Abstract
Intestinal mucosal inflammation is associated with epithelial wounds that rapidly reseal by migration of intestinal epithelial cells (IECs). Cell migration involves cycles of cell-matrix adhesion/deadhesion that is mediated by dynamic turnover (assembly and disassembly) of integrin-based focal adhesions. Integrin endocytosis appears to be critical for deadhesion of motile cells. However, mechanisms of integrin internalization during remodeling of focal adhesions of migrating IECs are not understood. This study was designed to define the endocytic pathway that mediates internalization of β1-integrin in migrating model IECs. We observed that, in SK-CO15 and T84 colonic epithelial cells, β1-integrin is internalized in a dynamin-dependent manner. Pharmacological inhibition of clathrin-mediated endocytosis or macropinocytosis and small-interfering RNA (siRNA)-mediated knock down of clathrin did not prevent β1-integrin internalization. However, β1-integrin internalization was inhibited following cholesterol extraction and after overexpression of lipid raft protein, caveolin-1. Furthermore, internalized β1-integrin colocalized with the lipid rafts marker cholera toxin, and siRNA-mediated knockdown of caveolin-1 and flotillin-1/2 increased β1-integrin endocytosis. Our data suggest that, in migrating IEC, β1-integrin is internalized via a dynamin-dependent lipid raft-mediated pathway. Such endocytosis is likely to be important for disassembly of integrin-based cell-matrix adhesions and therefore in regulating IEC migration and wound closure.
Keywords: mucosal restitution, endocytosis, recycling, dynamin, caveolin, flotillin
epithelial erosions and ulcerations are observed in active inflammatory disorders of the intestine. In response to injury, the epithelium migrates to rapidly cover denuded surfaces and reseal wounds (17, 38, 59). In this process, referred to as restitution, epithelial cells adjoining the wound migrate as a cohesive sheet of cells. Migrating cells transiently adhere to the matrix via an array of specialized basal structures that evolve from focal complexes to focal adhesions to fibrillar adhesions (42, 34, 52, 69, 71). Cell adhesion complexes contain a structural core of transmembrane integrins (25) that form heterodimers consisting of α- and β-subunits. Intestinal epithelial cells (IECs) are enriched in integrin dimers containing a β1-subunit that plays an important role in cell migration (38, 39). Integrins affiliate with the matrix via their extracellular domains and also interact with the cytoskeleton via cytosolic scaffolding proteins. Such dual interactions make integrin-based adhesion complexes not simply substrate anchors but critical mechanical sensors and signal transducers that orchestrate the entire process of cell movement (21, 57).
Cell migration involves cycles of cell attachment to and detachment from the underlying matrix that is mediated by the dynamic turnover (assembly and disassembly) of integrin-mediated adhesions. It is noteworthy that disassembly of integrin-based adhesion structures is critical for effective cell movement (16, 23, 30, 57). Mechanistically, this process is poorly understood; however, a current paradigm implies that cell matrix adhesions in migrating cells can be rapidly disrupted via endocytic removal of integrins from the cell surface (5, 6, 30). Furthermore, these internalized integrins are believed to recycle back to the plasma membrane to aid the formation of new adhesion complexes (9, 30, 53). Together, this suggests a critical role of integrin trafficking in motility of IEC during mucosal restitution.
The endocytic mechanisms that remove β1-integrin from the cell surface remain controversial (9). The majority of data on this subject were obtained by using individually migrating fibroblasts or fibroblast-like cells, and they implicated two major pathways, namely clathrin-mediated (4, 48, 49, 56) and lipid raft-mediated endocytosis (43, 61, 63) in β1-integrin internalization. It remains unknown if this discrepancy is based on peculiar behavior of different cell lines or it possibly reflects use of nonselective tools to probe different endocytic pathways. It is noteworthy that no studies examined mechanisms of β1-integrin internalization during collective migration of IECs as a sheet, despite the importance of this mechanism in intestinal mucosal restitution.
This study was designed to dissect endocytic pathways responsible for β1-integrin internalization in migrating human colonic epithelial cells. The involvement of major internalization pathways was probed by a combination of different molecular tools that include pharmacological inhibitors of endocytosis, colocalization with known pathway markers, use of dominant-negative constructs, and small-interfering RNA (siRNA)-mediated downregulation of target proteins. Our results suggest that, in motile intestinal epithelia, β1-integrin is internalized via a dynamin-dependent lipid raft-mediated pathway.
MATERIALS AND METHODS
Antibodies and other reagents.
The following polyclonal (pAb) and monoclonal (mAb) antibodies were used for immunofluorescence labeling and Western blotting: anti-β1-integrin mAb clone P5D2, cMyc pAb (Santa Cruz Biotechnology, Santa Cruz, CA), anti-β1-integrin mAb clone 18, flotillin-1 mAb clone 18, flotillin-2 mAb clone 29, caveolin-1 mAb clone 2234, caveolin-1 pAb (BD Biosciences, San Jose, CA), clathrin heavy chain pAb (Cell Signaling Technology, Danvers, MA), HA pAb (Covance, San Diego, CA), α-actin pAb (Sigma, St. Louis, MO), Alexa-phalloidin, fluorescein-labeled lysine-fixable dextran (3,000 Da), Alexa-546-labeled human transferrin, donkey anti-rabbit, goat anti-rat, and goat anti-mouse secondary antibodies conjugated to Alexa-488 or Alexa-568 dyes were obtained from Invitrogen (Carlsbad, CA). Fluorescein isothiocyanate (FITC)-labeled cholera toxin subunit B (CtxB) was purchased from Sigma, and horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse secondary antibodies were obtained from Jackson Immunoresearch Laboratories (West Grove, PA). Protease inhibitors cocktail (used 1:100) and phosphatase inhibitor cocktails 1 and 2 (both used 1:200) were obtained from Sigma.
Cell cultures, adenoviruses, siRNA transfections.
SK-CO15 cells [gift from Dr. Dr. E. Rodriguez-Boulan, Weill Medical College of Cornell University (35)] and T84 IEC (American Type Culture Collection, Rockville, MD) were grown as described previously (29, 64). Linear wounds were made in a monolayer of epithelial cells using a 20 μl micro-tip attached to a vacuum flask. Recombinant adenoviruses expressing chimeric tetracycline-regulated transcription activator, wild type dynamin-1, its K44A dominant-negative mutant, or the wild-type caveolin-1 under control of tetracycline-regulated (3) promoter (36, 72) were generous gift of Dr. I. Nabi, University of British Columbia (Vancouver, British Columbia, Canada). The confluent cells were incubated in serum-free DMEM media containing 2 mM EGTA for 30 min at 37°C to remove calcium and allow tight junction disassembly. The cells were then wounded and washed two times with normal serum-free DMEM medium, and the viruses were added to the cells for 16–43 h. For infection of the spreading cells, the EGTA step was omitted. RNA interference experiments were performed using SmartPool siRNA for human clathrin heavy chain, caveolin-1, flotillin-1, and flotillin-2 (Dharmacon, Lafayette, CO). Lamin A/C SmartPool and nontargeting human siRNA SmartPool #1 were used as controls. SK-CO15 cells were transfected in Opti-MEM-I medium (Invitrogen) with 50–100 nm siRNA using DharmaFect 1 transfection reagent and analyzed 68–93 h posttransfection. The efficiency of protein downregulation was verified by Western blot. The transfected cells were either wounded for immunolabeling or trypsinized and replated for surface biotinylation.
