Abstract
Fluctuations in circulating estrogen and progesterone levels associated with the estrous cycle alter circadian rhythms of physiology and behavior in female rodents. Endogenously applied estrogen shortens the period of the locomotor activity rhythm in rodents. We recently found that estrogen implants affect Period (Per) gene expression in the suprachiasmatic nucleus (SCN; central clock) and uterus of rats in vivo. To explore whether estrogen directly influences the circadian clock in the SCN and/or tissues of the reproductive system, we examined the effects of 17β-estradiol (E2) on PER2::LUCIFERASE (PER2::LUC) expression in tissue explant cultures from ovariectomized PER2::LUC knockin mice. E2 applied to explanted cultures shortened the period of rhythmic PER2::LUC expression in the uterus but did not change the period of PER2::LUC expression in the SCN. Raloxifene, a selective estrogen receptor modulator and known E2 antagonist in uterine tissues, attenuated the effect of E2 on the period of the PER2::LUC rhythm in the uterus. These data indicate that estrogen directly affects the timing of the molecular clock in the uterus via an estrogen receptor-mediated response.
Keywords: ovarian steroid hormone, period, PER2::LUCIFERASE, raloxifene, suprachiasmatic nucleus
in mammals, the master clock resides in the suprachiasmatic nucleus (SCN) of the hypothalamus and synchronizes peripheral oscillators to ensure temporally coordinated physiology (32). Circadian rhythms are generated within each cell by transcriptional and posttranscriptional feedback loops involving a small family of clock genes and their associated proteins. The molecular clock consists of interacting transcription factors, including both the enhancers CLOCK and BMAL and repressors including the Period proteins (PER1, PER2, and PER3) and Cryptochromes (CRY1 and CRY2) (12). In addition to these core proteins, an additional loop consisting of the repressor Rev-Erbα and the enhancer RORα plays a role in the timing of the molecular clock (12). It has become evident that the same or very similar molecular mechanisms function in most cells and tissues of the body (39) and that these peripheral clocks can continue to oscillate in the absence of the SCN (41).
Circadian clock genes are expressed in female reproductive organs, including the ovary (6, 17), oviduct (18), and uterus (15, 26), and their expression appears to be rhythmic. A recent study in quail revealed a 24-h rhythm of steroidogenic acute regulatory protein in preovulatory follicles, suggesting direct clock-mediated regulation of steroid biosynthesis in ovarian cells (28). Although the physiological function of clock genes in female reproductive tissues remains unclear, some reports demonstrate reproductive dysfunctions in mice with clock gene mutations (5, 21). Female Clock mutant mice, which carry a 51-amino acid deletion in the transcriptional activation domain of the CLOCK protein, fail to show either regular estrous cycles or circadian rhythms of clock gene expression in the uterus (5) and do not produce an LH surge on the day of proestrus (21).
Estrogen modifies circadian rhythms of wheel running by female hamsters and rats (1, 22, 23, 36). High levels of estrogen induce an early onset of locomotor activity of female rats and hamsters (i.e., a more positive phase-angle difference with the light-to-dark transition) on the evening of proestrus and increase the amplitude and bout length of locomotor activity (1, 22, 23, 37). This change in the locomotor activity rhythm throughout the estrous cycle may be caused by a direct effect of estrogen on the circadian clock, since the period of the locomotor activity rhythm shortens when estrogen is implanted in ovariectomized (OVX) rats (1) and hamsters (23, 36).
Although it is clear that ovarian steroid hormones affect circadian rhythms of locomotor activity and reproductive function, there is currently no evidence to suggest that the steroids “directly” regulate the timing of the molecular clock in the SCN and/or non-SCN tissues. Recently, it has been reported that estrogen implants modulate the expression of clock genes in the SCN, reproductive, and nonreproductive tissues in vivo (26, 27). We tested the hypothesis that estrogen has direct effects on the molecular clock in estrogen-responsive tissues. Here we demonstrate that 17β-estradiol (E2) shortens the period of PER2::LUCIFERASE (PER2::LUC) expression in uterine, but not SCN, tissue explants from OVX PER2::LUC knockin mice. In addition, we demonstrate that this effect can be attenuated by the addition of raloxifene (RLX), a well-known selective estrogen receptor (ER) modulator (SERM) with varying antiestrogenic effects in mammary, bone, and uterine tissues (for review, see Refs. 19 and 25). These results demonstrate that estrogen can directly regulate clock gene expression in the uterus and suggest that fluctuations of circulating steroid hormones during the estrous cycle may directly alter the timing of the molecular clock in non-SCN tissues.
