Abstract
Apelin is the endogenous ligand for the APJ receptor; both are expressed in the gastrointestinal tract. Experimental colitis in rodents and inflammatory bowel disease in humans are associated with increased intestinal apelin production. Our aim was to use LPS and proinflammatory cytokine-treated (IL-6 and IFN-γ) rodents or enteric cells to identify signaling mechanisms underlying inflammation-induced enteric apelin expression. LPS, IL-6, or IFN-γ treatment of rodents increased enteric apelin expression. Pharmacological blockade of Jak/Stat signaling or IL-6 antibody administration inhibited elevations in enteric apelin expression. Transient transfection experiments showed that LPS, IL-6, or IFN-γ increased apelin expression by stimulation of apelin promoter activity, and blockade of Jak/Stat signaling abolished elevations in apelin promoter activity. A chromatin immunoprecipitation assay showed that IL-6 induced binding of phospho-Stat3 to a putative Stat3 site in the apelin promoter; mutation of this site abrogated the LPS-induced elevation in apelin promoter activity. Together, our findings indicate that binding of phospho-Stat3 to the apelin promoter is the final step underlying proinflammatory cytokine-induced enteric apelin expression during intestinal inflammation.
Keywords: rodent, gastrointestinal tract, lipopolysaccharide
apelin is the endogenous ligand for the APJ receptor (3, 8, 16). Apelin and APJ are expressed widely in the body. Apelin and APJ are synthesized in the brain, kidney, adipose tissue, lung, mammary gland, gastrointestinal tract, and cardiovascular system (12, 16, 18, 25, 29, 32, 36, 41–43). In agreement with its distribution in the body, apelin exerts a broad range of activities that affect multiple organ systems including effects on heart contractility and blood pressure (6, 9), immune response (20), appetite and drinking behavior (22, 39), and secretion of gastric acid, insulin, and cholecystokinin (21, 35, 43).
Our laboratory has reported earlier that apelin expression in the rodent colon is increased during experimental colitis and that apelin immunostaining is elevated in the colonic epithelium in patients with inflammatory bowel disease (IBD) (14). The increase in intestinal apelin expression during inflammation may have a role in the regulation of epithelial proliferation (13) and in goblet cell function (44). Proinflammatory cytokines, including IL-6 and IFN-γ, play critical roles in the pathogenesis of IBD in humans and in experimental colitis in rodents (2, 27). The extent to which these proinflammatory cytokines regulate apelin expression in the colon during colonic inflammation is not known.
The aim of the present study, therefore, was to investigate the influence of IL-6 and IFN-γ on enteric apelin expression. Additionally, the influence of lipopolysaccharide (LPS) on enteric apelin expression was examined. LPS administration to rodents promotes systemic inflammation and will activate host immune and inflammatory cells, resulting in elevations of tissue and systemic levels of numerous cytokines, including TNF-α, IL-1, IL-6, and IFN-γ (4, 15, 24, 33, 40). The roles of the Jak/Stat signaling pathway and of a putative Stat3 binding site in the rat apelin core promoter in cytokine- and LPS-induced apelin transcriptional activity were also examined.
We show that IL-6 and IFN-γ activated enteric apelin expression is mediated by Jak/Stat signaling. Furthermore, a putative Stat3 binding site in the apelin core promoter may be the final common pathway underlying stimulation of enteric apelin expression by inflammation.
MATERIALS AND METHODS
Animals
All animal experiments were done in accordance with mandated standards of humane care and were approved by the University of Texas Medical Branch Institutional Animal Care and Use Committee. Sprague-Dawley rats, C57/BL6 mice, and nude mice were maintained in air-conditioned and light-regulated rooms (lights on 0600–1800) and given access to food and water ad libitum.
Chemicals
All chemicals were obtained from Sigma (St. Louis, MO) unless noted otherwise. Ambion RNAqueous RNA kits (Austin, TX) were used to prepare total cellular RNA. Reverse transcriptase-polymerase chain reaction (RT-PCR) kits were purchased from BD Biosciences Clontech (Palo Alto, CA). LPS; recombinant rat IFN-γ; human, rat, and mouse recombinant IL-6; and anti-mouse IL-6 antibody were obtained from Sigma. Collagenase A was from Roche (Indianapolis, IN), and the Stat3 expression vector was from Addgene (Cambridge, MA).
Primary Cell Cultures
As described previously (13), intestinal ileums of 6-day-old rat pups were harvested, and lumens were flushed and rinsed three times in PBS, minced, and digested by incubation with collagenase A (2 mg/ml, 37°C, 30 min). Cells were then washed with Dulbecco's modified Eagle's medium (DMEM), centrifuged at low speed, and resuspended in medium; this procedure was repeated twice before cell plating. Cells were plated onto plates or dishes in DMEM containing 10% fetal bovine serum to facilitate attachment of cells and crypts. Primary cultures consisted of monolayer cultures of a mixed enteric cell population. Confluent monolayers formed within 3–4 days. Cultured cells were then exposed to LPS, IL-6, or IFN-γ (24 h).
Thymus glands were removed from 24-day-old rat pups, and peripheral tissues were removed. Thymic fragments were minced into tissue pieces less than 1 mm3 and suspended by being passed though a syringe needle. After being washed with PBS, thymocytes were placed into dishes in DMEM containing 10% fetal bovine serum.
Generation of Conditioned Media From LPS-Treated Mouse Macrophages, Rat Thymocytes, and Mouse Enteroendocrine Cells
Mouse macrophages (RAW264.7) or rat primary thymocytes were plated and grown for ∼24 h in DMEM with 10% FBS in 5% CO2-95% humidified air at 37°C. Mouse intestinal enteroendocrine (STC-1) cells (5) were plated and grown in DMEM containing 15% horse serum and 5% fetal bovine serum for ∼24 h. Cells were then treated with vehicle or 200 ng/ml LPS in serum-free DMEM. Twenty-four hours later culture media were harvested, clarified by centrifugation (250 g), and used immediately.
Measurement of Apelin Expression Levels
For measurement of apelin expression levels in cell lines, total cellular RNA was extracted and purified by means of the RNAqueous kit (AB-Ambion, Foster City, CA) as described previously (45). Before real-time PCR analyses, total RNA was used as a template to synthesize first-strand cDNA by the random priming method using the Advantage RT-for-PCR kit (BD Biosciences Clontech). Briefly, 1 μg of total RNA was incubated with 1 μl random primer at 70°C for 2 min, chilled on ice and mixed with a solution containing 4 μl of reaction buffer (5×), 1 μl deoxynucleotide (dNTP) (10 mM), 0.5 μl recombinant RNase inhibitor (40 U/μl), and 1.0 μl of Moloney murine leukemia virus reverse transcriptase (200 U/μl). The mixture (20 μl) was incubated for 1 h at 42°C, and the reverse transcriptases were denatured at 94°C for 5 min. Apelin mRNA levels were then measured by real-time RT-PCR assays as described previously (43, 45). Assays were done by using an Applied Biosystems (Foster City, CA) 7000 sequence detection system. Applied Biosystems Assays-By-Design containing a 20× assay mix of primers and TaqMan MGB probes (6-FAM dye-labeled probe) were used for the target genes: rat apelin (accession no. AF179679); human apelin (accession no. NM_017413); mouse apelin (accession no. NM_013912); and a predeveloped 18S rRNA (VIC dye-labeled probe). TaqMan assay reagent (P/N 4319413E) was used for the internal control. Primers were designed to span exon-exon junctions.
