Abstract
Extracellular ATP regulates bile formation by binding to P2 receptors on cholangiocytes and stimulating transepithelial Cl− secretion. However, the specific signaling pathways linking receptor binding to Cl− channel activation are not known. Consequently, the aim of these studies in human Mz-Cha-1 biliary cells and normal rat cholangiocyte monolayers was to assess the intracellular pathways responsible for ATP-stimulated increases in intracellular Ca2+ concentration ([Ca2+]i) and membrane Cl− permeability. Exposure of cells to ATP resulted in a rapid increase in [Ca2+]i and activation of membrane Cl− currents; both responses were abolished by prior depletion of intracellular Ca2+. ATP-stimulated Cl− currents demonstrated mild outward rectification, reversal at ECl−, and a single-channel conductance of ∼17 pS, where E is the equilibrium potential. The conductance response to ATP was inhibited by the Cl− channel inhibitors NPPB and DIDS but not the CFTR inhibitor CFTRinh-172. Both ATP-stimulated increases in [Ca2+]i and Cl− channel activity were inhibited by the P2Y receptor antagonist suramin. The PLC inhibitor U73122 and the inositol 1,4,5-triphosphate (IP3) receptor inhibitor 2-APB both blocked the ATP-stimulated increase in [Ca2+]i and membrane Cl− currents. Intracellular dialysis with purified IP3 activated Cl− currents with identical properties to those activated by ATP. Exposure of normal rat cholangiocyte monolayers to ATP increased short-circuit currents (Isc), reflecting transepithelial secretion. The Isc was unaffected by CFTRinh-172 but was significantly inhibited by U73122 or 2-APB. In summary, these findings indicate that the apical P2Y-IP3 receptor signaling complex is a dominant pathway mediating biliary epithelial Cl− transport and, therefore, may represent a potential target for increasing secretion in the treatment of cholestatic liver disease.
Keywords: cholangiocyte; ATP; purinergic signaling; P2Y receptor; Cl− channel; inositol 1,4,5-triphosphate
extracellular ATP has emerged as an important signaling molecule regulating hepatobiliary function. Released into bile by both hepatocytes and cholangiocytes, ATP functions as a potent autocrine/paracrine stimulus for cholangiocyte secretion via activation of plasma membrane purinergic (P2) receptors (15). The concentration of ATP in human bile is within the physiological range required for activation of most P2 receptors (7). Upon P2 receptor binding, ATP rapidly increases intracellular Ca2+ concentration ([Ca2+]i) and activation of membrane Cl− channels in both rat and human biliary epithelial models (25, 35). The resulting increase in transepithelial Cl− secretion contributes importantly to transport of water and HCO3−, resulting in dilution and alkalinization of bile (18). Thus signaling through extracellular nucleotides represents an important mechanism regulating bile formation.
The recent identification of fluid flow or shear stress as a potent stimulus for biliary epithelial cell ATP release adds an important link to the P2-signaling pathway (44). The mechanical effect of fluid flow on the cholangiocyte apical membrane, by stimulating ATP release, increases [Ca2+]i and activates non-CFTR Cl− channels through a process requiring agonist binding to apical P2 receptors. Thus mechanosensitive ATP release and P2-signaling may complement, or even surpass, secretin-mediated cAMP-dependent secretion through CFTR. In fact, recent evidence suggests that a major function of biliary CFTR is to regulate ATP release from an intracellular site into the duct lumen and that ATP release per se represents a final common pathway transducing secretin and other secretagogue effects (27).
Although cholangiocytes express a repertoire of P2 receptors, including both P2X and P2Y, their relative contributions are unknown. P2X receptors are ligand-gated cation channels, where ATP binding leads to opening of a calcium-permeable pore and calcium influx from extracellular sites. In contrast, P2Y receptors are members of the superfamily of G protein-coupled receptors often coupled to phospholipase C and generation of inositol 1,4,5-triphosphate (IP3). In biliary epithelial cells, the pathways linking receptor binding to Cl− efflux are unknown. Consequently, the purpose of the present studies was to identify the intracellular pathways linking P2 receptor binding to increases in intracellular [Ca2+]i and Cl− channel activation. The findings support a dominant role for P2Y receptors leading to sequential activation of PLC generation, IP3 receptor stimulation, release of Ca2+ from intracellular stores, and Cl− channel activation. Together these elements constitute a P2-linked signaling complex in the apical domain of cholangiocytes regulating biliary secretion. Importantly, the pathway is independent of, and of greater magnitude than, CFTR-mediated secretion. Thus the apical P2Y-IP3 receptor signaling complex represents an important pathway mediating biliary epithelial cell secretion.
METHODS
Cell models.
Studies in isolated cells were performed using Mz-Cha-1 cells and in polarized monolayers were performed utilizing normal rat cholangiocytes (NRC). Mz-Cha-1, originally isolated from human adenocarcinoma of the gallbladder (23), were passaged at biweekly intervals and maintained in culture at 37°C in a 5% CO2 incubator in HCO3−-containing CMRL-1066 media (GIBCO-BRL, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum, penicillin (100 IU/ml), and streptomycin (100 μg/ml). Mz-Cha-1 cells exhibit phenotypic features of differentiated biliary epithelium (4, 23). When cultured as described, they have been utilized as models for biliary ATP release, purinergic signaling (16), and Ca2+-dependent secretion (14, 31, 43). Since Mz-ChA-1 cells do not form high-resistance monolayers in culture, additional Ussing chamber studies were performed utilizing NRC monolayers isolated from intrahepatic bile ducts (42). These cells express phenotypic features of differentiated biliary epithelium including receptors, signaling pathways, and ion channels similar to those found in primary cells (32, 35). Moreover, exposure to ATP is followed by opening of apical membrane Cl− channels, producing an increase in short-circuit current (Isc), the electrical signature of transepithelial secretion. NRC monolayers were cultured on rat tail collagen slabs as previously described (33, 35) and passaged onto collagen-coated semipermeable (24-mm diameter, 0.4-μm pore) Costar Transwell supports (Corning) 7–10 days before all electrophysiological and molecular studies. This protocol permits highly polarized cells, the development of a high transepithelial resistance (Rt > 1,000 Ω·cm2), and net apical Cl− secretion.
Ca2+ imaging.
Mz-Cha-1 cells were cultured for 48 h on 15-mm glass coverslips and then loaded with 2.5 μg/ml of fura 2-AM for 20–30 min (TEF Laboratories, Austin, TX) in isotonic extracellular buffer containing (in mM) 140 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 1 KH2PO4, 5 glucose, and 10 HEPES (pH 7.4) supplemented with 0.01% pluronic F127 for 30 min at 22°C. In selected studies, Ca2+ was removed from the bath solution by EGTA (2 mM). The coverslip was placed in a perfusion chamber (RC-25F/PHA, Warner Instruments) on the stage of an inverted fluorescence microscope (Nikon TE2000). Changes of [Ca2+]i were measured at excitation wavelength of 340 nm for calcium-bound fura 2-AM and 380 nm for calcium-free fura 2-AM, emission wavelength of 510 nm. After subtraction of background fluorescence, [Ca2+]i was calculated according to Grynkiewicz equation (36): [Ca2+]i (nM) = Kd × [(R − Rmin)/(Rmax − R)] × Sfb, where Kd at 22°C = 145 and Sfb is ratio of baseline fluorescence (380 nm) under Ca2+-free and Ca2+-bound conditions, R is the recorded (340/380) emission ratio, Rmin is the minimal value of R at zero [Ca2+]i, and Rmax is the value of R at saturation [Ca2+]i.
Measurement of membrane Cl− currents.
