Abstract
Portal hypertension (PHT) is a common complication of liver cirrhosis and significantly increases morbidity and mortality. Abrogation of PHT using NSAIDs has demonstrated that prostacyclin (PGI2), a direct downstream metabolic product of cyclooxygenase (COX) activity, is an important mediator in the development of experimental and clinical PHT. However, the role of COX isoforms in PGI2 biosynthesis and PHT is not fully understood. Prehepatic PHT was induced by portal vein ligation (PVL) in wild-type, COX-1−/−, and COX-2−/− mice treated with and without COX-2 (NS398) or COX-1 (SC560) inhibitors. Hemodynamic measurements and PGI2 biosynthesis were determined 1–7 days after PVL or sham surgery. Gene deletion or pharmacological inhibition of COX-1 or COX-2 attenuated but did not ameliorate PGI2 biosynthesis after PVL or prevent PHT. In contrast, treatment of COX-1−/− mice with NS398 or COX-2−/− mice with SC560 restricted PGI2 biosynthesis and abrogated the development of PHT following PVL. In conclusion, either COX-1 or COX-2 can mediate elevated PGI2 biosynthesis and the development of experimental prehepatic PHT. Consequently, PGI2 rather then COX-selective drugs are indicated in the treatment of PHT. Identification of additional target sites downstream of COX may benefit the >27,000 patients whom die annually from cirrhosis in the United States alone.
Keywords: gene-deficient mice, portal vein ligation
in the united states cirrhosis and chronic liver disease is the 12th leading cause of death and in 2005 accounted for more than 27,000 deaths (27). Portal hypertension (PHT) is a serious complication of liver cirrhosis, whereby increased resistance to portal flow is almost always the initial pathophysiological event and is followed by an increased portal venous flow through a hyperdynamic splanchnic system (47). Increased portal venous pressure promotes the formation of collateral venous circulation and esophageal and gastric varices (57). These events impart a significant increase in mortality and morbidity via a propensity for variceal hemorrhage and encephalopathy (4). About 25–40% of all cirrhotic patients have varices of which one-third will hemorrhage with a 20–30% mortality rate (34). In the absence of liver transplantation the current, recommended treatments for PHT and variceal formation are β-blockers to reduce heart rate and portal venous flow, variceal banding, and transhepatic intrajugular portal shunts (18, 28). However, these treatment schemes do not focus on the underlying etiology that increases portal venous pressure. In particular, they do not address the aberrations in systemic and splanchnic circulation that promote increased portal flow and increased portal venous pressure. A cohort of studies have shown that overexpression of prostacyclin (PGI2) reduces systemic and splanchnic resistance and the resultant increased flow is key to increased portal venous pressure (6, 22, 49, 61).
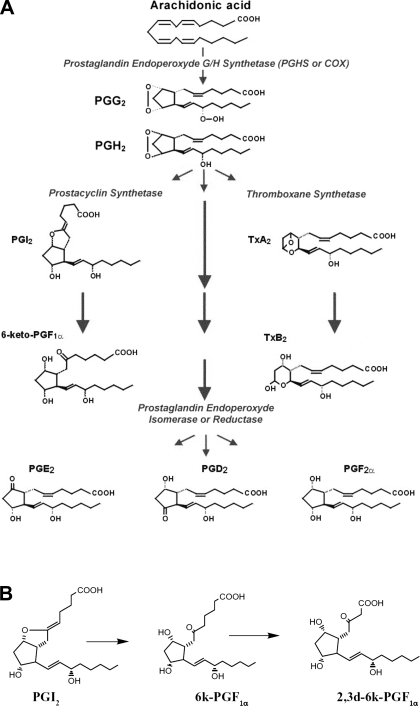
PGI2 is a potent vasodilator and antithrombotic agent and is a member of the prostanoid family. The biosynthesis of prostanoids, which include the prostaglandins and thromboxane, occurs in three steps: 1) the mobilization of arachidonic acid, from membrane phospholipids through the action of phospholipase A2; 2) the formation of prostaglandin endoperoxide H2 (PGH2) via prostaglandin endoperoxide H synthase (PGHS); and 3) the conversion of PGH2 to specific prostanoids through the action of a synthase such as PGI2 synthase (PGIS) to form PGI2. There are two PGHS isoforms that have similar enzymological and structural properties. These are commonly referred to as cyclooxygenase (COX) enzymes or COX-1 and COX-2 (Figure 1) (51, 52). Therefore, since COX-1 and -2 catalyze the first committed step in the production of PGI2, nonsteroidal anti-inflammatory drugs (NSAIDs), which inhibit prostaglandin biosynthesis, should limit PGI2 biosynthesis and the development of PHT. Indeed, PHT can be abrogated in rats and rabbits by reducing PGI2 levels following the blockade of COX activity with the NSAID indomethacin (6, 14). Unfortunately, gastric ulceration is a serious complication associated with the use of NSAIDs.
Fig. 1.
Prostaglandin synthesis pathway. A: cyclooxygenase is a heme-containing glycoprotein and is a key enzyme in prostanoid biosynthesis by catalyzing the conversion of arachidonic acid to PGH2. Subsequently, PGH2 is converted to active prostanoids via secondary prostanoid synthases. Prostacyclin has a half-life of ∼1 h before inactivation via a nonenzymatic hydration to form 6-keto-PGF1α. B: 6-keto-PGF1α is further metabolized in urine to form the 2,3-dinor derivative 2,3-dinor-6-keto-PGF1α.
Until recently the maintenance of gastric mucosal integrity was believed to be dependent on COX-1 derived protective PGE2 from gastric and duodenal epithelium (24, 48, 59). In contrast, under normal physiological conditions PGI2 biosynthesis is believed to be mediated by COX-2 (10, 37). This diversity argued that COX-1 and -2 have distinct functions in tissues and organs and that selective inhibition of COX isoforms may have useful therapeutic outcomes. Animal models and clinical studies have shown that COX-2 inhibitors do not induce gastric ulcers (35). Consequently, it was hoped that this new class of drug could be beneficial in the acute treatment of PHT. However, since then, COX-2 inhibitors have been further studied, leading to the withdrawal of rofecoxib (Vioxx) and valdecoxib (Bextra) (9), while celecoxib (Celebrex) remains available albeit with an FDA-mandated boxed warning.