Immunofluorescence labeling.
Cells grown on collagen-coated transwell filters or cover slips were fixed with 3.7% paraformaldehyde and either permeabilized with 0.5% Triton X-100 or postfixed with ethanol for immunolabeling with clathrin antibodies. Fixed cells were blocked in HEPES-buffered Hanks' balanced salt solution (HBSS+) containing 1% BSA (blocking buffer) and incubated for 60 min with primary antibodies in blocking buffer. Cell monolayers were washed, incubated for 60 min with Alexa dye-conjugated secondary antibodies, and mounted on slides using ProLong Antifade medium (Invitrogen). Nuclei were stained with TO-PRO-3 iodide (Invitrogen), and F-actin was labeled with Alexa-conjugated phalloidin. Stained monolayers were examined using a Zeiss LSM510 laser scanning confocal microscope (Zeiss Microimaging, Thornwood, NY) with ×63 or ×100 Pan-Apochromat oil lenses coupled to a Zeiss 100M axiovert. Fluorescent dyes were imaged sequentially in frame-interlace mode to eliminate cross talk between channels. Image analysis including pixel intensity measurement was done using an LSM 5 Pascal Image browser, version 3.2, from Zeiss Micromaging. The number of internalized vesicles per cell was counted using Metamorph analysis software, version 7.0r3.
Wound closure assay.
SK-CO15 cells were grown on plastic plates or Transwell membrane filters and wounded after reaching confluency. Wound closure was recorded at the indicated time points using a camera attached to an inverted microscope (Axiover 35 M; Zeiss), and the images were analyzed using Image J software.
Antibody and ligands internalization assays.
Migrating IECs were washed three times in cold serum-free culture medium and incubated in the same medium with azide-free mouse mAb specific for the extracellular domain of β1-integrin, clone P5D2, 2 μg/ml, for 30 min at 4°C. Internalization of β1-integrin-antibody complex was initiated by addition of prewarmed serum-free medium to the cells, followed by incubation at 37°C for indicated times. Surface-bound noninternalized antibodies were stripped from cells by incubation in acidified serum-free culture medium, pH 4.0 for 10 min. Cells were then rinsed with HBSS+, fixed, and permeabilized, and the internalized antibodies were immunostained with fluorophore-labeled secondary antibodies. Where indicated, FITC-CTxB (2 μg/ml) and Alexa 546-labeled human transferrin (20 μg/ml) were added together with the antibodies; fluoresceinated dextran (5.4 μg/ml) was added after antibody binding at the onset of internalization.
Biotinylated β1-integrin internalization assay.
Surface biotinylation experiments were conducted in sparsely plated spreading cells to enrich for a population of migrating cells. SK-CO15 cells were surface biotinylated using thiol-cleavable EZ-Link Sulfo-NHS-SS-Biotin (Pierce, Rockford, IL), 0.5 mg/ml, dissolved in PBS for 30 min on ice. Labeled cells were washed three times in cold PBS and then incubated at 37°C in prewarmed serum-free DMEM media for 20 min, after which biotin groups remaining on the cell surface were cleaved off by three 20-min washes with buffer containing reducing agent [100 mM MESNA (sodium-2-mercaptoethane sulfonate), 50 mM Tris (pH 8.6), 100 mM NaCl, 1 mM EDTA, and 0.2% BSA] at 4°C. The cells were rinsed two times in ice-cold PBS, and excess biotin was quenched with 60 mM iodoacetamide in PBS buffer for 5 min at +4°C. Cells were consequently washed three times in ice-cold PBS and lysed in RIPA buffer (20 mM Tris, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1% sodium deoxycholate, 1% SDS, and 1% Triton X-100) containing protease and phosphatase inhibitors. The lysates were clarified by centrifugation at 14,000 g for 10 min. Biotinylated β1-integrin was captured on streptavidin ELISA plates (NUNC IMMOBILIZER Streptavidin C8, Roskilde, Denmark) from the cell lysates diluted to 10 μg/ml total protein in PBS containing 0.5% Tween 20, pH 7.3 (PBST), during 2 h incubation at room temperature on a shaker. Plates were then washed three times with PBST, incubated with β1-integrin antibody P5D2 (Santa Cruz) (2 μg/ml) for 2 h at room temperature, washed, and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature; the plates were subsequently washed three times in PBST before adding color substrate (R&D Systems, Minneapolis, MN) for 10 min. Color development was stopped by adding an equal amount 4 M H2SO4 and analyzed at 450 nm.
Immunobloting.
Cells were washed with HBSS+, scraped off, and homogenized in Laemmli sample buffer from Bio-Rad (Hercules, CA) supplemented with phosphatase and proteinase inhibitors and boiled after addition of β-mercaptoethanol. SDS-PAGE and Western blotting were performed by standard protocols. The films were scanned, and the immunoreactive protein bands were quantified by densitometry using UN-SCAN-IT digitizing software.
Pharmacological inhibitors.
Wounded cell monolayers were pretreated with inhibitors at 37°C for 30 min with subsequent antibody binding and internalization in the presence of inhibitors. For potassium depletion, cells were first subject to osmotic shock by incubation in diluted twofold serum-free culture medium for 5 min followed by incubation in potassium-deficient medium (100 mM NaCl, 50 mM HEPES, pH 7.4, 1 mM CaCl2, and 1 mM MgCl2) The following concentrations of the inhibitors were used: 50 μM chlorpromazine; 10 mM methyl-β-cyclodextrin (MβCD), 50 μM 5-(N-ethyl-N-isopropyl)amiloride (EIPA), or 50 μM 5-(N,N-dimethyl)amiloride (DMA). Stock solutions of water-insoluble inhibitors were prepared in dimethyl sulfoxide (DMSO). The same concentration of DMSO (0.001%) was used in control experiments.
Statistical analysis.
Statistical significance of the difference between the means was analyzed by two-tailed Student's t-test for comparison between two groups of data, with statistical significance assumed at P < 0.05.
RESULTS
Dynamin regulates β1-integrin endocytosis in migrating epithelial cells.