Membrane currents were measured by whole cell and single-channel patch clamp techniques. Cells on a coverslip were mounted in a chamber (volume ∼400 μl) and whole cell currents measured with a standard extracellular solution containing (in mM) 140 NaCl, 4 KCl, 1 CaCl2, 2 MgCl2, 1 KH2PO4, 10 glucose, and 10 HEPES/NaOH (pH ∼7.40). The standard intracellular (pipette) solution for whole cell recordings contained (in mM) 130 KCl, 10 NaCl, 2 MgCl2, 10 HEPES/KOH, 0.5 CaCl2, 3 MgATP2− or NaATP4−, and 1 EGTA (pH 7.3), corresponding to a Ca2+ concentration of ∼100 nM (6). Patch pipettes were pulled from Corning 7052 glass and had a resistance of 2–5 or 7–10 MΩ for whole cell and single-channel recordings, respectively. Recordings were made with an Axopatch ID amplifier (Axon Instruments, Foster City, CA) and were filtered at 1–2 kHz and sampled at 4 kHz for storage on a computer and analyzed by using pCLAMP version 10 (Axon Instruments, Burlingame, CA) as previously described (19, 24). Two voltage protocols were utilized: 1) holding potential −40 mV, with 200-ms steps to 0 mV and −80 mV at 10 s intervals (for real-time tracings), and 2) holding potential −40 mV, with 400-ms steps from −100 mV to +100 mV in 20 mV increments. Current-voltage (I-V) relations were generated from the “step” protocol. Pipette voltages (Vp) are referred to the bath. In the whole cell configuration, Vp corresponds to the membrane potential, and upward deflections of the current trace indicate outward membrane current. In the cell-attached patch configuration, applied voltages represent −Vp values. Results are compared with control studies measured on the same day to minimize any effects of day-to-day variability and reported as current density (pA/pF) to normalize for differences in cell size (13). The average whole cell capacitance for the Mz-Cha-1 cells was 22.6 ± 1.1 pF (mean ± SE). For the majority of single-channel studies standard extracellular buffer (described above) was used for both bath and pipette solutions. For selected studies, a high-K+ bath and pipette solution was utilized (changes from standard extracellular buffer: 140 mM KCl, 4 mM NaCl, 5 mM BaCl2) to hold EK+ at ∼0 mV and minimize the effects of the interior negative membrane potential, where E is the equilibrium potential. Average open probability (NPo) was determined from 6- to 10-s recordings and mean open times were determined by using pClamp systems and OriginPro 7.0 software (MicroCal Software, Northampton, MA). The number of channels in each patch (N) was measured by using an all-points amplitude histogram and calculated as the peak current divided by the unitary current. The slope conductance was calculated from currents at positive voltages.
Transepithelial Cl− secretion.
NRC cells were utilized to study the relative contribution of P2 receptors and the pathways involved in extracellular nucleotide-stimulated transepithelial Cl− secretion. Cells were grown to confluence on collagen-treated polycarbonate filters with a pore size of 0.4 μm (Costar, Cambridge, MA) until resistance was >1,000 Ω·cm2 (EVOHM; World Precision Instruments, Sarasota, FL) (33). Cells were mounted in a Trans-24 miniperfusion system for tissue culture cups (Jim's Instrument Manufacturing, Iowa City, IA). All experiments were carried out at 37°C, and basolateral and apical (luminal) sides were bubbled with O2 through air-lift circulators. The standard extracellular buffer solution containing (in mM) 140 NaCl, 4 KCl, 1 KH2PO4, 2 MgCl2, 1 CaCl2, 5 glucose, and 10 HEPES/NaOH (pH 7.3). Transepithelial voltage was clamped to 0 mV and Isc was recorded through agar bridges (3% agar in 1 M KCl) connected to Ag-AgCl electrodes (cartridge electrodes, World Precision Instruments). The Isc represents the net sum of the transepithelial anion and cation fluxes and reflects the level of ion and fluid secretion (35). Studies included paired, same-day monolayers to minimize any potential effects of day-to-day variability.
Reagents.
Thapsigargin was obtained from Calbiochem/EMD Biosciences (La Jolla, CA). The phospholipase C inhibitor U73122, the inactive analog U73343, and the IP3 receptor inhibitor 2-APB were obtained from Calbiochem/EMD Biosciences and from MP Biomedicals (Solon, OH). Since 2-APB can affect gap junction channels in other cell models (3, 22) and, hence, alter cell capacitance during the whole cell patch clamp experiments, capacitance measurements were performed in the presence (21.5 ± 1.2 pF) and absence (22.6 ± 1.1 pF) of the reagent and were determined to be statistically without difference [P = 0.63, not significant (NS)], thus ensuring that 2-APB did not have unanticipated effects on cell capacitance. The cystic fibrosis transmembrane conductance regulator (CFTR) inhibitor, CFTRinh-172, was a kind gift from Dr. Nitin Sonawane and Dr. Alan Verkman (Univ. of California, San Francisco, CA). All other reagents were obtained from Sigma-Aldrich (St. Louis, MO).
Statistics.
Results are presented as means ± SE, with n representing the number of culture plates or repetitions for each assay as indicated. Student's paired or unpaired t-test or ANOVA for multiple comparisons was used to assess statistical significance as indicated, and P values <0.05 were considered to be statistically significant.
RESULTS
Characterization of ATP-stimulated Cl− channels.
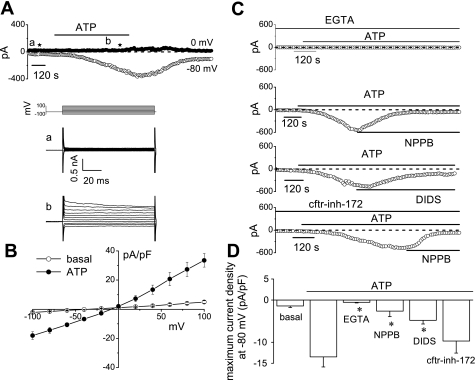
To develop a functional characterization of the Cl− channel(s) activated by ATP, whole cell patch clamp studies were performed in Mz-Cha-1 cells. Under basal conditions with standard intra- and extracellular buffers, I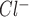 was small (−1.7 ± 0.3 pA/pF, n = 33). Exposure to ATP (50 μM) resulted in activation of currents within 2 min (representative trace shown in Fig. 1), increasing current density to −13.6 ± 1.2 pA/pF at −80 mV (n = 33, P < 0.001 compared with basal, Fig. 1). ATP-stimulated currents exhibited characteristic biophysical features with reversal at 0 mV (E
was small (−1.7 ± 0.3 pA/pF, n = 33). Exposure to ATP (50 μM) resulted in activation of currents within 2 min (representative trace shown in Fig. 1), increasing current density to −13.6 ± 1.2 pA/pF at −80 mV (n = 33, P < 0.001 compared with basal, Fig. 1). ATP-stimulated currents exhibited characteristic biophysical features with reversal at 0 mV (E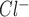 ), time-dependent inactivation, and outward rectification as described previously (25) (Fig. 1B). Currents were sustained for the duration of ATP exposure, followed by gradual return to basal levels within 5–10 min following removal of ATP from the extracellular buffer. ATP-stimulated currents were dependent on intracellular Ca2+ as chelation (2 mM EGTA in pipette) significantly decreased maximum current density to −0.5 ± 0.2 pA/pF (n = 6, P < 0.05, Fig. 1, C and D). Additionally, currents were reversibly inhibited by 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB, 100 μM), which decreased maximum current density measured at −80 mV to −2.6 ± 1.3 pA/pF (n = 8, P < 0.005, Fig. 1, C and D). The Ca2+-activated Cl− channel inhibitor 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS, 100 μM) also significantly inhibited ATP-stimulated Cl− currents (−4.8 ± 0.9 pA/pF, n = 5, P < 0.05); however, the CFTR inhibitor CFTRinh-172 (5 μM) did not (−10.8 ± 2.3 pA/pF, n = 11, P = NS, Fig. 1, C and D). Thus exposure to ATP stimulates a calcium-activated Cl− conductance and both biophysical and pharmacological approaches demonstrate that it is unrelated to CFTR.
), time-dependent inactivation, and outward rectification as described previously (25) (Fig. 1B). Currents were sustained for the duration of ATP exposure, followed by gradual return to basal levels within 5–10 min following removal of ATP from the extracellular buffer. ATP-stimulated currents were dependent on intracellular Ca2+ as chelation (2 mM EGTA in pipette) significantly decreased maximum current density to −0.5 ± 0.2 pA/pF (n = 6, P < 0.05, Fig. 1, C and D). Additionally, currents were reversibly inhibited by 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB, 100 μM), which decreased maximum current density measured at −80 mV to −2.6 ± 1.3 pA/pF (n = 8, P < 0.005, Fig. 1, C and D). The Ca2+-activated Cl− channel inhibitor 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS, 100 μM) also significantly inhibited ATP-stimulated Cl− currents (−4.8 ± 0.9 pA/pF, n = 5, P < 0.05); however, the CFTR inhibitor CFTRinh-172 (5 μM) did not (−10.8 ± 2.3 pA/pF, n = 11, P = NS, Fig. 1, C and D). Thus exposure to ATP stimulates a calcium-activated Cl− conductance and both biophysical and pharmacological approaches demonstrate that it is unrelated to CFTR.
Fig. 1.