Despite the increased risks, the biological basis for the cardiovascular consequences of COX-2 inhibition continues to be elucidated and research into COX-2 inhibitors and COX enzymology persists (13, 15, 20, 35, 63). In particular, two recent publications into the gastrointestinal advantages of COX-2 inhibitors shows that the role of COX inhibitors are still relevant and as such the role of COX isoforms in PGI2 biosynthesis and PHT is significant (17, 29). Previous experimental models investigating PGI2 biosynthesis in PHT have been inconclusive. Increased levels of COX-1 and -2 in the aorta and mesenteric vascular bed of portal vein-ligated (PVL) rats suggest no isoform preference (43). Conversely, Tsugawa et al. (56) have shown that NS398, a COX-2-specific inhibitor, prevented PHT in PVL rats. Moreover, pharmacological studies can sometimes be misleading. For example, 1) COX-1 gene-deleted mice have no gastric pathology and are resistant to indomethacin-induced gastric ulceration (30), 2) selective inhibition of COX-1 in healthy rats with SC560 does not induce gastric ulcers (19, 58), 3) COX heterodimers are resistant to COX isoform selective inhibition (63), and 4) both COX-1 and COX-2 contribute to increased PGI2 in non-PHT vascular disorders (3). Therefore, the COX isoform profile associated with elevated PGI2 in PHT is not clear and a better understanding is required.
To address which COX isoform is essential to the development of PHT, we utilized a murine prehepatic PVL model of PHT in wild-type and COX-1−/− and COX-2−/− mice. Within this study we investigate the effect of 1) selective inhibition of COX-2 (NS398) or COX-1 (SC560), 2) the targeted gene deletion of either COX-1 or COX-2, and 3) a combination of targeted COX isoform gene deletion with pharmacological COX inhibition (COX-1−/− ± NS398 or SC560 or COX-2−/− ± SC560 or NS398) on PGI2 levels, abdominal aortic flow (systemic hyperemia), or splenic pulp pressure following PVL. This data will improve our understanding of PGI2 biosynthesis and therapeutic targets needed to benefit cirrhotic patients at risk of PHT and variceal hemorrhage.
EXPERIMENTAL PROCEDURES
Prehepatic PHT Model; Partial Portal Vein Ligation
All studies were approved by the Indiana University institutional animal care and use committee and adhered to American Association for Accreditation of Laboratory Animal Care and federal guidelines for the humane care and treatment of animals. Mice were maintained in sterilized isolette cages on a 12:12-h light-dark cycle and were allowed access to food and water ad libitum. Mice were anesthetized by halothane inhalation. A midline laparotomy was performed and the portal vein was exposed. A blunt-ended 27-gauge needle was placed alongside the portal vein and a 4-0 silk suture was tied around the vein and needle, after which the needle was withdrawn, producing a standardized stenosis. In sham animals the procedure consisted of dissection and visual inspection of the portal vein without ligature. The abdomen was closed and the animals were allowed to recover under a heat lamp.
Physiological Measurements
Physiological measurements were performed as previously described by Theodorakis et al. (55). At the indicated times after sham operation or PVL, animals were anesthetized and subjected to laparotomy to allow physiological measurements to be taken. Portal pressure was determined by measuring the splenic pulp pressure. We have previously shown that portal venous pressure and splenic pulp pressure are directly proportional (55). To measure splenic pulp pressure a microtip pressure transducer (SPR-839, Millar Instruments) was inserted in the spleen. Aortic flow was measured by placing an ultrasonic Doppler flow probe (Transonic no. 11RB) around the abdominal aorta between the diaphragm and celiac artery. Flow rates were obtained with a Transonic T206 Blood Flow Meter (Transonic Instruments). Aortic blood flows were standardized per gram of body weight.
Gene-Deficient Mice
Mice containing targeted mutations in prostaglandin H synthase (ptghs)-1 gene (COX-1; strain B6;129P2-Ptgs1tm1) (30) and the ptghs-2 gene (COX-2, strain B6;129P2-Ptgs2tm1) (39) were purchased from Taconic (Germantown, NY). COX-1/2 double-knockout mice are not viable (33). Age-matched mice from congenic strains (B6;129p2) were used as wild-type controls. Mice genotypes were confirmed by PCR on DNA isolated from tail samples by using a Qiagen DNeasy kit (Qiagen, Valencia, CA) per manufacturer's instructions. Gene-specific primers [COX1, 5′-gagagaaggagatggctgctg 3′-tctgcatccatggctggcctagaa; COX-2, 5′-tcaacacactctatcactggcacc 3′-ccactgcttgtacagcaattggca (cycle = 1 min each of 94°C, 60°C and 74°C × 25)] are complementary to the site-specific mutations previously published (30, 39).
Plasma 6-keto-Prostaglandin F1α and Thromboxane B2 Levels
Prostacyclin (PGI2) and thromboxane (TXA2) have a relatively short half-life in vivo before they are converted to the biologically inactive 6-keto-PGF1α and thromboxane-B2 (TXB2). Both analytes were measured by commercially available competitive ELISA kits in accordance with manufacturer's instructions (Oxford Biomedical Research). Extraction procedure was identical for 6-keto-PGF1α and TXB2. Briefly, blood was collected by cardiac puncture, injected into heparinized tubes, centrifuged and plasma stored at 4°C. Urine was collected over 24 h by use of metabolic cages and stored at 4°C prior to analysis. Urine and plasma 6-keto-PGF1α and plasma TXB2 were isolated by using SEP-PAK C-18 cartridges (Applied Separations). Plasma was diluted with methanol to a final concentration of 15% and applied to a SEP-PAK C-18 cartridge that had been preequilibrated with 2 ml of methanol and 2 ml of H2O at 1 ml/min flow rate. After application of the plasma sample, cartridges were washed with 2 ml of 15% methanol and 2 ml of petroleum ether at 1 ml/min. 6-keto-PGF1α and TXB2 were eluted from the column with 2 ml of methyl formate at 1 ml/min. Eluent was dried down by centrifugal evaporation and resuspended in extraction buffer supplied within ELISA kits (Oxford Biomedical Research).