For measurement of apelin expression levels in the rat gastrointestinal tract, total cellular RNA was extracted and purified from ileal tissue homogenates and Northern blotting analyses were done using published procedures (10, 43, 45).
IL-6 ELISA
IL-6 concentrations in cell culture supernatants and in mouse plasma samples were determined by using an IL-6 enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences, San Jose, CA) according to the manufacturer's protocol.
Western Blotting Analysis
Cells were harvested and sonicated in cell lysis buffer (Cell Signaling, Beverly, MA) to extract total cellular proteins. Supernatants were harvested, and protein concentrations were quantitated. Total cellular protein extracts (30 μg) were then incubated at 70°C for 5 min in the presence of sample loading buffer (NuPAGE LDS sample buffer, Invitrogen, Carlsbad, CA) before separation on a 4–12% SDS-PAGE. After gel separation, proteins were transferred onto nitrocellulose membranes and incubated with either a Stat3, a phospho-Stat3, an Erk1/2, or a phospho-Erk1/2 antibody (Cell Signaling) overnight at 4°C. Membranes were then incubated for 45 min at room temperature with conjugated anti-rabbit (1:5,000) IgG-horseradish peroxidase antibody in washing buffer. Detection was done by using the enhanced chemiluminescent method according to the manufacturer's instructions (ECL, NEN Life Science Products, Boston, MA).
Site-Directed Mutagenesis of a Putative Stat3 Binding Site in the Rat Apelin Promoter
A putative Stat3 binding site (−198/−195 bp) in the rat apelin promoter was mutated by use of a Quick Change Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). The mutation reaction was done by using Pfu Turbo DNA polymerase at 95°C for 60 s followed by 18 cycles of PCR at 95°C for 50 s, 60°C for 50 s, and 68°C for 6.5 min, then at 68°C for 10 min. The parental, double-stranded DNA was digested with DpnI. Mutant sequences were confirmed by DNA sequencing.
Immunofluorescent Staining
Primary cultured rat ileal cells were treated with vehicle or IL-6 (100 ng/ml) for 5 min. After treatment, medium was removed and cells were fixed with 4% paraformaldehyde in 0.01 mM phosphate buffer solution (PBS) at room temperature for 20 min. Cells were washed three times with PBS (5 min each). Cells were permeabilized with 0.3% Triton X-100 in PBS for 15 min at room temperature. Nonspecific staining was blocked by a protein blocking solution (DAKO, Carpinteria, CA) at room temperature for 1 h. Sections were then incubated with a Stat3 or a phospho-Stat3 primary antibody (Cell Signaling) diluted in Antibody Diluent Buffer (DAKO) overnight at 4°C. Incubation was followed by three washes in PBS (5 min each) and a subsequent incubation with a secondary antibody (Alexa Fluor 594 goat anti-rabbit HCA, Invitrogen) at 1:400 for 1 h. After incubation, sections were washed three times with PBS. Nuclei were visualized by DAPI. All manipulations after addition of the fluorescent secondary antibodies were shielded from light.
ChIP Assay
Primary cultured rat ileal cells were treated with vehicle or IL-6 (100 ng/ml) for 5 min. Chromatin immunoprecipitation (ChIP) assays were done as described by our laboratory previously (45). A phospho-Stat3 antibody (Cell Signaling) was used in ChIP reactions. For an input control, 2% of the DNA amount used for immunoprecipitation was used for PCR. Precipitated DNA was subjected to PCR amplification by using the Hotstart PCR kit (Qiagen, Valencia, CA). PCR was done by using primers, 5′-TCCCGG GAGCAGTTGTGGGT-3′ and 5′-TTTGGCCGCGAGCCCACTTG-3′, that were designed to amplify a region containing the putative Stat3 binding site(−198/−195 bp) in the rat apelin promoter. PCR conditions included one cycle of 15 min at 95°C and 35 cycles of 30 s at 94°C, 50 s at 50°C and 1 min at 72°C with a final extension step of 10 min at 72°C.
Experiments
Experiment 1.
The aim of this experiment was to examine the effects of LPS on apelin expression levels in the rodent gastrointestinal tract. Either adult male rats (0.1 mg/kg ip) or 6-day-old rat pups (1 mg/kg ip) were given LPS and euthanized 4, 8, 24, and 48 h and 8, 24, and 48 h later, respectively. C57BL/6 mice were euthanized 8 h after LPS (1 mg/kg ip) administration, and nude mice were euthanized 8, 24, and 48 h after LPS administration. Gastrointestinal tissues were harvested; total cellular RNA extracts were prepared and apelin mRNA levels were measured by Northern blot or real-time RT-PCR analyses.
Experiment 2.
Human colon (HCT116, HT29, KM20, Caco2, NCM356), liver (HepG2), and breast epithelial (Hs578T); rat retinal capillary endothelial (TR-iBRB2); mouse intestinal enteroendocrine (STC-1) and hypothalamic (GT1-7); and primary rat ileal cells were treated with LPS (0.2–1 μg/ml) for 24 h. Cells were harvested; total cellular RNA was extracted and apelin mRNA levels were measured.
Experiment 3.
The aim of this experiment was to examine the effects of recombinant IL-6 administration on ileal apelin expression levels in the rat and in primary cultured ileal cells. Six-day-old rat pups were given vehicle or IL-6 (100 ng ip) and ileums were harvested 24 h later. An inhibitor of Jak/Stat signaling, AG490 (20 mg/kg), was given 1 h before IL-6 treatment. Ileal apelin mRNA levels were measured by Northern blot analyses. Primary cultured ileal cells were pretreated with a Jak Inhibitor I (EMD Chemicals, Gibbstown, NJ; 10 μM) before exposure to IL-6 (100 ng/ml, 24 h). Cells were harvested and apelin mRNA levels were measured.
Experiment 4.
The aim of this experiment was to examine the effects of IFN-γ treatment on ileal apelin expression levels in rats and the effects of either IFN-γ or IL-6 alone or in combination on apelin expression levels in primary cultures of rat ileal cells. Six-day-old rat pups were given a single IFN-γ (1 μg/rat) administration. The ileum was harvested 24 h later for measurement of apelin mRNA levels. Primary cultured ileal cells were exposed to graded doses of either IL-6 (1, 10, or 100 ng/ml) or IFN-γ (10 or 100 ng/ml or 1 μg/ml) alone or in combination for 24 h. Cells were harvested for measurement of apelin mRNA levels.
Experiment 5.