The endocytic mechanism that removes β1-integrin from the cell surface in migrating IECs was investigated using model SK-CO15 and T84 human colonic epithelial cell lines. Cells were induced to migrate by mechanical wounding of confluent epithelial monolayers. Because dynamin is a large GTPase critical for scission of plasma membrane vesicles generated by multiple internalization pathways (10), we first examined its role in endocytosis of β1-integrin in migrating IECs. To modulate dynamin function, SK-CO15 cells were infected with recombinant adenoviruses expressing wild-type dynamin-1 or its K44A mutant lacking GTPase activity (11). Endocytosis of β1-integrin was analyzed by antibody internalization assay and confocal microscopy. After 10 min of internalization, β1-integrin antibodies were observed in characteristic vesicular structures in the cytoplasm (Fig. 1A). In contrast, no intracellular antibodies were detected in cells incubated at 4°C to prevent endocytosis (data not shown). Overexpression of the K44A dynamin mutant substantially decreased the number of cytosolic vesicles containing internalized β1-integrin (Fig. 1A, arrow). Because antibody binding may cluster integrins on the cell surface and thus artificially promote their endocytosis, we also used surface biotinylation assay to independently examine steady-state internalization of β1-integrin. We observed that, in spreading SK-CO15 cells overexpressing the K44A dynamin-1 mutant but not wild-type dynamin-1, internalization of biotinylated β1-integrin was significantly decreased (by ∼40%) (Fig. 1B). Furthermore, the rate of cell migration and wound closure was decreased in the SK-CO15 monolayers expressing K44A dynamin mutant (Fig. 2). Together these results demonstrate that dynamin is involved in internalization of β1-integrin and that such a dynamin-dependent endocytosis is an important regulator of IEC migration.
Fig. 1.
Internalization of β1-integrin depends on the activity of dynamin GTPase. A: SK-CO15 cells grown on collagen-coated transwell filters were wounded and infected with adenoviral constructs encoding the hemagglutinin (HA)-tagged dominant-negative mutant of dynamin-1 (K44A) or wild-type dynamin-1. The β1-integrin antibody internalization assay (10 min) was performed 20 h postinfection. Cells were fixed, and localization of internalized β1-integrin antibody (red) and the expression tag (green) was determined by immunofluorescent labeling and confocal microscopy. Arrows point to the absence of β1-integrin-containing vesicles in cells overexpressing D/N dynamin-1. Scale bar, 20 μm. B: surface-biotinylated β1-integrin internalization assay. Spreading SK-CO15 cells were infected with the same adenoviral constructs as in A or with the control, transactivator-bearing virus, and 16 h postinfection cells were surface biotinylated. Surface proteins were internalized, and biotinylated β1-integrin was captured on enzyme-linked immunosorbent assay plates and detected with anti-β1-integrin antibody. Data are expressed as percentage of control (means ± SE, n = 8 experiments). *Statistically significant difference (P < 0.05).
Fig. 2.
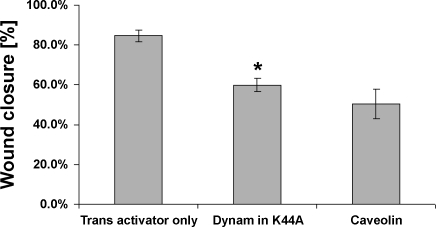
Effects of endocytosis inhibitors on migration of SK-CO15 cells. SK-CO15 cells cultured on collagen-coated transwell filters were grown to confluence, wounded, and infected with adenoviral constructs encoding HA-tagged dominant-negative mutant of dynamin-1 (K44A), transactivator plasmid only, or caveolin-1. Wound closure was recorded 48 h after wounding using a camera attached to an inverted microscope (Axiovert 35 M; Zeiss), and the images were analyzed using Image J software. Quantification results of data representing means ± SD from 4 independent experiments. *P < 0.001 compared with transactivator control.
Clathrin pathway is not critical for β1-integrin internalization at the leading edge of migrating IEC.
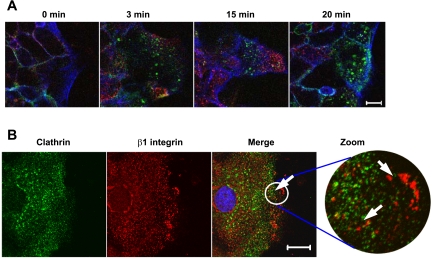
Because dynamin regulates both clathrin-dependent and lipid raft-mediated endocytosis (10), we analyzed the role of these pathways in regulating internalization of plasma membrane β1-integrin in migrating IECs. To examine involvement of the clathrin-mediated pathway, we first compared the dynamics of β1-integrin internalization with endocytosis of transferrin, a classical marker of the clathrin pathway. We performed antibody internalization assay in the presence of fluorescently labeled human transferrin. No significant colocalization of internalized transferrin and β1-integrin/antibody complex was observed within 3–20 min of endocytosis (Fig. 3A). In addition, we examined localization of internalized β1-integrin and clathrin by performing the antibody internalization assay followed by immunolabeling for clathrin heavy chain. As shown in Fig. 3B, β1-integrin-antibody complexes that were internalized at the leading edge of migrating cells did not colocalize with clathrin.
Fig. 3.
Internalized β1-integrin does not colocalize with markers of clathrin-mediated endocytosis. A: time course of cointernalization of β1-integrin and transferrin at the leading edge of migrating T84 cells. Confluent cell monolayers were wounded, and 20 h later β1-integrin antibody internalization assay was performed in the presence of Alexa 546-labeled human transferrin for indicated times. Cells were fixed, and localization of internalized β1-integrin antibody (green) and transferrin (red) was determined by immunofluorescence labeling and confocal microscopy. F-actin was visualized with Alexa-635-labeled phalloidin (blue). Scale bar, 10 μm. B: β1-integrin antibody internalization assay was performed for 4 min in migrating SK-CO15 cells. Cells were fixed and double immunolabeled for clathrin heavy chain (green) and β1-integrin antibody (red). Note that integrin antibodies at the leading edge do not colocalize with clathrin-containing vesicles (arrows). Scale bar, 20 μm.
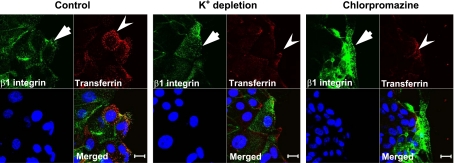
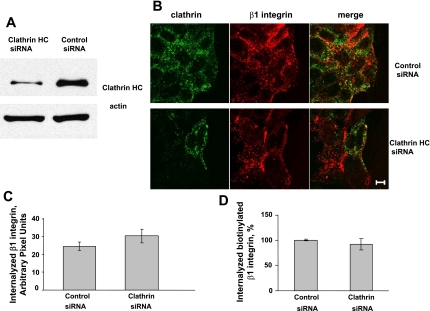
To further gain insight into the role of the clathrin pathway in regulating β1-integrin internalization, we used pharmacological inhibitors of this pathway such as potassium depletion and chlorpromazine (28) as well as siRNA-mediated downregulation of clathrin expression. First, internalization of β1-integrin antibody was compared in three different groups of migrating T84 cells, which included control cells, cells exposed to chlorpromazine (50 μM), and cells subjected to potassium depletion as described in materials and methods. In control experiments, these pharmacological inhibitors blocked internalization of transferrin in SK-CO15 cells (Fig. 4, arrowheads), which indicates their effectiveness in our experimental conditions. However, neither inhibitor of the clathrin pathway prevented β1-integrin antibody internalization (Fig. 4). To confirm our pharmacological observations, we inhibited clathrin expression by using a siRNA approach. Transfection of SK-CO15 cells with clathrin heavy chain-specific siRNA decreased expression of this coat protein by ∼80% compared with control siRNA-transfected cells (Fig. 5A). A control experiment demonstrated that clathrin knockdown efficiently inhibited transferrin endocytosis [Supplemental Fig. 1 (Supplemental data for this article may be found on the American Journal of Physiology: Gastrointestinal and Liver Physiology web site.)]. However, downregulation of clathrin expression failed to block internalization of β1-integrin, as determined by either antibody internalization assay (Fig. 5, B and C) or by β1-integrin biotinylation assay (Fig. 5D). Together, these data suggest that the clathrin-mediated pathway does not play a major role in β1-integrin endocytosis in migrating IECs.