Urine 2,3-dinor-6-keto-Prostaglandin F1α Levels
2,3-Dinor-6-keto-prostaglandin F1α (2,3-dinor-6-keto-PGF1α) is a stable β-oxidation metabolite of 6-keto-PGF1α and levels have previously been used to quantitate systemic PGI2 biosynthesis in mice and humans (3, 44). In contrast, urine 6-keto-PGF1α is predominantly a marker of renal COX-2 activity. Urine was collected for 7 days and 2,3-dinor-6-keto-PGF1α was extracted by a selective two-step solid-phase extraction as described previously (45). 2,3-Dinor-6-keto-PGF1α was quantitated by a competitive ELISA for 2,3-dinor-6-keto-PGF1α and 6-keto-PGF1α (Assay Designs, Ann Arbor, MI) as per manufacturer's instructions. Briefly, urine was first acidified with 0.1 M HCl to pH 3.0 using a mini-lab low volume pH meter (IQ Scientific) before incubation overnight at room temperature. Spe-ed C-1 methyl silica 500 mg/6 ml minicolumns (Applied Separations) were equilibrated with 5 ml of methanol and 5 ml of H2O at 2 ml/min. Acidified urine was applied to the column at 0.5 ml/min and the column was washed with 5 ml of H2O and 5 ml of n-hexane at 1 ml/min. 2,3-dinor-6-keto-PGF1α was eluted with 5 ml of diethyl ether-n-hexane (85:15, vol/vol). Eluent was resuspended in prostanoid-free urine (see below for preparation of prostanoid-free urine) and pH was altered to 10 with 0.1 M sodium hydroxide and incubated for 1 h. Spe-ed C-1 methyl silica 500 mg/6 ml minicolumns were equilibrated as before, and sample was applied at 0.5 ml/min. Column was washed as before and 2,3-dinor-6-keto-PGF1α was eluted with 5 ml of chloroform at 1 ml/min, centrifugally evaporated, and resuspended in extraction buffer supplied in the urine 2,3-dinor-6-keto-PGF1α assay kit. Prostanoid-free urine is prepared by adding activated charcoal to pooled urine from unadulterated B6129P2 mice (5% wt/vol) and stirred for 1 h. After centrifugation at 3,000 g for 15 min supernatant was taken and assayed for 2,3-dinor-6-keto-PGF1α and 6-keto-PGF1α.
Determination of In Vivo COX Activity Inhibition Using the COX-2 Inhibitor NS398 and COX-1 Inhibitor SC560
NS398 [N-(2-cyclohexyloxy-4-nitrophenyl)methanesulfonamide] (Cayman Chemical) is marketed as a selective COX-2 inhibitor (IC50 COX-2 = 3.8 μM), but it can, at high doses, inhibit COX-1 activity (IC50 COX-1 >100 μm) (41). Titration of NS398 to selectively inhibit COX-2 in vivo with minimal effect on COX-1 was determined by measurement of serum TXB2 and 6-keto-PGF1α and urine 6-keto-PGF1α levels 24 h following administration of 1–10 mg/ml ip NS398 or DMSO vehicle control in B6;129P2 wild-type mice (Taconic). Urine 6-keto-PGF1α is predominantly a marker of COX-2 activity whereas serum TXB2 is representative of COX-1 activity. NS398 at 2 mg/kg was chosen to selectively inhibit COX-2 activity. Please see results. To determine the duration of inhibition, 2 mg/kg NS398 was given to B6;129P2 mice and 24-h urine 6-keto-PGF1α levels were determined 0–72 h thereafter. To confirm that 2 mg/kg NS398 was COX-2 specific COX-1−/− and COX-2−/− gene-knockout mice (strains B6;129P2-Ptgs1tm1 and B6;129P2-Ptgs2tm1, respectively) (Taconic) were given 2 mg/kg ip NS398, and 24-h urine 6-keto-PGF1α levels were determined.
SC560 [5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluoromethyl)-1H-pyrazole] (Cayman Chemical) is marketed as a selective COX-1 inhibitor (COX-1 IC50 is 9 nM), but it can, at high doses, inhibit COX-2 activity (COX-2 IC50 is 6.3 μM) (50). Titration of SC560 to selectively inhibit COX-1 in vivo with minimal effect on COX-2 was determined by measurement of serum TXB2 and urine 6-keto-PGF1α levels 24 h following administration of 10–80 mg/ml ip SC560 or DMSO vehicle control in B6129P2 wild-type mice. Urine 6-keto-PGF1α is predominantly a marker of COX-2 activity whereas serum TXB2 is representative of COX-1 activity. SC560 at 20 mg/kg was chosen to selectively inhibit COX-1 activity. Please see results. To determine the duration of inhibition 20 mg/kg ip SC560 was given to B6129P2 mice and plasma TXB2 was determined 0–72 h thereafter. To confirm that 20 mg/kg SC560 was COX-1 specific, COX-1−/−- and COX-2−/− gene-knockout mice were given 20 mg/kg ip SC560 and plasma TXB2 was quantitated.
Effects of Portal Vein Ligation on PGI2 Biosynthesis and Portal Hemodynamics in Wild-Type and COX Isoform Gene Deleted Mice
To determine the effects of PVL on portal hemodynamics and PGI2 biosynthesis, wild-type (B6:129P2), COX-1−/− (B6;129P2-Ptgs1tm1), and COX-2−/− (B6;129P2-Ptgs2tm1) mice were subjected to either sham or PVL surgery as described above. In wild-type mice abdominal aortic flow, splenic pulp pressure, and plasma 6-keto-PGF1α and TXB2 levels were determined 1, 2, 4, and 7 days following sham or PVL (n = 5 per group). In addition, 7-day PVL and sham wild-type mice were analyzed for 7-day urine 2,3-dinor-6-keto-PGF1α levels. In COX-1−/− and COX-2−/− mice abdominal aortic flow, splenic pulp pressure, and 7-day urine 2,3-dinor-6-keto-PGF1α were determined 7 days following sham or PVL surgery (n = 5 per group).