Characterization of whole cell ATP-stimulated currents. Exposure to ATP stimulates currents in human Mz-Cha-1 biliary epithelial cells. Whole cell currents were measured during basal conditions and during exposure to ATP (50 μM) (methods). A: representative whole cell recording. Currents measured at −80 mV (○), representing I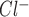 , and at 0 mV (•), representing I
, and at 0 mV (•), representing I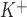 , are shown. ATP exposure is indicated by the bar. A voltage-step protocol (test potentials between −100 mV and +100 mV in 20-mV increments) was obtained at a★ (basal) and b★ (maximal current response) as indicated. The current-voltage (I-V) plot shown in B was generated from these protocols. B: I-V relationship of whole cell currents during basal (○) and ATP-stimulated (•) conditions. C: representative ATP-stimulated whole cell current tracings measured at −80 mV in the absence of intracellular Ca2+ (EGTA 2 mM in pipette solution) or presence of Cl− channel inhibitors. D: cumulative data demonstrating magnitude of ATP-stimulated currents in the presence or absence of the Cl− channel inhibitors 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB; 100 μM), DIDS (100 μM), or CFTRinh-172 (5 μM) or removal of intracellular Ca2+ (EGTA in pipette). Values represent maximum current density (pA/pF) measured at −80 mV (n = 5–11 each). *ATP-stimulated currents were significantly inhibited (P < 0.05 for each).
, are shown. ATP exposure is indicated by the bar. A voltage-step protocol (test potentials between −100 mV and +100 mV in 20-mV increments) was obtained at a★ (basal) and b★ (maximal current response) as indicated. The current-voltage (I-V) plot shown in B was generated from these protocols. B: I-V relationship of whole cell currents during basal (○) and ATP-stimulated (•) conditions. C: representative ATP-stimulated whole cell current tracings measured at −80 mV in the absence of intracellular Ca2+ (EGTA 2 mM in pipette solution) or presence of Cl− channel inhibitors. D: cumulative data demonstrating magnitude of ATP-stimulated currents in the presence or absence of the Cl− channel inhibitors 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB; 100 μM), DIDS (100 μM), or CFTRinh-172 (5 μM) or removal of intracellular Ca2+ (EGTA in pipette). Values represent maximum current density (pA/pF) measured at −80 mV (n = 5–11 each). *ATP-stimulated currents were significantly inhibited (P < 0.05 for each).
ATP-receptor binding releases intracellular Ca2+ stores.
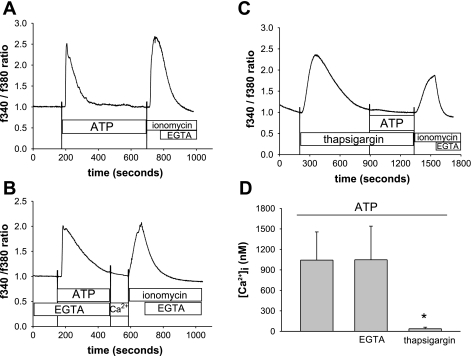
Because the ATP-stimulated Cl− currents are dependent on intracellular Ca2+, further studies were performed using fura 2-loaded Mz-Cha-1 cells to determine the contributions of extra- vs. intracellular Ca2+. Exposure to ATP (100 μM) resulted in a rapid increase in Ca2+ fluorescence reaching a maximum at ∼30 s (1,042 ± 341 nM, Fig. 2, A and D). Removal of extracellular Ca2+ (EGTA, 2 mM) had no effect on this response (1,047 ± 492 nM, n = 5, P = NS, Fig. 2, B and D), but prior depletion of intracellular Ca2+ stores by exposure to thapsigargin (200 nM × 12 min) significantly decreased the ATP-induced release of Ca2+ (37 ± 22 nM, n = 7, P < 0.005, Fig. 2, C and D). Thus ATP-receptor binding stimulates release of Ca2+ from intracellular stores.
Fig. 2.
ATP stimulates an increase in intracellular Ca2+ concentration ([Ca2+]i). Mz-ChA-1 cells grown on a coverglass were loaded with fura 2-AM, washed with PBS, and exposed to ATP (100 μM). Values represent an increase in the ratio of fluorescence at 340 and at 380. A: in this representative study, fura 2 fluorescence increased within seconds of ATP exposure (indicated by the bar) rapidly reaching a maximal value and then decreased to basal levels. Maximal and minimal Ca2+ fluorescence was obtained by exposure to ionomycin (2 μM) and EGTA (10 mM), respectively. B: representative study demonstrating ATP-stimulated fura 2 fluorescence after removal of extracellular Ca2+ (EGTA). C: representative study demonstrating ATP-stimulated fura 2 fluorescence after depletion of intracellular Ca2+ stores (thapsigargin 200 nM × 12 min). D: cumulative data demonstrating maximum [Ca2+]i (nM) for ATP alone (n = 12), ATP in presence of EGTA (n = 5), or ATP following previous exposure to thapsigargin (n = 7). *Preincubation with thapsigargin significantly inhibited ATP-stimulated [Ca2+]i (P < 0.005).
Agonist profile of nucleotide-stimulated Cl− currents and Ca2+ fluorescence.
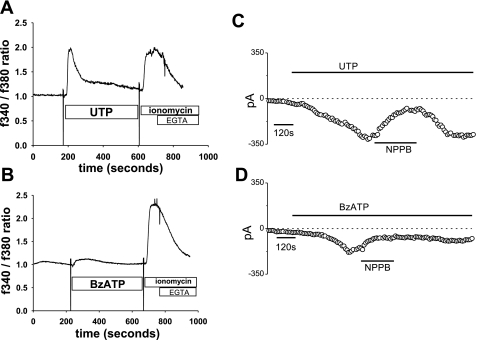
To establish a pharmacological profile of the P2 receptors involved in ATP-stimulated responses, Mz-Cha-1 cells were exposed to different extracellular nucleotides, and changes in Ca2+ fluorescence and Cl− permeability were measured (Table 1). First, to characterize the pharmacological profile of the P2 receptors involved in Ca2+ signaling, Mz-Cha-1 cells were loaded with fura 2 and [Ca2+]i was measured. Exposure to UTP (50 μM), a preferential agonist for P2Y vs. P2X receptors, resulted in an increase in [Ca2+]i (819 ± 231 nM, n = 5, P < 0.05, Fig. 3A). Exposure of cells to benzoyl-benzoyl ATP (Bz-ATP), a potent agonist for P2X receptors (P2X4, P2X7), resulted in a more modest increase in Ca2+ fluorescence (21 ± 17 nM, n = 4, P < 0.05, 3B).
Table 1.
Maximum [Ca2+]i and current density (measured at −80 mV) for each agonist
| [Ca2+]i, nM | Current Density, pA/pF | |
|---|---|---|
| Basal | 16±9, n=5 | −1.3±0.1, n=33 |
| ATP | 944±422, n=6, *P<0.01 | −13.5±2.4, n=33, †P<0.001 |
| UTP | 819±272, n=5, *P<0.01 | −13.1±2.7, n=6, †P<0.01 |
| BzATP | 21±17, n=4, *P=n.s. | −7.5±2.3, n=5, †P<0.05 |
[Ca2+]i, intracellular Ca2+ concentration.
Signficant differences: vs. basal [Ca2+]i;
vs. basal current density.
Fig. 3.
Agonist profile of nucleotide-stimulated intracellular Ca2+ fluorescence and Cl− currents. A and B: representative Ca2+ fluorescence in fura 2-loaded cells. Maximal and minimal Ca2+ fluorescence was obtained by exposure to ionomycin (2 μM) and EGTA (10 mM), respectively. C and D: representative whole cell current tracings measured at −80 mV performed according to the protocol described in Fig. 1A. Fura 2 fluorescence increases rapidly in response to UTP (100 μM). B: benzoyl-benzoyl ATP (Bz-ATP; 100 μM) resulted in only a small increase in fura 2 fluorescence. C: UTP (50 μM) (indicated by the bar) activates currents that were reversibly inhibited by the Cl− channel blocker NPPB (100 μM). D: Bz-ATP (50 μM) activated currents of low amplitude that were partially inhibited by NPPB (100 μM).
Similarly, whole cell patch-clamp measurements of Cl− currents showed parallel responses. Exposure to UTP (50 μM) increased current density from −1.3 ± 0.4 to −13.1 ± 2.7 pA/pF (n = 6, P < 0.01). The UTP-induced currents exhibited identical properties to the ATP-stimulated currents with a reversal potential at 0 mV, time-dependent inactivation at potentials >+60 mV, and inhibition by NPPB (100 μM) (−3.7 ± 1.1 pA/pF, n = 5 each, P < 0.05, Fig. 3C). In contrast, Bz-ATP (50 μM) resulted in current activation in only ∼47% of cells, and the currents were of lower magnitude (maximum current density measured at −80 mV of −8.8 ± 3.6 pA/pF at 10 min, n = 4), more linear, without time-dependent inactivation, and were only partially inhibited by NPPB (Fig. 3D). This pharmacological profile of nucleotide-stimulated increases in [Ca2+]i and Cl− currents, ATP = UTP ≫ Bz-ATP, is consistent with P2Y receptors playing a prominent role in transducing extracellular ATP into secretory responses in biliary epithelial cells, in agreement with molecular data demonstrating that P2Y receptors are abundant in biliary epithelium (11, 35).
