Abstract
Aldosterone-induced intestinal Na+ absorption is mediated by increased activities of apical membrane Na+/H+ exchange (aNHE3) and basolateral membrane Na+-K+-ATPase (BLM-Na+-K+-ATPase) activities. Because the processes coordinating these events were not well understood, we investigated human intestinal Caco-2BBE cells where aldosterone increases within 2–4 h of aNHE3 and α-subunit of BLM-Na+-K+-ATPase, but not total abundance of these proteins. Although aldosterone activated Akt2 and serum glucorticoid kinase-1 (SGK-1), the latter through stimulation of phosphatidylinositol 3-kinase (PI3K), only the SGK-1 pathway mediated its effects on Na+-K+-ATPase. Ouabain inhibition of the early increase in aldosterone-induced Na+-K+-ATPase activation blocked most of the apical NHE3 insertion, possibly by inhibiting Na+-K+-ATPase-induced changes in intracellular sodium concentration ([Na]i). Over the next 6–48 h, further increases in aNHE3 and BLM-Na+-K+-ATPase activity and total protein expression were observed to be largely mediated by aldosterone-activated SGK-1 pathway. Aldosterone-induced increases in NHE3 mRNA, for instance, could be inhibited by RNA silencing of SGK-1, but not Akt2. Additionally, aldosterone-induced increases in NHE3 promoter activity were blocked by silencing SGK-1 as well as pharmacological inhibition of PI3K. In conclusion, aldosterone-stimulated intestinal Na+ absorption involves two phases. The first phase involves stimulation of PI3K, which increases SGK-dependent insertion and function of BLM-Na+-K+-ATPase and subsequent increased membrane insertion of aNHE3. The latter may be caused by Na+-K+-ATPase-induced changes in [Na] or transcellular Na flux. The second phase involves SGK-dependent increases in total NHE3 and Na+-K+-ATPase protein expression and activities. The coordination of apical and BLM transporters after aldosterone stimulation is therefore a complex process that requires multiple time- and interdependent cellular processes.
Keywords: Na+ transport; intestine; intracellular Na+, intestinal adaptation; fluid and electrolyte transport; diarrhea; malabsorption; Na+-H+ exchange
for efficient vectorial Na+ absorption in the intestine, membrane transporters at opposite poles of the epithelial cell must be coordinately regulated, a process referred to as homocellular regulation (43). The mechanisms mediating this phenomenon are poorly understood. In Na+-absorbing epithelia, for instance, an increase in the rate of apical membrane Na+ influx is accompanied by increased conductance of the basolateral membrane to K+ (42, 44) or Cl− (8) that are postulated as necessary to accommodate K+ recycling for increased Na+-K+-ATPase activity. Similar findings have been observed in salivary duct epithelium (8) and intestinal T84 cells (45).
In the small intestine, the major pathway for non-nutrient-dependent sodium absorption is Na+/H+ exchange (NHE) (11), specifically involving apical membrane NHE2 and NHE3 (3, 24, 52) that are responsible for the initial step in vectorial Na+ absorption (28, 54). After apical transport, absorbed sodium ion is then pumped out the intestinal epithelial cell by the basolateral Na+-K+-ATPase pump (53) although how this is coordinated with increased Na+ influx is unclear. This ATPase is also pivotal since it maintains the electrochemical driving force necessary for sustained vectorial Na+ absorption.
NHE2 and NHE3 activities are rapidly regulated (within minutes) by a wide variety of second messenger systems including cAMP, cGMP, intracellular calcium, PI3-kinase (PI3K), serum glucorticoid kinase-1 (SGK-1), and protein kinase C (25, 27, 29, 30, 31, 32, 35, 49, 50, 57). This acute regulation appears to involve both changes in functional activity (30, 51, 59) and membrane trafficking (25, 30, 31, 57). In contrast, less is known about the chronic regulation of NHE2 and NHE3 expression by gluco- and mineralocorticoids (6, 7, 40, 56) as well as by nutrients such as short chain fatty acids (28, 37). Homocellular regulation in these instances likely involves complex interplay and coordination between apical and basolateral membrane transporters. Glucocorticoids have both acute and long-term effects in stimulating NHE3 (7, 49, 50, 56). The acute effects on NHE3 may be mediated by the glucocorticoid receptor, which stimulates SGK-1, resulting in NHE3 phosphorylation at serine 663, NHE3 exocytosis, and increased NHE activity (49, 50).
The present studies demonstrate that physiological actions of mineralocorticoid (aldosterone) on net intestinal Na+ absorption consists of two phases. The initial phase involves activation of PI3K that stimulates insertion of the α-subunit of Na+-K+-ATPase through a SGK-1-dependent pathway, increasing Na+-K+-ATPase activity and subsequent increased apical membrane NHE3 (aNHE3). The second phase involves SGK-1-dependent increases in total NHE3 and Na+-K+-ATPase (α-subunit) expression and activity although a contribution by aldosterone-mediated gene transcription of NHE3 cannot be ruled out. These actions reveal a complex, but orderly sequence of events that are essential for coordination of transport processes in both poles of epithelial cells and efficient net Na absorption.
MATERIALS AND METHODS
Cell culture.
Caco-2BBE intestinal epithelial cells, provided by Dr. Mark Mooseker (39) (Yale University, New Haven, CT), were grown as confluent monolayers on rat tail collagen-coated Transwells in DMEM supplemented with 10% vol/vol fetal bovine serum, 2 mM glutamine, 10 μg/ml transferrin, 50 U/ml penicillin, and 50 μg/ml streptomycin in a humidified atmosphere of air containing 5% CO2. Cells were seeded onto the collagen-coated Transwells at a density of 105 cells/cm2 and cultured for 14 days before each experiment. Differentiation of Caco-2BBE cells in culture was determined by expression of villin and alkaline phosphatase.
Apical membrane unidirectional 22Na+ influx as a measure of NHE activity.
For influx studies, Caco-2BBE cells were grown on Transwells for 14 days in regular DMEM with HCO3 supplemented with 10% fetal bovine serum (vol/vol), glutamine, penicillin, streptomycin, and transferrin in a 5% CO2 incubator. Monolayers were washed once in 150 mM choline Cl, 10 mM HEPES pH 7.4, and then unidirectional apical membrane sodium uptakes were determined in flux buffer (130 mM Choline Cl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 15 mM HEPES pH 7.4, 20 mM NaCl with 1 μCi/ml [22NaCl]) for 10 min. Sodium influx was stopped by 4 washes in cold buffer (140 mM NaCl, 5 mM KCl, 15 mM HEPES pH 7.4, and 1 mM Na3PO4) and was calculated by dividing the accumulated disintegrations per minute by the specific Na activity in the medium. Dimethylamiloride (DMA) (500 μM) and HOE 694 (30 μM) were used to distinguish NHE2 and NHE3 activities, as previously described (35). NHE2 activity was defined as the HOE-694-inhibitable portion of the flux and NHE3 activity as the difference of the DMA- and HOE-694 inhibitable portions of the unidirectional 22Na influx.
For studies on aldosterone-induced apical NHE3 exocytosis, cell monolayers were treated with ouabain (100 μM) for 2 h before aldosterone (1 nM) stimulation when appropriate. For low Na+ medium, two media of varying [Na+] were made, one of 140 mM and one of 5 mM Na+ (composition in mmol/l: 5 or 140 NaCl, 135 or 0 N-methyl-d-glucamine Cl, 5 KCl, 1.25 MgCl2, 2 CaCl2, 10 HEPES pH 7.4, both essential and nonessential amino acid mixtures, vitamin mixture, and 10% fetal bovine serum dialyzed using a 3,000-kDa cutoff membrane). Monolayers were incubated with the 140 mM Na+ medium on the apical side and 5 mM Na+ medium on the basolateral side. Monolayers were treated with the NHE monensin (3 μM) or vehicle (2.5 μl of ethanol into 2,500 μl medium) on the basolateral medium at the same time as change of the medium. Monolayers were incubated with low basolateral Na+ medium with or without monensin and normal apical medium Na+, the latter to insure the presence of an apical membrane Na gradient for driving NHE3 activity. Monolayers were incubated with these altered Na+ medium with or without basolateral monensin (or ethanol vehicle) for 2 h, and then monolayers were rapidly cooled and apical surface NHE3 biotinylated. Biotinylated apical surface proteins, as well as total NHE3, were analyzed by Western blotting. In previous studies, we have demonstrated that this protocol results in specific surface biotinylation on one side of the cell (37). Apical sulfo-normal horse serum (NHS)-biotin over 30-min incubation labels NHE3 on the apical side of the cell but does not biotinylate the basolateral α-subunit of the Na+-K+-ATPase, nor heat shock cognate 73, an endoplasmic reticulum chaperone (Hsc70). Basolateral addition of this form of biotin over 30 min labels the basolateral α-ATPase subunit but not apical NHE3 nor the intracellular Hsc70.