Effects of Pharmacological COX Inhibition in Wild-Type and COX Isoform Gene Knockout Mice on PGI2 Biosynthesis and Portal Hemodynamics
To determine the effects of COX-1 or COX-2 selective inhibitors on PGI2 biosynthesis and portal hemodynamics following PVL wild-type mice (B6;129P2) were treated with either 2 or 10 mg/kg NS398 or 20 or 80 mg/kg SC560 12 h prior to PVL or sham surgery. Both NS398 and SC560 have previously been used as COX-selective inhibitors in the rat PVL model of PHT (1). The two doses will show the effects of each compound when given at selective and non-isoform-selective doses. After surgery NS398 was given daily whereas SC560 was given every 48 h. Urine was collected throughout and stored at 4°C. At 7 days following surgery, abdominal aortic flow, splenic pulp pressure, and 7-day urine 2,3-dinor-6-keto-PGF1α were determined. To determine the effect of pharmacological COX inhibition in COX isoform gene-knockout mice, COX-1−/− and COX-2−/− mice were given 2 mg/ml NS398 or 20 mg/kg ip SC560 12 h before and after PVL or sham operation. After surgery NS398 was given daily whereas SC560 was given every 48 h. Urine was collected throughout and stored at 4°C. On day 7 after PVL or sham operation, abdominal aortic flow, splenic pulp pressure, and 7-day urine 2,3-dinor-6-keto-PGF1α were determined.
Statistics
The data shown are means ± SE, with five animals per experimental group. Statistical significance was estimated by one-way ANOVA statistical analysis. A value of P < 0.05 was considered significant.
RESULTS
Portal Vein Ligation Increases PGI2 Biosynthesis and Portal Hemodynamics in Wild-Type and COX-1−/− and -2−/− Gene-Deficient Mice
Mortality.
Sham and PVL surgery was associated with a 0 and 29% mortality rate, respectively, 7 days following surgery. There was no significant difference in 7-day mortality rates between B6;129P2 wild-type and COX-1−/− or COX-2−/− mice following either sham or PVL surgery.
Wild-type mice.
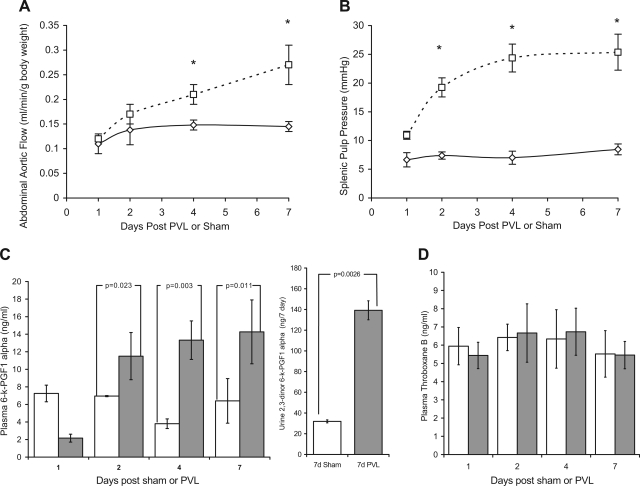
In B6;129P2 wild-type mice portal hemodynamics and PGI2 biosynthesis were significantly increased following PVL compared with sham-operated controls. The abdominal aortic flow increased steadily 1 and 2 days following PVL but was not significantly different from shams. After 4 and 7 days the abdominal aortic flow was significantly raised 23.5 and 58.8%, respectively, in PVL-treated mice compared with sham-operated controls (Fig. 2A). The splenic pulp pressure progressively increased immediately following PVL and was elevated 64.2, 189.3, 266.3, and 281.5% at 1, 2, 4, and 7 days post-PVL, respectively, compared with sham-operated controls (Fig. 2B). To determine PGI2 biosynthesis plasma 6-keto-PGF1α and urine 2,3-dinor-6-keto-PGF1α were quantitated. PGI2 rapidly converts to the stable and biologically inert 6-keto-PGF1α and after β-oxidation to 2,3-dinor-6-kPGF1α is excreted in the urine. 2,3-dinor-6-keto-PGF1α and plasma 6-keto-PGF1α levels have previously been used to quantitate systemic PGI2 biosynthesis in mice and humans (3, 44). Plasma 6-keto-PGF1α was increased twofold 2 days after PVL and was maximally increased by 2.5-fold after 7 days compared with shams (Fig. 2C). Correspondingly, 7-day urine 2,3-dinor-6-keto-PGF1α excretion was significantly increased 336% following PVL compared with shams (Fig. 2C). In comparison, plasma level of TXB2 was not altered following PVL. TXB2 is the stable in vivo hydrolyzed product of TXA2, which is the unstable and bioactive metabolic product coupled to COX-1 activity. Not at any time following surgery was plasma TXB2 level significantly different between sham and PVL mice (Fig. 2D).
Fig. 2.
Portal vein ligation (PVL) increases splenic pulp pressure, abdominal aortic flow, and prostacyclin levels but does not increase thromboxane levels. Prehepatic portal hypertension (PHT) was induced in B6;129P2 wild-type mice by PVL. Control mice received a sham operation. At 1–7 days after surgery splenic pulp pressure and abdominal aortic flow were measured and plasma was analyzed for metabolites of prostacyclin (6-keto-PGF1α) and thromboxane (TXB2). In addition, total urine output from the 7-day sham and PVL mice was collected and measured for 2,3-dinor-6-keto-PGF1α (β-oxidative metabolite of 6-keto-PGF1α). A: abdominal aortic flow increased progressively following PVL (□) and was significantly different to shams (⋄) after 4 and 7 days. B: splenic pulp pressure rapidly increased in the PVL group (□) but was not increased following sham surgery (⋄). C: plasma 6-keto-PGF1α levels increased significantly following PVL (shaded bars) but not following sham operation (open bars). The urine 2,3-dinor-6-keto-PGF1α 7-day level was also significantly increased in 7-day PVL mice compared with 7-day shams. D: plasma TXB2 levels were unaltered by either PVL (shaded bars) or sham (open bars) operation. *PVL vs. sham, P < 0.05; means ± SE; n = 5 per group.
COX-1−/− and -2−/− gene-deleted mice.
Although COX-1−/− and COX-2−/− mice were screened by the supplier (Taconic Laboratories). COX gene deficiency was confirmed by PCR. Wild-type genomic DNA used as a PCR template generated products corresponding to COX-1- and COX-2-specific sequences. When genomic DNA from COX-1−/− and COX-2−/− mice were used as a template, PCR products for COX-1 and COX-2, respectively, were absent (data not shown).
COX-1−/− mice.
