.5% Triton X-100 in PBS, neutralized in 0.1 M Na2B4O7 (pH 8.5), and washed in 1% BSA 0.5% Tween-20 PBS for 30 min. FITC-conjugated anti-BrdU antibody was then added for 60 min, and the fluorescence intensity of the cell suspension was measured using 96-well multiwell dishes at laser excitation of 488 nm with a TECAN Safire2 multiwell reader.
RNA isolation and quantitative RT-PCR.
Total RNA from isolated adipocytes was extracted with Trizol Reagent. After RNase-free DNase I digestion, RNA was purified with Qiagen RNeasy MinElute cleanup kit. The purified RNA was then used to synthesize cDNA (iScript cDNA Synthesis kit). Real-time PCR was performed with iQ SYBR Green Supermix on MyiQ single-color real-time PCR detection system from Bio-Rad. The 2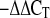 method was used to analyze the relative mRNA level. Ferritin heavy chain or actin mRNAs were used routinely as internal controls. Primers were designed using Universal Probe Library (Roche Diagnostics) and are available upon request.
method was used to analyze the relative mRNA level. Ferritin heavy chain or actin mRNAs were used routinely as internal controls. Primers were designed using Universal Probe Library (Roche Diagnostics) and are available upon request.
Western blotting.
Equal amounts of protein (20 μg for VE-Cadherin analysis) or equal amount of medium (40 μl for ANGPTL4 analysis) were loaded on 15 or 10% polyacrylamide gels, respectively, and transferred into nitrocellulose membrane (PerkinElmer Life Science) according to a standard procedure. The membranes were blocked with 5% dry nonfat milk and 1% BSA for 1 h at room temperature and blotted with relevant primary antibodies overnight at 4°C (anti-VE-Cadherin diluted 1:200 and anti-ANGPTL4 diluted 1:500). Secondary antibodies used were anti-rabbit IgG horseradish peroxidase conjugated (1:75,000; Promega, Madison, WI) for ANGPTL4 and rabbit anti-rat IgG (H + L) horseradish peroxidase conjugated for VE-Cadherin (Zymed). Blots were developed using Western Lightning Chemiluminiscent reagent (PerkinElmer Life Science).
Immunohistochemical staining and analysis.
Epididymal fat pads were fixed for 24 h in Zinc fixative (BD Pharmingen), washed in PBS, and embedded in paraffin. Longitudinal sections of 5-μm thickness were cut, mounted on Superfrost Plus microscope slides (Fisher Scientific), and stained with rat anti-mouse CD31 antibody, and indirect immunoperoxidase method (LSAB+ system; DakoCytomation) was employed to visualize endothelial cells. Sections were processed simultaneously with isotype controls. All sections were counterstained with hematoxylin.
Affymetrix GeneChip expression analysis.
Total RNA was prepared from three 150-mm dishes of 3T3-L1 cells on each day of differentiation. Affymetrix protocols were followed for the preparation of cRNA from total RNA, which was hybridized according to Affymetrix instructions for an MOE430-2 chip. The GeneChips were washed with a GeneChip Fluidics Station 400 and scanned with an HP GeneArray Scanner (Affymetrix). Raw expression data were analyzed with the Bioconductor statistical environment using RMA and MAS5, a Bioconductor implementation of the MAS 5.0 algorithm (Affymetrix). The “fold change” for each gene was determined by dividing the mean of the average difference from three independent experiments.
Statistical analysis.
Means of two populations were compared by Student's t-test. To compare means of several populations, one-way ANOVA analysis of variance was used, followed by Tukey's multiple comparison posttest. Data are presented as means ± SE. Differences were considered statistically significant at P < 0.05.
RESULTS
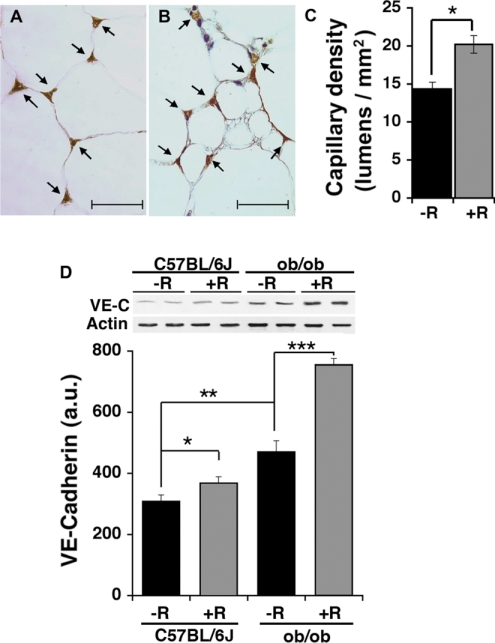
To determine whether RSG treatment affects the adipose tissue capillary network, we performed an immunohistochemical analysis of excised fat pads using antibody to CD31, a marker of endothelial cells. It has been described previously that TZDs stimulate the formation of small adipocyte clusters within subcutaneous adipose tissue of rats (13, 40). Consistent with these reports, we find that RSG-treated mice contain a larger proportion of small (<20 μm diameter) adipocytes (11 vs. 39%, untreated ob/ob vs. RSG-treated ob/ob mice). We compared capillary density in randomly chosen areas containing both small and large adipocytes in RSG-treated ob/ob mice with the capillary density of fat tissue from untreated ob/ob mice (Fig. 1, A and B). The epididymal fat pad capillary density was 25% higher in RSG-treated ob/ob mice compared with untreated controls (Fig. 1C).