Western blotting.
Caco-2BBE monolayers were scraped off the Transwells in PBS, pelleted, and resuspended in lysis buffer (10 mM Tris, 5 mM MgCl2, the complete protease inhibitor cocktail, 50 U/ml DNase, and RNAse). An aliquot was removed for protein determination and enzyme assays, and the remaining cell protein was then solubilized in Laemmli stop buffer at 65°C for 10 min. Proteins (generally 20 μg) were separated by 7.5% SDS-PAGE and immediately transferred to polyvinylidene difluoride membranes (Polyscreen; Perkin Elmer Life Sciences, Boston, MA) in 1× Towbin buffer (25 mM Tris, 192 mM glycine, pH 8.8 with 15% vol/vol methanol). Membranes were blocked in Tween 20-Tris buffered saline (T-TBS; 150 mM NaCl, 5 mM KCl, 10 mM Tris pH 7.2 with 0.1% vol/vol Tween 20) containing 5% wt/vol nonfat dry milk (Carnation, Solon, OH) for 60 min at room temperature. Blots were incubated overnight at 4°C with primary antibodies (specific polyclonal antisera developed and characterized in our laboratory) to NHE2 and NHE3 (3, 35) diluted in T-TBS with 1% wt/vol bovine serum albumin. For measurement of levels of α- and β-subunits of the Na+-K+-ATPase, specific murine monoclonal antibodies from Upstate Biotechnology (Charlottesville, VA) were used. Blots were then developed using an enhanced chemiluminescence system (Supersignal; Pierce Chemical, Rockford, IL).
RNA isolation, reverse transcription, and real-time PCR.
Caco-2BBE cells were treated with aldosterone (1 nM) for varying times. Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA). A sample (1 μg) of RNA was reverse transcribed by random priming (Superscript II RT, Invitrogen) and 1/20th used for real-time PCR performed on an I Cycler (Bio-Rad, Hercules, CA) using SybrGreen Mix and primers for human NHE3 (bases 2070-2199, NM_004174) and human GAPDH (NM_002046, bases 498-625). Relative mRNA levels were calculated using the comparative threshold cycle (ΔΔCt) method (41). Each PCR reaction was performed in triplicate, and all experiments were repeated three times. For each sample, mRNA levels of both NHE3 and GAPDH were measured and the cycle threshold of NHE3 subtracted from that of GAPDH. This value was set to one for untreated control conditions at zero time, and other time points are calculated relative to this change.
Luciferase reporter activity.
A 2,200-bp region of the rat NHE3 promoter (5) was a generous gift of Dr. A. Cano (Univ. of Texas Southwestern Medical Center, Dallas, TX). This promoter was linked to firefly luciferase in the plasmid pGL3 (Promega, Madison, WI). Monolayers were transiently transfected with 2 μg of the NHE3 promoter-firefly luciferase plasmid along with 100 ng of a thymidine kinase promoter linked to Renilla luciferase reporter plasmid using 10 μl of the transfection reagent LT-1 (Mirus, Madison, WI) using the manufacturer's directions. The transfection was done in monolayers that were 14 days postplating. Twenty-four hours after transfection, monolayers were treated with aldosterone. When appropriate, monolayers were treated with ouabain or spironolactone or SGK or Akt2 kinases siRNA as described above. Cell monolayers were harvested in lysis buffer provided with the dual-luciferase assay kit, and firefly and Renilla luciferase were measured in a Berthold Lumat luminometer (Berthold, NJ) using the protocol provided with the Dual Luciferase assay system (Promega).
Na+-K+-ATPase activity.
Monolayers were also treated with physiologically relevant (pM and nM) to pharmacological (μM) concentrations of aldosterone for times from 1–48 h. Agents were added directly to the culture medium. Na+-K+-ATPase activity in the cells was measured using a K+-stimulated phosphatase assay in postmitochondrial membranes (13, 45). Cells were scraped into ice-cold saline and pelleted (500 g for 2 min at room temperature). Cell pellets were resuspended in 5 ml of lysis buffer [10 mM Tris pH 7.4, 3 mM EDTA, 80 mM mannitol with the complete protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN)] and homogenized for 30 s at 15,000 revolution/min in an Ultra-Turrax homogenizer. Unbroken cells, nuclei, and mitochondria were pelleted (10,000 g for 10 min at 4°C), and the supernatant was spun at 45,000 g for 30 min at 4°C to obtain the postmitochondrial membranes. Membranes were resuspended in lysis buffer, an aliquot removed for protein determination by the bicinchoninic acid procedure, and ATPase activities measured in Na+- or K+-containing buffers (20 mM Tris pH 7.8, 5 mM MgCl2, and 50 mM NaCl or KCl) using paranitrophenyl phosphate (p-NPP) (5 mM) as substrate and paranitrophenyl (p-NP) as the standard as described (13). Reactions were allowed to proceed for 30 min, stopped by addition of trichloroacetic acid (to 1% wt/vol) and then NaOH (to 10 mM). Absorbance values were measured at 410 nm, and K+-stimulated phosphatase activity was calculated as the difference between activities in the Na+- and K+- containing buffers. Activities were expressed as nanomoles p-NP hydrolyzed per hour per milligram protein.
To determine surfaced expression of the basolateral Na+-K+-ATPase α-subunits or apical NHE3, monolayers (when appropriate treated with 1 nM aldosterone) were treated with the cell impermeant sulfo-NHS-biotin (1 mg/ml, Pierce Chemicals) for 30 min at 4°C in phosphate-buffered saline on the basolateral side for the ATPase subunit or apical for NHE3 (37). Reactions were stopped by the addition of Tris (10 μl of 1 M stock, pH 7.4), after which cells were scraped off the filters in ice-cold saline and pelleted (500 g for 20 s at 4°C). Pellets were resuspended in RIPA buffer (150 mM NaCl, 10 mM Tris pH 7.4, 2 mM EDTA, 1 mM PMSF, with 1% vol/vol Triton X-100, 0.5% wt/vol sodium deoxycholate, and 0.1% wt/vol SDS). Samples were solubilized for 30 min on ice, insoluble material pelleted (14,000 g for 2 min at 4°C), and the supernatant was added to 50 μl of a 50% wt/vol slurry of streptavidin agarose (Pierce Chemicals). Samples were rotated overnight at 4°C, biotinylated material bound to beads pelleted (500 g for 20 s at 4°C), and washed four times with RIPA buffer. Samples were eluted from the beads using Laemmli stop solution at 65°C and analyzed for the ATPase subunit or NHE3 by Western blotting as described above.
Akt and SGK activities.
For measurement of Akt and SGK activities, cells were treated as appropriate and then scraped into ice-cold saline and resuspended in immunoprecipitation buffer (150 mM NaCl, 10 Tris pH 7.4, 2 mM EDTA, 1 mM Na orthovanadate, 10 mM Na fluoride, 5 M Na pyrophosphate, 1% vol/vol Triton X-100 with the complete protease inhibitor cocktail). Cells from one 60-mm Petri dish were solubilized in 500 μl of the buffer and anti-SGK or anti-Akt (both from Upstate Biotechnology) antibody added along with 25 μl protein G-Sepharose (Pierce Chemical). Samples were rotated for 2 h in the cold, and then beads were pelleted and washed three times (14,000 g for 20 s at 4°C) with immunoprecipitation buffer with additional 350 mM NaCl and 0.1% 2-mercaptoethanol. Beads were washed once with 50 mM Tris pH 7.5 0.35% Brij-35, 0.1 mM EGTA, and 0.1% 2-mercaptoethanol and twice with assay buffer (20 mM MOPS pH 7.2, 25 mM β-glycerol phosphate, 5 mM EGTA, 1 mM Na orthovanadate, 1 mM dithiothreitol). A sample (10 μl) of assay buffer was added to the beads along with 2 μCi γ-32[P]-ATP and the peptide substrate for both SGK and Akt (Upstate Biotechnology). Reactions were terminated after 15 min by addition of 20 μl 50% trichloroacetic acid and spotting on P81 phosphocellulose paper (Whatman). Papers were gently rinsed with two washes, 5 min each in 0.75% phosphoric acid. Papers were dried and radioactivity quantified by liquid scintillation spectroscopy.