The aim of this experiment was to examine the effect of soluble factors secreted by LPS-treated immune cells on enteric apelin expression levels when injected into rats. Previous reports indicate that mouse macrophages (RAW264.7) and T cells release significant amounts of cytokines in response to LPS exposure (11, 28). Rat pups were given conditioned media (200 μl ip) harvested from either vehicle or LPS-treated cells (RAW267.4, thymocytes, STC-1). Rat ileums were collected 24 h later and processed for measurement of apelin mRNA levels by Northern blot analyses. IL-6 secretion into the media by RAW267.4 and STC-1 cells in response to LPS was monitored by an IL-6 ELISA kit.
Experiment 6.
The aim of this experiment was to examine the effect of soluble factors released into the media by LPS-treated immune cells (RAW267.4, thymocytes) on apelin expression levels in colon cells or in primary cultures of rat ileal cells. Colon cells or primary cultures of rat ileal cells were exposed for 24 h to vehicle, conditioned medium, or conditioned medium containing an anti-mouse IL-6 antibody. Media were incubated with an anti-mouse IL-6 antibody at room temperature for 30 min. The effects of IL-6 and Jak/Stat signaling blockade were also examined in rat ileal cells exposed to conditioned medium. Cells were pretreated with a Jak Inhibitor I (10 μM), 1 h prior to exposure to conditioned medium. Cells were harvested, total cellular RNA was extracted, and apelin expression levels were measured.
Experiment 7.
The aim of this experiment was to examine the influence of LPS treatment on rat apelin promoter activity. In transient transfection experiments, mouse macrophages (RAW264.7) or human colon cancer (HCT116) cells were transfected with 5′-upstream rat apelin promoter fragment-luciferase reporter constructs (−3000/−1; −2500/−1; −2000/−1; −1500/−1; −1000/−1; −506/−1; −407/−1; −302/−1; −207/−1; −106/−1 bp) [1.6 μg/well (12-well plate)] plus an internal control expression vector (pRL-TK, 0.1 μg/well) as described by our laboratory previously (43, 45). Eighteen hours after transfection, cells were exposed to LPS for 24 h. Cells were then harvested for measurement of luciferase activities as described (43, 45).
Experiment 8.
The influence of IL-6 or IFN-γ on rat apelin promoter activity was examined in transient transfection experiments. The 5′-flanking region (−207/−1, −106/−1 bp) of the rat apelin gene fused to a luciferase reporter gene was transfected into mouse macrophages (RAW264.7 cells) and treated with IL-6 (100 ng/ml) or IFN-γ (100 ng/ml) alone or with a Jak inhibitor I. The Jak inhibitor I was given 1 h before IL-6 or IFN-γ treatments.
Experiment 9.
Sequence analysis identified a putative Stat3 binding site (−198/−195 bp) in the rat apelin core promoter. The influence of Stat3 overexpression and of mutation of this putative Stat3 binding site on apelin core promoter activity were tested in transient transfection experiments. The effect of Stat3 overexpression (0.8 μg/well) on apelin promoter activity in RAW264.7 cells was examined by using wild-type (−407/−1; −302/−1; −207/−1; −106/−1 bp) (0.8 μg/well) and mutated (−207/−1 bp) apelin promoter-luciferase reporter constructs. The effect of LPS treatment (200 ng/ml) on apelin promoter activity was also examined by using the mutated apelin promoter-luciferase reporter construct.
Experiment 10.
The extent to which IL-6 increases Stat3 phosphorylation and binding of phospho-Stat3 to a putative Stat3 site in apelin promoter was examined by Western blotting analysis, immunofluorescent staining, and ChIP assay.
RESULTS
LPS Treatment Increases Enteric Apelin Expression In Vivo
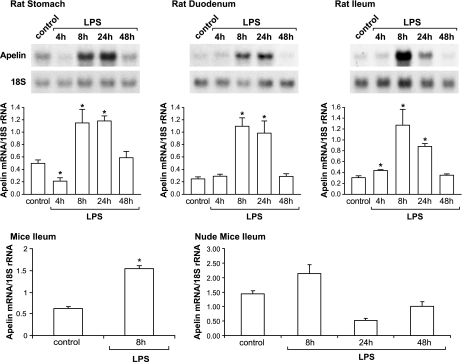
The aim of this experiment was to examine the effect of LPS treatment on apelin expression levels in the rodent gastrointestinal tract. In the stomach, duodenum, and ileum of adult male rats, apelin expression levels increased approximately two- to fourfold, 8 and 24 h after LPS treatment compared with control rats (Fig. 1, top and middle). LPS treatment also increased apelin expression levels in the gastrointestinal tract of 6-day-old rat pups with a similar profile (data not shown). In mice, apelin expression levels increased ∼2.5-fold 8 h after LPS administration compared with control mice. In nude mice, although apelin expression levels increased ∼50% 8 h after LPS administration, the elevation failed to achieve statistical difference compared with controls (P > 0.05) (Fig. 1, bottom).
Fig. 1.
LPS treatment increases apelin expression levels in the rodent gastrointestinal tract. Top: Northern blotting analysis shows apelin mRNA levels in the rat stomach, duodenum, and ileum at different times after LPS treatment. Apelin hybridization is shown for only a single animal at each time point. Middle: means ± SE of densitometric readings of apelin mRNA levels in the stomach, duodenum, and ileum of control and LPS-treated rats; n = 5 rats/group. Bottom: in mice, ileal apelin mRNA levels increased significantly 8 h after LPS administration compared with control mice. In nude mice, apelin expression levels did not increase significantly after LPS administration; n = 5–7 mice/group. In all experiments, apelin expression levels are normalized to 18S ribosomal RNA expression levels. *P < 0.05 vs. control levels.
In contrast to the stimulatory effect of LPS on apelin expression in vivo, LPS treatment failed to affect apelin expression levels in cultured somatic cells (Table 1). LPS exposure did not increase apelin expression levels in cultured cells derived from the intestine, liver, breast, eye, and hypothalamus.
Table 1.
LPS treatment does not increase apelin expression in nonimmune cells
| Primary Cultured Rat Ileal Cells | Enteroendocrine STC-1 | Colon* HCT116 | Liver HepG2 | Breast Hs578T | Capillary Endothelial TR-iBRB2 | Hypothalamic GT1-7 | |
|---|---|---|---|---|---|---|---|
| Control | 3.6±0.2 | 1.0±0.2 | 1.0±0.1 | 1.6±0.1 | 121.7±6.3 | 1.2±0.1 | 0.8±0.1 |
| LPS treated | 3.7±0.2 | 1.0±0.3 | 1.0±0.3 | 1.7±0.1 | 133.2±14.9 | 1.5±0.1 | 0.7±0.03 |
Values are apelin expression levels for the cell types listed, normalized to 18S ribosomal RNA expression levels. LPS dose for primary rat ileal, STC-1, HCT116 cells = 1 μg/ml; LPS dose for HepG2, Hs578T, TR-iBRB2, GT1-7 cells = 200 ng/ml.