Antagonist profile of ATP-stimulated Ca2+ fluorescence and Cl− currents.
To further characterize the receptors involved in ATP-stimulated signaling, the response to ATP was measured in Mz-Cha-1 cells in the presence or absence of P2 receptor antagonists as summarized in Table 2. First, compared with control cells (1,101 ± 438 nM, n = 5), both suramin (50 ± 21 nM, n = 7, P < 0.05) and reactive blue 2 (25 μM), a structurally unrelated P2Y inhibitor, (24 ± 7 nM, n = 7, P < 0.05) significantly inhibited ATP-stimulated [Ca2+]i (Fig. 4, A and B). Conversely, the P2X receptor antagonist brilliant blue G had little effect on ATP-stimulated increases in [Ca2+]i (733 ± 79 nM, n = 4, P = NS, Fig. 4C). Similarly, in separate studies, ATP-stimulated Cl− currents were inhibited by either suramin (100 μM, −2.2 ± 0.8 pA/pF, n = 6, P < 0.001) or reactive blue 2 (25 μM, −2.8 ± 0.5 pA/pF, n = 4, P < 0.001, Fig. 4, D and E) compared with control cells (−14.7 ± 1.7 pA/pF, n = 7). However, the P2X receptor antagonist brilliant blue G (1 μM) had little effect on ATP-stimulated Cl− currents (−14.6 ± 1.8 pA/pF, n = 4, P = NS, Fig. 4F). These studies provide further evidence that P2Y receptors mediate the ATP-stimulated increase in [Ca2+]i and Cl− conductance.
Table 2.
Effect of P2 receptor antagonists on ATP-stimulated [Ca2+]i and current density
| Maximum ATP-Stimulated [Ca2+]i, nM | Maximum ATP-Stimulated Current Density, pA/pF | |
|---|---|---|
| Control | 1101±438, n=5 | −14.73±1.74, n=7 |
| Suramin | 50±21, n=7, *P<0.05 | −2.25±0.8, n=6, †P<0.001 |
| Reactive blue 2 | 24±7, n=7, *P<0.05 | −2.77±0.51, n=4, †P<0.001 |
| Brilliant blue G | 733±79, n=4, *P=n.s. | −14.83±1.81, n=4, †P=n.s |
Significant differences: vs. maximum control [Ca2+]i;
Significant differences: vs. maximum control current density.
Fig. 4.
Effect of P2 receptor antagonists on ATP-stimulated intracellular [Ca2+] and Cl− currents. A–C: representative Ca2+ fluorescence in fura 2-loaded cells. In each representative sample, cells were exposed to ATP (100 μM) as indicated by the bar. Maximal and minimal Ca2+ fluorescence was obtained by exposure to ionomycin (2 μM) and EGTA (10 mM), respectively. D–F: representative whole cell current tracings (pA) measured at −80 mV performed according to the protocol described in Fig. 1 after exposure to ATP (50 μM). A–C: effect of the P2Y receptor antagonists suramin (100 μM) (A) and reactive blue 2 (RB-2, 25 μM) (B), or the P2X receptor antagonist brilliant blue G (BBG, 10 μM) (C) on fura 2 fluorescence. D–F: representative whole cell patch clamp recordings of ATP-stimulated currents are shown in the presence of suramin (D), reactive blue 2 (E), or brilliant blue G (F).
ATP responses are transduced through a PLC-IP3 pathway.
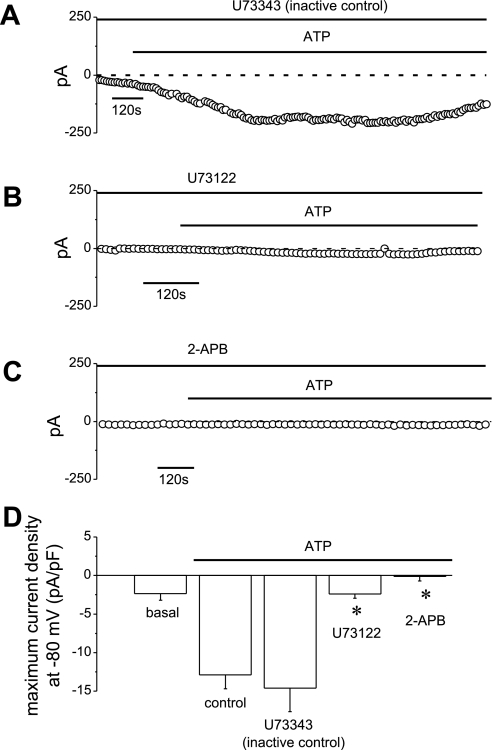
P2Y receptors are G protein-coupled receptors that activate phospholipase C (PLC). PLC activation hydrolyzes the membrane phospholipids phosphatidylinositol 4,5-bisphosphate, resulting in the formation of diacylglycerol and IP3. Interaction of IP3 with specific IP3 receptors results in Ca2+ release from intracellular stores. Consequently, the effect of PLC inhibition by U73122 (10 μM) and IP3 receptor inhibition by 2-APB (100 μM) on ATP-stimulated Ca2+ fluorescence and Cl− currents were assessed. The characteristic increase in [Ca2+]i following ATP exposure (1,513 ± 419 nM, n = 4) was unaffected by the inactive U73343 analog (1,760 ± 497 nM, n = 5, P = NS vs. control, Fig. 5, A and D) but significantly inhibited by U73122 (17 ± 14 nM, n = 9, P < 0.05, Fig. 5, B and D). Likewise, 2-APB also abolished increases in ATP-stimulated [Ca2+]i (34 ± 14 nM, n = 4, P < 0.05, Fig. 5, C and D). In parallel patch-clamp studies, ATP (50 μM) activated Cl− currents that were unaffected by inactive U73343 (−14.6 ± 3.1 pA/pF, n = 6, Fig. 6, A and D) but completely abolished by either U73122 (−2.4 ± 0.5 pA/pF, n = 4, P < 0.01, Fig. 6, B and D) or 2-APB (−0.1 ± 0.5 pA/pF, n = 6, P < 0.01, Fig. 6, C and D). Continued release from intracellular stores is necessary for continued ATP-stimulated Cl− currents as addition of 2-APB during maximum current activation also decreased current magnitude significantly (−3.2 ± 1.2 pA/pF, n = 5, P < 0.05, data not shown).
Fig. 5.
Effect of pharmacological inhibition of PLC or inositol 1,4,5-triphosphate (IP3) receptors on ATP-stimulated changes in [Ca2+]i. Cells were loaded with fura 2-AM, according to the protocol described in Fig. 2, and exposed to ATP (100 μM) in the presence or absence of PLC or IP3 receptor inhibitors (methods). Values represent an increase in the ratio of fluorescence at 340 and at 380. Maximal and minimal Ca2+ fluorescence was obtained by exposure to ionomycin (2 μM) and EGTA (10 mM), respectively. A: effect of the inactive analog U73343 (10 μM) on fura 2 fluorescence. B: effect of the PLC inhibitor U73122 (10 μM) on fura 2 fluorescence. C: effect of the IP3 receptor inhibitor 2-APB (100 μM) on fura 2 fluorescence. D: cumulative data demonstrating effect of PLC or IP3 receptor inhibition on ATP-stimulated intracellular [Ca2+]i. *U73122 (n = 9) or 2-APB (n = 4) significantly inhibited (P < 0.05 for each) ATP-stimulated increases in [Ca2+]i.
Fig. 6.
Effect of pharmacological inhibition of PLC or IP3 receptors on ATP-stimulated Cl− currents. Whole cell patch clamp studies were performed according to the protocol described in Fig. 1. A–C: representative whole cell patch clamp recordings of ATP-stimulated (50 μM) currents in the presence of U73343 (10 μM) (A), U73122 (10 μM) (B), or 2-APB (100 μM) (C). D: cumulative data demonstrating effects of PLC or IP3 inhibition on ATP-stimulated current density. *U73122 or 2-APB significantly (P < 0.001) inhibited ATP-stimulated currents. The inactive analog U73343 had no effect. Values represent maximum current density (pA/pF) measured at −80 mV (n = 4–9 each).
Intracellular dialysis with purified IP3 activates membrane Cl− currents.