To silence SGK-1 or Akt-2, predesigned siRNA were purchased from Ambion (Austin, TX). Silencing RNA specific for human SGK-1 (NM_005627, no. 710) or Akt-2 (NM_001626, no. 150) were introduced into Caco-2BBE monolayers using SilentFect reagent (BioRad). An aliquot to yield concentration of silencing oligonucleotide of 50 nM was complexed with SilentFect in OptiMEM (Invitrogen) for 30 min at room temperature. This was added to monolayers where the medium had been just replaced with OptiMEM. Incubations were allowed to proceed for 30 min, and then complete medium with 20% vol/vol FBS added. In initial experiments, a second introduction of silencing oligonucleotide after 24 h and subsequent harvesting at 24–36 h later proved to be most successful at decreasing SGK-1 or Akt-2 (data not shown). At the time of the second application of silencing oligonucleotide, aldosterone was added when appropriate. Monolayers were harvested either 2 h later for exocytosis of the α-subunit of the Na+-K+-ATPase or 22Na fluxes measured after 36 h.
PI3K assay.
Samples were harvested after treatment with aldosterone, in some cases after cells were pretreated with various inhibitors for 2 h. Cells were solubilized in nondenaturing immunoprecipitation buffer (150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 2 mM Na orthovanadate, 1 mM NaF, 20 mM Tris pH 7.4, 1% vol/vol NP-40, 10% vol/vol glycerol, 1 mM PMSF, and the complete protease inhibitor cocktail) and placed on ice for 5 min. The insoluble material was then pelleted at 14,000 g for 10 min at 4°C. An aliquot was removed for protein determination, and 500 μg were subsequently used. A sample (2 μg) of mouse monoclonal anti-phosphotyrosine antibody 4G10 (Upstate Biotechnology) was used to immunoprecipitate PI3K. After addition of 20 μl protein G agarose, immunoprecipitations were allowed to proceed 2 h, and then beads were pelleted, washed in above buffer, and washed once with assay buffer (10 mM Tris pH 7.4, 100 mM NaCl, 1 mM EDTA). The beads were resuspended in 30 μl of assay buffer that included 10 μg sonicated phosphatidyl inositol (Avanti Polar Lipids, Alabaster, AL) and 1 μCi γ-ATP (Perkin Elmer), and reactions were incubated at 30°C for 30 min. Reactions were stopped by the addition of 20 μl 3 N HCl and 150 μl chloroform:methanol (1:1). Samples were centrifuged (14,000 g at room temperature for 5 min), and the chloroform phase was removed, dried, and resolved on silica gel thin layer chromatography plates (Analtech, Newark, DE) in a solvent system of chloroform:methanol:water:ammonium hydroxide (60:45:12:3). Plates were autoradiographed to identify monophosphorylated phosphatidyl inositol, which was scraped from the plates and radioactivity quantified by liquid scintillation spectroscopy.
Statistical analysis and densitometry.
For all statistical comparisons, Instat software for the Macintosh (GraphPad, San Diego, CA) was used. For multiple comparisons, analysis of variance using a Bonferroni correction for the number of comparisons was used. To quantitate differences in images of protein or mRNA expression, films were scanned and densitometry performed using NIH Image 1.54 software.
RESULTS
Aldosterone, at physiological concentrations, stimulates a time-dependent increase in NHE3 expression and activity.
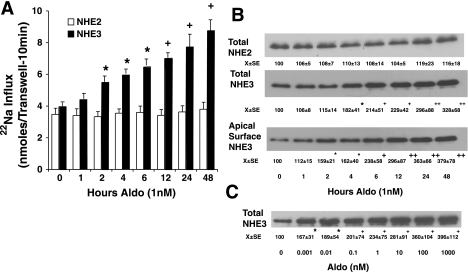
Aldosterone, at a physiological concentration of 1 nM, increases apical NHE3 but not NHE2 activity in Caco-2BBE monolayers (Fig. 1A). Aldosterone increased NHE3 activity as early as 2 h and continued to increase for up to 48 h. To determine whether total NHE3 expression and apical surface localization were altered by aldosterone, Western blots of cell lysates (total) and apical surface membrane expression were performed. Aldosterone-increased surface NHE3 expression could be seen as early as 1 h, and these changes were clearly significant by 2 h. In contrast, significant changes in total NHE3 protein expression could only be appreciated at 4 h. Thereafter, both total and apical NHE3 expression continued to increase up to 48 h (Fig. 1B). No changes in total NHE2 expression were noted (Fig. 1B). Next, the concentration-dependent aldosterone-induced increases in total cellular NHE3 protein expression were determined (Fig. 1C). Following 24-h stimulation, aldosterone-induced increases in total NHE3 expression could be observed even at 1 pM (0.001 nM) with a maximal effect near 100 nM. The concentration that half maximally increased NHE3 was ∼3 nM (Fig. 1C).
Fig. 1.
Aldosterone induces apical membrane and total Na+/H+ exchange (NHE)3, but not total NHE2. A: unidirectional 22Na uptakes were used to measure apical membrane NHE activities as described in materials and methods following treatment with 1 nM aldosterone (Aldo) for varying times. Fluxes shown are means ± SE for 6 separate experiments. *P < 0.05, +P < 0.01 compared with 0 time by analysis of variance using a Bonferroni correction. B: aldosterone increased surface and total expression of NHE3 but did not affect total cellular expression of NHE2. Cells were treated with 1 nM aldosterone, and NHE expression was determined in cell lysates (total) and apical surface (apical biotinyl) NHE3 determined using apical surface biotinylation. C: aldosterone stimulates a concentration-related increase in total NHE3 expression. Cells were harvested for determination after 48-h treatment. Images shown are representative of those of 6 separate experiments for B and C. *P < 0.05, +P < 0.01, ++P < 0.001 compared with no treatment within this set (B or C) by analysis of variance using a Bonferroni correction.
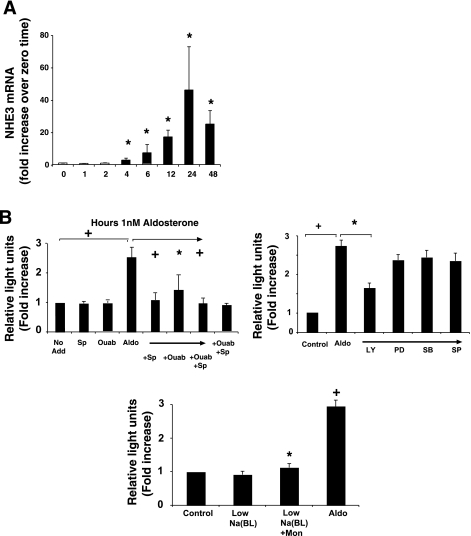
To determine the mechanism(s) of aldosterone-induced increases in total NHE3 expression, NHE3 mRNA was determined at varying times after incubation with 1 nM aldosterone. As shown in Fig. 2A, aldosterone stimulated a time-dependent increase in NHE3 mRNA expression, evident at 4 h and peaking at 24 h. NHE3 mRNA was measured relative to GAPDH mRNA by real-time PCR and analyzed using the ΔΔCt method and expressed as fold increases from untreated cells at 0 h (41).
Fig. 2.