Fig. 4.
Pharmacological inhibition of the clathrin pathway blocks transferrin internalization but does not influence β1-integrin endocytosis. Confluent SK-CO15 monolayers were wounded and 20 h later subjected to the β1-integrin antibody internalization assay in the presence of labeled transferrin (red, arrowheads). The internalization was performed for 7 min in control cell monolayers and in cells either subjected to potassium depletion or treated with chlorpromazine (50 μM). Cells were immunolabeled for internalized β1-integrin (green, arrows), whereas nuclei were highlighted with ToPro3 (blue). Note that inhibitors of the clathrin pathway prevent transferring internalization but do not affect β1-integrin endocytosis. Scale bar, 20 μm.
Fig. 5.
Small-interfering RNA (siRNA)-mediated knockdown of clathrin heavy chain does not prevent β1-integrin internalization. A: SK-CO15 cells were transfected with siRNA targeted to the clathrin heavy chain or control Lamin A/C siRNA. Western blot analysis demonstrates efficient knockdown of clathrin heavy chain. B: control or clathrin heavy chain-depleted SK-CO15 cell monolayers were wounded and 20 h later subjected to the anti-β1-integrin antibody internalization assay for 10 min. Cells were fixed and double-immunolabeled for internalized β1-integrin antibody (red) and clathrin heavy chain (green). Note efficient internalization of β1-integrin in clathrin-depleted cells. Scale bar, 10 μm. C: quantification of pixel intensity of β1-integrin fluorescence on images presented in B and shown as means ± SE, n = 12. D: surface-biotinylated β1-integrin internalization assay. SK-CO15 cells were replated 3 days post-siRNA transfection, allowed to migrate for 16 h, and subjected to the surface-biotinylation assay. Data are expressed as a percentage of control and shown as means ± SE, n = 4.
Internalization of β1-integrin occurs via lipid rafts.
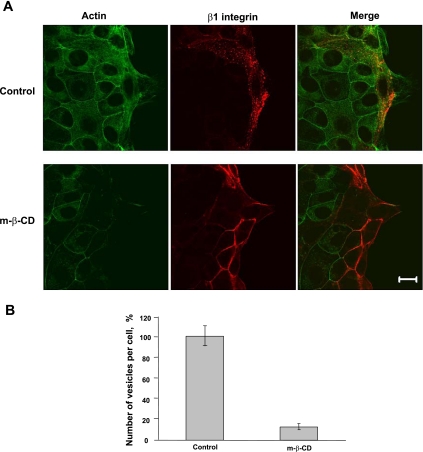
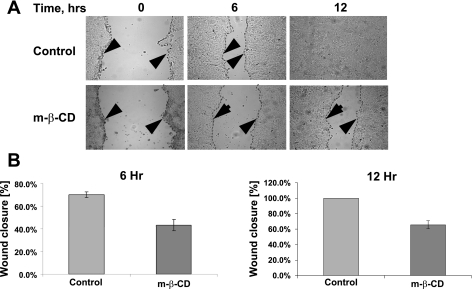
We next investigated participation of lipid rafts in β1-integrin endocytosis. Lipid rafts are broadly defined as low-density cholesterol-enriched membrane subdomains that are also enriched in proteins such as glycosylphosphatidyl inositol (GPI)-linked proteins, caveolins, flotillins, and sphingolipids such as ganglioside GM1. GM1 is a natural receptor for cholera toxin; therefore, we used CtxB as marker for lipid raft-mediated endocytosis. We analyzed cointernalization of β1-integrin antibody with FITC-labeled CtxB and examined intracellular localization of these two ligands. As shown in Fig. 6, internalized β1-integrin and FITC-CTxB colocalize in vesicular structures at the migrating front of SK-CO15 cells. Because raft-mediated endocytosis is sensitive to cholesterol depletion (33, 36), we examined the effect of cholesterol depletion on β1-integrin internalization and cell migration by using MβCD, that sequesters cholesterol from cell membranes (27, 31, 62). Wounded SK-CO15 cell monolayers were incubated with 10 mM MβCD for 30 min, and β1-integrin internalization was determined using the antibody internalization assay. As shown in Fig. 7, MβCD treatment significantly (∼87%) decreased accumulation of β1-integrin in cytosolic vesicles at the leading edge of migrating cells. Importantly, MβCD also decreased the velocity of wound closure at 6 and 12 h after wounding (Fig. 8). To further define the contribution of membrane rafts, we modulated the expression of caveolin-1 and flotillins that are considered resident proteins in membrane rafts (18, 51). Because it has been previously shown that caveolin-1 overexpression negatively regulates internalization of lipid raft ligands (33, 36) we first examined the effect of caveolin-1 overexpression on β1-integrin endocytosis in migrating IECs. Infection of SK-CO15 cells with an adenovirus expressing myc-tagged caveolin-1 induced a dramatic increase in intracellular myc-tagged caveolin-1 (Fig. 9A). Increased expression of caveolin-1 resulted in ∼57% inhibition of β1-integrin endocytosis as determined by the antibody internalization assay (Fig. 9, B and C). In addition, overexpression of caveolin-1, which exerts a dominant-negative effect on caveolin function, inhibited epithelial cell migration and wound closure (Fig. 2).
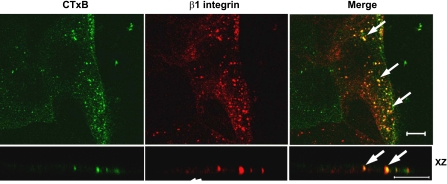
Fig. 6.
Cholera toxin subunit B (CTxB) cointernalizes with β1-integrin at the leading edge of the migrating intestinal epithelial cells (IECs). Migrating T84 cells were subjected to the β1-integrin antibody internalization assay for 20 min in the presence of fluorescein isothiocyanate-labeled CtxB (green). Cells were then fixed, immunolabeled for β1-integrin (red), and analyzed by confocal microscopy. Yellow color (arrows) indicates colocalization of internalized β1-integrin and cholera toxin. Scale bar, 10 μm.
Fig. 7.
Cholesterol-sequestering drug methyl-β-cyclodextrin (MβCD) attenuates internalization of β1-integrin. A: wounded SK-CO15 monolayers were subjected to the β1-integrin antibody internalization assay for 10 min with or without MβCD (10 mM). Cells were fixed, and localization of internalized β1-integrin antibody (red) and F-acting (green) was determined by immunofluorescent labeling and confocal microscopy. Note a significant decrease in intracellular β1-integrin labeling after MβCD treatment. Scale bar, 20 μm. B: quantification of number of β1-integrin vesicles on the cell leading edge from images represented in A. Data are expressed as a percentage of control and shown as means ± SE, n = 31. P < 0.01.