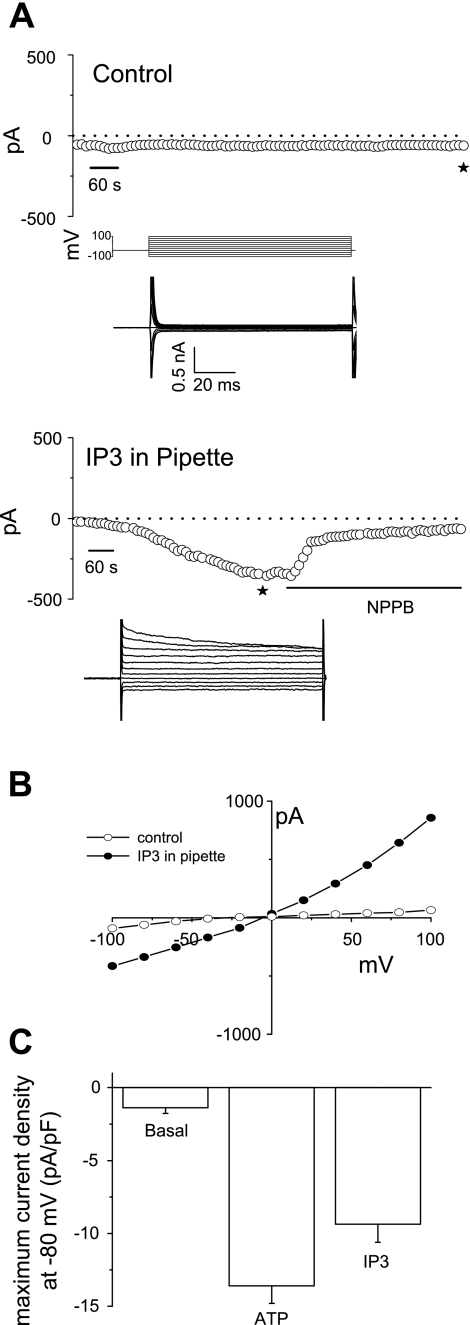
To confirm the role of IP3 on membrane Cl− currents, purified IP3 was delivered to the cell interior during whole cell patch clamp. Whole cell currents (measured at −80 mV) remained small in control cells (−2.9 ± 0.8 pA/pF, n = 4, Fig. 7, A and C). In contrast, when IP3 was included in the patch pipette, spontaneous activation of currents was observed as IP3 diffused into the cell interior with a maximum current density measured at −80 mV of −9.4 ± 1.2 pA/pF (n = 4, P < 0.005, Fig. 7A, bottom, and 7C). These currents demonstrated identical characteristics to the ATP-stimulated currents with reversal at 0 mV, outward rectification, and time-dependent inactivation at depolarizing potentials above +60 mV (Fig. 7, A–C).
Fig. 7.
Intracellular dialysis with recombinant IP3 activates membrane Cl− currents. Under whole cell patch clamp conditions (performed according to protocol described in Fig. 1), recombinant IP3 (20 μM) was delivered to the cell interior by inclusion in the patch pipette. A: in control cells (without IP3), no spontaneous currents are noted (top tracing). Dialysis with IP3 resulted in spontaneous activation of membrane currents as IP3 diffused into the cell interior (bottom tracing). NPPB inhibited membrane currents. A voltage-step protocol (test potentials between −100 mV and +100 mV in 20-mV increments) was obtained at times marked with ★ during both basal and maximal current responses and shown below the trace, respectively. The I-V plot shown in B was generated from these protocols. B: I-V relationship of whole cell currents during basal (○) and intracellular dialysis with IP3 (•). C: cumulative data demonstrating maximum current density (pA/pF) measured at −80 mV under basal conditions, in response to extracellular ATP (50 μM), or intracellular dialysis with IP3 (20 μM). *Extracellular ATP (n = 33) or intracellular IP3 (n = 4) significantly increased current density (P < 0.001 and P < 0.005 vs. basal, respectively).
Single-channel characterization.
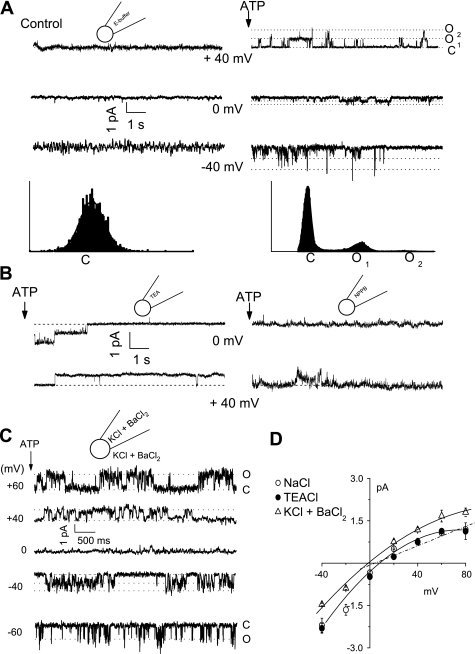
To characterize the channel types contributing to ATP-stimulated Cl− currents, unitary currents were measured in the cell-attached configuration. Under basal conditions, few spontaneous openings were evident (Fig. 8). Exposure to ATP (50 μM) activated currents (Fig. 8A), increasing NPo from 0.04 ± 0.02 to 0.74 ± 0.02 (n = 6, P < 0.05). Representative amplitude histograms are shown under each tracing, demonstrating the effect of ATP on NPo. In this example, two open levels are apparent. Because a nonselective cation (NSC) conductance could also contribute to currents, additional studies were performed to establish that the ATP-stimulated currents were mediated by Cl−. First, replacement of the pipette monovalent cations by tetraethylammonium did not affect the magnitude or reversal potential of the ATP-stimulated currents (n = 5, P = NS), thus excluding a contribution from an NSC conductance (Fig. 8B, left). Second, in the presence of the Cl− channel blocker NPPB (100 μM), NPo of ATP-stimulated currents decreased significantly from 0.60 ± 0.10 to 0.13 ± 0.10 (n = 5, P < 0.01, Fig. 8B, right). Additional studies were performed with a high-K+ bath and pipette solution designed to hold E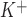 at 0 mV and minimize the effects of the interior negative membrane potential (Fig. 8C). To exclude the possibility that unitary currents under these conditions were carried by K+, Ba2+ was included in both bath and pipette solutions to effectively block K+ channels. Under these conditions, ATP induced similar currents with an observed reversal at 0 mV (E
at 0 mV and minimize the effects of the interior negative membrane potential (Fig. 8C). To exclude the possibility that unitary currents under these conditions were carried by K+, Ba2+ was included in both bath and pipette solutions to effectively block K+ channels. Under these conditions, ATP induced similar currents with an observed reversal at 0 mV (E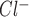 , Fig. 8, C and D). Single-channel conductance was calculated at positive potentials and was 17.1 ± 0.1 pS (n = 7, Fig. 8D). To our knowledge these represent the first single-channel recordings of ATP-stimulated currents in biliary epithelial cells. In summary, exogenous ATP stimulates a 17-pS Cl− channel dependent on intracellular Ca2+ and distinct from CFTR.
, Fig. 8, C and D). Single-channel conductance was calculated at positive potentials and was 17.1 ± 0.1 pS (n = 7, Fig. 8D). To our knowledge these represent the first single-channel recordings of ATP-stimulated currents in biliary epithelial cells. In summary, exogenous ATP stimulates a 17-pS Cl− channel dependent on intracellular Ca2+ and distinct from CFTR.
Fig. 8.
ATP exposure activates unitary Cl− currents. A: single ion channel currents were measured in the cell-attached configuration. Currents are shown at the resting membrane potential (0 mV) and at pipette potentials [pipette voltages (Vp) as indicated]. An all-points amplitude histogram is shown for Vp +40 mV. Under basal conditions few spontaneous openings were measured (C = closed level), left. Exposure to ATP (50 μM) caused rapid appearance of channels carrying outward membrane currents (at Vp +40 mV), right. The presence of 2 open levels (O1 and O2) in each patch was characteristic. B: replacement of the monovalent cations (Na+, K+) in the patch pipette with tetraethylammonium (TEA) did not affect mean open probability (NPo) in response to ATP (NPo 0.66 ± 0.1) shown at left, whereas inclusion of the Cl− channel blocker NPPB significantly inhibited ATP-stimulated currents (NPo 0.13 ± 0.1) shown at right. C: representative current trace showing single-channel events at different voltages (indicated at left of each trace) in presence of high K+ (KCl 140 mM) and simultaneous inclusion of Ba2+ (BaCl2 5 mM), in bath and pipette solution. Open (O) and closed (C) states are represented by dotted lines. D: effect of cation substitution on the single-channel current-voltage relation of the cell-attached patch recordings. Currents were recorded with standard NaCl− containing buffer (○), replacement of monovalent cations with TEA (•) or with high-K+ and -Ba2+ solutions (▵). Replacement of monovalent cations with TEA did not affect membrane currents, whereas the high-K+ solutions resulted in a shift in reversal to 0 mV (ECl−). Each point represents the mean ± SE connected by polynomial fit. The dotted line represents the linear best-fit at the positive potentials recorded with standard extracellular and pipette solutions. The single-channel conductance was calculated from this slope.