MATERIALS AND METHODS
Analysis of rhythmic PER2::LUC expression in SCN and uterine tissue explants.
Eight-week-old female PER2::LUC knockin mice [originally from the colony of Dr. J. Takahashi (Northwestern University, Evanston, IL) (41)] from our breeding colony were used for all experiments. Animals were OVX under isoflurane anesthesia 2 wk prior to culture experiments and maintained under controlled environmental conditions (temperature 22 ± 2°C, lights on at 0500-1700), with food and water available ad libitum. All procedures and standards of care were approved by the University of Virginia Institutional Animal Care and Use Committee and were conducted according to the National Institutes of Health guidelines for the use of experimental animals.
Culture procedures are identical to those described by Yamazaki and Takahashi (40). Briefly, 1–2 h before lights off, mice were euthanized by deep CO2 anesthesia and rapidly decapitated. To prepare SCN cultures, brain tissue was removed and placed in chilled Hanks' buffered salt solution (HBSS). The brain was sliced in the coronal plane on a vibratome at a thickness of 300 μm. The bilateral SCN and a minimum of surrounding tissue were isolated from the slice using scalpels. The uterine horns were removed, placed in chilled HBSS, and isolated from the surrounding adipose tissue under a dissecting microscope. The uteri were cut in round slices, with 4–5 cross-sections taken from each mouse. The sections of SCN and uterus were placed at the liquid interface on membranes (Millicell-CM, PICM030-50; Millipore, Billerica, MA) in 35-mm dishes (BD, Franklin Lakes, NJ) containing 1.2 ml of recording medium [serum-free, no sodium bicarbonate, no phenol red, Dulbecco's modified Eagle's medium (D-2902; Sigma-Aldrich, St. Louis, MO)] supplemented with 0.35 g/l sodium bicarbonate, 10 mM HEPES (pH 7.2), B27 (2%, 17504-010; Invitrogen, Rockville, MD), and 0.1 mM luciferin (beetle luciferin, potassium salt; Promega) and antibiotics (25 U/ml penicillin, 25 mg/ml streptomycin; Invitrogen).
β-Cyclodextrin (βCD)-caged E2 or progesterone (P4) was diluted in sterile millipore double-distilled water with due consideration for actual steroid amounts and added directly to the recording medium at the onset of the culture. βCD-coated steroids were chosen for these experiments due to their ability to dissolve readily in cell culture medium. RLX was dissolved in dimethyl sulfoxide (DMSO) and also added directly to the recording medium. For controls, cultures were treated with DMSO vehicle or βCD without steroid or a combination of DMSO and βCD. We have confirmed previously that βCD treatment had no effect on clock gene expression in vitro (33). All steroid compounds were acquired from Sigma-Aldrich. Following the addition of steroid or vehicle, the dishes were sealed with a cover glass using vacuum grease and placed under photomultiplier tubes (H6240; Hamamatsu, Bridgewater, NJ) or into a LumiCycle (Actimetrics, Wilmette, IL) inside light-tight 36.5°C environmental chambers. Bioluminescence was counted each minute from every dish for ≥6 days.
Data analysis and statistics.
Bioluminescence records were normalized by subtraction of the 24-h running average from the raw data and then smoothed with a 2-h running average (4). Smoothed and detrended data were imported into the LumiCycle analysis software (Actimetrics), and the period of the PER2::LUC expression rhythms was determined with a sine-fit curve (13, 14). A minimum of 6 days of bioluminescent data was included in the analysis. Statistical significance between groups was determined by one-way ANOVA followed by Tukey's post hoc statistical test. All results are presented as means ± SE and were considered significant at P < 0.05.