Fig. 8.
MβCD inhibits migration of IEC. A: confluent SK-CO15 cells were wounded and allowed to migrate for 6 and 12 h. MβCD (2.5 mM) or vehicle was added on the onset of cell migration. The dashed lines and the arrowheads highlight the leading edge of cells migrating in the wound. B: velocity of the wound closure is presented as a percentage of the initial wound widths. Note a significant attenuation of wound closure in the presence of MβCD (n = 4, P < 0.0004).
Fig. 9.
Overexpression of caveolin-1 inhibits internalization of β1-integrin. A: Western blot analysis of cells overexpressing Myc-tagged caveolin-1 in SK-CO15 cells 43 h postinfection compared with control cells. B: SK-CO15 cells were wounded and infected with the caveolin-1-bearing adenovirus. β1-Integrin antibody internalization assay was performed 20 h postinfection for 7 min. Cells were fixed, and localization of internalized β1-integrin antibody (red) and the expression tag (green) was determined by immunofluorescent labeling and confocal microscopy. Note effective internalization of anti-β1-integrin antibodies in the noninfected cell (arrow) and decreased internalization in the adjacent infected cell (arrowhead). Scale bar, 20 μm. C: quantification of the intensity of internalized β1-integrin in images presented in B. Data are shown as means ± SE, n = 22. *Statistically significant difference (P < 0.01).
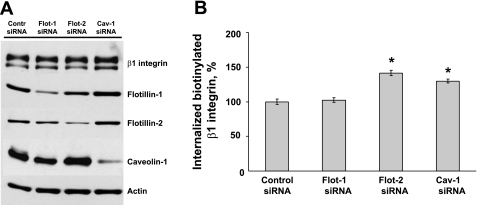
We next investigated if decreased expression of caveolin and flotillin affects β1-integrin uptake from the plasma membrane. Caveolin-1, flotilin-1, and flotillin-2 expression was downregulated in SK-CO15 cells using protein-specific siRNA SmartPools. Figure 10A shows that siRNA-mediated knockdown dramatically decreased protein levels of flotilin-1, flotillin-2, and caveolin-1 by ∼88, 85, and 89%, respectively, compared with Lamin A/C siRNA control. Knockdown of these proteins did not change the total levels of β1-integrin, as shown by Western blot using clone 18 monoclonal β1-integrin antibody (Fig. 10A). Effect of flotillin and caveolin-1 knockdown on β1-integrin internalization was analyzed by an ELISA-based biotinylation assay. This assay was validated in two types of experiments. First, we verified specificity of the used P5D2 β1-integrin antibody by demonstrating that, by immunoblotting, this antibody recognized a single protein band with molecular weight ∼120 kDa corresponding to β1-integrin (Supplemental Fig. 2A). Next, we demonstrated that the ELISA assay shows a linear increase in the amount of internalized β1-integrin during 30 min of internalization (Supplemental Fig. 2B). Using this assay, we observed that downregulation of flotillin-2 and caveolin-1 expression significantly increased β1-integrin endocytosis by ∼1.4- and 1.3-fold, respectively (Fig. 10B). Last, we determined if β1-integrin is distributed in lipid rafts. T84 epithelial cell lysates were fractionated in continuous 5–30% sucrose density gradients, and distribution of β1-integrin and caveolin-1 in gradient fractions was determined by immunoblotting. Supplemental Fig. 3 shows accumulation of β1-integrin and caveolin-1 in light-density lipid raft fractions.
Fig. 10.
Downregulation of lipid raft-associated proteins increases internalization of β1-integrin. A: Western blot analysis of SK-CO15 cells transfected with flotillin-1, flotillin-2, caveolin-1, and Lamin A/C siRNA (control). Note efficient downregulation of all targeted proteins. B: surface biotinylation and β1-integrin internalization assay in control and caveolin-1-, flotilin-1-, and flotilin-2-depleted SK-CO15 cells. Note a significant increase of β1-integrin in caveolin-1- and flotilin-2-deficient cells. Data are expressed as a percentage of internalization in Lamin A/C control and are shown as means ± SE, n = 4. *Statistically significant difference compared with control (P < 0.05).
Together, these data suggest that, in migrating epithelial cells, β1-integrin is internalized via a lipid raft-mediated pathway and that lipid raft proteins caveolin-1 and flotillin-2 negatively regulate β1-integrin endocytosis.
Macropinocytosis is not involved in β1-integrin internalization.
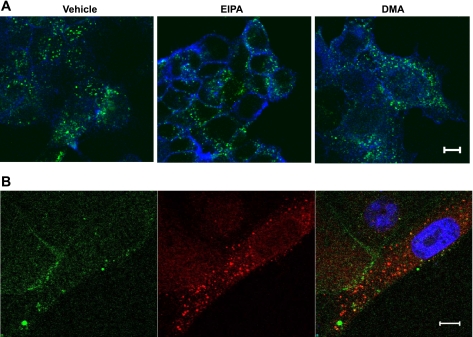
Last, we examined a possible role of macropinocytosis in β1-integrin internalization. We used pharmacological inhibitors of macropinocytosis, EIPA and DMA. Both drugs are derivative of amiloride, and they reportedly block macropinocytosis by inhibiting plasma membrane Na+/H+ exchanger (40, 45, 66, 68). As shown in Fig. 11A, treatment of SK-CO15 cells with 50 μM of EIPA or DMA failed to prevent β1-integrin endocytosis, which was evaluated by the antibody internalization assay. It should be noted that these inhibitors prevented internalization of the macropinocytosis marker fluoresceinated dextran, which indicates their efficiency (Supplemental Fig. 4). Furthermore, cointernalization experiments showed lack of colocalization of internalized β1-integrin and dextran in the migrating front of SK-CO15 cells (Fig. 11B). Together, these results do not support the role of macropinocytosis in β1-integrin internalization in model IECs.
Fig. 11.
Macropinocytosis is not involved in β1-integrin internalization. A: wounded SK-CO15 cells were subjected to the β1-integrin antibody internalization assay for 10 min in the presence of either vehicle or the pharmacological inhibitors of macropinocytosis 5-(N-ethyl-N-isopropyl)amiloride (EIPA) or 5-(N,N-dimethyl)amiloride (DMA) (each, 50 μM). Cells were fixed, and localization of internalized antibody (green) and F-actin (blue) was determined by fluorescence labeling and confocal microscopy. Note that macropinocytosis inhibitors failed to prevent internalization of β1-integrin. Scale bar, 10 μm. B: β1-integrin antibody internalization assay was carried out in migrating SK-CO15 cell in the presence of fluorescein-labeled dextran. Cells were fixed, and localization of internalized antibodies (red) and dextran (green) was determined. The nucleus is stained with ToPro3 (blue). Note lack of colocalization of internalized β1-integrin and fluoresceinated dextran. Scale bar, 5 μm.