Aldosterone increases NHE3 mRNA and NHE3 gene promoter activity. A: RNA was harvested from cells at varying times after aldosterone (1 nM) and analyzed by real-time PCR for human NHE3 and GAPDH. Ct values were calculated, and ΔΔCt was derived from these values and the value at 0 time set to 1. Data are means ± SE for 3 separate experiments. *P < 0.05, +P < 0.01 compared with 0 time by analysis of variance using a Bonferroni correction. B: aldosterone-stimulated NHE3 promoter-luciferase reporter activity, an effect inhibited by the receptor antagonist, spironolactone (Sp) (10 μM). Interestingly, the Na+-K+-ATPase inhibitor, ouabain (Ouab) (100 μM), significantly inhibited aldosterone-induced reporter activity as well (third bar from far right), an effect that was not additive to the inhibition caused by spironolactone. Neither agent alone nor a combination of ouabain and spironolactone affected baseline reporter activity. Some cells were treated with the PI3K kinase inhibitor (L294002, LY, 30 μM) or MEK-1 inhibitor (PD98059, PD, 30 μM), the p38 MAP kinase inhibitor (SB203580, SB, 30 μM) or the SAPK/JNK MAP kinase inhibitor (SP600125, SP, 30 μM), all for 2 h before aldosterone treatment (1 nM) of NHE3 promoter-luciferase reporter-transfected cells. Cells were also harvested after 6-h aldosterone stimulation. Cell monolayers were grown for 14 days, transfected with rat NHE3 promoter firefly luciferase and Rous sarcoma virus promoter Renilla luciferase constructs. Twenty-four hours later, cells were treated with aldosterone (1 nM) and harvested for luciferase activities 6 h later. Values are means ± SE for 3 separate determinations. *P < 0.05, +P < 0.01 compared with untreated control by analysis of variance with a Bonferroni correction.
To determine whether aldosterone-induced increases in NHE3 mRNA were due to stimulation of NHE3 gene transcription, a 2200-bp rat NHE3 promoter (a generous gift of Dr. A. Cano, Ref. 5) linked to firefly luciferase was transfected into Caco-2BBE along with a construct of Rous sarcoma viral promoter linked to Renilla luciferase, the latter used to normalize transfection. Twenty-four hours after transfection, cells were treated with aldosterone at varying concentrations and harvested 6 h later for luciferase assay. Aldosterone significantly increased firefly luciferase activity (Fig. 2B), which is consistent with the known transcriptional activating effects of the aldosterone (36). Aldosterone-stimulated NHE3 promoter-luciferase reporter activity was inhibited by the receptor antagonist, spironolactone (10 μM) (Fig. 2B). Interestingly, the Na+-K+-ATPase inhibitor, ouabain (100 μM for 2 h before aldosterone), significantly inhibited aldosterone-induced reporter activity as well (Fig. 2B, third bar from far right), an effect that was not additive to the inhibition caused by spironolactone. Neither agent had effects on baseline reporter activity. These data suggest that aldosterone-induced increases in Na+-K+-ATPase activity has some role in aldosterone-induced transcriptional activation of NHE3. However, since ouabain has been reported to affect PI3K, Src tyrosine kinases, ERK1/2, and effects on cytosolic calcium (10, 19, 26, 33, 46, 55), we cannot rule out the possibility that these pathways have some contribution to the observed effects, and subsequent experiments will help elucidate the mechanism(s) of action of ouabain.
To further investigate the mechanisms for aldosterone stimulation of NHE3 gene promoter activity, cells were treated with inhibitors of various signal transduction pathways that have been reported to be activated by aldosterone. PI3K was inhibited by LY294002 (30 μM). PD98059 (30 μM) was used to inhibit the kinase MEK-1, which is “upstream” and activates p44/42 (ERK1/2) MAP kinase. Although SAPK/JNK and p38 are not known targets for aldosterone, the inhibitors SP600125 and SB203580, respectively, were included to complete the analysis. Additionally, to determine whether intracellular Na+ might be involved, cell monolayers were treated with the Na+ ionophore, monensin. For these monolayers, medium for the apical side was made with 140 mM Na+ and for the basolateral, 5 mM. Twenty-four hours after transfection, one monolayer was treated with basolateral monensin, whereas another monolayer remained untreated as a control. Aldosterone was not used for these monolayers because the goal was to determine whether monensin-induced alterations of cell Na+ might stimulate NHE3 gene promoter activity. NHE3 gene promoter activity stimulated by aldosterone was inhibited by PI3K inhibition but not by inhibition of MEK-1, p38 MAP kinase, or SAPK/JNK (Fig. 2B). Incubation in media with mucosal Na+ concentration of 140 mM and serosal of 5 mM did not alter NHE3 gene promoter activity alone; however, addition of monensin (3 μM) to the basolateral side increased reporter activity (Fig. 2B).
Aldosterone, at physiological concentrations, regulates membrane insertion and total expression of Na+-K +-ATPase.
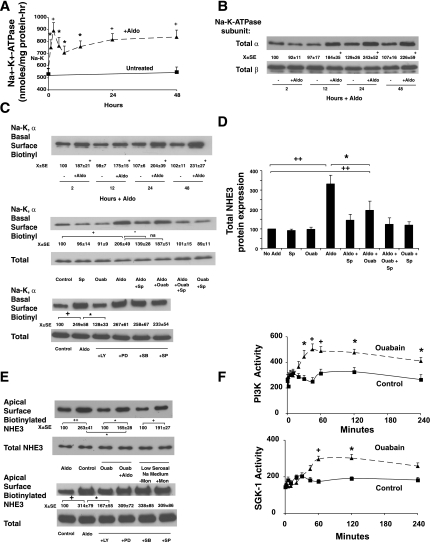
To further explore the role of Na+-K+-ATPase in aldosterone-induced changes in NHE3 expression, we examined the effects of aldosterone stimulation on total and basolateral membrane Na+-K+-ATPase expression. The increased Na+ influx through NHE3 resulting in increased intracellular Na+ activity or cell swelling could potentially stimulate homocellular changes in the expression or activity of the basolateral Na+-K+-ATPase. The ability of aldosterone to stimulate Na+-K+-ATPase activity was therefore examined to determine a potential role for increased basolateral Na+ transport to increase apical NHE3 expression. Aldosterone significantly stimulated Na+-K+-ATPase activity as early as 1 h after stimulation and was maximal at 2 h of stimulation. Following a brief decline, there was a persistent increase in Na+-K+-ATPase activity over the next 48 h (Fig. 3A). The aldosterone stimulation of Na+-K+-ATPase activity therefore appeared to occur before the induction of NHE3 expression and activity. To determine whether Na+-K+-ATPase stimulation was due to expression of ATPase subunits, both total and surface-expressed α- and β-subunits of the Na+-K+-ATPase were measured. At the time of maximal “acute” stimulation (2 h), total expression of both the α- and β-subunits were unchanged (Fig. 3B). At longer times of aldosterone incubation (from 12–48 h), total expression of the α-subunit, but not the β-subunit, increased (Fig. 3B). To determine whether increased surface expression of the α-subunit occurs after aldosterone, (the β-subunit should not be anticipated since this subunit is intracellular and cannot be labeled using the cell-impermeant form of biotin on the outside of the cell), basolateral surface biotinylations were performed. Aldosterone stimulated basolateral surface expression of the α-subunit of the Na+-K+-ATPase, an effect that could be inhibited by spironolactone but not ouabain treatment of the cells (Fig. 3C). The concentration of ouabain used (100 μM) inhibits the Na+-K+- ATPase activity in Caco-2BBE cells over 95% since these cells are human and have high sensitivity to ouabain compared with rodent Na+-K+-ATPase. Ouabain treatment did not affect cell morphology or alter the number of Trypan blue-positive cells (always less than 0.8% at all time points).
Fig. 3.