Other colon cell lines (HT29, KM20, Caco2, and NCM356) gave similar results.
IL-6 and IFN-γ-Induced Apelin Expression and Blockade of Jak/Stat Signaling Abolished IL-6 Induced Apelin Expression
LPS administration in vivo increases systemic levels of numerous inflammatory cytokines, including TNF-α, IL-1, IL-6, and IFN-γ (4, 15, 40). A rise in plasma IL-6 levels was confirmed in our experiments (Table 2).
Table 2.
IL-6 levels in LPS-treated mice and cells
|
IL-6 Levels, pg/ml |
||
|---|---|---|
| Control | LPS Treated | |
| Mice plasma | Undetectable | 230.8±56.3* |
| Macrophages (RAW2647) | 3.3±1.9 | 1747.7±4.0* |
| Enteroendocrine (STC-1) | Undetectable | Undetectable |
LPS dose for mice = 1 μg/kg, n = 5 mice/group; LPS dose for cells = 200 ng/ml, n = 3 dishes/group.
P < 0.05 vs. control levels.
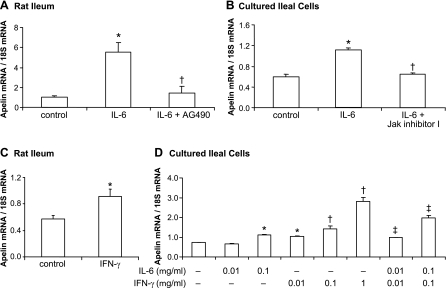
The aim of this experiment, therefore, was to examine the effect of IL-6 and IFN-γ treatments on apelin expression in the rat ileum. IL-6 administration increased ileal apelin expression levels approximately sixfold in rat pups compared with control levels (Fig. 2A). In cell culture experiments, IL-6 treatment (100 ng/ml, 24 h) increased apelin expression levels ∼50% compared with control-treated ileal cells (P < 0.05) (Fig. 2B). IFN-γ treatment of rats increased ileal apelin expression levels ∼50% (Fig. 2C). In primary cultured rat ileal cells, IFN-γ treatment alone increased apelin expression levels in a dose-dependent fashion (Fig. 2D). IFN-γ plus IL-6 treatment in combination, at 10 and 100 ng/ml, increased apelin expression levels additively (P < 0.05). Blockade of the Jak/Stat signaling pathway by pretreatment with AG490 in vivo or with a Jak Inhibitor I in vitro abolished the IL-6-stimulated elevations in ileal apelin expression levels (Fig. 2, A and B).
Fig. 2.
IL-6 and IFN-γ-induced apelin expression in vivo and in vitro, blockade by inhibition of a Jak/Stat signaling. A and B: blockade of Jak/Stat signaling reduces IL-6-induced apelin expression in the rat ileum and in primary cultured rat ileal cells. Apelin expression levels increased significantly 24 h after IL-6 treatment compared with control groups. Inhibitors of Jak/Stat signaling (AG490; Jak inhibitor I) were given 1 h before IL-6 treatment (in vivo: 100 ng/rat; in vitro: 100 ng/ml). *P < 0.05 vs. control group; †P < 0.05 vs. IL-6-treated group. C: in rats, ileal apelin mRNA levels increased significantly 24 h after IFN-γ treatment (100 ng/rat). D: in primary cultured rat ileal cells, IFN-γ treatment increased apelin mRNA levels in a dose-dependent fashion (0.01, 0.1, 1 μg/ml). IFN-γ plus IL-6 treatment in combination increased apelin expression levels additively. *P < 0.05 vs. controls; †P < 0.05 vs. control or lower dose; ‡P < 0.05 vs. control or IL-6 or IFN-γ treatment alone. In vivo n = 5 rats/group; in vitro n = 3 dishes/group.
Conditioned Media Harvested From LPS-Treated Immune Cells Stimulates Ileal Apelin Expression
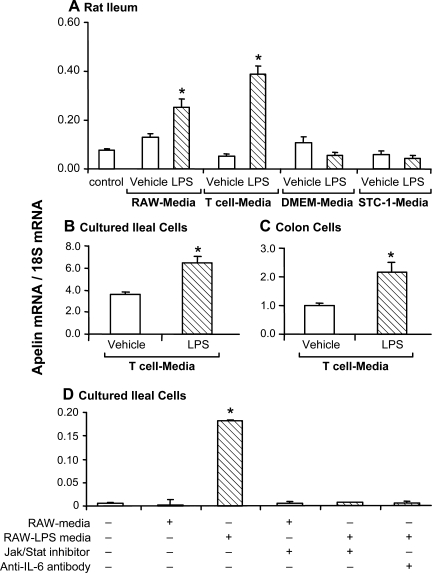
In rats, ileal apelin mRNA levels increased significantly in response to intraperitoneal administration of media harvested from either LPS-treated mouse macrophages (RAW264.7) or primary cultured T cells (Fig. 3A). Administration of fresh DMEM or of conditioned media harvested from LPS-treated STC-1 cells failed to affect ileal apelin expression levels. LPS administration in vivo, at a dose equivalent to that used to treat immune cells, did not affect ileal apelin expression levels (Fig. 3A).
Fig. 3.
Apelin expression levels increase in response to exposure to media harvested from LPS-treated immune cells. A: in rats, ileal apelin mRNA levels increased significantly in response to intraperitoneal administration of media (200 μl) harvested from either LPS-treated mouse macrophages [RAW264.7 (RAW)] or primary cultured T cells, but not in response to DMEM alone, DMEM containing LPS, or medium harvested from LPS-treated STC-1 cells. In all cases, medium harvested from vehicle-treated cells was ineffective; n = 5 rats/group. B and C: compared with vehicle-treated cells, media harvested from LPS-treated T cells increased apelin expression in cultured ileal and colon cells. D: in cultured ileal cells, the increase in apelin expression induced by media harvested from LPS-treated macrophages was blocked either by treating the media with an IL-6 antibody or by pretreating ileal cells with a Jak/Stat inhibitor. Apelin expression levels are normalized to 18S ribosomal RNA expression levels. *P < 0.05 vs. control levels; n = 3 dishes/group.
IL-6 levels in media harvested from macrophages (RAW264.7), but not from STC-1 cells exposed to LPS, increased significantly (Table 2).
Apelin expression levels increased significantly in primary cultured rat ileal cells and in colon cancer cells exposed to conditioned media harvested from LPS-treated T cells (Fig. 3, B and C). In addition, apelin expression levels increased in primary cultured rat ileal cells exposed to media harvested from LPS-treated macrophages (RAW264.7) (Fig. 3D). In cultured ileal cells, the elevation in apelin expression induced by media harvested from LPS-treated macrophages was blocked by treating either ileal cells with a Jak/Stat inhibitor or the medium with an IL-6 antibody.