DISCUSSION
In this study, we have identified a mechanism responsible for the uptake of cell surface β1-integrin in migrating IECs. This mechanism involves dynamin-dependent endocytosis of β1-integrin via lipid a raft-mediated pathway. A previous study has reported that expression of mutant dynamin protein (K44A) in NIH-3T3 fibroblasts resulted in inhibition of endocytosis at the stage of smooth invaginations that were unable to bud from the plasma membrane (44). These findings are consistent with the current view that dynamin acts at the late stages of endocytosis being responsible for vesicle scission from the cell membrane (24). Our loss of function experiment (expression of inactive K44A dynamin mutant) showed decreased integrin endocytosis in migrating IECs (Fig. 1). This result is consistent with two previously published studies that implicated dynamin in β1-integrin internalization. One study demonstrated that dominant-negative K44A dynamin mutant attenuated β1-integrin endocytosis in breast carcinoma cells (47). Another study found that dynamin inhibition blocked internalization of echovirus, which uses β1-integrin to enter cells (54). It is noteworthy that dynamin has been previously observed in focal adhesions in fibroblasts (16) and another integrin-rich adhesion structure, podosomes, in osteoclast cells (7), and overexpression of inactive K44A dynamin mutant was shown to decrease focal adhesion disassembly (16). Importantly, dynamin inhibition attenuates cell migration (Fig. 2; see Ref. 7 and 16). These data strongly suggest a causal connection between dynamin-dependent β1-integrin internalization, disassembly of focal adhesions, and cell motility.
Several lines of evidence support our conclusion that endocytosis of β1-integrin in migrating IECs occurs via a lipid rafts pathway. First, internalized β1-integrin colocalized with the lipid raft marker CTxB (Fig. 6). Second, β1-integrin endocytosis (Fig. 7) and cell motility (Fig. 8) were suppressed after disruption of lipid rafts by cholesterol depletion. Third, internalization of β1-integrin was influenced by modulating expression of lipid raft-associated proteins (Figs. 9 and 10). Overall, our data suggest that β1-integrin uptake requires cholesterol in the plasma membrane rafts but is negatively regulated by the resident raft proteins caveolin-1 and flotillin-2. This surprising conclusion is supported by a number of studies that have shown β1-integrin distribution in lipid rafts of various cell types. For example, the raft fraction isolated from oligodendrocytes contained α6β1-integrin, and treatment of the cells with sphingolipid synthesis inhibitor fumosin-B1 resulted in disappearance of integrin dimers from lipid rafts (14). Similarly, β1-integrin was detected in lipid rafts isolated from embryonic neural precursor cells, but not in the corresponding fraction isolated from the cells after cholesterol depletion with MβCD (70). More importantly, it was recently demonstrated that MβCD treatment suppressed internalization of β1-integrin at cell-cell contacts of human cutaneous squamous carcinoma cells HSC-1 (43). Lipid raft association of β1-integrin was shown to be a dynamic process that can be modulated by integrin-binding partners or external stimuli. Indeed, recruitment of β1-integrin to lipid rafts by tetraspanin CD36 or by GPI-anchored protein CD24 was demonstrated in melanoma cells (63) and lung adenocarcinoma cells (58). Furthermore, shear stress was shown to trigger accumulation of β1-integrin within caveolin-containing lipid rafts in aortic endothelial cells (55). β1-Integrins are not the only members of the integrin superfamily associated with lipid rafts. Other members such as αLβ2-, ανβ2-, and α6β4-integrins have been found within these membrane structures (reviewed in Ref. 13). Interestingly, not only can rafts regulate integrin internalization and signaling, but integrins themselves modulate lipid raft/caveolar-dependent endocytosis (60). Such modulation may be due to integrin-dependent alterations in the level and distribution of plasma membrane cholesterol (50) and changes in fluidity of membrane lipid domains (20). Overall, our findings are in line with an emerging concept implicating the interplay between integrin-mediated adhesion and lipid raft-dependent endocytosis in regulation of various stages of cell cycle, including cell migration (8, 14, 15, 60).
Our results support a role of lipid rafts in β1-integrin endocytosis and yet reveal a negative regulation of this process by resident lipid raft proteins caveolin-1 and flotillin-2 (Fig. 10). Two different mechanisms may explain inhibition of β1-integrin internalization in caveolin-1-overexpressing cells. The first mechanism involves caveolin-1-dependent alterations in lipid rafts structure and endocytic activity. Indeed, caveolin-1 overexpression was shown to disperse lipid raft microdomains and to inhibit raft-mediated endocytosis (33, 46). This inhibition may be a consequence of caveolin-mediated stabilization of raft invagination at the plasma membrane or sequestration of key endocytosis components, including cholesterol and dynamin. The second mechanism is based on a proposed role of caveolin-1 in regulation of integrin adhesion structures at the plasma membrane. Indeed, caveolin and particularly its Tyr14 phosphorylated form is localized at integrin-based focal adhesions (67). Functional consequences of such localization remain incompletely understood, but one can suggest that caveolin-1 overexpression stabilizes integrin complexes at the plasma membrane and prevents their disassembly and endocytosis. Indirect support for this suggestion is provided by a study that demonstrated a negative role of caveolin-1 in endothelial cell migration (22).
Some of the above-discussed mechanisms may also be valid in explaining negative influence of flotillin-2 on β1-integrin internalization. It should be mentioned that flotillins and caveolins participate in formation of different membrane microdomains as was suggested by recent microscopic analysis of intact cells (18, 19) and biochemical fractionation of cell membranes (41). However, analogous to caveolin-1, flotillins were shown to easily oligomerize and to form stable proteolipid structures at the plasma membrane (19). It is reasonable to suggest that such stable membrane microdomains may sequester a component of lipid rafts and thus decrease their endocytic activity. Hence, destabilization and increased endocytic activity of lipid rafts in flotillin-deficient cells is likely to be responsible for increased internalization of β1-integrin.
In addition to providing evidence in support of involvement of lipid rafts in β1-integrin endocytosis, we also excluded the role of alternative internalization pathways such as macropinocytosis and clathrin-mediated endocytosis. The role of macropinocytosis was ruled out based on three lines of evidence. First, we did not find colocalization of internalized β1-integrin and the macropinocytosis marker dextran. Second, integrin endocytosis was not affected by pharmacological inhibitors of macropinocytosis (Fig. 11). Finally, the observed involvement of dynamin in integrin internalization argued against macropinocytosis, which is known to be dynamin independent (32). It is noteworthy that macropinocytosis would be a rather unusual pathway for removal of integrins from the plasma membrane. It was reported for coendocytosis of adenovirus type 2 and its receptors αν-integrins (26). However, even this integrin internalization did not occur via classical macropinocytosis but through some unusual chimeric pathway that is also dependent on lipid rafts (26).