Fig. 1.
Analysis of the effect of rosiglitazone (RSG) on epididymal fat pad capillary density. Endothelial cells were visualized by indirect immunohistochemistry with anti-CD31 antibody. Representative photomicrographs (A and B) and quantification of capillary density (C) of epididymal fat pad sections of untreated (A) and RSG-treated (B) ob/ob mice. Arrows indicate cross sections of capillary lumens. In each experiment, ∼20 fields from 6 separate sections/mouse were analyzed, and ≥3 mice for each experimental group were used. Statistical analysis was by 2-tailed unpaired Student t-test. *P < 0.05. D: analysis of VE-cadherin (VE-C) content in epididymal fat pad extracts by immunobloting. Shown side by side are samples from 2 separate animals from each experimental group as indicated. Actin immunobloting of the same gel was used as a loading control. Band intensity was quantified by densitometry, and the average ± SE of 4 independent experiments is plotted. Significance was determined using paired 1-tailed Student t-tests. *P < 0.05; **P < 0.01; ***P < 0.001. AU, arbitrary units.
The histological analysis of increased capillary density in adipose tissue from RSG-treated animals is complicated by the heterogeneity in adipose cell size. Thus, as an alternative method to determine capillary abundance, equal amounts of protein from adipose tissue extracts were analyzed by Western blotting with antibodies to VE-cadherin, an endothelial cell-specific marker (Fig. 1D). The concentration of VE-cadherin was increased in response to both obesity and RSG treatment. Together these results indicate that increases in fat mass, as well as RSG treatment, are accompanied by increased capillary density in adipose tissue.
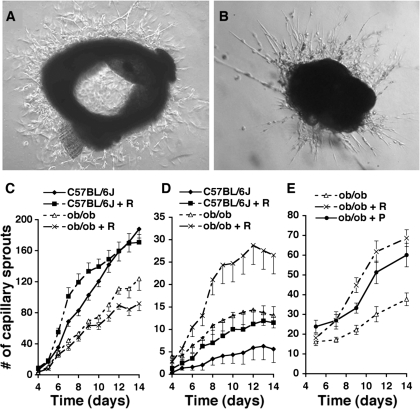
To determine whether the increased capillary density in adipose tissue seen in obesity and after treatment with RSG was due to an angiogenic response to the drug, we sought to develop a specific angiogenesis assay. Angiogenesis can be studied ex vivo by culturing aorta rings in proangiogenic medium. We used this approach to determine whether RSG treatment affects angiogenic activity. We also determined whether this assay could be used to measure angiogenic potential of the adipose tissue capillary network by embedding adipose tissue fragments previously depleted of adipocytes, as described in materials and methods. Capillary sprout formation from both aorta and adipose tissue explants could be observed (Fig. 2, A and B). Maximum capillary density was seen after 13–14 days in culture (not illustrated). This maximum capillary density was maintained for 5–7 more days, after which cells began to lose their original morphological appearance and entered apoptosis. Thus, the extent of capillary sprout formation was measured before 14 days.
Fig. 2.
Analysis of capillary sprout formation from aorta and epididymal fat pad explants. A and B: representative photomicrographs of capillary sprouts formed from aorta explants of C57BL/6J mice (A) on day 7 or epididymal fat pad explants of ob/ob mice (B) on day 14 of culture. Magnification is ×40. C–E: quantitative analysis of capillary sprout formation from aorta (C) or epididymal fat pad explants (D and E) from mice treated with RSG (+R) or pioglitazone (+P). Sprout formation was significantly (P < 0.05) decreased in aortas from ob/ob mice compared with C57BL/6J mice. No significant effect of RSG feeding of either C57BL/6J or ob/ob mice on aorta sprout formation was seen. In each experiment, each aorta was divided to 7–12 ring explants, and 4 independent experiments were performed. Total number of explants was n = 41 for C57BL/6J, n = 39 for C57BL/6J + RSG, n = 46 for ob/ob, and n = 45 for ob/ob + RSG. Sprout formation from fat pad explants was significantly increased by RSG feeding of both C57BL/6J (P < 0.01) and ob/ob mice (P < 0.001). A significant increase (P < 0.01) in sprout formation from explants from C57BL/6J compared with ob/ob mice was seen. For each experiment, ∼20 explants from each experimental group were embedded and counted, and 3–5 independent experiments were performed. Total number of explants in D was n = 72 for C57BL/6J, n = 69 for C57BL/6J + R, n = 123 for ob/ob, and n = 117 for ob/ob + R. Total number of explants in E was n = 94 for ob/ob, n = 88 for ob/ob + R, and n = 92 for ob/ob + P. Data were analyzed by 1-way ANOVA, followed by Tukey's multiple comparison test.