LPS Increases Apelin Promoter Activity
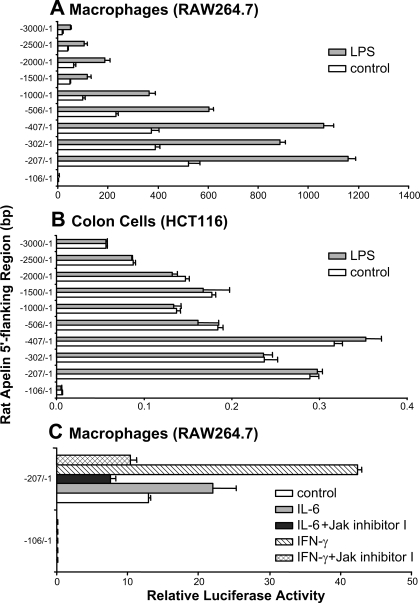
To test the extent to which LPS activates apelin promoter activity and identify LPS sensitive regions in the rat apelin promoter, transient transfection experiments were done using scanning deletion-reporter constructs of the rat apelin 5′-flanking region (−3000/−1; −2500/−1; −2000/−1; −1500/−1; −1000/−1; −506/−1; −407/−1; −302/−1; −207/−1, −106/−1 bp) fused to a luciferase reporter gene. Transfections were done in mouse macrophages (RAW264.7) or human colon cancer (HCT116) cells. Cells were treated with either vehicle (control) or LPS (1 μg/ml, 24 h). LPS treatment increased rat apelin promoter activity two- to threefold in macrophages but not in colon cells (Fig. 4, A and B). Compared with the −207/−1 bp construct, basal transcriptional activity (control) decreased substantially in the longer constructs, suggesting the presence of inhibitory regions in upstream fragments. Transcriptional activity of the −106/−1 bp apelin-luciferase reporter construct in the presence of vehicle (control) or LPS treatment was negligible (Fig. 4, A and B); this deletion construct lacks a putative Stat3 binding site (−198/−195 bp).
Fig. 4.
LPS, IL-6, and IFN-γ increase apelin promoter activity; blockade of IL-6- and IFN-γ-stimulated apelin promoter activity by a Jak/Stat inhibitor. A and B: LPS treatment increases rat apelin promoter activity 2- to 3-fold in macrophages but not in colon (HCT116) cells. The 5′-flanking region (−3000/−1; −2500/−1; −2000/−1; −1500/−1; −1000/−1; −506/−1; −407/−1; −302/−1; −207/−1; −106/−1 bp) of the rat apelin gene fused to a luciferase reporter gene was transfected into macrophage or colon cells and treated with LPS (1 μg/ml). Luciferase activities were measured 18 h after start of LPS treatment. C: IL-6- and IFN-γ-stimulated apelin promoter activity is mediated by the Jak/Stat pathway. The 5′-flanking region (−207/−1; −106/−1 bp) of the rat apelin gene fused to a luciferase reporter gene was transfected into mouse macrophages and treated with IL-6 (100 ng/ml) or IFN-γ (100 ng/ml) alone or in combination with a Jak inhibitor I (10 μM). IL-6 and IFN-γ treatments increased apelin promoter activity; the stimulatory effects of IL-6 and IFN-γ on apelin promoter activity were blocked by pretreatment with a Jak inhibitor I.
IL-6 and IFN-γ-Stimulated Apelin Promoter Activity Is Mediated by the Jak/Stat Pathway
In transient transfection experiments the influence of pharmacological blockade of the Jak/Stat pathway on IL-6 (100 ng/ml) or IFN-γ (100 ng/ml)-stimulated apelin promoter activity was examined in macrophages. IL-6 or IFN-γ treatment increased apelin promoter activity by 50 and 300%, respectively. The stimulatory effects of IL-6 and IFN-γ on apelin promoter activity were blocked by pretreatment with a Jak inhibitor I (Fig. 4C). Transcriptional activities of the −106/−1 bp apelin-luciferase reporter construct in the presence of vehicle, IL-6, or IFN-γ treatments were negligible (Fig. 4C).
Stat3 Overexpression Increases Apelin Promoter Activity
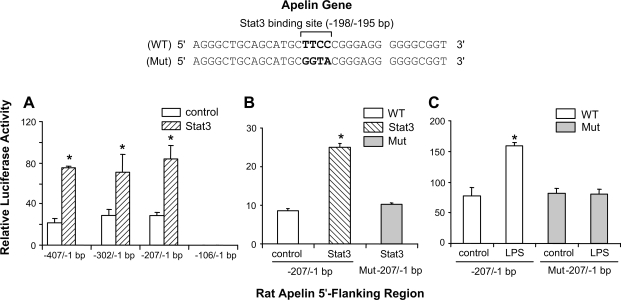
In transient transfection experiments, Stat3 overexpression in macrophages increased apelin promoter activity approximately threefold (P < 0.05 vs. control) (Fig. 5A). Stat3 overexpression did not affected transcriptional activity of the −106/−1 bp apelin-luciferase reporter construct (Fig. 5A). Site-directed mutagenesis of a putative Stat3 binding site (−198/−195 bp) in the apelin core promoter abolished the elevation in apelin promoter activity induced by Stat3 overexpression (Fig. 5B). Additionally, mutation of the putative Stat3 binding site inhibited the elevation in apelin promoter activity induced by LPS exposure (Fig. 5C).
Fig. 5.
A putative Stat3 binding site regulates apelin promoter activity. Top schematic: a putative Stat3 binding site is identified in the wild-type (WT) apelin promoter sequence of the rat (−198/−195 bp); mutated nucleotides are indicated in bold in the mutated (Mut) sequence. Only partial sequences of WT and Mut promoters are shown. A: transient transfection experiments show that Stat3 overexpression increases apelin promoter activity in apelin promoter deletion-luciferase reporter constructs (−407/−1, −302/−1, −207/−1 bp). Promoter activity of the −106/−1 bp apelin promoter reporter construct was not influenced by Stat3 overexpression. B: mutation of a putative Stat3 binding site (−198/−195 bp) in the rat apelin core promoter abolished the elevation in apelin promoter activity induced by Stat3 overexpression. C: mutation of the putative Stat3 binding site inhibited the elevation in apelin promoter activity induced by LPS treatment. *P < 0.05 vs. control levels.
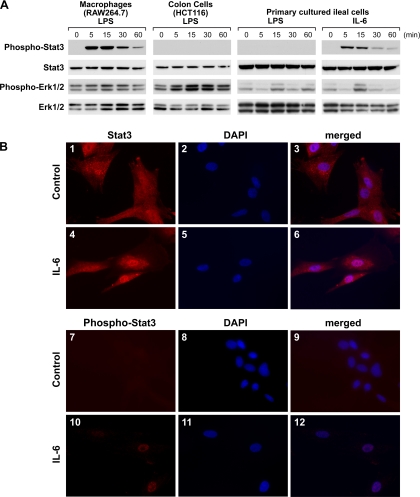
IL-6 Treatment Increases Phospho-Stat3 Levels in Ileal Cells
The influence of LPS or IL-6 treatments on phospho-Stat3 levels in macrophages, colon cells, or primary cultured rat ileal cells were determined by Western blotting (Fig. 6A). Phospho-Stat3 levels increased in LPS-treated macrophages and in IL-6-treated ileal cells. LPS-stimulated phosphorylation of Stat3 was not detected in colon and ileal cells. Levels of phospho-Erk1/2 increased in LPS-treated colon and ileal cells. In macrophages, levels of phospho-Erk1/2 increased marginally in response to LPS treatment. Immunofluorescent staining shows that Stat3 is uniformly distributed throughout ileal cells with control treatment (Fig. 6B1). IL-6 treatment increased Stat3 and phospho-Stat3 immunostaining intensity in cell nuclei (Fig. 6, B4 and B10). Immunostained phospho-Stat3 was not detectable in control-treated cells (Fig. 6B7).