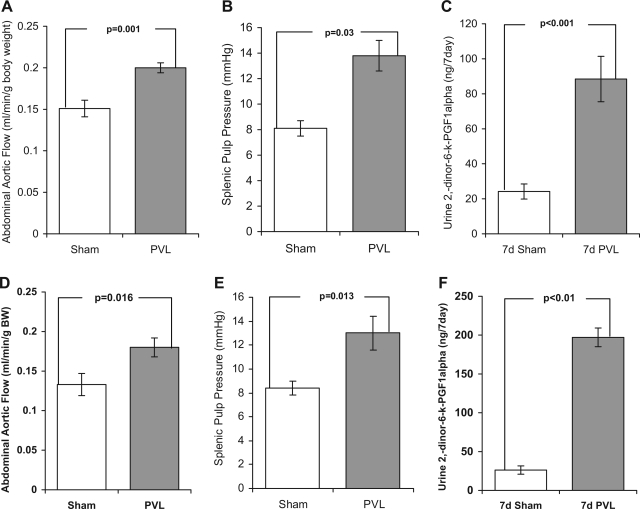
In sham-operated COX-1−/− mice abdominal aortic flow, splenic pulp pressure, and 7-day urine 2,3-dinor-6-keto-PGF1α were not statistically different from those observed in wild-type shams. Following PVL abdominal aortic flow, splenic pulp pressure, and 7-day urine 2,3-dinor-6-keto-PGF1α were increased 32.4, 70, and 265%, respectively, in 7-day PVL COX-1−/− mice compared with 7-day sham-operated COX-1−/− controls (Fig. 3, A–C). With the exception of 2,3-dinor-6-keto-PGF1α these increases were significantly less than those observed between wild-type 7-day shams and 7-day PVL-operated mice.
Fig. 3.
Effect of COX-1 or COX-2 gene deficiency on abdominal aortic flow, splenic pulp pressure, and urine 2,3-dinor-6-keto-PGF1α1 levels following partial PVL or sham surgery. B6;129P2-Ptgs1tm1 (COX-1−/−) (A–C) and B6;129P2-Ptgs2tm1 (COX-2−/−) (D–F) mice were subjected to either a partial PVL or sham surgery, and urine was collected for 7 days. After this time the abdominal aortic flow and splenic pulp pressure were measured and the urine was analyzed for the prostacyclin metabolite 2,3-dinor-6-keto-PGF1α. Abdominal aortic flow (A and D), splenic pulp pressure (B and E), and urine 7-day 2,3-dinor-6-keto-PGF1α (C and F) were all significantly increased 7 days following PVL compared with sham controls. Data represents means ± SE, n = 5 per group.
COX-2−/− mice.
In 7-day sham-operated COX-2−/− mice, there was no significant difference in abdominal aortic flow, splenic pulp pressure, and 7-day urine 2,3-dinor-6-keto-PGF1α compared with wild-type 7-day shams. Following PVL, abdominal aortic flow, splenic pulp pressure, and 7-day urine 2,3-dinor-6-keto-PGF1α were increased 35, 56, and 315%, respectively, in 7-day PVL COX-2−/− mice compared with 7-day sham-operated COX-2−/− controls (Fig. 3, D–F). With the exception of 2,3-dinor-6-keto-PGF1α these increases were significantly less than those observed between wild-type 7-day shams and 7-day PVL-operated mice but were not when compared with those between COX-1−/− 7-day shams and COX-1−/− 7-day PVL-operated mice.
COX Isoform Inhibition Is Dose Dependent in Wild-Type and COX Gene-Deficient Mice
Plasma 6-keto-PGF1α levels were not statistically different among unadulterated B6;129P2, COX-1−/−, and COX-2−/− mice (11.1 ± 0.9, 9.1 ± 1.8, and 12.4 ± 1.2 ng/ml, respectively). In contrast, plasma TXB2 levels were significantly lower in unadulterated COX-1−/− mice compared with B6;129P2 and COX-2−/− mice (1.01 ± 0.3, 4.8 ± 0.4, and 4.1 ± 0.9 ng/ml, respectively) (P = 0.003 and 0.44, B6;129P2 vs. COX-1−/− and COX-2−/−, respectively).
Mortality.
There was no mortality associated with 2–10 mg/kg NS398, 10–80 mg/kg SC560, or DMSO vehicle control in unadulterated B6;129P2, COX-1−/−, or COX-2−/− mice.
Wild-type mice.
Urine 6-keto-PGF1α and plasma TXB2 levels were dose dependently reduced by NS-398 and SC560 compared with DMSO controls.
NS398.
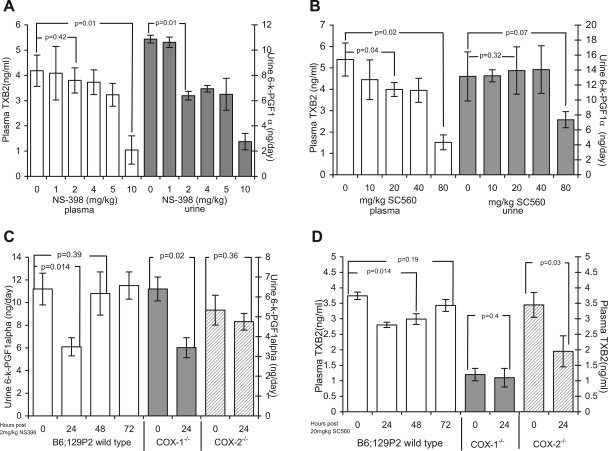
Urine 6-keto-PGF1α (marker of COX-2 activity) was reduced 41% by 2 mg/kg NS398 and was maximally reduced 75.8% at 10 mg/kg (Fig. 4A). Plasma TXB2 (marker of COX-1 activity) was not altered at 1–4 mg/kg but was reduced 75.1% by 10 mg/ml NS398 (Fig. 4A). From this data, 2 mg/kg NS398 was determined as a COX-2-specific inhibitory dose. To determine the length of inhibition, 2 mg/kg NS398 was given to B6;129P2 mice and 24-h urine 6-keto-PGF1α levels were determined for 72 h. NS398 at 2 mg/kg reduced urine 6-keto-PGF1α 24 h following administration. After 24 h levels were not significantly different compared with 0 mg/ml DMSO vehicle control levels; therefore it was administered daily (Fig. 4C).
Fig. 4.