Aldosterone stimulates Na+-K+-ATPase activity by stimulating exocytosis of the α-subunit, contributing to NHE3 induction. A: cells were treated with 1 nM aldosterone, cells were harvested at varying times, and K+-stimulated phosphatase activity, a measure of Na+-K+-ATPase activity, was measured in postmitochondrial membranes as described in materials and methods. Values shown are means ± SE of 3 separate experiments. *P < 0.05 and +P < 0.01 compared with activity at zero time by analysis of variance. B: aldosterone stimulates a time-dependent increase in the α-subunit, but not β-subunit, of Na+-K+-ATPase protein expression. Western blots shown are representative of 3 experiments. Densitometry values shown are means ± SE. C: aldosterone stimulates basolateral membrane (BLM) insertion of the α-subunit of the Na+-K+-ATPase as early as 2 h, which continues up to 48 h (top, Western blot). Samples at 2 h were analyzed further (middle, Western blot) to determine the basis of increased Na+ pump activity (see A). Surface expression of the α-subunit was assessed through BLM biotinylation and total expression of α-subunit and β-subunit measured in lysates. Cells were stimulated with 1 nM aldosterone for 2–48 h, and surface expressed proteins on the basolateral side were labeled with Sulfo-normal horse serum (NHS)-biotin as described in materials and methods; total cellular expression was determined in total cell lysates. To determine effects of transduction kinase inhibitors, cells were pretreated with the PI3K kinase inhibitor (L294002, 30 μM) or MEK-1 inhibitor (PD98059, 30 μM), the p38 MAP kinase inhibitor (SB203580, 30 μM), or the SAPK/JNK MAP kinase inhibitor (SP600125, 30 μM) for 2 h before aldosterone treatment (1 nM), and basolateral surface biotinylation was performed after 2-h aldosterone stimulation. +P < 0.01 compared with untreated at same time point by analysis of variance for B and C, top (time course). D: spironolactone and ouabain inhibited aldosterone-induced increases in total cellular NHE3 expression at 24 h. Cells were treated with ouabain and/or spironolactone alone or before aldosterone, and total cell NHE3 expression was determined 24 h later. E: aldosterone-stimulated apical NHE3 insertion is inhibited by ouabain and could be mimicked by low Na+ basolateral medium and the Na+/H+ ionophore monensin. Monolayers were stimulated with aldosterone as previously; in those cases noted monolayers were treated with ouabain (100 μM) for 2 h before 2-h aldosterone stimulation or treated only with ouabain for 4 h. For monolayers and low serosal Na+ medium, media were changed to HCO3− medium with 140 mM Na+ apically and 5 mM Na+ basolaterally for 2 h, and, when appropriate, monensin (3 μM) was added to the basolateral medium (or ethanol vehicle). To determine effects of transduction kinase inhibitors, cells were pretreated with the PI3K kinase inhibitor (LY294002, 30 μM), MEK-1 inhibitor (PD98059, 30 μM), the p38 MAP kinase inhibitor (SB203580, 30 μM), or the SAPK/JNK MAP kinase inhibitor (SP600125, 30 μM) for 2 h before aldosterone treatment (1 nM), and apical surface biotinylations were performed after 2 h of stimulation. F: cells were untreated (control) or stimulated with ouabain (100 μM) and harvested at varying times, and PI3K and SGK-1 activities were measured as described in materials and methods. Images shown are representative of 5 separate experiments and are means ± SE. *P < 0.05, + P < 0.01, and ++P < 0.001 statistical comparisons by analysis of variance with a Bonferroni correction with groups designed by bars in C, bottom (2-h inhibitor data) and D and E. For F, comparisons were made by paired Student's t-test.
The effect of aldosterone to stimulate basolateral insertion of the α-subunit of the Na+-K+-ATPase was due to stimulation of PI3K because the inhibitor LY294002 blocked the aldosterone-induced basolateral insertion of the α-subunit (Fig. 3C). Inhibitors of three MAP kinases described for NHE3 gene promoter activity were also tested, and none blocked the aldosterone-induced basolateral insertion of the α-subunit of the ATPase. Thus it appears that the initial increased in Na+-K+-ATPase can be accounted for by enhanced insertion of its α-subunit into the basolateral membrane that is dependent upon aldosterone activation of PI3K. Subsequent increases in total and surface α-subunit of Na+-K+-ATPase protein may explain the longer, more sustained increase in Na pump activity observed up to 48 h (see Fig. 3A).
To determine whether the aldosterone stimulation of Na+-K+-ATPase activity was involved in NHE3 induction, cells were pretreated with 100 μM ouabain 2 h before treatment with aldosterone. To determine whether aldosterone stimulation of Na+-K+-ATPase activity was involved in NHE3 gene promoter activation, cells were treated with ouabain as described above (100 μM, 2 h before aldosterone). As shown in Fig. 3D, ouabain inhibition of Na+-K+-ATPase blocked aldosterone induction of NHE3 expression at 24 h, suggesting that regulation of Na+ movement through the cells or the cell concentration of Na+ could affect NHE3 gene expression. The aldosterone receptor antagonist spironolactone significantly inhibited the ability of aldosterone to induce NHE3 to a similar extent as ouabain treatment.
To determine the role that increased basolateral insertion of the α-subunit of the Na+-K+-ATPase might have on acute (2 h) aldosterone-stimulated NHE3 insertion at the apical membrane, monolayers were treated with aldosterone with and without ouabain (100 μM for 2 h) pretreatment. Ouabain alone had no effect on apical surface NHE3 expression but inhibited the aldosterone-induced increase in apical membrane NHE3 at 2 h of stimulation. After 2 h pretreatment with ouabain, the aldosterone-induced induction of apical NHE3 expression was blocked on average 50% (Fig. 3E). Since increased Na+-K+-ATPase activity might be anticipated to increase Na+ movement through the cells from apical to basolateral media, the conditions of 140 mM mucosal Na+ and 5 mM serosal Na+ media were used in the presence and absence of basolateral monensin. Only when monensin (3 μM) was added to the basolateral side was an increase in apical membrane NHE3 observed (Fig. 3E). No change was observed in total NHE3 expression at this time.
The findings with monensin suggest that the effect of ouabain on aldosterone induction of acute apical NHE3 insertion and longer term (24 h and longer) NHE3 expression are mediated by inhibition of Na+-K+-ATPase. However, as mentioned above, ouabain has been reported to have other effects (10, 19, 26, 33, 46, 55). Therefore, the effect of ouabain to stimulate PI3K or ERK1/2 activity was measured. Ouabain (100 μM; 5 min to 6 h) did not stimulate ERK1/2 (not shown) but did stimulate a significant increase in PI3K activity (Fig. 3F). A later activation of SGK-1 was observed (Fig. 3F); however, Akt activity was not increased (data not shown).
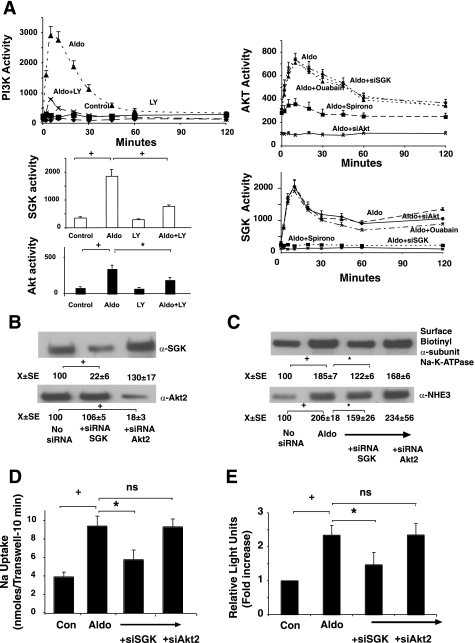
Aldosterone stimulates both SGK and Akt, but SGK is pivotal for activation of Na+-K+-ATPase activity and the subsequent induction of NHE3 expression.
PI3K is an upstream regulator of SGKs and protein kinase B (Akt). To determine whether these pathways were involved in aldosterone-regulation of Na+-K+-ATPase, we inhibited these pathways using selection pharmacological agents. In vitro assays were used to measure PI3K, SGK, and Akt kinase activities. Aldosterone-stimulated PI3K activity was inhibited by LY294002 (LY, 30 μM pretreatment for 2 h, Fig. 4A). Inhibiting PI3K with LY294002 blocked the aldosterone-stimulated activity of both SGK and Akt kinases. To determine whether SGK or Akt kinases were involved in stimulation of exocytosis of the α-subunit of the Na+-K+-ATPase and the induction of NHE3 dependent upon Na+-K+-ATPase activation (due to ouabain sensitivity), silencing of SGK-1 and Akt-2 (the major forms of these kinases found in Caco-2BBE cells) was employed. Cells were treated with silencing oligonucleotides, and the ability of each to inhibit aldosterone induction of NHE3 determined by measuring 22Na+ influx. Specific silencing of SGK and Akt-2 was confirmed both by Western blotting (Fig. 4B) and specific inhibition of the kinase activities (Fig. 4A). Silencing SGK-1 dramatically decreased the aldosterone-stimulated increase of NHE3 expression (Fig. 4C) and activity (Fig. 4D), whereas silencing Akt-2 had no effect (Fig. 4, C and D). Silencing SGK-1 did not alter aldosterone stimulation of Akt-2, whereas Akt-2 silencing did not silence aldosterone stimulation of SGK-1, demonstrating that the silencing oligonucleotides was specific for their designated targets (Fig. 4A). Ouabain pretreatment (100 μM for 2 h) did not inhibit aldosterone activation of either kinase, whereas spironolactone blocked activation of both aldosterone-stimulated SGK-1 and Akt-2 (Fig. 4A).