RESULTS
Effect of estrogen on the period of PER2::LUC expression rhythm in the SCN.
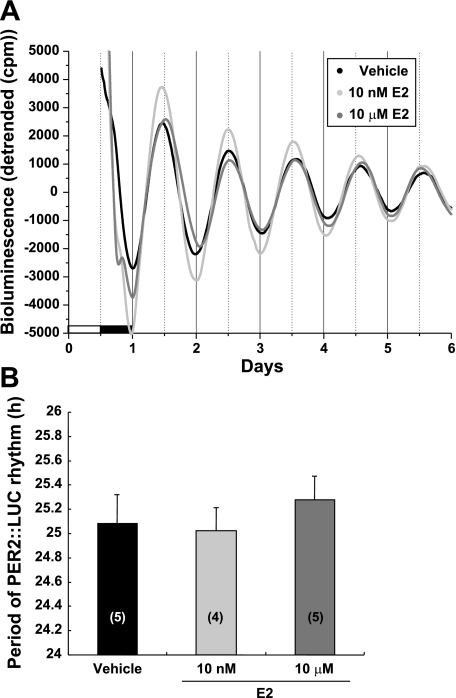
We hypothesized that E2 can modulate rhythms of locomotor activity through direct actions on the molecular clock in SCN neurons. We investigated the direct effects of E2 on the period of PER2::LUC rhythm in cultured SCN explanted from OVX PER2::LUC mice. Our previous in vitro data indicated that E2, at several concentrations between 200 nM and 500 μM, did not affect the period of the Per1-luciferase (Per1-luc) expression rhythm in the SCN (33). In the current experiment, we treated SCN cultures with a single low (10 nM) and high (10 μM) concentration of E2. Cultured SCN explants demonstrated a robust rhythm of PER2::LUC expression in medium containing vehicle with a period of 25.08 ± 0.24 h (Fig. 1A). Treatment with E2 at 10 nM or 10 μM did not significantly alter the period of PER2::LUC expression in the SCN [10 nM period = 25.03 ± 0.19 h and 10 μM period = 25.28 ± 0.19 h; 1-way ANOVA, F(2,11) = 0.402; Fig. 1B]. These results are consistent with our previous study on Per1-luc expression in isolated SCN tissue explants from OVX rats (33) and do not support the hypothesis of a direct action of E2 on the molecular oscillator in SCN neurons.
Fig. 1.
Effects of estrogen on the period of PER2::LUCIFERASE (PER2::LUC) expression in the suprachiasmatic nucleus (SCN) of ovariectomized (OVX) mice. A: representative bioluminescence traces showing the effects of 17β-estradiol (E2) on circadian rhythms of PER2::LUC expression in cultured SCN explants from OVX mice. The SCN was treated with vehicle [100 μM β-cyclodextrin (βCD)], 10 nM E2, or 10 μM E2. B: mean period values (±SE) for each group. Numbers in parentheses indicate the number of animals in each treatment group.
Estrogen shortens the period of PER2::LUC expression rhythm in the uterus.