The number of capillary sprouts developed from aorta explants from ob/ob mice was significantly lower than in aortas from control C57BL/6J mice (Fig. 2C), a result consistent with a previous report showing decreased angiogenic competence of aorta in Zucker diabetic fatty rats (8). Treatment of either ob/ob or C57BL/6J mice with RSG in vivo had no significant effect on the angiogenic potential of aortic explants from these animals, although the kinetics of sprout formation in aortas from C57BL/6J mice treated with RSG displayed a trend to accelerated growth at early time points in culture and a leveling of growth at later time points. However, none of these differences were statistically significant. In contrast to results in aorta, the number of capillary sprouts developed from adipose tissue explants from ob/ob mice was three times that from control C57BL/6J mice (Fig. 2D), consistent with previous reports that indicate increased adipose tissue angiogenesis in obesity (7, 37, 48) and probably reflecting the increased angiogenesis necessary for the expansion of adipose tissue with the onset of obesity (5, 12). When either ob/ob or C57BL/6J mice were treated with RSG, twice as many capillary sprouts developed from adipose tissue explants (Fig. 2D), indicating that RSG increases the angiogenic potential of the adipose tissue capillary network. Together these results indicate that the angiogenic response of different vascular beds to changing physiological conditions varies, with aortic angiogenic potential being decreased and adipose tissue angiogenic potential increased by obesity, with further increase in adipose tissue angiogenic potential induced by RSG.
The effect of RSG to increase angiogenesis in adipose tissue is likely to be due to the activation of its major target, PPARγ. To gain further evidence for the specificity of this effect, we investigated the effect of pioglitazone, another clinically relevant PPARγ ligand. Similarly to the results seen with RSG, significantly more capillary sprouts developed from adipose tissue explants from ob/ob mice treated for 15 days with pioglitazone compared with untreated mice (Fig. 2E). These results indicate that the increased angiogenic potential of adipose tissue from treated animals is likely to result from activation of PPARγ.
The increased angiogenic potential of the adipose tissue capillary network ex vivo is likely to reflect an increased density of endothelial cells or endothelial cell precursors in adipose tissue in vivo. However, the direct effect of RSG on endothelial cells is to inhibit growth (24, 41, 52). Thus, its effects to cause a net increase in adipose tissue angiogenic potential could best be explained by a PPARγ-stimulated secretion of adipocyte-specific factors capable of overcoming the direct antiangiogenic effects of RSG on endothelial cells. To search for such factors, we analyzed Affymetrix databases derived from 3T3-L1 adipocytes undergoing differentiation (Table 1). Gene Ontology terms “angiogenesis” and “extracellular space” were used to filter databases derived from 3T3-L1 adipocytes before (day 0) and after 6 days of differentiation (day 6). Eleven annotated probes were found to significantly increase during differentiation, the top most abundant comprising adiponectin, ANGPTL4, VEGFA, and VEGFB. Both adiponectin and ANGPTL4 are expressed predominantly in adipose tissue and are known to be direct targets of PPARγ (27, 33, 53), making them candidate mediators for the net proangiogenic effect of RSG. Neutralizing antibodies to VEGFA can inhibit adipogenesis induced by obesity (37), suggesting that enhanced adipocyte VEGF expression may also be involved in the angiogenic response to RSG. The levels of ANGPTL6 mRNA were below detection level by quantitative real-time PCR in primary adipocytes, and thus this factor was not further investigated.
Table 1.
Probes identified with GO terms “angiogenesis” and “extracellular space” displaying a >1.30-fold increase during 3T3-L1 adipocyte differentiation
| Symbol |
Signal |
Fold Change | |
|---|---|---|---|
| Day 0 | Day 6 | ||
| Acdc | 894 | 23,631 | 26.43 |
| Angptl4 | 1,933 | 2,629 | 1.36 |
| Vegfa | 1,157 | 2,579 | 2.23 |
| Vegfb | 1,210 | 2,520 | 2.08 |
| Vegfa | 1,200 | 2,368 | 1.97 |
| Angptl6 | 416 | 950 | 2.28 |
| Mmp19 | 480 | 684 | 1.43 |
| Mmp19 | 351 | 503 | 1.43 |
| Arts1 | 137 | 262 | 1.92 |
| Fgf1 | 112 | 155 | 1.37 |
| Arts1 | 60 | 93 | 1.54 |
GO, Gene Ontology; Acdc, adiponectin; Angpt14 and -6, angiopoietin-like protein 4 and -6; Mmp19, matrix metalloproteinase 19; Arts1, endoplasmic reticulum aminopeptidase 1; Fgf1, fibroblast growth factor 1. Probes contained in the MOE430-2 Affymetrix gene chip annotated with GO terms “angiogenesis” and “extracellular space” were used to filter databases derived from confluent 3T3-L1 preadipocyte cultures (day 0) and from cells after 6 days of differentiation (day 6). Probe signal and fold change are derived from 3 independent replicates, as described in materials and methods.
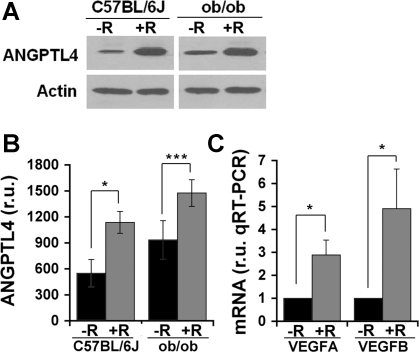
To determine the possible role of these factors, we analyzed their protein and mRNA levels in adipocytes from RSG-treated animals. Adipocytes were isolated to ensure that observed changes were indeed produced in adipose cells and not in associated stroma. Consistent with previous studies using pioglitazone (19, 53), ANGPTL4 mRNA levels were increased by RSG, although the effect was small (not shown). However, protein levels were found to be significantly increased in adipocytes isolated from RSG-treated mice (Fig. 3, A and B). In addition, a significant increase in VEGFA and VEGFB expression was observed (Fig. 3C), consistent with results seen in rat whole adipose tissue (45).