Fig. 4.
Aldosterone stimulates PI3K, serum glucorticoid kinase (SGK), and Akt-2 activities, but only SGK via Na+-K+-ATPase stimulation contributes to induction of NHE3. A: cells were stimulated with 1 nM aldosterone, and cell lysates were harvested at varying times; PI3K, SGK, and Akt-2 kinase activities were measured at varying times by immunoprecipitating the kinases and using a peptide substrate. When appropriate, cells were treated with the PI3K inhibitor LY294002 (30 μM for 2 h), ouabain (100 μM for 2 h), or spironolactone (10 μM for 2 h) or had SGK-1 or Akt-2 kinases silenced (siRNA). Values are activity to transfer [32P] from ATP to the phosphatidyl inositol acceptor (PI3K assay) or synthetic peptide substrates and are means ± SE of 3 separate experiments. B–D: silencing SGK but not Akt-2 activity inhibits aldosterone-stimulated insertion of Na+-K+-ATPase α-subunit into the basolateral membrane. SGK-1 or Akt-2 expression were silenced with silencing oligonucleotides (confirmed by Western blots presented in B and activity silenced presented in A), and, 24 h after second oligo introduction, cells were stimulated by 1 nM aldosterone and basolateral surface Na+-K+-ATPase α-subunit expression determined 2 h later as described in materials and methods (C), total cell NHE3 expression (C), and activity (D) as well as NHE3 promoter activity determined by luciferase transfections (E) measured 24 h later. Images shown are representative of 3 separate experiments. Flux or luciferase values are means ± SE for 3 separate experiments. *P < 0.05, +P < 0.01, and ++P < 0.001 for comparisons as indicated by bars between groups by analysis of variance with a Bonferroni correction for B–E.
To determine whether this might be due to inhibition of aldosterone-stimulated exocytosis of the α-subunit of the Na+-K+-ATPase and increased activity of this ATPase, cells where SGK-1 was silenced were harvested 2 h after aldosterone treatment. Silencing SGK-1 decreased the aldosterone stimulation of Na+-K+-ATPase activity and the exocytosis of the α-subunit of this protein (Fig. 4C). To determine whether increased Na+-K+-ATPase activity stimulated by aldosterone was inhibited by silencing SGK, Na+-K+-ATPase activity was measured. Basal Na+-K+-ATPase activity was 512 ± 48 nmol PNPP hydrolyzed per hour per mg protein, which increased to 891 ± 67 after 1 nM aldosterone (1 nM). Silencing SGK-1 or Akt-2 alone did not alter baseline activity (498 ± 39 and 507 ± 42 nmol PNPP hydrolyzed per hour per mg protein); however, SGK-1 silencing inhibited the stimulation of aldosterone to 616 ± 74, whereas silencing Akt-2 had no effect on aldosterone stimulation of Na+-K+-ATPase activity (885 ± 102 nmol PNPP hydrolyzed per hour per mg protein, n = 3 for all groups). The ability of aldosterone to increase NHE3 promoter activity was also decreased but not completely inhibited by silencing SGK (Fig. 4E). These results support a model where SGK activation by aldosterone increases Na+-K+-ATPase membrane insertion (α-subunit) and activity and NHE3 gene transcription, all contributing to the stimulation of Na absorption by aldosterone. Aldosterone-induced genomic effects independent of Na+-K+-ATPase activation may also be involved.
DISCUSSION
Mineralocorticoids have an essential role in maintaining net Na+ balance through stimulation of renal reabsorption and intestinal absorption of sodium (4, 6). In conditions where there are physiological demands for Na+ and restoration of vascular volume, endogenous release of mineralocorticoids increases net intestinal Na+ absorption through region-specific processes. In the distal colon of human and mammals, the expression and apical membrane insertion of the epithelial Na+ channel occurs and may also be associated with enhanced Na+-K+-ATPase activity in the basolateral membrane.
The coordination of increased basolateral Na+-K+-ATPase activity in regulation of apical transport pathways has been most clearly characterized in small intestinal transport of solute. In piglets, IGF-1-stimulated uptake of luminal glucose by the Na+-glucose cotransporter (SGLT) does not involve increased SGLT protein abundance but increased enterocyte Na+-K+-ATPase activity (1). In addition, in vitro treatment of piglet jejunum with IGF-1 was associated with similar rapid changes in the Na+-dependent apical transport of glucose. The latter studies also demonstrated a role for the PI3K pathway although the role of SGK-1 was not investigated. For our studies, it is important to recognize that aldosterone has been demonstrated to transactivate the IGF-1 receptor (21), and this may explain some of its nongenomic effects. Thus IGF-1 stimulation of both basolateral Na+-K+-ATPase activity and apical solute absorption is an excellent example of homocellular regulation whereby luminal and contraluminal transporter activities are coordinately regulated to bring about efficient vectorial transport of water and electrolytes (43). Aldosterone has also been noted to have nongenomic effects in mammalian colon; however, the precise nature of how these pathways regulate colonic Na+ absorption, particularly with relevance to homocellular regulation, was not the focus of these studies (20).
In the small intestine, aldosterone increases net Na+ absorption primarily through the stimulated expression of the apical membrane transporter, NHE3 (6, 56). The present studies demonstrate that aldosterone action on intestinal enterocytes involves multiple pathways. Aldosterone stimulates PI3K-dependent SGK-1 activity, which then activates a number of processes involved in NHE3 regulation and expression. Previous studies have demonstrated that activation of the glucocorticoid receptor in Caco-2BBE cells through phosphorylation of serine 663 may stimulate exocytosis of aNHE3 (49, 50). The present studies suggest that, for mineralocorticoid receptor stimulation, SGK-1 also promotes exocytosis of the α-subunit of the Na+-K+-ATPase and that the activity of this ATPase plays a key role in the acute stimulation of apical NHE3 activity. At later time points, mineralocorticoids may induce NHE3 gene and protein expression, and a portion of this longer-term effect also appears to be influenced by activation of the Na+-K+-ATPase activity. The results using ouabain to inhibit aldosterone-induced induction of apical NHE3 insertion and the ability of monensin to stimulate apical NHE3 insertion (Fig. 3E) suggest that one of the ways that aldosterone contributes to NHE3 regulation may be through regulation of Na+-K+-ATPase activity and cellular Na+ homeostasis. The results using the Na ionophore monensin to alter acute aNHE3 insertion suggest that aldosterone-induced increases in basolateral Na+-K+-ATPase activity, which would affect intracellular Na or transcellular Na+ movement, play a role in mediating apical membrane insertion of NHE3. It is notable that ouabain does have other effects, notably in cardiac myocytes where it stimulates ERK1/2 activity through stimulation of Src kinase and the EGF receptor, as well as PI3K and Akt activities (10, 19, 26, 33, 46, 55). However, in Caco-2BBE cells, ouabain had minimal effects on PI3K and SGK activities, and no change in ERK1/2 activity was observed. Thus the effects of ouabain on PI3K and SGK are not likely to contribute to its observed inhibition of aldosterone-stimulated apical NHE3 insertion.
This cellular Na+ homeostasis may regulate aldosterone-induced NHE3 insertion into the apical membrane at shorter incubations, but is likely not the sole contributory event for aldosterone to increase aNHE3 activity. The ability of aldosterone to increase surface expression of the α-subunit of the Na+-K+-ATPase and increase Na+-K+-ATPase activity through exocytosis is not limited to intestinal epithelial cells. Increased surface expression of the α-subunit of the Na+-K+-ATPase also occurs in Xenopus oocytes stimulated with aldosterone, which through activated SGK-1 translocates and activates Na+-K+-ATPase (58) as well as stimulation of this ATPase in amphibian renal epithelial cells by SGK-1 (2).