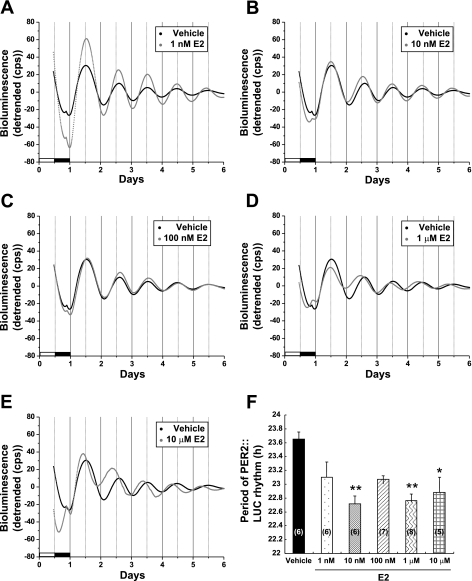
To determine whether ovarian steroid hormones can modulate the molecular clock in the uterus, we examined the effects of E2 and P4 on the period of PER2::LUC expression in uterine explants from OVX PER2::LUC mice. We chose to examine the response to steroid hormones in the uterus because 1) the uterus exhibits robust circadian rhythms of PER2::LUC expression similar to the central circadian clock in the SCN (see Figs. 1 and 2), 2) our previous results suggest that E2 has marked effects on uterine Per2 gene expression in vivo (26), and 3) the uterus is one of the major target organs for E2 and P4 (3, 24). Figure 2 shows the effects of E2 on the expression of PER2::LUC in the uterus. We treated uterine explants with 1 nM, 10 nM, 100 nM, 1 μM, and 10 μM E2 and calculated the period of the PER2::LUC expression rhythm. We observed a robust rhythm of PER2::LUC expression in vehicle-treated uterine cultures with a mean period of 23.65 ± 0.11 h (Fig. 2, A and F). Post hoc statistical tests revealed that 1 nM E2 did not significantly affect the period (period = 23.10 ± 0.22 h); however, 10 nM (period = 22.71 ± 0.12 h, P < 0.05), 1 μM (period = 22.76 ± 0.10, P < 0.01), and 10 μM E2 (period = 22.88 ± 0.32 h, P < 0.05) significantly shortened the period of the PER2::LUC expression rhythm in the uterus when compared with vehicle controls [1-way ANOVA, F(5,32) = 6.05, P < 0.05; Fig. 2F]. Surprisingly, we did not see a statistically significant effect of 100 nM E2 treatment, although the period (23.07 ± 0.05 h) was shorter than that of the vehicle controls by ∼0.5 h (Fig. 2F).
Fig. 2.
Estrogen shortens the period of PER2::LUC expression in the uterus of OVX mice. A–E: representative bioluminescence traces showing the effects of E2 on circadian rhythms of PER2::LUC expression in cultured uterine explants from OVX mice. The uterus was treated with vehicle (100 μM β-cyclodextrin) or 1 nM (A), 10 nM (B), 100 nM (C), 1 μM (D), or 10 μM E2 (E). The black trace in each graph is the same vehicle control trace. F: mean period values (±SE) for each group. Numbers in parentheses indicate the number of animals in each group. **P < 0.01 and *P < 0.05 when compared with vehicle group (Tukey's test).
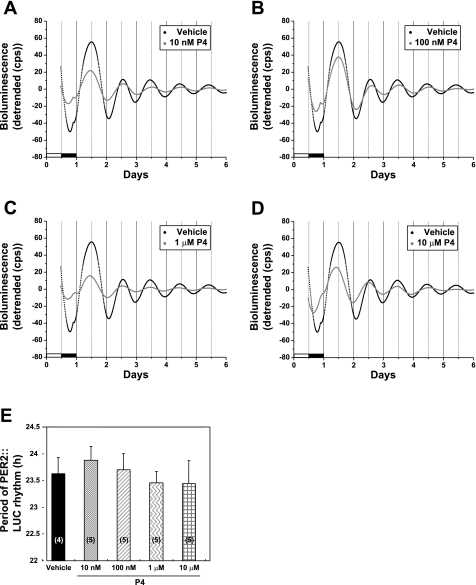
Our previous study indicated that a physiological concentration of P4 fails to affect the period of Per1-luc expression in SCN tissue explants from OVX Per1-luc rats (33). This result suggests that there are few P4 receptors (PR) within the SCN. We chose to examine the effects of P4 on the uterus, a tissue known to express PR, to determine whether the steroid would affect the rhythm of PER2::LUC expression in this tissue. In contrast to the effects of E2, treatment with P4 alone did not alter the period of PER2::LUC expression in the uterus, regardless of the concentration of steroid applied [1-way ANOVA, F(4,19) = 0.36; Fig. 3, A–E].
Fig. 3.
Effects of progesterone (P4) on the period of PER2::LUC expression in the uterus of OVX mice. A–D: representative bioluminescence traces showing the effects of P4 on circadian rhythms of PER2::LUC expression in cultured uterine explants from OVX mice. The uterus was treated with vehicle (100 μM βCD) or 10 nM (A), 100 nM (B), 1 μM (C), or 10 μM P4 (D). The black trace in each graph is the same vehicle control trace. E: mean period values (±SE) for each group. Numbers in parentheses indicate the number of animals in each group.