Fig. 3.
Expression of angiopoietin-like protein 4 (ANGPTL4) and VEGFA in isolated adipocytes. A: representative blots showing ANGPTL4 protein levels in isolated adipocytes from C57BL/6J and ob/ob mice without (−R) or with (+R) RSG feeding. B: quantification of ANGPTL4 levels from Western blots by scanning densitometry. C: real-time quantitative PCR analysis of VEGFA and VEGFB mRNA levels in isolated adipocytes from ob/ob mice treated in vivo without (−R) or with (+R) RSG. Averages from 3–5 independent experiments are shown. Statistical significance was calculated using 1-tailed paired Student t-tests. *P < 0.05; ***P < 0.001.
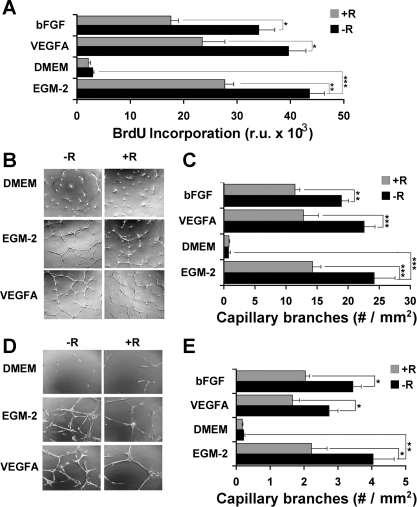
The finding that VEGFA, VEGFB, and ANGPTL4 are increased in adipocytes in response to systemic RSG treatment prompted us to analyze their capacity to stimulate endothelial cell growth and differentiation and to counteract the inhibitory effects of RSG on endothelial cell growth in vitro. For this purpose, the growth and ability to form capillary branches of HUVEC was analyzed (Fig. 4). The highest rate of proliferation of HUVEC was seen in EGM-2, the proprietary medium designed to promote proliferation and growth of endothelial cells, and no proliferation was observed in DMEM (Fig. 4A). Supplementation of DMEM with VEGFA [or basic fibroblast growth factor (bFGF)] increased proliferation to a level similar to that seen in EGM-2. Consistent with prior studies, RSG had a significant inhibitory effect on endothelial cell proliferation in the presence of these growth factors (41, 44). The formation of capillary tubes by HUVEC (Fig. 4, B and C), as well as by purified microvascular endothelial cells derived from epididymal fat pads (Fig. 4, D and E), was also analyzed. Both cell types formed capillary tubes when cultured in EGM-2 or in DMEM supplemented with purified VEGFA or bFGF. However, in all cases, addition of RSG to the medium significantly impaired tube formation.
Fig. 4.
Analysis of the effect of RSG on endothelial cell proliferation and tube formation. A: cell proliferation in response to VEGFA or basic fibroblast growth factor (bFGF) in the absence (−R) or presence (+R) of 1 μM RSG was measured by BrdU incorporation, as described in materials and methods. Shown are means ± SE of 3 independent experiments performed in triplicate (total n = 9). Representative photomicrographs showing formation of capillary tubes by human umbilical endothelial cells (HUVEC; B) or microvascular endothelial cells (D) incubated for 24 h under the conditions indicated. Original magnification for HUVEC is ×40 and for microvascular endothelial cells is ×100. C and E: quantitative analysis of capillary tube formation by HUVEC (C) or microvascular endothelial cells (E). In each experiment, 4 fields of each of 3 wells/condition were analyzed, and the experiment was repeated 3 times (total, n = 36). Statistical analysis was performed by ANOVA followed by Tukey's multiple comparison test. *P < 0.05; **P < 0.01; ***P < 0.001. EGM-2, endothelial cell basal medium-2 supplemented with endothelial growth factor. RU, relative units.
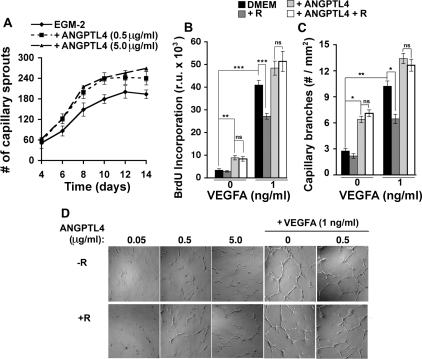
The role of ANGPTL4 on endothelial cells appears to be highly dependent on the methodology used, displaying proangiogenic activity when secreted from Chinese hamster ovary cells, or produced as a recombinant histidine-tagged protein (23, 31) and antiangiogenic activity when used as a partially purified Flag-tagged construct (25). Under our conditions, and consistent with prior findings (23, 31), recombinant ANGPTL4 produced by cell-free translation (Abnova) significantly enhanced capillary sprout formation from aorta rings cultured in proangiogenic medium (Fig. 5A) and, in the absence of any additional proangiogenic factor, stimulated proliferation (Fig. 5B) and induced capillary branch formation (Fig. 5, C and D) of HUVEC. Although highly significant, the effects of ANGPTL4 were smaller than that of VEGFA but, importantly, were qualitatively different in that they were not inhibited by RSG (Fig. 5, B and C). When VEGFA and ANGPTL4 were used in combination, an additive effect on proliferation was observed in the absence of RSG (Fig. 5B). However, in the presence of RSG the effect was more than additive (Fig. 5B), suggesting that ANGPTL4 rendered cells refractory to the growth inhibitory effects of RSG. Similar results were seen when capillary branch formation was analyzed (Fig. 5, C and D). These results suggest that the simultaneous secretion of VEGFA and ANGPTL4 by adipocytes in response to RSG could result in robust stimulation of angiogenesis by counteracting the inhibitory effects of RSG on endothelial cells in the vicinity of adipocytes.