Aldosterone stimulation of SGK has received considerable attention in both renal and intestinal epithelial physiology because of its genomic and nongenomic effects. Aldosterone has been demonstrated to rapidly increase Akt activity (21, 47) and stimulate SGK-1 activity (48). Mineralocorticoid activation of SGK-1, however, is pivotal in the homocellular regulation of electroneutral Na+ transport because RNAi silencing of SGK-1 (Fig. 4) effectively abrogates aldosterone-stimulated insertion of the Na+-K+-ATPase α-subunit into the basolateral membrane and increased NHE3 expression, whereas Akt-2 silencing did not inhibit this effect of aldosterone.
In certain systems, aldosterone rapidly stimulates MAP kinases, particularly ERK1/2, which may decrease NHE3 activity (in medullary thick ascending limb cells, Ref. 17), increase NHE1 activity (in Madin-Darby canine kidney cells, Ref. 14) and Na+-K+-ATPase activity and expression (17), potentiate vitamin D action in renal thick ascending limb cells (16), and, in vascular smooth muscle cells, modulate the level of protein tyrosine phosphorylation (34). However, we were unable to detect changes in ERK1/2, p38, or SAPK/JNK MAP kinases after aldosterone in Caco-2BBE cells.
On the basis of our studies, aldosterone and potentially other agents appear to increase vectorial Na+ absorption through multiple mechanisms, among them changes in cell Na+ actuated by increased Na+-K+-ATPase activity. It is possible that alteration of cellular Na+ homeostasis by increasing basolateral Na+ efflux could lower cell Na+ or increase transcellular Na flux, which then promotes increased activity of the Na+-absorptive transporting apical NHEs. For aldosterone, SGK1 plays a central role. The increased vectorial Na+ transport that might be stimulated by increased Na+-K+-ATPase activity could also play a role for other agents that increase intestinal Na+-K+-ATPase activity such as vitamin D or thyroid hormones (9, 15), altered intestinal Na+ delivery (12), as well as bowel resection, which increases Na+-K+-ATPase activity of the remnant intestine (23). Increases in Na+-K+-ATPase may allow adaptation to increase vectorial Na+ absorption and help maintain Na+ homeostasis.
Acknowledgments
This work was supported by NIH Grant DK-35810 (E. B. Chang), Digestive Disease Center Grant (DK-42086), and support of the Gastrointestinal Research Foundation of Chicago.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alexander AN, Carey HV. Involvement of PI-3-kinase in IGF-1 stimulation of jejunal Na+-K+-ATPase activity and nutrient absorption. Am J Physiol Gastrointest Liver Physiol 280: G222–G228, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez de la Rosa DA, Gimenez I, Forbush B, Canessa CM. SGK1 activates Na-K-ATPase in amphibian renal epithelial cells. Am J Physiol Cell Physiol 290: C492–C498, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bookstein C, DePaoli AM, Xie Y, Niu P, Musch MW, Rao MC, Chang EB. Na+/H+ exchangers NHE1 and NHE3, of rat intestine. J Clin Invest 93: 106–113, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth RE, Johnson JP, Stockland JD. Aldosterone. Adv Physiol Educ 26: 8–20, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Cano A Characterization of the rat NHE3 promoter. Am J Physiol Renal Fluid Electrolyte Physiol 271: F629–F636, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Cho JH, Musch MW, Bookstein C, McSwine RL, Rabenau K, Chang EB. Aldosterone stimulates intestinal Na+ absorption in rats by increasing NHE3 expression of the proximal colon. Am J Physiol Cell Physiol 274: C586–C594, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Cho JH, Musch MW, DePaoli AM, Bookstein C, Xie Y, Burant CF, Rao MC, Chang EB. Glucocorticoids regulate Na/H exchange expression and activity in region- and tissue specific manner. Am J Physiol Cell Physiol 267: C796–C803, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Cook DI, Dinudom A, Komwatana P, Young JA. Control of Na+ transport in salivary duct epithelial cells by cytosolic Cl- and Na+. Eur J Morphol 36: 67–73, 1998. [PubMed] [Google Scholar]
- 9.Cross HS, Peterlik M. Vitamin D stimulates (Na+ − K+)-ATPase activity in chick small intestine. FEBS Lett 153: 141–145, 1983. [DOI] [PubMed] [Google Scholar]
- 10.Edwards A, Pallone TL. Ouabain modulation of cellular calcium stores and signaling. Am J Physiol Renal Physiol 293: F1518–F1532, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Field M Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 111: 931–943, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gal-Garber O, Mabjeesh SJ, Sklan D, Uni Z. Nutrient transport in the small intestine: Na+,K+-ATPase expression and activity in the small intestine of the chicken as influenced by dietary sodium. Poult Sci 82: 1127–1133, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Garrahan PJ, Pouchan MI, Rega AF. Potassium activated phosphatase from human red blood cells. The mechanism of potassium activation. J Physiol 202: 305–327, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gekle M, Freudinger R, Mildenberger S, Schenk K, Marschitz I, Schramek H. Rapid activation of Na+/H+ exchange in MDCK cells by aldosterone involves MAP kinase ERK1/2. Pflügers Arch 441: 781–786, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Giannella RA, Orlowski J, Jump ML, Lingrel JB. Na(+)-K(+)-ATPase gene expression in rat intestine and Caco-2 cells: response to thyroid hormone. Am J Physiol Gastrointest Liver Physiol 265: G775–G782, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Good DW, Geroge T, Watts BS 3rd. Aldosterone potentiates 1,25-dihydroxyvitamin D3 action in renal thick ascending limb via a nongenomic, ERK-dependent pathway. Am J Physiol Cell Physiol 285: C1122–C1130, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Good DW, George T, Watts BA 3rd. Nongenomic regulation by aldosterone of the epithelial NHE3 Na(+)/H(+) exchanger. Am J Physiol Cell Physiol 290: C757–C763, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Granitzer M, Nagel W, Crabbe J. K+ recirculation in A6 cells at increased Na+ transport rate. Pflügers Arch 422: 546–555, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Haas M, Wang H, Tian J, Xie Z. Src-mediated inter-receptor crosstalk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen activated protein kinase. J Biol Chem 277: 18694–18702, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Harvey BJ, Doolan CM, Condiffe SB, Renard SB, Alzamora R, Urbach V. Non-genomic convergent and divergent signaling of rapid responses to aldosterone and estradiol in mammalian colon. Steroids 67: 483–491, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Holtzman JL, Liu L, Duke BJ, Kemendy AE, Eaton DC. Transactivation of the IGF-1R by aldosterone. Am J Physiol Renal Physiol 292: F1219–F1228, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Hong G, Lockhart A, Davis B, Rahmoune H, Baker S, Ye L, Thompson P, Shou Y, O'Shaughnessy K, Ronco P, Brown J. PPAR γ activation enhances cell surface ENaCα via upregulation of SGK1 in human collecting duct cells. FASEB J 17: 1966–1968, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Hines OJ, Bilchik AJ, McFadden DW, Skotzko MJ, Whang EE, Zinner MJ, Ashley SW. Up-regulation of Na+,K+ adenosine triphosphatase after massive intestinal resection. Surgery 116: 401–407, 1994. [PubMed] [Google Scholar]