RLX attenuates the effect of estrogen on the period of the PER2::LUC expression rhythm in the uterus.
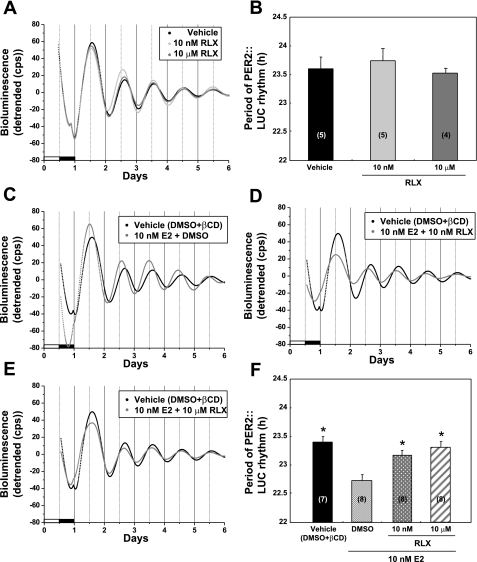
To further examine the mechanism by which E2 modulates the molecular clock in the uterus, we assessed the effects of RLX on the period of uterine PER2::LUC expression. We examined the effects of RLX alone or in the presence of 10 nM E2. RLX alone at concentrations of 10 nM and 10 μM did not alter the period of PER2::LUC expression in the uterus when compared with DMSO (vehicle)-treated controls (Fig. 4, A and B). In the presence of 10 nM E2, RLX at concentrations of 10 nM or 10 μM all but eliminated the robust effect of E2 on the period of PER2::LUC expression [1-way ANOVA, F(3,27) = 8.62, P < 0.05; Fig. 4, C–F].
Fig. 4.
Raloxifene (RLX) attenuates the effect of estrogen on the period of PER2::LUC expression in the uterus of OVX mice. A, C, D, and E: representative bioluminescence traces showing the effects of RLX on circadian rhythms of PER2::LUC expression in untreated and E2-treated uterine explants from OVX mice. A: uterine cultures treated with vehicle (DMSO) or 2 concentrations (10 nM or 10 μM) of RLX in DMSO. B: Mean period values (±SE) for cultures treated with RLX alone. C: uterine cultures treated with vehicle containing both DMSO and 100 μM βCD. D and E: cultures treated with 10 nM E2 + 10 nM RLX (D) or 10 nM E2 + 10 μM RLX (E). The plot of vehicle control data is duplicated in C–E. F: mean period values (±SE) for vehicle-, E2-, and E2 + RLX-treated cultures. Numbers in parentheses indicate the number of animals in each group. *P < 0.05 when compared with DMSO + 10 nM E2 treatment group (Tukey's test).
DISCUSSION
Our results demonstrate that estrogen has marked effects on the rhythm of PER2 expression in the isolated uterus. In uterine explants from PER2::LUC mice, E2 shortened the period of PER2::LUC expression and RLX attenuated the effects of E2. However, we did not observe significant effects of E2 and P4 treatment on the amplitude of PER2::LUC rhythm in the uterus (data not shown). These data suggest that estrogen can directly affect the timing of the molecular clock in the uterus.
We observed that 10 nM E2 significantly shortened the period of the PER2::LUC expression rhythm in the uterus, whereas neither 10 nM nor even 10 μM E2 affected the period of PER2::LUC expression in the SCN. Our previous study using Per1-luc transgenic rats also indicated that isolated SCN tissue explants do not respond to E2 treatment in the physiological range (33). Fatehi and Fatehi-Hassanabad (7) have recently shown that in vitro application of 30 nM to 3 μM E2 significantly increased the spontaneous firing frequency and depolarized the cell membrane of SCN neurons. These data suggest that estrogen has a direct influence on neural activity, but not on clock gene expression, in SCN pacemaker neurons. Interestingly, these data do not agree with our previous in vivo data, which indicated that estrogen slightly advanced the acrophase of Per2 mRNA expression in the SCN of OVX rats housed in constant darkness for 3 days (26). The effects of E2 in vivo may be the result of an interaction between steroid-responsive neural oscillators outside of the SCN and the SCN itself. As an example, steroid hormones might affect the timing of the molecular clock in neurons of the mediobasal hypothalamus that can in turn modulate the activity of SCN neurons.