Fig. 5.
Effect of recombinant ANGPTL4 on capillary sprout and tube formation. A: effect of ANGPTL4 on capillary sprout formation from aorta explants. Approximately 7 rings were analyzed per experiment per experimental condition, and the experiment was repeated 3 times. One-way ANOVA followed by Tukey's multiple comparison test yielded P < 0.05 for EGM-2 vs. EGM-2 + 0.5 μg/ml ANGPTL4 and for EGM-2 vs. EGM-2 + 5 μg/ml ANGPTL4. VEGFA and ANGPTL4 induced HUVEC proliferation (B) and capillary tube formation (C) in the absence or presence (+R) of 1 μM RSG. B: shown are the mean ± SE of 3 independent experiments performed in triplicate (total, n = 9). C: 4 fields of each of 3 wells/condition were analyzed, and the experiment was repeated 3 times (total, n = 36). Comparison between experimental groups was performed by Tukey's multiple comparison tests. *P < 0.05; **P < 0.01; ns, Not significant. D: representative photomicrographs of capillary tube formation by HUVEC on Matrigel after 24-h incubation in the presence of the factors indicated. Original magnification is ×40.
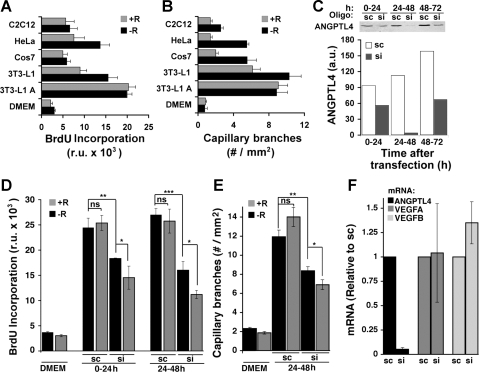
To further test this hypothesis, we examined whether ANGPTL4 is proangiogenic in the context of its secretion by adipocytes. As shown previously by others (15), DMEM conditioned for 24 h by incubation with confluent cultures of 3T3-L1 adipocytes stimulated HUVEC proliferation (Fig. 6A) and formation of capillary branches (Fig. 6B). Interestingly, in contrast to the effects of EGM-2 or purified VEGFA or bFGF, the effects of adipocyte-conditioned DMEM were not inhibited by RSG (Fig. 6, A and B). Supplementation of DMEM through conditioning with other cell types, including nondifferentiated 3T3-L1 preadipocytes, also increased proliferation and capillary branch formation, but generally less than that seen after conditioning with 3T3-L1 adipocytes and in a manner sensitive to inhibition by RSG (Fig. 6, A and B). To determine whether ANGPTL4 contributes to the proangiogenic effects of 3T3-L1 adipocyte-conditioned medium, we silenced its expression in 3T3-L1 adipocytes using siRNA. On day 5 of differentiation, cells were electroporated with either scrambled or ANGPTL4-directed siRNA oligonucleotides. Medium was collected and exchanged on days 6 (0–24 h posttransfection), 7 (24–48 h posttransfection), and 8 (48–72 h posttransfection), and the amount of ANGPTL4 that accumulated in the medium during each 24-h interval was quantified by Western blotting (Fig. 6C). ANGPTL4 accumulated in the medium of cells electroporated with scrambled oligonucleotides at all 24-h intervals. Accumulated levels were slightly higher on day 7 compared with day 6 (0–24 h posttransfection) and highest at day 8 (48–72 h posttransfection). However, much less ANGPTL4 accumulated in medium of cells transfected with ANGPTL4-directed siRNA at every interval studied (Fig. 6C).
Fig. 6.
Angiogenic effect of 3T3-L1 adipocyte-conditioned medium and secreted ANGPTL4. A and B: confluent cultures of differentiated (3T3-L1 A) or nondifferentiated 3T3-L1 cells as well as other cell types indicated were used to condition DMEM for 24 h. Collected medium was then placed on HUVEC cells, and proliferation (A) and capillary tube formation (B) were assayed in the absence (−R) or presence (+R) of 1 μM RSG, as described in Fig. 4. C: 3T3-L1 adipocytes were transfected with scrambled (sc) or ANGPTL4-directed (si) siRNA oligonucleotides on day 5 of differentiation. Medium was collected and exchanged every 24 h and analyzed by Western blotting with anti-ANGPLT4 antibody. Conditioned medium was analyzed for its ability to stimulate growth (D) or induce capillary branch formation (E) by HUVEC in the absence (−R) or presence (+R) of 1 μM RSG. F: mRNA isolated from 3T3-L1 adipocytes 48 h following transfection with sc or si siRNA oligonucleotides was analyzed by RT-PCR for the expression of the factors indicated. Bars represent the average of 3 experiments preformed in duplicate. Comparison between experimental groups was performed by 1-way ANOVA followed by Tukey's multiple comparison tests. *P < 0.05; **P < 0.01; ***P < 0.001.