Fig. 6.
IL-6 treatment increases phosphorylated Stat3 (phospho-Stat3) levels in rat ileal cells. A: Western blotting analysis shows phospho-Stat3 or phospho-Erk1/2 levels at various times after LPS or IL-6 treatment of macrophages (RAW264.7), colon (HCT116) cells, and primary cultured rat ileal cells. Total Stat3 and Erk1/2 protein levels are also shown. LPS treatment increased phospho-Stat3 protein levels in macrophages but not in colon or primary ileal cells. IL-6 treatment increased phospho-Stat3 protein levels in primary ileal cells. LPS treatment increased phospho-Erk1/2 protein levels in colon cells and cultured rat ileal cells and in macrophages marginally. IL-6 treatment increased phospho-Erk1/2 protein levels in primary ileal cells. Equal protein loading of lanes was confirmed by reprobing blots with antibodies against total Stat3 and Erk1/2 protein levels. B: immunofluorescent staining (red fluorescence) for Stat-3 and phospho-Stat3 in primary cultured rat ileal cells. DAPI staining (blue fluorescence) indicates localization of nuclei. Stat3 is uniformly distributed throughout the cells with control treatment (B1). IL-6 treatment increases intensity of immunostained Stat3 and phospho-Stat3 in nuclei (B4, B10). Immunostained phospho-Stat3 is not detectable in control-treated cells (B7).
Evidence That IL-6-Induced Phospho-Stat3 Interacts With a Putative Stat3 Binding Site in the Apelin Promoter
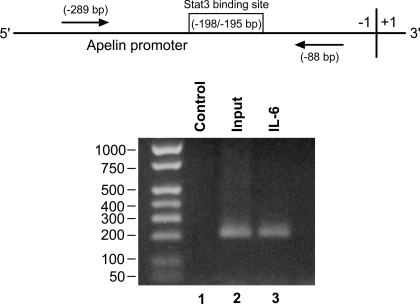
To investigate the extent to which phospho-Stat3 binds to a putative Stat3 binding site in the core promoter of rat apelin, ChIP assays were done using chromatin isolated from vehicle- or IL-6-treated rat ileal cells. Chromatin was cross-linked, sheared, and immunoprecipitated by a phospho-Stat3 antibody. The apelin 5′-upstream region containing the putative Stat3 binding site was amplified by PCR of DNA precipitated by the phospho-Stat3 antibody (Fig. 7). As a positive input control, cross-linked and sheared genomic DNA from ileal cells served as a template. These results indicate that IL-6 treatment causes recruitment of phospho-Stat3 to the rat apelin promoter.
Fig. 7.
Chromatin immunoprecipitation (ChIP) assay indicates that IL-6 induced phospho-Stat3 is recruited to a putative Stat3 site in the rat apelin promoter in primary cultured rat ileal cells. Top: schematic of the proximal promoter region depicting location of a putative Stat3 binding site and start sites of distal and proximal primers (arrows) used in PCR amplification. −289 and −88 bp = start sites. ChIP assays were done using chromatin isolated from vehicle (lane 1) or IL-6-treated (lane 3) primary cultured rat ileal cells. Chromatin was cross-linked, sheared, and immunoprecipitated by a phospho-Stat3 antibody. Immunoprecipitations were followed by PCR amplification of the promoter region containing the putative Stat3 binding site (−198/−195 bp). Positive input DNA (lane 2) used for PCR was 2% of the amount used for immunoprecipitation. DNA size markers are shown.
DISCUSSION
Earlier studies by our laboratory indicate that intestinal inflammation is associated with elevations in expression of a regulatory peptide called apelin (13, 14). In patients with IBD and in rodents with experimental colitis colonic apelin production is increased (14). The principal aim of this study was to identify mediators and a molecular mechanism underlying the increased enteric apelin expression.
The major finding of these studies is that the increased enteric apelin expression measured during inflammation is dependent on a Stat3-mediated elevation in apelin promoter activity that is triggered by increased inflammatory cytokine activity.
In these studies, LPS treatments of rodents in vivo and of macrophages and T cells in vitro were used as experimental models to identify potential mediators behind the increase in enteric apelin expression during inflammation. LPS treatment of rodents increased enteric apelin expression levels, and apelin expression levels in the ileum of rat pups or in cultured rat ileal cells increased in response to media harvested from LPS-treated macrophages or T cells. It is well known that LPS administration in vivo activates host immune and inflammatory cells, particularly mononuclear phagocytes. In the intestinal muscularis, LPS administration will activate macrophages, triggering, in part, increased secretion of IL-6 and IFN-γ (33, 38). IL-6 and IFN-γ production are also elevated during IBD in humans and in rodents with experimental colitis (1, 30, 46). Involvement of secreted T cell mediators in the increased apelin production was supported further by reductions in ileal apelin expression levels in LPS-treated nude mice. The importance of inflammatory mediators in stimulating apelin production is bolstered by the failure of LPS administration to increase apelin expression in cultured cells derived from noninflammatory tissues and the failure of media harvested from LPS-treated intestinal enteroendocrine (STC-1) cells to increase ileal apelin expression in rat pups.
Our findings demonstrate that the inflammation-induced elevation in enteric apelin expression is mediated by IL-6 and IFN-γ. In terms of a molecular mechanism, IL-6 and IFN-γ increase enteric apelin mRNA levels by a stimulation of apelin promoter activity, which is mediated by the Jak/Stat signaling pathway. Treatment of animals or of cultured cells with IL-6 and IFN-γ stimulated enteric apelin expression, and pharmacological blockade of Jak/Stat signaling abolished IL-6-induced elevations in enteric apelin expression in vivo and in vitro. Blockade of Jak/Stat signaling also suppressed IL-6- and IFN-γ-induced apelin promoter activity and elevations in enteric apelin expression in cultured ileal cells exposed to media harvested from LPS-treated macrophages. More importantly, transient transfection experiments demonstrated that the LPS-induced elevation in apelin promoter activity in macrophages was abolished by mutation of a putative Stat3 binding site in the rat apelin core promoter. Unlike the stimulatory effect of LPS on apelin promoter activity in macrophages, LPS treatment of colon cancer (HCT116) cells failed to increase apelin promoter activity. Together, these findings indicate the dependence of increased enteric apelin expression during inflammation on an enhanced apelin transcriptional activity stimulated by inflammatory mediators.