Transepithelial Cl− secretion.
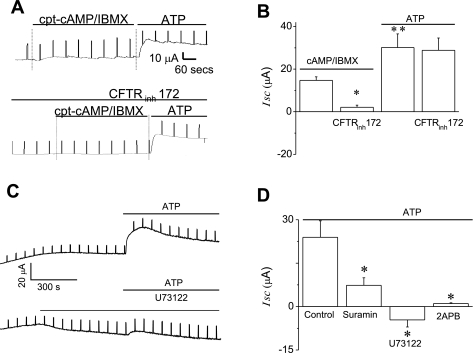
NRC form high-resistance monolayers (Rt > 1,000 Ω·cm2) and respond to secretory agonists by increasing their apical Cl− conductance (35). Addition of 8-(4-chlorophenylthio)-adenosine-3′,5-cyclic monophosphate, sodium salt (cpt)-cAMP (500 μM) and 3-isobutyl-1-methylxanthine (IBMX; 100 μM) to the apical chamber (to activate CFTR) increased Isc from 4.2 ± 2.4 μA/cm2 (n = 8) to 14.7 ± 1.7 μA/cm2 (n = 4, P < 0.05, Fig. 9, A and B). Subsequent addition of ATP to the apical chamber further increased the Isc to 30.1 ± 6.4 μA/cm2 (n = 4, P < 0.05 vs. maximal increase with cAMP-IBMX). In the presence of CFTRinh-172 (5 μM), the cpt-cAMP-IBMX-induced ΔIsc was inhibited (2.1 ± 1.1 μA/cm2, P < 0.01 vs. maximal cAMP-IBMX-induced ΔIsc), but the ATP-induced Isc was unaffected (28.7 ± 5.8 μA/cm2, n = 4 each, P = NS vs. maximal ATP-induced ΔIsc, Fig. 9, A and B). This important finding demonstrates that ATP stimulates transepithelial Cl− secretion from NRC monolayers through CFTR-independent pathways. To determine whether the P2Y-IP3 receptor pathway contributes to the ATP-stimulated increase in transepithelial secretion, further individual studies were performed in the presence or absence of the P2Y receptor antagonist suramin (100 μM), the PLC inhibitor U73122 (10 μM), or the IP3 receptor inhibitor 2-APB (100 μM). In control cells, ATP-stimulated a large increase in Isc (21.9 ± 5.4 μA/cm2, n = 9, Fig. 9, C and D), which was significantly inhibited in the presence of suramin (3.2 ± 0.9 μA/cm2, n = 4, P < 0.05), U73122 (−4.6 ± 2.4 μA/cm2, n = 7, P < 0.01), or 2-APB (1.1 ± 0.3 μA/cm2, n = 6, P < 0.05, Fig. 9D). Taken together these findings are analogous to those in single cells and demonstrate that P2Y receptors contribute to the increase in ATP-stimulated transepithelial secretion through a CFTR-independent, but IP3-dependent, pathway.
Fig. 9.
ATP increases transepithelial Cl− secretion in polarized biliary monolayers. Short-circuit current (Isc) across normal rat cholangiocyte (NRC) monolayers was measured under voltage-clamp conditions in an Ussing chamber. In these representative recordings agonists are added to the apical chamber. A: simultaneous addition of cpt-cAMP (500 μM) and 3-isobutyl-1-methylxanthine (IBMX, 100 μM) increased Isc, and subsequent addition of ATP (100 μM) resulted in a further significant increase in the magnitude of the Isc (top tracing). In the presence of CFTRinh-172 (5 μM) cpt-cAMP-IBMX failed to increase Isc; however, the ATP-stimulated increase in Isc was unaffected (bottom tracing). B: cumulative data showing the average change in Isc after addition of cpt-cAMP-IBMX or ATP in the presence or absence of CFTRinh-172. The y-axis values are reported as ΔIsc (maximum Isc − basal Isc). *CFTRinh-172 significantly inhibited the cpt-cAMP-IBMX-induced increase in Isc (P < 0.01, n = 4 each) **Extracellular ATP added to the apical chamber significantly increased Isc vs. cpt-cAMP-IBMX (P < 0.05, n = 4). C: in this representative recording, addition of ATP (100 μM) to the apical bath increases Isc (top tracing). In the presence of U73122, the ATP-stimulated Isc is inhibited. D: cumulative data demonstrating the average change in ATP-stimulated Isc in the presence of suramin, U73122, or 2-APB. The y-axis values are reported as ΔIsc (maximum Isc − basal Isc). *Suramin (n = 4, P < 0.05), U73122 (n = 7, P < 0.01), or 2-APB (n = 6, P < 0.05) each significantly inhibited the ATP-stimulated increase in Isc.
DISCUSSION
Biliary epithelial cells play a prominent role in regulating the volume and composition of bile through secretion of fluid and electrolytes. Recently, ATP has emerged as an important autocrine/paracrine signaling molecule that is present in bile and integrates the diverse signals controlling responses. For example, increases in intracellular cAMP, cGMP, and Ca2+ all increase ATP release, and elimination of extracellular ATP blocks the secretory response to cAMP (27). In the present studies, the mechanisms responsible for the cellular response to ATP are examined. From combined pharmacological and functional approaches, the principal findings are that 1) ATP activates a 17-pS anion channel distinct from CFTR; 2) the mechanism involves receptor binding and PLC- and IP3-dependent release of calcium from intracellular stores; 3) pharmacological sensitivity of the response is most consistent with a dominant role for P2Y vs. P2X receptors localized to the apical domain of polarized monolayers; and 4) the resulting increase in transepithelial Cl− secretion is quantitatively important. In association with recent findings suggesting that the primary function of CFTR in biliary cells involves ATP release (27), these findings suggest that the ATP release and P2Y receptor activation may represent a dominant pathway for regulation of biliary secretion.
Seven P2X receptors and eight P2Y receptors are currently recognized in mammals (1). Freshly isolated rat cholangiocytes express P2Y1, P2Y2, P2Y4, P2Y6, and P2X4 receptors (11). Mz-Cha-1 cells and NRC monolayers express a similar pattern of P2 receptors including P2Y2, P2X2, P2X4, P2X6 (10, 35, 39). In these biliary preparations, P2X4 and P2Y2 are expressed in highest abundance and are present and functional on the apical membrane (10, 35). P2Y2 and P2X4 can be distinguished by their distinct pharmacological profiles with a rank order of potency for P2Y2 of UTP = ATP > 2meSATP > ADP and P2X4 of Bz-ATP > ATP > αβmeATP. Although there are no specific antagonists for the two P2 receptor subtypes, each demonstrates a different sensitivity to receptor blockers, with P2Y inhibited by suramin and RB-2 but relatively insensitive to brilliant blue G; conversely, P2X are inhibited by brilliant blue G and relatively insensitive to suramin or RB-2. Thus our findings demonstrating 1) a rank order potency of ATP = UTP ≫ Bz-ATP for both the nucleotide-stimulated increase in [Ca2+]i and I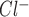 , 2) inhibition of both the ATP-stimulated increase in [Ca2+]i and ICl.ATP by suramin and RB-2 but unaffected by brilliant blue G; and 3) disappearance of the [Ca2+]i and I
, 2) inhibition of both the ATP-stimulated increase in [Ca2+]i and ICl.ATP by suramin and RB-2 but unaffected by brilliant blue G; and 3) disappearance of the [Ca2+]i and I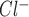 responses to extracellular ATP by prior depletion of intracellular Ca2+ are all consistent with primary signaling through a P2Y receptor.
responses to extracellular ATP by prior depletion of intracellular Ca2+ are all consistent with primary signaling through a P2Y receptor.
In the majority of secretory epithelium, agonist binding to P2Y initiates signaling through Gq/11 to activate the PLCβ/IP3 pathway and release of intracellular Ca2+ stores. Thus P2Y-linked ion channel activation potentially can occur through two pathways, including 1) direct interaction with activated G protein subunits or 2) an increase in [Ca2+]i mediated by IP3 receptors. Direct G protein-gated anion channels have been identified in cholangiocytes and are negatively regulated by Giα-2 and/or Giα-3 and demonstrate voltage-dependent closure (26), properties quite dissimilar from the nucleotide-stimulated channel described here. Additionally, the present studies demonstrate that ATP-activated Cl− current (ICl.ATP) requires intracellular Ca2+ and intact PLC-IP3 signaling pathways. Moreover, intracellular dialysis with purified IP3 activates Cl− currents with identical properties to ICl.ATP in the absence of a P2 agonist, providing further evidence of the involvement of the PLC-IP3 pathway in Cl− channel activation. Thus nucleotide binding to apical P2 receptors initiates a Ca2+ signaling cascade, through PLC-β generation of IP3, resulting in Cl− channel activation in single-cell studies and transepithelial secretion in polarized preparations.