In wild-type and COX-knockout mice, NS398 and SC560 are dose-dependent COX-selective inhibitors. To confirm the selectivity of commercially available COX inhibitors, B6;129P2 or COX gene-knockout mice were given either the COX-2 inhibitor NS398 (A and C) or the COX-1 inhibitor SC560 (B and D). In vivo COX inhibition was determined by quantitating plasma TXB2 (marker of COX-1 activity) or urine 6-keto-PGF1α (marker of COX-2 activity) levels. A: 2 mg/kg NS398 dose dependently reduced urine 6-keto-PGF1α levels (shaded bars) with no significant change in plasma TXB2 levels (open bars). At 10 mg/kg NS398 decreased plasma TXB2 levels also. B: 20 mg/kg SC560 reduced plasma TXB2 levels (open bars) with no significant change in urine 6-keto-PGF1α (shaded bars); 80 mg/kg SC560 decreased plasma TXB2 levels also, and 2 mg/kg NS398 and 20 mg/kg SC560 were determined to achieve selective inhibition of COX-2 and COX-1 isoforms, respectively. C: 2 mg/kg NS398 reduced urine 6-keto-PGF1α level (open bars) for only 24 h. Urine 6-keto-PGF1α was significantly reduced by 2 mg/kg NS398 in COX-1−/− (shaded bars) but not in COX-2−/− mice (hatched bars). D: 20 mg/kg SC560 reduced plasma 6-keto-PGF1α (open bars) for 24 and 48 h. Plasma 6-keto-PGF1α was not altered by 20 mg/kg SC560 in COX-1−/− (shaded bars) but was significantly reduced in COX-2−/− mice (hatched bars) (A–D). Data represents means ± SE, n = 5 per group.
SC560.
Plasma TXB2 was reduced by 26% by 20 mg/kg SC560 and was maximally reduced by 71.9% at 80 mg/kg. In contrast, urine 6-keto-PGF1α was not altered at 20 mg/ kg but was reduced 44% by 80 mg/ml SC560 (Fig. 4B). To determine the length of inhibition, 20 mg/kg SC560 was given to B6;129P2 mice and plasma TXB2 levels were determined every 24 h for 72 h thereafter; 20 mg/kg SC560 reduced plasma TxB2 levels 26 and 23% at 24 and 48 h, respectively. After 48 h there was no difference compared with 0 mg/kg DMSO vehicle controls; therefore it was administered every second day (Fig. 4D).
Hemodynamic measurements.
No significant hemodynamic changes were observed following administration of either NS398 or SC560. Abdominal aortic flow and splenic pulp pressure trended downward with NS398 and high doses of SC560 but were not significantly altered compared with DMSO vehicle controls (data not shown).
COX-1−/− and COX-2−/−mice.
To confirm COX isoform selective inhibition 2 mg/kg NS398 or 20 mg/kg SC560 were given to COX-2−/− and COX-1−/− mice. 6-keto-PGF1α and TXB2 levels were determined in urine and plasma, respectively. NS398 at 2 mg/kg reduced urine 6-keto-PGF1α levels by 42.3% in COX-1−/− mice but had no effect in COX-2−/− gene-deleted mice compared with 0 h controls (Fig. 4C). Conversely, 20 mg/kg SC560 had no effect on plasma TXB2 levels in COX-1−/− mice, whereas in COX-2−/− mice plasma TXB2 was reduced 45.1% (Fig. 4D).
Pharmacological Inhibition of COX With NS398 and SC560 To Prevent PHT Is Dose Dependent
To determine the effectiveness of COX inhibitors to limit PGI2 biosynthesis and the development of PHT, wild-type B6;129P2 mice were treated with 2 and 10 mg/kg NS398 or 20 and 80 mg/kg SC560 prior to and following PVL or sham surgery.
Effect of NS398.
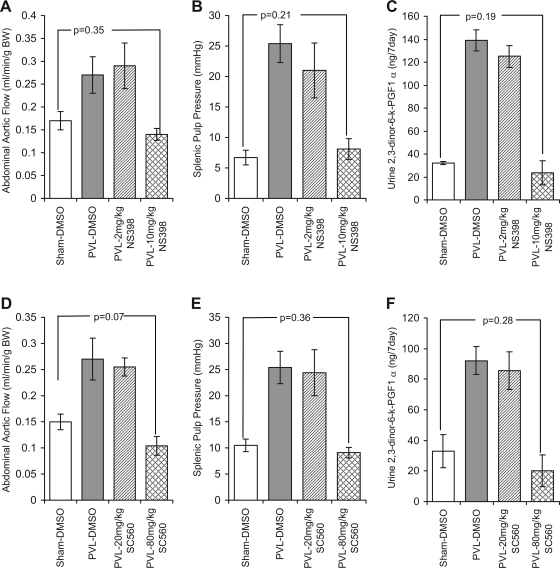
In DMSO-treated vehicle controls, abdominal aortic flow, splenic pulp pressure, and 7-day urine 2,3-dinor-6-keto-PGF1α were significantly increased by 59, 279, and 336%, respectively, 7 days following PVL compared with 7-day sham controls. These increases were not significantly altered by daily administration of 2 mg/kg NS398 and remained increased by 70.6, 213, and 291%, respectively. However, when NS398 was given at 10 mg/kg, which is not COX-2 selective, abdominal aortic flow, splenic pulp pressure, and 7-day urine 2,3-dinor-6-keto-PGF1α were not significantly increased in PVL mice compared with either DMSO shams or 10 mg/kg NS398 shams (Fig. 5, A–C). There were no differences in abdominal aortic flow or splenic pulp pressure among DMSO shams, 2 mg/kg NS398 shams, and 10 mg/kg NS398 shams.
Fig. 5.