Medium from cells electroporated with scrambled or ANGPTL4-directed siRNA was then tested for its ability to support growth (Fig. 6D) and capillary branch formation (Fig. 6E) by HUVEC in the absence or presence of RSG. As seen with nontransfected cells (Fig. 6, A and B), medium collected from cells transfected with scrambled oligonucleotides supported HUVEC cell growth and capillary branch formation, and this effect was not suppressed by RSG (Fig. 6, D and E). Medium collected from cells transfected with ANGPTL4-directed siRNA was significantly less effective at supporting cell proliferation and capillary branch formation, and importantly, cells cultured in ANGPTL4-deficient medium were significantly sensitive to the inhibitory effects of RSG (Fig. 6, D and E).
The possibility that the effects of silencing ANGPTL4 might result from a parallel decreased secretion of other proangiogenic factors was considered. However, ANGPTL4 silencing had no detectable effect on VEGFA and VEGFB expression (Fig. 6F). These results, together with the observation that ANGPTL4 alone stimulates endothelial cell growth and differentiation (Fig. 5), support a model where ANGPTL4 is at least in part directly responsible for the proangiogenic properties of adipocyte-conditioned medium and that it exerts a protective effect against the antiangiogenic actions of RSG.
DISCUSSION
In this study we find that, despite being a strong inhibitor of endothelial cell growth and differentiation, RSG exerts an adipose tissue-specific proangiogenic effect that increases adipose tissue microvasculature. The ability to stimulate adipose tissue angiogenesis is likely to be necessary for the therapeutic effect of TZDs, because adipose tissue expansion requires angiogenesis, and the generation of new active adipocytes capable of storing and catabolizing free fatty acids may be essential for the insulin-sensitizing effects of PPARγ activators (3). Neovascularization could also lead to changes in blood flow, which have been reported to be decreased in obese and type 2 diabetic patients and enhanced by RSG and to positively correlate with glucose uptake into adipose tissue (47).
The finding that the effects of RSG on angiogenesis are tissue dependent and positively influenced by the presence of adipocytes has several important ramifications. One of these is that the effect of PPARγ ligands on the risk of cancer may vary markedly with the specific tissues involved. For example, the use of TZDs has been associated with a reduced risk of lung cancer (20) but an increased risk of cancer among women (42). It is possible that the effects of TZDs to suppress, for example, mammary epithelial tumor cell growth may be superseded by the proangiogenic effects of TZDs in the mammary gland, where adipose tissue is abundant. Also, in diabetes, the angiogenic potential of different vascular beds is altered in different ways. For example, the increased incidence of morbidity and mortality from atherosclerosis in type 2 diabetes is associated with an impaired ability to form collateral vessels (1, 49). In this context, the antiangiogenic effects of RSG may supersede proangiogenic effects, resulting in enhanced morbidity. However, excessive neovascularization is a hallmark of diabetic retinopathy (28), and diabetic nephropathy can be considered a disorder of excessive angiogenesis (38). In this context, antiangiogenic effects of RSG may mitigate the development of complications.
The considerations discussed above suggest that the analysis of the effects of TZD treatment on each specific vascular system in diabetes is required to better define the impact of TZD therapy on the diverse complications of this disease. The findings presented here suggest that these responses can be measured and further studied in ex vivo systems. Ex vivo angiogenic potential of explants from adipose tissue reflects observed changes in adipose tissue in vivo in response to both obesity and treatment with RSG. In both cases, expansion of adipose tissue correlates with increased angiogenic potential ex vivo. Further development and characterization of this assay, and its adaptation to other tissues, will result in a better understanding of the cellular and molecular mechanisms that underlie tissue-specific angiogenic potential, including the role of recruited angiogenic precursor cells, the potential role of adipose tissue infiltrating macrophages (39), and the stable secretion of pro- and antiangiogenic factors.
Experiments in vitro and in vivo shown here suggest that the adipose tissue-specific proangiogenic effect of RSG may result from the stimulated production by adipocytes of VEGFA and ANGPTL4. ANGPTL4 secreted by adipocytes has a proangiogenic effect that differs qualitatively from the effects of VEGFA in that it renders endothelial cells refractory to the growth-inhibitory effects of PPARγ activation. This unique interplay between VEGFA and ANGPTL4 may mediate the increase in vascularization necessary for adipose tissue expansion under conditions of enhanced adipocyte differentiation induced by PPARγ activators.
ANGPTL4 has previously been identified as being expressed predominantly in adipose tissue (27) in a manner sensitive to nutritional changes and adipose tissue mass (53). ANGPTL4 has also been studied extensively due to its unusual effect to directly inhibit lipoprotein lipase, an effect that leads to hypertriglyceridemia upon enforced overexpression of ANGPTL4 in animals (30, 54, 55), and to decreased levels of triglycerides in animals in which ANGPTL4 is knocked out or neutralized (14, 30). ANGPTL4 may be an important factor for the stimulation of endothelial cell growth necessary for adipose tissue expansion. However, the multiple effects of ANGPTL4 make the genetic testing of this hypothesis contingent upon identification of the receptor for ANGPTL4 and its disruption in a tissue-specific manner. The results shown here provide the impetus for this line of research.