The plasma concentration of E2 in female rats reaches a peak, ∼50 pg/ml (0.2 nM) on the morning of proestrus, and P4 reaches ∼60 ng/ml (200 nM) on the evening of proestrus (35). At its peak, the circulating estrogen concentration reaches a plateau approximately seven times higher than baseline levels (35). Although we did not observe a significant effect of 1 nM E2 treatment, we observed a significant effect on the period of PER2::LUC expression with 10 nM E2, a concentration within 1–2 orders of magnitude of the physiological range. Furthermore, the dose-response curve for the effects of E2 plateaued at concentrations >10 nM (with the notable exception of the 100 nM group; see Fig. 2F). There are several ways to explain the fact that 1 nM E2 failed to shorten the period of PER2::LUC expression in the uterus: 1) due to experimental limitations, we may be unable to detect a subtle change in period within the 6 days of tissue culture data we have analyzed or, 2) the concentrations of E2 we have used in vitro may lie below the physiological range for the uterus and thus below the threshold for effects on clock gene expression. It is possible that the level of E2 in the mouse uterus may be considerably higher than the level in the general circulation, and therefore, measurements of E2 in plasma may underestimate the effective dose at the tissue level. It is well known that estrogen shows variable tissue and concentration-dependent effects (38). In fact, E2 levels in human endometrium are ∼10 times higher than the level found in plasma (2). In OVX rats, the E2 concentration in uterine tissue is ∼100 pg/100 mg tissues (31), suggesting that the level of E2 in rodent uterine tissue may be as much as 20 times higher than the levels found in serum.
The fully developed uterus is composed of many heterogeneous cell types comprising four major anatomical compartments, the luminal epithelium, stroma, myometrium, and glandular epithelium. There is evidence of significant variation in the cell type-specific distribution of estrogen-induced or constitutively expressed ER, PR, and steroid receptor coactivators in the uterus of rats (29, 30). We have reported recently that Per1 mRNA expression peaks at projected ZT 8 in the whole uterus of OVX rats in constant darkness (26). Similar results have been observed by others in anestrous rats under light-dark conditions (10). The rhythm of Per1 mRNA expression, however, is not observed in the uterine glandular epithelium compartment. Taken together, these data suggest that the molecular clocks in the uterus may exhibit variations of sensitivity to circulating steroid hormones with cell type specificity.
Although RLX alone did not affect the period of PER2::LUC expression, it suppressed E2-induced shortening of the period of PER2::LUC expression. While our understanding of the molecular mechanisms underlying estrogen and SERM actions is growing, many details remain obscure. In particular, little is known regarding the activity and contribution of membrane-bound ERs in the uterus (16). It is now clear that at least two ERs (ERα and ERβ) can modify estrogen or SERM action. ERα and ERβ are differentially distributed throughout the body and may modify ligand interactions by dimerization or interactions with other proteins, such as coactivators and corepressors (16). There are some reports suggesting that only ERβ is present in the SCN, whereas ERα appears to be the dominant ER in the uterus (9, 11, 20, 34). Moreover, RLX is 20-fold selective for ERα (42). Gery et al. (8) recently demonstrated the interaction of ERα and Per2 via estrogen-responsive elements in the Per2 promoter using electrophoretic mobility shift assays and chromatin immunoprecipitation. These data strongly support our hypothesis that estrogen directly regulates Per2 expression rhythms in cells containing ERα. Given the fact that E2 treatment in vitro alters the period of PER2::LUC rhythm in the uterus but not the SCN, we propose that the effects of E2 on the circadian rhythm of PER2 expression in the uterus are mediated by ERα.