Non-isoform-selective inhibition of COX-1 and COX-2 with NS398 and SC560 prevents PHT, hyperemia, and elevated prostacyclin in wild-type mice. B6;129P2 mice were given either DMSO (vehicle control) 2–10 mg·kg−1·day−1 NS-398 or 20–80 mg/kg ip SC560 24 h prior to partial PVL or sham surgery. DMSO and 2–10 mg/kg NS398 were given daily thereafter for 7 days, and SC560 was given at 20–80 mg/kg every 48 h thereafter for 7 days. During this time total urine output was collected. After 7 days the abdominal aortic flow (A and D) and splenic pulp pressure (B and E) were measured and the urine was analyzed for the prostacyclin metabolite 2,3-dinor-6-keto-PGF1α (C and F). Histograms show data for 7-day sham-DMSO since there was no effect of 2–10 mg/kg NS398 or 20–80 mg/kg SC560 in shams. A–C: abdominal aortic flow, splenic pulp pressure, and 7-day urine 2,3-dinor-6-keto-PGF1α were increased 7 days following PVL (shaded bars) compared with 7-day shams (open bars). Treatment with 2 mg/kg NS398 (hatched bars) did not prevent these increases, whereas treatment with 10 mg/kg NS398 (cross-hatched bars) significantly reduced all three. D–F: similarly, abdominal aortic flow, splenic pulp pressure, and urine 7-day 2,3-dinor-6-keto-PGF1α remained elevated in 7-day PVL mice treated with the low, 20 mg/kg, dose of SC560 (hatched bars) compared with PVL-DMSO and sham-DMSO controls, whereas 80 mg/kg SC560 (cross-hatched bars) prevented an increase in abdominal aortic flow, splenic pulp pressure, and 2,3-dinor-6-keto-PGF1α. Data represents means ± SE; n = 5 per group. BW, body weight.
Effect of SC560.
In DMSO-treated vehicle controls abdominal aortic flow, splenic pulp pressure, and 7-day urine 2,3-dinor-6-keto-PGF1α were significantly increased by 80, 141, and 188%, respectively, 7 days following PVL compared with 7-day sham controls. These increases were not significantly altered by daily administration of 20 mg/kg SC560 and remained increased by 70, 132, and 160%, respectively. However, when SC560 was given at 80 mg/kg, which is not COX-1 selective, abdominal aortic flow, splenic pulp pressure, and 7-day urine 2,3-dinor-6-keto-PGF1α were not significantly increased in PVL mice compared with either DMSO shams or 80 mg/kg SC560 shams (Fig. 5, D–F). There were no significant differences in abdominal aortic flow or splenic pulp pressure among DMSO shams, 20 mg/kg SC560 shams, and 80 mg/kg SC560 shams.
COX Targeted Gene Deletion in Combination With Pharmacological COX Inhibition Inhibits PGI2 Biosynthesis and PHT
To determine the effect of COX-1 or COX-2 activity inhibition in combination with COX gene deletion on PGI2 biosynthesis and PHT development, COX-1−/− and COX-2−/− mice were treated with 20 mg/kg SC560 or 2 mg/kg NS398 prior to and following PVL or sham surgery. Unfortunately, COX-1/2 double knockouts are not viable (33).
Mortality.
Mortality rates following administration of NS398, SC560, or DMSO to sham- or PVL-operated COX-1−/− or COX-2−/− mice was 0 and 29%, respectively. There was no difference in mortality rates among B6;129p2, COX-1−/−, and COX-2−/− mice. However, PVL-COX-2−/− mice treated with 20 mg/kg SC560 were distressed compared with B6;129P2 or COX-1−/− PVL mice treated with 20 mg/kg SC560. Mice were less active, hunched, and had largely distended stomachs 7 days following PVL. This observation is in agreement with Akahoshi et al. (1), who have previously reported that SC560 (10–40 mg/kg) causes gastric mucosal damage in PHT rats but not in shams.
COX-1−/− mice.
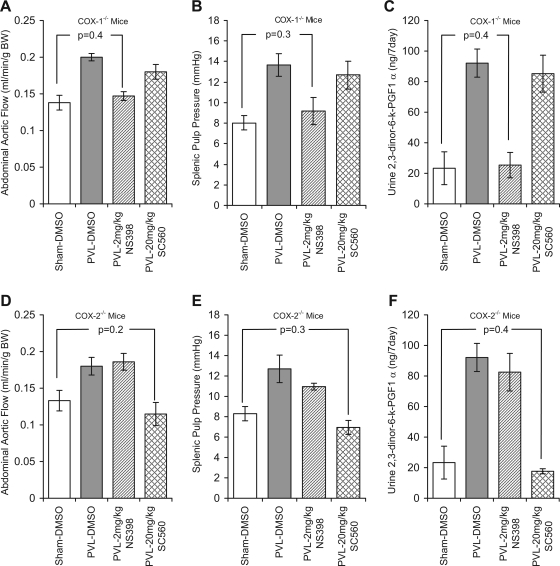
There were no significant differences in abdominal aortic flow, splenic pulp pressure, or 2,3-dinor-6-keto-PGF1α among 7-day DMSO shams, 2 mg/kg NS398 shams, and 20 mg/kg SC560 shams. At 7 days following PVL surgery, abdominal aortic flow, splenic pulp pressure, and 2,3-dinor-6-keto-PGF1α were increased 45, 70, and 295%, respectively, in PVL-DMSO mice compared with 7-day sham-DMSO controls (Fig. 6, A–C). NS398 at 2 mg/kg significantly reduced these increases, whereas 20 mg SC560 did not. Abdominal aortic flow, splenic pulp pressure, and 2,3-dinor-6-keto-PGF1α were minimally increased 7, 14, and 9%, respectively, in 7-day PVL-NS398 COX-1−/− mice compared with 7-day sham-NS398 COX-1−/− mice. In COX-1−/− mice treated with 20 mg/kg SC560, abdominal aortic flow, splenic pulp pressure, and 2,3-dinor-6-keto-PGF1α were increased 30, 58, and 265%, respectively, in 7-day PVL-SC560 mice compared with 7-days sham-SC560 mice (Fig. 6, A–C).
Fig. 6.