GRANTS
This work was funded by a Pilot Project Program Award from the University of Massachusetts Medical School Clinical and Translational Sciences Initiative. Informatics and Morphology core services were supported by University of Massachusetts DERC Grant DK-32520.
Acknowledgments
We thank Jyotsna Vinayak for dedicated assistance during the progress of this work and members of the Corvera laboratory for valuable suggestions on the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinc H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation 99: 2239–2242, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Akazawa S, Sun F, Ito M, Kawasaki E, Eguchi K. Efficacy of troglitazone on body fat distribution in type 2 diabetes. Diabetes Care 23: 1067–1071, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med 53: 409–435, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bogacka I, Xie H, Bray GA, Smith SR. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes 54: 1392–1399, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bouloumie A, Lolmede K, Sengenes C, Galitzky J, Lafontan M. Angiogenesis in adipose tissue. Ann Endocrinol (Paris) 63: 91–95, 2002. [PubMed] [Google Scholar]
- 6.Bouskila M, Pajvani UB, Scherer PE. Adiponectin: a relevant player in PPARgamma-agonist-mediated improvements in hepatic insulin sensitivity? Int J Obes (Lond) 29, Suppl 1: S17–S23, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Brakenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P, Cao Y. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res 94: 1579–1588, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Brodsky SV, Gealekman O, Chen J, Zhang F, Togashi N, Crabtree M, Gross SS, Nasjletti A, Goligorsky MS. Prevention and reversal of premature endothelial cell senescence and vasculopathy in obesity-induced diabetes by ebselen. Circ Res 94: 377–384, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Carey DG, Cowin GJ, Galloway GJ, Jones NP, Richards JC, Biswas N, Doddrell DM. Effect of rosiglitazone on insulin sensitivity and body composition in type 2 diabetic patients [corrected]. Obes Res 10: 1008–1015, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, Yoon YS, Yoon G, Choi KM, Ko YG. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia 49: 784–791, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 46: 2347–2355, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Crandall DL, Hausman GJ, Kral JG. A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation 4: 211–232, 1997. [DOI] [PubMed] [Google Scholar]
- 13.de Souza CJ, Eckhardt M, Gagen K, Dong M, Chen W, Laurent D, Burkey BF. Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes 50: 1863–1871, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Desai U, Lee EC, Chung K, Gao C, Gay J, Key B, Hansen G, Machajewski D, Platt KA, Sands AT, Schneider M, Van Sligtenhorst I, Suwanichkul A, Vogel P, Wilganowski N, Wingert J, Zambrowicz BP, Landes G, Powell DR. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc Natl Acad Sci USA 104: 11766–11771, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobson DE, Kambe A, Block E, Dion T, Lu H, Castellot JJ Jr, Spiegelman BM. 1-Butyryl-glycerol: a novel angiogenesis factor secreted by differentiating adipocytes. Cell 61: 223–230, 1990. [DOI] [PubMed] [Google Scholar]
- 16.Fenner MH, Elstner E. Peroxisome proliferator-activated receptor-gamma ligands for the treatment of breast cancer. Expert Opin Investig Drugs 14: 557–568, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Frye CA, Patrick CW Jr. Isolation and culture of rat microvascular endothelial cells. In Vitro Cell Dev Biol Anim 38: 208–212, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, Chatterjee K, Garkavtsev I, Jain RK. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res 93: e88–e97, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge H, Cha JY, Gopal H, Harp C, Yu X, Repa JJ, Li C. Differential regulation and properties of angiopoietin-like proteins 3 and 4. J Lipid Res 46: 1484–1490, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, Kim PJ, Owens RJ, Lang NP. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol 25: 1476–1481, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Gray SL, Vidal-Puig AJ. Adipose tissue expandability in the maintenance of metabolic homeostasis. Nutr Rev 65: S7–S12, 2007. [DOI] [PubMed] [Google Scholar]
- 22.He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA 100: 15712–15717, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermann LM, Pinkerton M, Jennings K, Yang L, Grom A, Sowders D, Kersten S, Witte DP, Hirsch R, Thornton S. Angiopoietin-like-4 is a potential angiogenic mediator in arthritis. Clin Immunol 115: 93–101, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Inoue K, Kawahito Y, Tsubouchi Y, Yamada R, Kohno M, Hosokawa Y, Katoh D, Bishop-Bailey D, Hla T, Sano H. Expression of peroxisome proliferator-activated receptor (PPAR)-gamma in human lung cancer. Anticancer Res 21: 2471–2476, 2001. [PubMed] [Google Scholar]
- 25.Ito Y, Oike Y, Yasunaga K, Hamada K, Miyata K, Matsumoto S, Sugano S, Tanihara H, Masuho Y, Suda T. Inhibition of angiogenesis and vascular leakiness by angiopoietin-related protein 4. Cancer Res 63: 6651–6657, 2003. [PubMed] [Google Scholar]