Findings also show that binding of phospho-Stat3 to the apelin promoter is the final step underlying the stimulatory effects of inflammatory cytokines on enteric apelin expression. Sequence scanning identified multiple Stat binding sites, including a putative Stat3 binding site, in the rat apelin promoter. Functionality for the Stat3 binding site was demonstrated by the increased apelin promoter activity in response to Stat3, but not to Stat1 or Stat5 overexpression, and by a complete reduction in apelin promoter activity by using an apelin promoter-luciferase reporter construct having a mutated Stat3 site in LPS-treated macrophages. Furthermore, an apelin promoter-luciferase reporter construct (−106/−1 bp) that lacks a putative Stat3 binding site is not responsive to LPS, IL-6, or IFN-γ treatment. ChIP assay and immunofluorescent staining findings verify that IL-6 induced phospho-Stat3 is bound to the putative Stat3 site in the rat apelin core promoter.
Macrophages will secrete a variety of inflammatory cytokines in response to LPS exposure (23, 24, 31). We show that the stimulatory effect of media harvested from LPS-treated macrophages on apelin expression is blocked by pretreatment either with an anti-IL-6 antibody or with a Jak/Stat inhibitor. Our findings agree with and extend an earlier report showing that increased IL-6 activity during intestinal inflammation in rodents with DSS-induced colitis and in humans with IBD stimulates Stat3 signaling (26, 37). Another report refers to IL-6 as the final common or “master cytokine” in the stimulation of Stat3 in the initiation of intestinal inflammation (30). Additionally, blockade of IL-6-induced Stat3 activity by IL-6 receptor antibodies lessens the severity of intestinal inflammation in patients with Crohn's disease and in rodents with experimental colitis (1, 17, 46).
It should be pointed out that the proinflammatory cytokine-induced elevation in apelin expression may have a functional relevance during obesity. In obese patients, a mild inflammatory condition exists with increased systemic levels of proinflammatory cytokines (34, 47). A proinflammatory cytokine-induced elevation in apelin production most likely participates in the expansion of adipose mass during obesity since a recent report shows that apelin is involved in development of the vascular network in adipose tissue (19).
Interestingly, a previous report identified phosphatidylinositol 3-kinase, JNK, and MAPK as signaling pathways behind TNF-α-induced elevations in apelin expression in human and mouse fat cells (7). It should be pointed out that the elevation in apelin expression may be mediated by an increased Stat3 phosphorylation since TNF-α treatment can cause IL-6 secretion in fat cells (7). Additionally, our studies show that a rise in phosphorylated Erk1/2 levels is not accompanied by an elevation in apelin expression in either cultured rat ileal or human colon cells. The failure by apelin expression to increase in nonimmune system derived cells is most likely explained by the absence of an increased Stat3 phosphorylation.
In summary, our findings indicate that the increased enteric apelin expression levels measured in in vitro and in vivo models of inflammation are triggered by an IL-6-induced phosphorylation of Stat3, which then causes an elevation in apelin transcriptional activity.
GRANTS
This work is supported by grants from the National Institutes of Health (5 P01 DK035608), Crohn's and Colitis Foundation of America (ref. no. 1821), and Broad Medical Research Program (IBD-0118).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, Schutz M, Bartsch B, Holtmann M, Becker C, Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schurmann G, Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S, Neurath MF. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med 6: 583–588, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Brown SJ, Mayer L. The immune response in inflammatory bowel disease. Am J Gastroenterol 102: 2058–2069, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Cayabyab M, Hinuma S, Farzan M, Choe H, Fukusumi S, Kitada C, Nishizawa N, Hosoya M, Nishimura O, Messele T, Pollakis G, Goudsmit J, Fujino M, Sodroski J. Apelin, the natural ligand of the orphan seven-transmembrane receptor APJ, inhibits human immunodeficiency virus type 1 entry. J Virol 74: 11972–11976, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan AS, Yip EC, Yung LY, Pang H, Luk SC, Pang SF, Wong YH. CKBM stimulates MAPKs but inhibits LPS-induced IFN-gamma in lymphocytes. Phytother Res 20: 725–731, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Chang CH, Chey WY, Sun Q, Leiter A, Chang TM. Characterization of the release of cholecystokinin from a murine neuroendocrine tumor cell line, STC-1. Biochim Biophys Acta 1221: 339–347, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Dai T, Ramirez-Correa G, Gao WD. Apelin increases contractility in failing cardiac muscle. Eur J Pharmacol 553: 222–228, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daviaud D, Boucher J, Gesta S, Dray C, Guigne C, Quilliot D, Ayav A, Ziegler O, Carpene C, Saulnier-Blache JS, Valet P, Castan-Laurell I. TNFα up-regulates apelin expression in human and mouse adipose tissue. FASEB J 20: 1528–1530, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Edinger AL, Hoffman TL, Sharron M, Lee B, Yi Y, Choe W, Kolson DL, Mitrovic B, Zhou Y, Faulds D, Collman RG, Hesselgesser J, Horuk R, Doms RW. An orphan seven-transmembrane domain receptor expressed widely in the brain functions as a coreceptor for human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol 72: 7934–7940, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farkasfalvi K, Stagg MA, Coppen SR, Siedlecka U, Lee J, Soppa GK, Marczin N, Szokodi I, Yacoub MH, Terracciano CM. Direct effects of apelin on cardiomyocyte contractility and electrophysiology. Biochem Biophys Res Commun 357: 889–895, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Gomez G, Zhang T, Rajaraman S, Thakore KN, Yanaihara N, Townsend CM Jr, Thompson JC, Greeley GH. Intestinal peptide YY: ontogeny of gene expression in rat bowel and trophic actions on rat and mouse bowel. Am J Physiol Gastrointest Liver Physiol 268: G71–G81, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Habara T, Nakatsuka M, Konishi H, Asagiri K, Noguchi S, Kudo T. The biological effects of antiadhesion agents on activated RAW264.7 macrophages. J Biomed Mater Res 61: 628–633, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S, Kitada C, Nishizawa N, Murosaki S, Kurokawa T, Onda H, Tatemoto K, Fujino M. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim Biophys Acta 1452: 25–35, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Han S, Wang G, Qi X, Lee HM, Englander EW, Greeley GH Jr. A possible role for hypoxia-induced apelin expression in enteric cell proliferation. Am J Physiol Regul Integr Comp Physiol 294: R1832–R1839, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Han S, Wang G, Qiu S, de la Motte C, Wang HQ, Gomez G, Englander EW, Greeley GH Jr. Increased colonic apelin production in rodents with experimental colitis and in humans with IBD. Regul Pept 142: 131–137, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Hirohashi N, Morrison DC. Low-dose lipopolysaccharide (LPS) pretreatment of mouse macrophages modulates LPS-dependent interleukin-6 production in vitro. Infect Immun 64: 1011–1015, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosoya M, Kawamata Y, Fukusumi S, Fujii R, Habata Y, Hinuma S, Kitada C, Honda S, Kurokawa T, Onda H, Nishimura O, Fujino M. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem 275: 21061–21067, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, Matsumoto T, Yamamura T, Azuma J, Nishimoto N, Yoshizaki K, Shimoyama T, Kishimoto T. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn's disease. Gastroenterology 126: 989–996, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Kawamata Y, Habata Y, Fukusumi S, Hosoya M, Fujii R, Hinuma S, Nishizawa N, Kitada C, Onda H, Nishimura O, Fujino M. Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta 1538: 162–171, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Kunduzova O, Alet N, Delesque-Touchard N, Millet L, Castan-Laurell I, Muller C, Dray C, Schaeffer P, Herault JP, Savi P, Bono F, Valet P. Apelin/APJ signaling system: a potential link between adipose tissue and endothelial angiogenic processes. FASEB J, 2008. Aug 15 [Epub ahead of print]. [DOI] [PubMed]