The clathrin-mediated pathway was excluded based on colocalization and functional inhibition analyses. The former showed segregation of internalized β1-integrin from endosomes containing clathrin itself or the canonical marker of clathrin-dependent endocytosis, transferrin (Fig. 3). The later analysis showed that β1-integrin internalization was not prevented by either pharmacological inhibitors of the clathrin pathway (Fig. 4) or by siRNA-mediated downregulation of clathrin heavy chain (Fig. 5). Our conclusion regarding clathrin-independent internalization of β1-integrin in model IEC agrees with some published studies and contradicts other reports. Previous studies have revealed that inhibition of clathrin pit formation by potassium depletion did not block internalization of the integrin ligand fibronectin in fibroblasts (1, 2). Likewise, in a recent study, siRNA-mediated knockdown of clathrin heavy chain did not affect β1-integrin internalization in HeLa cells (37). Finally, biochemical experiments failed to detect binding of β1-integrins to clathrin (12). On the other hand, studies performed with MDA-MB-231 breast carcinoma cells (53) and HeLa cells (48) demonstrated clathrin dependence of β1-integrin endocytosis. Although it is difficult to explain these contradictory data, they might simply reflect the fact that different cell types or even different clonal variants of the same cell line (HeLa) use different endocytic mechanisms to downregulate their integrin-based matrix adhesions.
In conclusion, our study revealed that dynamin-dependent lipid raft-mediated endocytosis is involved in the internalization of β1-integrin from the plasma membrane in migrating IECs. Such a mechanism is likely to be important for disassembly of integrin-based cell-matrix adhesions and therefore for regulating cell movement. Pharmacological modulation of β1-integrin endocytosis could be beneficial in accelerating wound healing during intestinal inflammation and for preventing dissemination and metastasis of colorectal tumor.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-55679 and DK-59888 (to A. Nusrat), a Career Development Award from the Crohn's and Colitis Foundation (to A. I. Ivanov), and Digestive Diseases Minicenter Grant DK-64399.
Supplementary Material
Acknowledgments
We thank Drs. S. Voss, S. Samarin, and Kyle DenBeste for technical assistance and data analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Altankov G, Grinnell F. Depletion of intracellular potassium disrupts coated pits and reversibly inhibits cell polarization during fibroblast spreading. J Cell Biol 120: 1449–1459, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altankov G, Grinnell F. Fibronectin receptor internalization and AP-2 complex reorganization in potassium-depleted fibroblasts. Exp Cell Res 216: 299–309, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Altschuler Y, Barbas SM, Terlecky LJ, Tang K, Hardy S, Mostov KE, Schmid SL. Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms. J Cell Biol 143: 1871–1881, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd ND, Chan BM, Petersen NO. Beta1 integrins are distributed in adhesion structures with fibronectin and caveolin and in coated pits. Biochem Cell Biol 81: 335–348, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Bretscher MS Circulating integrins: alpha 5 beta 1, alpha 6 beta 4 and Mac-1, but not alpha 3 beta 1, alpha 4 beta 1 or LFA-1. Embo J 11: 405–410, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bretscher MS Endocytosis and recycling of the fibronectin receptor in CHO cells. Embo J 8: 1341–1348, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruzzaniti A, Neff L, Sanjay A, Horne WC, De Camilli P, Baron R. Dynamin forms a Src kinase-sensitive complex with Cbl and regulates podosomes and osteoclast activity. Mol Biol Cell 16: 3301–3313, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burridge K Foot in mouth: do focal adhesions disassemble by endocytosis? Nat Cell Biol 7: 545–547, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Caswell PT, Norman JC. Integrin trafficking and the control of cell migration. Traffic 7: 14–21, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature 422: 37–44, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol 127: 915–934, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Deyne PG, O'Neill A, Resneck WG, Dmytrenko GM, Pumplin DW, Bloch RJ. The vitronectin receptor associates with clathrin-coated membrane domains via the cytoplasmic domain of its beta5 subunit. J Cell Sci 111: 2729–2740, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Decker L, Baron W, Ffrench-Constant C. Lipid rafts: microenvironments for integrin-growth factor interactions in neural development. Biochem Soc Trans 32: 426–430, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Decker L, Ffrench-Constant C. Lipid rafts and integrin activation regulate oligodendrocyte survival. J Neurosci 24: 3816–3825, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Echarri A, Muriel O, Del Pozo MA. Intracellular trafficking of raft/caveolae domains: Insights from integrin signaling. Semin Cell Dev Biol 18: 627–637, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol 7: 581–590, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Fenteany G, Janmey PA, Stossel TP. Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr Biol 10: 831–838, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Fernow I, Icking A, Tikkanen R. Reggie-1 and reggie-2 localize in non-caveolar rafts in epithelial cells: cellular localization is not dependent on the expression of caveolin proteins. Eur J Cell Biol 86: 345–352, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Frick M, Bright NA, Riento K, Bray A, Merrified C, Nichols BJ. Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Curr Biol 17: 1151–1156, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Gaus K, Le Lay S, Balasubramanian N, Schwartz MA. Integrin-mediated adhesion regulates membrane order. J Cell Biol 174: 725–734, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilcrease MZ Integrin signaling in epithelial cells. Cancer Lett 247: 1–25, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez E, Nagiel A, Lin AJ, Golan DE, Michel T. Small interfering RNA-mediated down-regulation of caveolin-1 differentially modulates signaling pathways in endothelial cells. J Biol Chem 279: 40659–40669, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Grose R, Hutter C, Bloch W, Thorey I, Watt FM, Fassler R, Brakebusch C, Werner S. A crucial role of beta 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 129: 2303–2315, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Hinshaw JE Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol 16: 483–519, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hynes RO Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Imelli N, Meier O, Boucke K, Hemmi S, Greber UF. Cholesterol is required for endocytosis and endosomal escape of adenovirus type 2. J Virol 78: 3089–3098, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irie T, Fukunaga K, Pitha J. Hydroxypropylcyclodextrins in parenteral use. I: Lipid dissolution and effects on lipid transfers in vitro. J Pharm Sci 81: 521–523, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Ivanov AI Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol Biol 440: 15–33, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Ivanov AI, Bachar M, Babbin BA, Adelstein RS, Nusrat A, Parkos CA. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions (Abstract). PLoS ONE 2: e658, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones MC, Caswell PT, Norman JC. Endocytic recycling pathways: emerging regulators of cell migration. Curr Opin Cell Biol 18: 549–557, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Kilsdonk EP, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, Phillips MC, Rothblat GH. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem 270: 17250–17256, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Kirkham M, Parton RG. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta 1746: 349–363, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Lajoie P, Nabi IR. Regulation of raft-dependent endocytosis. J Cell Mol Med 11: 644–653, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laukaitis CM, Webb DJ, Donais K, Horwitz AF. Differential dynamics of alpha 5 integrin, paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol 153: 1427–1440, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Bivic A, Real FX, Rodriguez-Boulan E. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc Natl Acad Sci USA 86: 9313–9317, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le PU, Guay G, Altschuler Y, Nabi IR. Caveolin-1 is a negative regulator of caveolae-mediated endocytosis to the endoplasmic reticulum. J Biol Chem 277: 3371–3379, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Peters PJ, Bai M, Dai J, Bos E, Kirchhausen T, Kandror KV, Hsu VW. An ACAP1-containing clathrin coat complex for endocytic recycling. J Cell Biol 178: 453–464, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lotz MM, Nusrat A, Madara JL, Ezzell R, Wewer UM, Mercurio AM. Intestinal epithelial restitution. Involvement of specific laminin isoforms and integrin laminin receptors in wound closure of a transformed model epithelium. Am J Pathol 150: 747–760, 1997. [PMC free article] [PubMed] [Google Scholar]