- 24.Hoogerwerf WA, Tsao SC, Devuyst O, Levine SA, Yun CH, Yip JW, Cohen ME, Wilson PD, Lazenby AJ, Tse CM, Donowitz M. NHE2 and NHE3 are human and rabbit intestinal brush-border proteins. Am J Physiol Gastrointest Liver Physiol 270: G29–G41, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Janecki AJ, Montrose MH, Zimniak P, Zweibaum A, Tse CM, Khurana S, Donowitz M. Subcellular redistribution is involved in acute regulation of the brush border Na+/H+ exchanger isoform 3 in human colon adenocarcinoma cell line Caco-2. Protein kinase C-mediated inhibition of the exchanger. J Biol Chem 273: 8790–8798, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Khundmiri SJ, Metzler MA, Ameen M, Amin V, Rane MJ, Delamere NA. Ouabain induced cell proliferation through calcium-dependent phosphorylation of Akt (protein kinase B) in opossum kidney proximal tubule cells. Am J Physiol Cell Physiol 291: C1247–C1257, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Kim JH, Lee-Kwon W, Park JB, Ryu SH, Yun CH, Donowitz M. Ca(2+)-dependent inhibition of Na+/H+ exchanger 3 (NHE3) requires an NHE3–E3KARP-alpha-actinin-4 complex for oligomerization and endocytosis. J Biol Chem 277: 23714–23724, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan S, Rajendran VM, Binder HJ. Apical NHE isoforms regulate butyrate-stimulated Na absorption in rat distal colon. Am J Physiol Cell Physiol 285: C1246–C1254, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Kurashima K, Yu FH, Cabado AG, Szabo EZ, Grinstein S, Orlowski J. Identification of sites required for down-regulation of Na+/H+ exchanger NHE3 activity by cAMP-dependent protein kinase phosphorylation-dependent and -independent mechanisms. J Biol Chem 272: 28672–28679, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Lamprecht G, Weinman EJ, Yun CHC. The role of NHERF and E3KARP in the c-AMP mediated inhibition of NHE3. J Biol Chem 273: 29972–29978, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Lee-Kwon W, Kawano K, Choi JW, Kim JH, Donowitz M. Lysophosphatidic acid stimulates brush border Na+/H+ exchanger 3 (NHE3) activity by increasing its exocytosis by an NHE3 kinase A regulatory protein-dependent mechanism. J Biol Chem 278: 16494–16501, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Galli T, Leu S, Wade JB, Weinman EJ, Leung G, Cheong A, Louvard D, Donowitz M. Na+-H+ exchanger 3 (NHE3) is present in lipid rafts in the rabbit ileal brush border: a role for rafts in trafficking and rapid stimulation of NHE3. J Physiol 537: 537–552, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Zhao X, Pierre SV, Askari A. Association of PI3K-Akt signaling pathway with digitalis induced hypertrophy of cardiac myocytes. Am J Physiol Cell Physiol 293: C1489–C1497, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Manegold JC, Falkenstein E, Wehling M, Christ M. Rapid aldosterone effects on tyrosine phosphorylation in vascular smooth muscle cells. Cell Mol Biol 45: 805–813, 1999. [PubMed] [Google Scholar]
- 35.McSwine RL, Musch MW, Bookstein C, Xie Y, Rao MC, Chang EB. Regulation of apical membrane Na+/H+ exchangers NHE2 and NHE3 in intestinal epithelial cell line CACO-2BBE/bbe. Am J Physiol Cell Physiol 275: C693–C701, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Mick VE, Itani OA, Loftus RW, Husted RF, Schmidt TJ, Thomas CP. The a-subunit of the epithelial sodium channel is an aldosterone induced transcript in mammalian collecting ducts, and this transcriptional response is mediated by distinct cis-elements in the 5′-flanking region of the gene. Mol Endocrinol 15: 575–588, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Musch MW, Bookstein C, Rocha F, Lucioni A, Ren H, Daniel J, Xie Y, McSwine RL, Rao MC, Alverdy J, Chang EB. Region-specific adaptation of apical Na/H exchangers after extensive proximal small bowel resection. Am J Physiol Gastrointest Liver Physiol 283: G975–G985, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Musch MW, Arvans DL, Walsh-Reitz MM, Uchiyama K, Fukuda M, Chang EB. Synaptotagmin I binds intestinal epithelial NHE3 and mediates cAMP and Ca++-induced endocytosis by recruitment of AP2 and clathrin. Am J Physiol Gastrointest Liver Physiol 292: G1549–G1588, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Peterson MD, Mooseker MS. An in vitro model for the analysis of intestinal brush border assembly. I. Ultrastructural analysis of cell contact-induced brush border assembly in Caco-2BBe cells. J Cell Sci 105: 445–460, 1993. [DOI] [PubMed] [Google Scholar]
- 40.Rajendran VM, Kashgarian M, Binder HJ. Aldosterone induction of electrogenic sodium transport in the apical membrane vesicles of rat distal colon. J Biol Chem 264: 18638–18644, 1989. [PubMed] [Google Scholar]
- 41.Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real time methods. Anal Biochem 285: 194–204, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Schultz SG Pump-leak parallelism in sodium-absorbing epithelia: the role of ATP-regulated potassium channels. J Exp Zool 279: 476–483, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Schultz SG, Dubinsky WP, Lapointe JY. Volume regulation and ‘cross-talk’ in sodium-absorbing epithelial cells. Contrib Nephrol 123: 205–219, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Schultz SG, Dubinsky WP. Sodium absorption, volume control and potassium channels: in tribute to a great biologist. J Membr Biol 184: 255–256, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Sugi K, Musch MW, Field M, Chang EB. Inhibition of Na+,K+-ATPase by interferon gamma down-regulates intestinal epithelial transport and barrier function. Gastroenterology 120: 1393–1403, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, Maksimova E, Huang XY, Xie ZJ. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Cell Biol 17: 317–326, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tong Q, Booth RE, Worrell RT, Stockland JD. Regulation of Na+ transport by aldosterone: signaling convergence and cross talk between the PI3-K and MAPK1/2 cascades. Am J Physiol Renal Physiol 286: F1232–F1238, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Verrey F, Summa V, Heitzmann D, Mordasini D, Vandewalle A, Feraille E, Zecevic M. Short-term aldosterone action on Na,K-ATPase surface expression: role of aldosterone-induced SGK1. Ann NY Acad Sci 986: 554–561, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Wang D, Sun H, Lang F, Yun CC. Activation of NHE3 by dexamethasone requires phosphorylation of NHE3 at Ser663 by SGK1. Am J Physiol Cell Physiol 289: C802–C810, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang D, Zhang H, Lang F, Yun CC. Acute activation of NHE3 by dexamethasone correlates with activation of SGK1 and requires a functional glucocorticoid receptor. Am J Physiol Cell Physiol 292: C396–C404, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiederkehr M, Zhao H, Moe OW. Acute regulation of Na+/H+ exchanger NHE3 activity by protein kinase C: role of NHE3 phosphorylation. Am J Physiol Cell Physiol 276: C1205–C1217, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Wormmeester L, Sanchez de Medina F, Kokke F, Tse CM, Khurana S, Bowser J, Cohen ME, Donowitz M. Quantitative contribution of NHE2 and NHE3 to rabbit ileal brush-border Na+/H+ exchange. Am J Physiol Cell Physiol 274: C1261–C1272, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Wright EM, Harms V, Mircheff AK, van Os CH. Transport properties of intestinal basolateral membranes. Ann NY Acad Sci 372: 626–636, 1981. [DOI] [PubMed] [Google Scholar]
- 54.Yeo C, Barry K, Gontarek D, Donowitz M. Na+-H+ exchange mediates meal-stimulated ileal absorption. Surgery 116: 338–394, 1994. [PubMed] [Google Scholar]
- 55.Yudowski GA, Efendiev R, Pedemonte CH, Katz AI, Berggren PO, Bertorella AM. Phosphoinositide-3-kinase binds to a proline rich motif in the Na+-K+-ATPAse α subunit and regulates its trafficking. Proc Natl Acad Sci USA 97: 6556–6561, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yun CH, Gurubhagavatula S, Levine SA, Montgomery JL, Brant SR, Cohen ME, Cragoe EJ Jr, Pouyssegur J, Tse CM, Donowitz M. Glucocorticoid stimulation of ileal Na+ absorptive cell brush border Na+/H+ exchange and association with an increase in message for NHE-3, an epithelial Na+/H+ exchanger isoform. J Biol Chem 268: 206–211, 1993. [PubMed] [Google Scholar]
- 57.Yun CHC, Oh S, Zizak M, Steplock D, Tsao S, Tse CM, Weinman EJ, Donowitz M. cAMP-mediated inhibition of the epithelial brush border Na+-H+ exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci USA 94: 3010–3015, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zecevic M, Heitzmann D, Camargo SM, Verrey F. SGK1 increases Na,K-ATP cell-surface expression and function in Xenopus laevis oocytes. Pflügers Arch 448: 29–35, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Zhao H, Wiederkehr MR, Fan L, Collazo RL, Crowder LA, Moe OW. Acute inhibition of Na/H exchanger NHE-3 by cAMP. Role of protein kinase A and NHE-3 phosphoserines 552 and 605. J Biol Chem 274: 3978–3987, 1999. [DOI] [PubMed] [Google Scholar]