Although it is generally accepted that the timing of events in the female reproductive system, such as gonadotropin secretion, is dependent on a functioning circadian clock in the SCN, it remains to be seen whether peripheral hormone secretion plays a role in the timing of central and peripheral clocks. We have shown that E2 affects the timing of PER2::LUC expression in the uterus but not the SCN. Thus, our data do not support a direct feedback effect of ovarian E2 on the molecular clock in SCN neurons. However, our data do suggest that steroid hormones, perhaps in conjunction with neural input, pituitary gonadotropins, or other humoral factors, can modulate reproductive function through direct effects on clocks in target tissues such as the uterus.
GRANTS
This work was supported by National Institute of Mental Health Grant RO1-MH-062517 to G. D. Block.
Acknowledgments
We acknowledge the technical assistance of Naomi Ihara, Denise T. Holmes, and Jeff Hager. We also thank Takashi Kudo for comments on the manuscript.
Current address of T. J. Nakamura and G. D. Block: Dept. of Psychiatry and Biobehavioral Sciences, Univ. of California Los Angeles, Los Angeles, CA 90024-1759.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Albers HE, Gerall AA, Alexson JF. Effect of reproductive state on circadian periodicity in the rat. Physiol Behav 26: 21–25, 1981. [DOI] [PubMed] [Google Scholar]
- 2.Cortes-Gallégos V, Gallegos AJ, Basurto CS, Rivadeneyra J. Estrogen peripheral levels vs estrogen tissue concentration in the human female reproductive tract. J Steroid Biochem 6: 15–20, 1975. [DOI] [PubMed] [Google Scholar]
- 3.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20: 358–417, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Davidson AJ, Poole AS, Yamazaki S, Menaker M. Is the food-entrainable circadian oscillator in the digestive system? Genes Brain Behav 2: 32–39, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Dolatshad H, Campbell EA, O'Hara L, Maywood ES, Hastings MH, Johnson MH. Developmental and reproductive performance in circadian mutant mice. Hum Reprod 21: 68–79, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Fahrenkrug J, Georg B, Hannibal J, Hindersson P, Gras S. Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary. Endocrinology 147: 3769–3776, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Fatehi M, Fatehi-Hassanabad Z. Effects of 17beta-estradiol on neuronal cell excitability and neurotransmission in the suprachiasmatic nucleus of rat. Neuropsychopharmacology 33: 1354–1364, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Gery S, Virk RK, Chumakov K, Yu A, Koeffler HP. The clock gene Per2 links the circadian system to the estrogen receptor. Oncogene 26: 7916–7920, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res Mol Brain Res 76: 191–204, 2000. [DOI] [PubMed] [Google Scholar]
- 10.He PJ, Hirata M, Yamauchi N, Hattori MA. Up-regulation of Per1 expression by estradiol and progesterone in the rat uterus. J Endocrinol 194: 511–519, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Hileman SM, Handa RJ, Jackson GL. Distribution of estrogen receptor-beta messenger ribonucleic acid in the male sheep hypothalamus. Biol Reprod 60: 1279–1284, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Hirayama J, Sassone-Corsi P. Structural and functional features of transcription factors controlling the circadian clock. Curr Opin Genet Dev 15: 548–556, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Izumo M, Johnson CH, Yamazaki S. Circadian gene expression in mammalian fibroblasts revealed by real-time luminescence reporting: temperature compensation and damping. Proc Natl Acad Sci USA 100: 16089–16094, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izumo M, Sato TR, Straume M, Johnson CH. Quantitative analyses of circadian gene expression in mammalian cell cultures. PLoS Comput Biol 2: e136, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson MH, Lim A, Fernando D, Day ML. Circadian clockwork genes are expressed in the reproductive tract and conceptus of the early pregnant mouse. Reprod Biomed Online 4: 140–145, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Jordan VC The science of selective estrogen receptor modulators: concept to clinical practice. Clin Cancer Res 12: 5010–5013, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Karman BN, Tischkau SA. Circadian clock gene expression in the ovary: Effects of luteinizing hormone. Biol Reprod 75: 624–632, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Kennaway DJ, Varcoe TJ, Mau VJ. Rhythmic expression of clock and clock-controlled genes in the rat