It should be noted that, in addition to the large increase in Cl− permeability measured during whole cell patch clamp studies, in ∼65% of recordings ATP activated a small and transient current measured at 0 mV, consistent with the small-conductance Ca2+-activated K+ channel previously identified in biliary cells (14). This outward K+ conductance may be necessary to maintain the electrical driving force for continued Cl− efflux and hence biliary secretion (37). The role of these calcium-activated K+ channels, as well as other channels and transporters, mediating the secretory response to extracellular nucleotides deserves further investigation.
Further characterization of this integrated secretory response, utilizing polarized NRC monolayers, demonstrated that the ATP-stimulated increase in transepithelial secretion was two- to threefold greater than that induced by activation of CFTR by cAMP-IBMX. This has significant, physiological implications, because targeting the ATP-stimulated secretory pathway in cholangiocytes may potentially provide a therapeutic strategy for the treatment of cystic fibrosis-associated liver disease in which abnormal Cl− transport is felt to contribute to a decrease in bile flow, bile duct plugging, and progressive liver injury (17). Strategies that target the P2-signaling pathway may increase fluid and electrolyte transport, augment bile flow, and therefore provide therapeutic benefit for cholestatic liver disorders such as cystic fibrosis associated with abnormal bile flow.
If these studies, performed in human and rat biliary epithelial models, translate to in vivo conditions, several points, as well as uncertainties, deserve highlighting. First, the molecular identity of the ATP-stimulated Cl− channel is unknown. The biophysical properties of ICl.ATP with mild outward rectification, reversal at 0 mV, time-dependent inactivation at positive potentials, and dependence on intracellular calcium, are similar to the swelling-activated Cl− channel (16, 30, 31) and the flow-stimulated Cl− channel (44) previously described in biliary cells, which are both dependent on autocrine stimulation of membrane P2 receptors by extracellular ATP. ICl.ATP, swell-activated ICl−, and flow-activated ICl−, all require intracellular Ca2+ for activation and are similar to the Ca2+-activated Cl− channels previously described (34); however, the molecular identity of these as well as other epithelial Ca2+-activated Cl− channels remains unresolved. A Cl− channel activated by calcium/calmodulin protein kinase II (CaMKII) has previously been cloned from a cDNA library derived from bovine trachea and termed CaCC (or CLCA-1) (8, 21). Although the cloned protein acts as a CaMKII-modulated Cl− channel when reconstituted in a lipid bilayer (29), RT-PCR failed to detect transcripts for CaCC in biliary epithelia (unpublished observations), and it therefore appears unlikely that this gene product is related to Ca2+-activated Cl− channels in liver cells.
Second, although the present studies demonstrate a predominant role of P2Y in mediating the secretory response to extracellular ATP, they do not exclude a possible contribution of P2X receptors. Previous studies of Mz-Cha-1 cells and NRC monolayers demonstrated that P2X4 stimulation results in a rapid inward current followed by a more sustained secretory response (10), and Bz-ATP activates whole cell Cl− currents even in the absence of consistent changes in bulk cytosolic Ca2+ concentration. In other secretory cells, homomeric P2X4 receptors function as Ca2+ influx pathways to replenish intracellular Ca2+ stores needed for sustained Cl− channel activation (45). However, the permeability of Ca2+ through the pore depends on the presence and concentration of other cations. For example, in homomeric receptors Na+ competes with Ca2+ for permeation (5, 38). Additionally, divalent cations, such as Mg2+, may either cause a fast channel block and/or decrease mean open times indicating an effect on channel gating of heterologously expressed P2X4 receptors (12, 28). Thus the present studies utilizing an extracellular isotonic solution with Na+ and divalent cations, in concentrations similar to that of bile, may have underestimated a potential contribution of P2X4 under these experimental conditions. In fact, both P2X and P2Y probably contribute to calcium-dependent signal transduction cascades to form an integrated and sustained secretory response in biliary epithelium. For example, upon agonist binding, P2Y2 receptor coupling to G proteins generates IP3, through PLC-β, resulting in release of Ca2+ from IP3 receptors, whereas simultaneous agonist binding to P2X4 receptors results in cation and Ca2+ influx to replenish Ca2+ stores necessary for continued secretion. As an additional source of intricacy, P2X and P2Y isoforms can each assemble into heteromultimers, adding an additional layer of complexity to the cellular responses to extracellular nucleotides.
Third, the expression of distinct P2 receptors on the apical membrane may vary in response to changing physiological conditions. Cholangiocytes demonstrate a rapid rate of constitutive exocytosis capable of replacing 1.3% of the plasma membrane each minute and ∼80% of the plasma membrane each hour (9), and therefore the repertoire of P2 receptors available to bind nucleotides in bile may demonstrate minute-to-minute regulation. In fact, it has been shown that elements of the cholangiocyte cAMP-stimulated secretory apparatus, including CFTR, AE2, and AQP-1, may be packaged, transported, and inserted together during stimulated exocytosis (40), presumably to achieve rapid and efficient secretory responses. If a similar paradigm exists for P2-mediated secretion, regulated exo- and endocytosis may rapidly modulate the expression of plasma membrane P2 receptors, channels, and/or other transporters, to achieve integrated responses during changing physiological conditions.
Lastly, it is interesting to note that there is significant functional heterogeneity between small and large cholangiocytes in terms of secretory responses. In the rat, small cholangiocytes, from the “upstream” small intrahepatic bile ducts, do not express CFTR and have very little secretory response to cAMP, whereas large cholangiocytes, from “downstream” large ducts, express CFTR and respond to cAMP with large increases in fluid and electrolyte transport (2). Although small cholangiocytes contribute only modestly to secretion, they nonetheless release hormones and growth factors capable of signaling to large cholangiocytes (2, 20, 41). Thus a functional axis may exist along the bile duct to coordinate organ-level responses to physiological and pathological stimuli affecting the liver. Whether a P2-signaling axis exists along the bile duct, wherein ATP released from small cholangiocytes upstream may contribute importantly to local purinergic signaling, serve as a source for ATP in bile, and represent an important paracrine signal to the downstream P2 receptor-expressing large cholangiocytes, is unknown.
These considerations regarding multiple receptors in multiple configurations suggest that biliary purinergic signaling responses are likely to be shaped in response to changing physiological demands by changes in ATP release and even the types of receptors expressed. However, under the conditions of these studies, the P2Y pathway activating intracellular calcium release and ∼17-pS anion channels predominates. Since there is increasing evidence that the cAMP-dependent secretory responses also depend on ATP release, this is likely to represent a final common pathway for the dominant conductance controlling transepithelial Cl− secretion. Accordingly, regulation of local P2 signaling pathways, through effects on ATP availability and/or delivery of receptor agonists, represents an attractive therapeutic target for treatment of cholestasis and other disorders associated with impaired bile formation.