- 20.Lago F, Dieguez C, Gomez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev 18: 313–325, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Lambrecht NW, Yakubov I, Zer C, Sachs G. Transcriptomes of purified gastric ECL and parietal cells: identification of a novel pathway regulating acid secretion. Physiol Genomics 25: 153–165, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, Osmond DH, George SR, O'Dowd BF. Characterization of apelin, the ligand for the APJ receptor. J Neurochem 74: 34–41, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Liu YW, Chen CC, Tseng HP, Chang WC. Lipopolysaccharide-induced transcriptional activation of interleukin-10 is mediated by MAPK- and NF-kappaB-induced CCAAT/enhancer-binding protein delta in mouse macrophages. Cell Signal 18: 1492–1500, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Matsuura M, Saito S, Hirai Y, Okamura H. A pathway through interferon-gamma is the main pathway for induction of nitric oxide upon stimulation with bacterial lipopolysaccharide in mouse peritoneal cells. Eur J Biochem 270: 4016–4025, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Medhurst AD, Jennings CA, Robbins MJ, Davis RP, Ellis C, Winborn KY, Lawrie KW, Hervieu G, Riley G, Bolaky JE, Herrity NC, Murdock P, Darker JG. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J Neurochem 84: 1162–1172, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Mitsuyama K, Matsumoto S, Rose-John S, Suzuki A, Hara T, Tomiyasu N, Handa K, Tsuruta O, Funabashi H, Scheller J, Toyonaga A, Sata M. STAT3 activation via interleukin 6 trans-signalling contributes to ileitis in SAMP1/Yit mice. Gut 55: 1263–1269, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mudter J, Neurath MF. Il-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm Bowel Dis 13: 1016–1023, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Murakami K, Bujo H, Unoki H, Saito Y. Effect of PPARalpha activation of macrophages on the secretion of inflammatory cytokines in cultured adipocytes. Eur J Pharmacol 561: 206–213, 2007. [DOI] [PubMed] [Google Scholar]
- 29.O'Carroll AM, Selby TL, Palkovits M, Lolait SJ. Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochim Biophys Acta 1492: 72–80, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Pizarro TT, De La Rue SA, Cominelli F. Role of interleukin 6 in a murine model of Crohn's ileitis: are cytokine/anticytokine strategies the future for IBD therapies? Gut 55: 1226–1227, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem 281: 33019–33029, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Reaux-Le Goazigo A, Alvear-Perez R, Zizzari P, Epelbaum J, Bluet-Pajot MT, Llorens-Cortes C. Cellular localization of apelin and its receptor in the anterior pituitary: evidence for a direct stimulatory action of apelin on ACTH release. Am J Physiol Endocrinol Metab 292: E7–E15, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz NT, Engel B, Eskandari MK, Kalff JC, Grandis JR, Bauer AJ. Lipopolysaccharide preconditioning and cross-tolerance: the induction of protective mechanisms for rat intestinal ileus. Gastroenterology 123: 586–598, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Sempere L, Martinez J, de Madaria E, Lozano B, Sanchez-Paya J, Jover R, Perez-Mateo M. Obesity and fat distribution imply a greater systemic inflammatory response and a worse prognosis in acute pancreatitis. Pancreatology 8: 257–264, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Sorhede Winzell M, Magnusson C, Ahren B. The apj receptor is expressed in pancreatic islets and its ligand, apelin, inhibits insulin secretion in mice. Regul Pept 131: 12–17, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Susaki E, Wang G, Cao G, Wang HQ, Englander EW, Greeley GH Jr. Apelin cells in the rat stomach. Regul Pept 129: 37–41, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki A, Hanada T, Mitsuyama K, Yoshida T, Kamizono S, Hoshino T, Kubo M, Yamashita A, Okabe M, Takeda K, Akira S, Matsumoto S, Toyonaga A, Sata M, Yoshimura A. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med 193: 471–481, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki M, Hisamatsu T, Podolsky DK. Gamma interferon augments the intracellular pathway for lipopolysaccharide (LPS) recognition in human intestinal epithelial cells through coordinated up-regulation of LPS uptake and expression of the intracellular Toll-like receptor 4-MD-2 complex. Infect Immun 71: 3503–3511, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taheri S, Murphy K, Cohen M, Sujkovic E, Kennedy A, Dhillo W, Dakin C, Sajedi A, Ghatei M, Bloom S. The effects of centrally administered apelin-13 on food intake, water intake and pituitary hormone release in rats. Biochem Biophys Res Commun 291: 1208–1212, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Takasuka N, Tokunaga T, Akagawa KS. Preexposure of macrophages to low doses of lipopolysaccharide inhibits the expression of tumor necrosis factor-alpha mRNA but not of IL-1 beta mRNA. J Immunol 146: 3824–3830, 1991. [PubMed] [Google Scholar]
- 41.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 251: 471–476, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Tatemoto K, Takayama K, Zou MX, Kumaki I, Zhang W, Kumano K, Fujimiya M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept 99: 87–92, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Wang G, Anini Y, Wei W, Qi X, O'Carroll AM, Mochizuki T, Wang HQ, Hellmich MR, Englander EW, Greeley GH Jr. Apelin, a new enteric peptide: localization in the gastrointestinal tract, ontogeny, and stimulation of gastric cell proliferation and of cholecystokinin secretion. Endocrinology 145: 1342–1348, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Wang G, Kundu RK, Han S, Qi X, Englander EW, Quertermous T, Greeley GH. Regulation of colonic trefoil factor 3 by apelin. Gastroenterology 134: A392, 2008. [Google Scholar]
- 45.Wang G, Qi X, Wei W, Englander EW, Greeley GH Jr. Characterization of the 5′-regulatory regions of the rat and human apelin genes and regulation of breast apelin by USF. FASEB J 20: 2639–2641, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol 164: 4878–4882, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, D'Andrea F, Molinari AM, Giugliano D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation 105: 804–809, 2002. [DOI] [PubMed] [Google Scholar]