oviduct. Mol Hum Reprod 9: 503–507, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Levenson AS, Jordan VC. Selective oestrogen receptor modulation: molecular pharmacology for the millennium. Eur J Cancer 35: 1628–1639, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Lindberg MK, Weihua Z, Andersson N, Moverare S, Gao H, Vidal O, Erlandsson M, Windahl S, Andersson G, Lubahn DB, Carlsten H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. Estrogen receptor specificity for the effects of estrogen in ovariectomized mice. J Endocrinol 174: 167–178, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol 14: 1367–1373, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morin LP Effect of ovarian hormones on synchrony of hamster circadian rhythms. Physiol Behav 24: 741–749, 1980. [DOI] [PubMed] [Google Scholar]
- 23.Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science 196: 305–307, 1977. [DOI] [PubMed] [Google Scholar]
- 24.Moutsatsou P, Sekeris CE. Estrogen and progesterone receptors in the endometrium. Ann NY Acad Sci 816: 99–115, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Musa MA, Khan MO, Cooperwood JS. Medicinal chemistry and emerging strategies applied to the development of selective estrogen receptor modulators (SERMs). Curr Med Chem 14: 1249–1261, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura TJ, Moriya T, Inoue S, Shimazoe T, Watanabe S, Ebihara S, Shinohara K. Estrogen differentially regulates expression of Per1 and Per2 genes between central and peripheral clocks and between reproductive and nonreproductive tissues in female rats. J Neurosci Res 82: 622–630, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura TJ, Shinohara K, Funabashi T, Kimura F. Effect of estrogen on the expression of Cry1 and Cry2 mRNAs in the suprachiasmatic nucleus of female rats. Neurosci Res 41: 251–255, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Nakao N, Yasuo S, Nishimura A, Yamamura T, Watanabe T, Anraku T, Okano T, Fukada Y, Sharp PJ, Ebihara S, Yoshimura T. Circadian clock gene regulation of steroidogenic acute regulatory protein gene expression in preovulatory ovarian follicles. Endocrinology 148: 3031–3038, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Nephew KP, Long X, Osborne E, Burke KA, Ahluwalia A, Bigsby RM. Effect of estradiol on estrogen receptor expression in rat uterine cell types. Biol Reprod 62: 168–177, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Nephew KP, Ray S, Hlaing M, Ahluwalia A, Wu SD, Long X, Hyder SM, Bigsby RM. Expression of estrogen receptor coactivators in the rat uterus. Biol Reprod 63: 361–367, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Resko JA, Boling JL, Brenner RM, Blandau RJ. Sex steroids in reproductive tract tissues: regulation of estradiol concentrations by progesterone. Biol Reprod 15: 153–157, 1976. [DOI] [PubMed] [Google Scholar]
- 32.Schibler U, Sassone-Corsi P. A web of circadian pacemakers. Cell 111: 919–922, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Sellix MT, Nakamura TJ, Davidson AJ, Menaker M, Block GD. Effects of ovarian steroid hormones on Per1 expression in the SCN (Abstract 126). Program of the 10th Meeting of the Society for Research on Biological Rhythms, Sandestin, FL, 2006.
- 34.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 388: 507–525, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96: 219–226, 1975. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi JS, Menaker M. Interaction of estradiol and progesterone: effects on circadian locomotor rhythm of female golden hamsters. Am J Physiol Regul Integr Comp Physiol 239: R497–R504, 1980. [DOI] [PubMed] [Google Scholar]
- 37.Wollnik F, Turek FW. Estrous correlated modulations of circadian and ultradian wheel-running activity rhythms in LEW/Ztm rats. Physiol Behav 43: 389–396, 1988. [DOI] [PubMed] [Google Scholar]
- 38.Woolley CS Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol 47: 657–680, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288: 682–685, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Yamazaki S, Takahashi JS. Real-time luminescence reporting of circadian gene expression in mammals. Methods Enzymol 393: 288–301, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101: 5339–5346, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zou A, Marschke KB, Arnold KE, Berger EM, Fitzgerald P, Mais DE, Allegretto EA. Estrogen receptor beta activates the human retinoic acid receptor alpha-1 promoter in response to tamoxifen and other estrogen receptor antagonists, but not in response to estrogen. Mol Endocrinol 13: 418–430, 1999. [DOI] [PubMed] [Google Scholar]