GRANTS
This study was supported by the Cystic Fibrosis Foundation (FERANC08G0), the Children's Medical Center Foundation, and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health grant RO1 DK078587 (A. P. Feranchak).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58: 281–341, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpini G, Roberts S, Kuntz SM, Ueno Y, Gubba S, Podila PV, Lesage G, LaRusso NF. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology 110: 1636–1643, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Bai D, del Corsso C, Srinivas M, Spray DC. Block of specific gap junction channel subtypes by 2-aminoethoxydiphenyl borate (2-APB). J Pharmacol Exp Ther 319: 1452–1458, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Basavappa S, Middleton JP, Mangel A, McGill J, Cohn JA, Fitz JG. Cl− and K+ transport in human biliary cell lines. Gastroenterology 104: 1796–1805, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Buell G, Lewis C, Collo G, North RA, Surprenant A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J 15: 55–62, 1996. [PMC free article] [PubMed] [Google Scholar]
- 6.Chang D, Hsieh PS, Dawson DC. Calcium: a program in basic for calculating the composition of solutions with specified free concentrations of calcium, magnesium and other divalent cations. Comput Biol Med 18: 351–366, 1988. [DOI] [PubMed] [Google Scholar]
- 7.Chari RS, Schutz SM, Haebig JA, Shimokura GH, Cotton PB, Fitz JG, Meyers WC. Adenosine nucleotides in bile. Am J Physiol Gastrointest Liver Physiol 270: G246–G252, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham SA, Awayda MS, Bubien JK, Ismailov II, Arrate MP, Berdiev BK, Benos DJ, Fuller CM. Cloning of an epithelial chloride channel from bovine trachea. J Biol Chem 270: 31016–31026, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Doctor RB, Dahl R, Fouassier L, Kilic G, Fitz JG. Cholangiocytes exhibit dynamic, actin-dependent apical membrane turnover. Am J Physiol Cell Physiol 282: C1042–C1052, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Doctor RB, Matzakos T, McWilliams R, Johnson S, Feranchak AP, Fitz JG. Purinergic regulation of cholangiocyte secretion: identification of a novel role for P2X receptors. Am J Physiol Gastrointest Liver Physiol 288: G779–G786, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Dranoff JA, Masyuk AI, Kruglov EA, LaRusso NF, Nathanson MH. Polarized expression and function of P2Y ATP receptors in rat bile duct epithelia. Am J Physiol Gastrointest Liver Physiol 281: G1059–G1067, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Evans RJ Single channel properties of ATP-gated cation channels (P2X receptors) heterologously expressed in Chinese hamster ovary cells. Neurosci Lett 212: 212–214, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Feranchak AP, Berl T, Capasso J, Wojtaszek PA, Han J, Fitz JG. p38 MAP kinase modulates liver cell volume through inhibition of membrane Na+ permeability. J Clin Invest 108: 1495–1504, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feranchak AP, Doctor RB, Troetsch M, Brookman K, Johnson SM, Fitz JG. Calcium-dependent regulation of secretion in biliary epithelial cells: the role of apamin-sensitive SK channels. Gastroenterology 127: 903–913, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Feranchak AP, Fitz JG. Adenosine triphosphate release and purinergic regulation of cholangiocyte transport. Semin Liver Dis 22: 251–262, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Feranchak AP, Roman RM, Doctor RB, Salter KD, Toker A, Fitz JG. The lipid products of phosphoinositide 3-kinase contribute to regulation of cholangiocyte ATP and chloride transport. J Biol Chem 274: 30979–30986, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Feranchak AP, Sokol RJ. Cholangiocyte biology and cystic fibrosis liver disease. Semin Liver Dis 21: 471–488, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Fitz JG Cellular mechanisms of bile secretion. In: Hepatology, edited by Zakim D and Boyer TD. Philadelphia, PA: Saunders, 1996, p. 362–376.
- 19.Fitz JG, Sostman A. Nucleotide receptors activate cation, potassium, and chloride currents in a liver cell line. Am J Physiol Gastrointest Liver Physiol 266: G544–G553, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Glaser S, Francis H, Demorrow S, Lesage G, Fava G, Marzioni M, Venter J, Alpini G. Heterogeneity of the intrahepatic biliary epithelium. World J Gastroenterol 12: 3523–3536, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruber AD, Elble RC, Ji HL, Schreur KD, Fuller CM, Pauli BU. Genomic cloning, molecular characterization, and functional analysis of human CLCA1, the first human member of the family of Ca2+-activated Cl− channel proteins. Genomics 54: 200–214, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Harks EG, Camina JP, Peters PH, Ypey DL, Scheenen WJ, van Zoelen EJ, Theuvenet AP. Besides affecting intracellular calcium signaling, 2-APB reversibly blocks gap junctional coupling in confluent monolayers, thereby allowing measurement of single-cell membrane currents in undissociated cells. FASEB J 17: 941–943, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Knuth A, Gabbert H, Dippold W, Klein O, Sachsse W, Bitter-Suermann D, Prellwitz W, Meyer zum Buschenfelde KH. Biliary adenocarcinoma. Characterisation of three new human tumor cell lines. J Hepatol 1: 579–596, 1985. [DOI] [PubMed] [Google Scholar]
- 24.Lidofsky SD, Xie MH, Sostman A, Scharschmidt BF, Fitz JG. Vasopressin increases cytosolic sodium concentration in hepatocytes and activates calcium influx through cation-selective channels. J Biol Chem 268: 14632–14636, 1993. [PubMed] [Google Scholar]
- 25.McGill J, Basavappa S, Shimokura GH, Middleton JP, Fitz JG. Adenosine triphosphate activates ion permeabilities in biliary epithelial cells. Gastroenterology 107: 236–243, 1994. [DOI] [PubMed] [Google Scholar]
- 26.McGill J, Gettys TW, Basavappa S, Fitz JG. GTP-binding proteins regulate high conductance anion channels in rat bile duct epithelial cells. J Membr Biol 133: 253–261, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Minagawa N, Nagata J, Shibao K, Masyuk AI, Gomes DA, Rodrigues MA, Lesage G, Akiba Y, Kaunitz JD, Ehrlich BE, LaRusso NF, Nathanson MH. Cyclic AMP regulates bicarbonate secretion in cholangiocytes through release of ATP into bile. Gastroenterology 133: 1592–1602, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Negulyaev YA, Markwardt F. Block by extracellular Mg2+ of single human purinergic P2X4 receptor channels expressed in human embryonic kidney cells. Neurosci Lett 279: 165–168, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Ran S, Fuller CM, Arrate MP, Latorre R, Benos DJ. Functional reconstitution of a chloride channel protein from bovine trachea. J Biol Chem 267: 20630–20637, 1992. [PubMed] [Google Scholar]
- 30.Roman RM, Feranchak AP, Salter KD, Wang Y, Fitz JG. Endogenous ATP regulates Cl− secretion in cultured human and rat biliary epithelial cells. Am J Physiol Gastrointest Liver Physiol 276: G1391–G1400, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Roman RM, Wang Y, Fitz JG. Regulation of cell volume in a human biliary cell line: calcium-dependent activation of K+ and Cl− currents. Am J Physiol Gastrointest Liver Physiol 271: G239–G248, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Salter KD, Fitz JG, Roman RM. Domain-specific purinergic signaling in polarized rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol 278: G492–G500, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Salter KD, Roman RM, LaRusso NR, Fitz JG, Doctor RB. Modified culture conditions enhance expression of differentiated phenotypic properties of normal rat cholangiocytes. Lab Invest 80: 1775–1778, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Schlenker T, Fitz JG. Calcium-activated chloride channels in a human biliary cell line: regulation by calcium/calmodulin-dependent protein kinase. Am J Physiol Gastrointest Liver Physiol 271: G304–G310, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Schlenker T, Romac JMJ, Sharara A, Roman RM, Kim S, LaRusso N, Liddle R, Fitz JG. Regulation of biliary secretion through apical purinergic receptors in cultured rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol 273: G1108–G1117, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Simpson A Fluorescent measurement of [Ca2+]. In: Methods in Molecular Biology: Calcium Signaling Protocols, edited by Lambert D. Totowa, NJ: Humana, 1999.
- 37.Singh SK, Mennone A, Gigliozzi A, Fraioli F, Boyer JL. Cl−-dependent secretory mechanisms in isolated rat bile duct epithelial units. Am J Physiol Gastrointest Liver Physiol 281: G438–G446, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, Stuhmer W. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc Natl Acad Sci USA 93: 3684–3688, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor AL, Schwiebert LM, Smith JJ, King C, Jones JR, Sorscher EJ, Schwiebert EM. Epithelial P2x purinergic receptor channel expression and function. J Clin Invest 104: 875–884, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tietz PS, Marinelli RA, Chen XM, Huang B, Cohn J, Kole J, McNiven MA, Alper S, LaRusso NF. Agonist-induced coordinated trafficking of functionally related transport proteins for water and ions in cholangiocytes. J Biol Chem 278: 20413–20419, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Ueno Y, Alpini G, Yahagi K, Kanno N, Moritoki Y, Fukushima K, Glaser S, Lesage G, Shimosegawa T. Evaluation of differential gene expression by microarray analysis in small and large cholangiocytes isolated from normal mice. Liver Int 23: 449–459, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Vroman B, LaRusso N. Development and characterization of polarized primary cultures of rat intrahepatic bile duct epithelial cells. Lab Invest 74: 303–313, 1996. [PubMed] [Google Scholar]
- 43.Wang Y, Roman RM, Schlenker T, Hannun YA, Raymond JR, Fitz JG. Cytosolic calcium and protein kinase C(alpha) couple cellular metabolism to membrane K+ permeability in a human biliary cell line. J Clin Invest 99: 2890–2897, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woo K, Dutta AK, Patel V, Kresge C, Feranchak AP. Fluid flow induces mechanosensitive ATP release, calcium signalling and Cl− transport in biliary epithelial cells through a PKCzeta-dependent pathway. J Physiol 586: 2779–2798, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zsembery A, Boyce AT, Liang L, Peti-Peterdi J, Bell PD, Schwiebert EM. Sustained calcium entry through P2X nucleotide receptor channels in human airway epithelial cells. J Biol Chem 278: 13398–13408, 2003. [DOI] [PubMed] [Google Scholar]