- 39.Lussier C, Basora N, Bouatrouss Y, Beaulieu JF. Integrins as mediators of epithelial cell-matrix interactions in the human small intestinal mucosa. Microsc Res Tech 51: 169–178, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Marechal V, Prevost MC, Petit C, Perret E, Heard JM, Schwartz O. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J Virol 75: 11166–11177, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mellgren RL Detergent-resistant membrane subfractions containing proteins of plasma membrane, mitochondrial, and internal membrane origins. J Biochem Biophys Methods 70: 1029–1036, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol 8: 957–969, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukoyama Y, Utani A, Matsui S, Zhou S, Miyachi Y, Matsuyoshi N. T-cadherin enhances cell-matrix adhesiveness by regulating beta1 integrin trafficking in cutaneous squamous carcinoma cells. Genes Cells 12: 787–796, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol 161: 673–677, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakase I, Niwa M, Takeuchi T, Sonomura K, Kawabata N, Koike Y, Takehashi M, Tanaka S, Ueda K, Simpson JC, Jones AT, Sugiura Y, Futaki S. Cellular uptake of arginine-rich peptides: roles for macropinocytosis and actin rearrangement. Mol Ther 10: 1011–1022, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Nakashima H, Hamamura K, Houjou T, Taguchi R, Yamamoto N, Mitsudo K, Tohnai I, Ueda M, Urano T, Furukawa K, Furukawa K. Overexpression of caveolin-1 in a human melanoma cell line results in dispersion of ganglioside GD3 from lipid rafts and alteration of leading edges, leading to attenuation of malignant properties. Cancer Sci 98: 512–520, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ng T, Shima D, Squire A, Bastiaens PI, Gschmeissner S, Humphries MJ, Parker PJ. PKCalpha regulates beta1 integrin-dependent cell motility through association and control of integrin traffic. Embo J 18: 3909–3923, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell 13: 15–28, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Panicker AK, Buhusi M, Erickson A, Maness PF. Endocytosis of beta1 integrins is an early event in migration promoted by the cell adhesion molecule L1. Exp Cell Res 312: 299–307, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Pankov R, Markovska T, Hazarosova R, Antonov P, Ivanova L, Momchilova A. Cholesterol distribution in plasma membranes of beta1 integrin-expressing and beta1 integrin-deficient fibroblasts. Arch Biochem Biophys 442: 160–168, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol 8: 185–194, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Partridge MA, Marcantonio EE. Initiation of attachment and generation of mature focal adhesions by integrin-containing filopodia in cell spreading. Mol Biol Cell 17: 4237–4248, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pellinen T, Arjonen A, Vuoriluoto K, Kallio K, Fransen JA, Ivaska J. Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J Cell Biol 173: 767–780, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pietiainen V, Marjomaki V, Upla P, Pelkmans L, Helenius A, Hyypia T. Echovirus 1 endocytosis into caveosomes requires lipid rafts, dynamin II, and signaling events. Mol Biol Cell 15: 4911–4925, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radel C, Carlile-Klusacek M, Rizzo V. Participation of caveolae in beta1 integrin-mediated mechanotransduction. Biochem Biophys Res Commun 358: 626–631, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raub TJ, Kuentzel SL. Kinetic and morphological evidence for endocytosis of mammalian cell integrin receptors by using an anti-fibronectin receptor beta subunit monoclonal antibody. Exp Cell Res 184: 407–426, 1989. [DOI] [PubMed] [Google Scholar]
- 57.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 302: 1704–1709, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Runz S, Mierke CT, Joumaa S, Behrens J, Fabry B, Altevogt P. CD24 induces localization of beta1 integrin to lipid raft domains. Biochem Biophys Res Commun 365: 35–41, 2008. [DOI] [PubMed] [Google Scholar]
- 59.Rutten MJ, Ito S. Morphology and electrophysiology of guinea pig gastric mucosal repair in vitro. Am J Physiol Gastrointest Liver Physiol 244: G171–G182, 1983. [DOI] [PubMed] [Google Scholar]
- 60.Salanueva IJ, Cerezo A, Guadamillas MC, Del Pozo MA. Integrin regulation of caveolin function. J Cell Mol Med 11: 969–980, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma DK, Brown JC, Cheng Z, Holicky EL, Marks DL, Pagano RE. The glycosphingolipid, lactosylceramide, regulates beta1-integrin clustering and endocytosis. Cancer Res 65: 8233–8241, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Smart EJ, Anderson RG. Alterations in membrane cholesterol that affect structure and function of caveolae. Methods Enzymol 353: 131–139, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Thorne RF, Marshall JF, Shafren DR, Gibson PG, Hart IR, Burns GF. The integrins alpha3beta1 and alpha6beta1 physically and functionally associate with CD36 in human melanoma cells. Requirement for the extracellular domain OF CD36. J Biol Chem 275: 35264–35275, 2000. [DOI] [PubMed] [Google Scholar]
- 64.Tong Q, Vassilieva EV, Ivanov AI, Wang Z, Brown GT, Parkos CA, Nusrat A. Interferon-gamma inhibits T84 epithelial cell migration by redirecting transcytosis of beta1 integrin from the migrating leading edge. J Immunol 175: 4030–4038, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Upla P, Marjomaki V, Kankaanpaa P, Ivaska J, Hyypia T, Van Der Goot FG, Heino J. Clustering induces a lateral redistribution of alpha 2 beta 1 integrin from membrane rafts to caveolae and subsequent protein kinase C-dependent internalization. Mol Biol Cell 15: 625–636, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Delwig A, Bailey E, Gibbs DM, Robinson JH. The route of bacterial uptake by macrophages influences the repertoire of epitopes presented to CD4 T cells. Eur J Immunol 32: 3714–3719, 2002. [DOI] [PubMed] [Google Scholar]
- 67.Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 94: 625–634, 1998. [DOI] [PubMed] [Google Scholar]
- 68.West MA, Bretscher MS, Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol 109: 2731–2739, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiseman PW, Brown CM, Webb DJ, Hebert B, Johnson NL, Squier JA, Ellisman MH, Horwitz AF. Spatial mapping of integrin interactions and dynamics during cell migration by image correlation microscopy. J Cell Sci 117: 5521–5534, 2004. [DOI] [PubMed] [Google Scholar]
- 70.Yanagisawa M, Nakamura K, Taga T. Roles of lipid rafts in integrin-dependent adhesion and gp130 signalling pathway in mouse embryonic neural precursor cells. Genes Cells 9: 801–809, 2004. [DOI] [PubMed] [Google Scholar]
- 71.Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci 116: 4605–4613, 2003. [DOI] [PubMed] [Google Scholar]
- 72.Zhang W, Razani B, Altschuler Y, Bouzahzah B, Mostov KE, Pestell RG, Lisanti MP. Caveolin-1 inhibits epidermal growth factor-stimulated lamellipod extension and cell migration in metastatic mammary adenocarcinoma cells (MTLn3). Transformation suppressor effects of adenovirus-mediated gene delivery of caveolin-1. J Biol Chem 275: 20717–20725, 2000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.