- 26.Jiang ZY, Zhou QL, Coleman KA, Chouinard M, Boese Q, Czech MP. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci USA 100: 7569–7574, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, Gonzalez FJ, Desvergne B, Wahli W. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem 275: 28488–28493, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Khan ZA, Chakrabarti S. Growth factors in proliferative diabetic retinopathy. Exp Diabesity Res 4: 287–301, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kintscher U, Law RE. PPARγ-mediated insulin sensitization: the importance of fat versus muscle. Am J Physiol Endocrinol Metab 288: E287–E291, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Koster A, Chao YB, Mosior M, Ford A, Gonzalez-DeWhitt PA, Hale JE, Li D, Qiu Y, Fraser CC, Yang DD, Heuer JG, Jaskunas SR, Eacho P. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology 146: 4943–4950, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Le Jan S, Amy C, Cazes A, Monnot C, Lamande N, Favier J, Philippe J, Sibony M, Gasc JM, Corvol P, Germain S. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am J Pathol 162: 1521–1528, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell 123: 993–999, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Mandard S, Zandbergen F, Tan NS, Escher P, Patsouris D, Koenig W, Kleemann R, Bakker A, Veenman F, Wahli W, Muller M, Kersten S. The direct peroxisome proliferator-activated receptor target fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J Biol Chem 279: 34411–34420, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Murata T, He S, Hangai M, Ishibashi T, Xi XP, Kim S, Hsueh WA, Ryan SJ, Law RE, Hinton DR. Peroxisome proliferator-activated receptor-gamma ligands inhibit choroidal neovascularization. Invest Ophthalmol Vis Sci 41: 2309–2317, 2000. [PubMed] [Google Scholar]
- 35.Nakamura T, Funahashi T, Yamashita S, Nishida M, Nishida Y, Takahashi M, Hotta K, Kuriyama H, Kihara S, Ohuchi N, Nishimura T, Kishino BI, Ishikawa K, Kawamoto T, Tokunaga K, Nakagawa C, Mineo I, Watanabe F, Tarui S, Matsuzawa Y. Thiazolidinedione derivative improves fat distribution and multiple risk factors in subjects with visceral fat accumulation—double-blind placebo-controlled trial. Diabetes Res Clin Pract 54: 181–190, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Neels JG, Thinnes T, Loskutoff DJ. Angiogenesis in an in vivo model of adipose tissue development. FASEB J 18: 983–985, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes 56: 1517–1526, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Nyengaard JR, Rasch R. The impact of experimental diabetes mellitus in rats on glomerular capillary number and sizes. Diabetologia 36: 189–194, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Eagle AR, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447: 1116–1120, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, Umesono K, Akanuma Y, Fujiwara T, Horikoshi H, Yazaki Y, Kadowaki T. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest 101: 1354–1361, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panigrahy D, Singer S, Shen LQ, Butterfield CE, Freedman DA, Chen EJ, Moses MA, Kilroy S, Duensing S, Fletcher C, Fletcher JA, Hlatky L, Hahnfeldt P, Folkman J, Kaipainen A. PPARgamma ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesis. J Clin Invest 110: 923–932, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramos-Nino ME, MacLean CD, Littenberg B. Association between cancer prevalence and use of thiazolidinediones: results from the Vermont Diabetes Information System. BMC Med 5: 17, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci USA 99: 10730–10735, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheu WH, Ou HC, Chou FP, Lin TM, Yang CH. Rosiglitazone inhibits endothelial proliferation and angiogenesis. Life Sci 78: 1520–1528, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Sotiropoulos KB, Clermont A, Yasuda Y, Rask-Madsen C, Mastumoto M, Takahashi J, Della Vecchia K, Kondo T, Aiello LP, King GL. Adipose-specific effect of rosiglitazone on vascular permeability and protein kinase C activation: novel mechanism for PPARgamma agonist's effects on edema and weight gain. FASEB J 20: 1203–1205, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Spiegelman BM PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes 47: 507–514, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Viljanen AP, Virtanen KA, Jarvisalo MJ, Hallsten K, Parkkola R, Ronnemaa T, Lonnqvist F, Iozzo P, Ferrannini E, Nuutila P. Rosiglitazone treatment increases subcutaneous adipose tissue glucose uptake in parallel with perfusion in patients with type 2 diabetes: a double-blind, randomized study with metformin. J Clin Endocrinol Metab 90: 6523–6528, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology 146: 4545–4554, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Waltenberger J Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovasc Res 49: 554–560, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Wilson-Fritch L, Burkart A, Bell G, Mendelson K, Leszyk J, Nicoloro S, Czech M, Corvera S. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol Cell Biol 23: 1085–1094, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest 114: 1281–1289, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xin X, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem 274: 9116–9121, 1999. [DOI] [PubMed] [Google Scholar]
- 53.Yoon JC, Chickering TW, Rosen ED, Dussault B, Qin Y, Soukas A, Friedman JM, Holmes WE, Spiegelman BM. Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol Cell Biol 20: 5343–5349, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshida K, Shimizugawa T, Ono M, Furukawa H. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J Lipid Res 43: 1770–1772, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Yu X, Burgess SC, Ge H, Wong KK, Nassem RH, Garry DJ, Sherry AD, Malloy CR, Berger JP, Li C. Inhibition of cardiac lipoprotein utilization by transgenic overexpression of Angptl4 in the heart. Proc Natl Acad Sci USA 102: 1767–1772, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang J, Fu M, Cui T, Xiong C, Xu K, Zhong W, Xiao Y, Floyd D, Liang J, Li E, Song Q, Chen YE. Selective disruption of PPARgamma 2 impairs the development of adipose tissue and insulin sensitivity. Proc Natl Acad Sci USA 101: 10703–10708, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]