Selective inhibition of COX-1 or COX-2 prevents PHT in COX-2−/− and COX-1−/− mice, respectively. B6;129P2-Ptgs1tm1 (COX-1−/−) (A–C) and B6;129P2-Ptgs2tm1 (COX-2−/−) (D–F) mice were given either DMSO (vehicle control) 2 mg·kg−1·day−1 ip NS-398 or 20 mg/kg ip SC560 prior to and following partial PVL or sham surgery. DMSO and 2 mg/kg NS398 was given daily for 7 days, and SC560 was given at 20 mg/kg every 48 h for 7 days. During this time total urine output was collected. After 7 days the abdominal aortic flow (A and D) and splenic pulp pressure (B and E) were measured and the urine was analyzed for the prostacyclin metabolite 2,3-dinor-6-keto-PGF1α. Histograms show data for 7-day sham-DMSO since there was minimal effect of 2 mg/kg NS398 or 20 mg/kg SC560 in shams (A–F). In both COX-1−/− and COX-2−/− mice abdominal aortic flow, splenic pulp pressure, and 7-day urine 2,3-dinor-6-keto-PGF1α were increased 7 days following PVL (shaded bars) compared with 7-day shams (open bars). Treatment with 2 mg/kg NS398 (hatched bars) prevented this increase in 7-day PVL-COX-1−/− mice, whereas treatment with 20 mg/kg SC560 had no effect. In contrast, abdominal aortic flow, splenic pulp pressure, and urine 7 days 2,3-dinor-6-keto-PGF1α were significantly reduced in 7-day PVL-COX-2−/− mice treated with 20 mg/kg SC560 but remained elevated in mice treated with 2 mg/kg NS398. Data represents means ± SE; n = 5 per group.
COX-2−/− mice.
There were no significant differences in abdominal aortic flow, splenic pulp pressure, or 2,3-dinor-6-keto-PGF1α among 7 days DMSO shams, 2 mg/kg NS398 shams, and 20 mg/kg SC560 shams. In contrast, 7-day urine 2,3-dinor-6-keto-PGF1α was reduced 32.8% in 20 mg/kg SC560 sham mice compared with DMSO sham controls. At 7 days following PVL surgery, abdominal aortic flow, splenic pulp pressure, and 2,3-dinor-6-keto-PGF1α were increased 35.3, 53, and 295%, respectively, in PVL-DMSO mice compared with 7-day sham-DMSO controls (Fig. 6, D and E). NS398 2 mg/kg did not significantly reduce these increases, whereas 20 mg/kg SC560 significantly reduced any increase. Abdominal aortic flow, splenic pulp pressure, and 2,3-dinor-6-keto-PGF1α were increased 40, 32, and 254%, respectively, in 7-day PVL-NS398 COX-2−/− mice compared with 7-day sham-NS398 COX-2−/− mice. In COX-2−/− mice treated with 20 mg/kg SC560 abdominal aortic flow and splenic pulp pressure were reduced 14 and 16%, respectively, whereas 2,3-dinor-6-keto-PGF1α was increased 36.4% in 7-day PVL-SC560 mice compared with 7-day sham-SC560 mice (Fig. 6, D–F).
DISCUSSION
To investigate the role of COX isoforms in PGI2 biosynthesis and PHT we utilized commercially available COX gene-deficient mice and COX isoform-selective inhibitors and documented changes in PGI2 biosynthesis and the development of PHT following PVL. We found no COX isoform predominance in PGI2 biosynthesis or PHT development, which argues that COX-selective inhibitors would not be beneficial in the treatment of PHT. PGI2 biosynthesis, abdominal aortic flow, and splenic pulp pressure were increased in COX-1−/− and COX-2−/− mice and in wild-type mice treated with COX-1 (20 mg/kg SC560)- or COX-2 (2 mg/kg NS398)-selective inhibitors, whereas when both COX isoforms were inhibited PGI2 biosynthesis was reduced, thus preventing vasodilatation and the formation of a hyperdynamic circulation. This prevents the development of PHT in the PVL model because in the absence of hyperemia portal systemic shunting negates the resistance caused by the mechanical stenosis.
There are a couple of reasons why no COX isoform predominates in this model: 1) COX-1 and COX-2 coexist in the vasculature and may be compensating for each other. Typical of most studies using gene-knockout mice, data must be interpreted with caution because redundant and/or compensatory mechanisms can appear. Indeed, this may explain why PGI2 levels were not reduced in COX-2−/− mice until they were treated with the COX-1 inhibitor SC560. Moreover, PGH2 can be synthesized in platelets via COX-1 and transferred to endothelial cells and converted to PGI2 (35). 2) COX heterodimers are resistant to COX isoform selective inhibition (63). In COX gene-deficient mice heterodimers cannot form but PGI2 biosynthesis was unaltered, showing that both COX-1 and COX-2 homodimers can mediate PGI2 biosynthesis in both basal and elevated PGI2 biosynthesis. These findings argue that additional targets, downstream of COX, need to be considered and investigated. Alternative targets for PGI2 include 1) inhibition of PGIS and 2) antagonism of prostacyclin signaling.
The synthesis of stable PGH2 analogs has generated a potent inhibitor for PGIS that has been used experimentally to investigate the role of PGI2 in multiple pathophysiological events (5, 21, 42). To our knowledge, PGIS inhibitors have not been used to reduce PGI2 biosynthesis and PHT in vivo. Following synthesis, PGI2 activates the G protein-coupled cell surface prostacyclin receptor (IP). Activation of IP stimulates adenylyl cyclase, leading to increased cAMP, vasodilation, inhibition of cell proliferation, and release of inflammatory mediators (60). Alternatively, PGI2 is also capable of activating peroxisome proliferator activated receptor β (PPARβ) (32), which is reported to have anti-inflammatory properties (31). Targeted gene-deficient mice for both IP and PPARβ have been generated (7, 54). To our knowledge, the development of PHT has not been investigated in these animals. With that said, these mice have multiple pathologies associated with a lack of normal PGI2 signaling and as such any investigation would be problematic. Specifically, deletion of IP elevates blood pressure, modulates vascular remodeling, and was found to promote atherosclerosis in mice via impaired activation of neutrophils and platelets (7, 12, 25).
COX isoforms are also important in regulating renal function. In the kidney PGIS and IP are mainly found in the mesangial cells and afferent arterial endothelial cells (26, 40). PGI2 regulates blood flow and therefore filtration rate of the kidney (2, 16). In PGI2-deficient mice, progressive morphological abnormalities develop in the kidney and mice have increased blood pressure and elevated plasma urea, nitrogen, and creatinine levels. However, these abnormalities have not been reported in IP-deficient mice (62). In this study we found that urine output is significantly reduced immediately following PVL in wild-type and COX-1 and -2−/− mice, after which urine output increases but does not exceed presurgical levels (data not shown). This is counter to an increase in PGI2 increasing renal output. The initial reduction is probably linked to the temporary drop in systemic blood flow that follows PVL. Therefore, serious consideration is required to evaluate the renal complications associated with PGI2 inhibition. Such an evaluation was not included in this study and would need to be covered separately. Inasmuch, the interaction between COX and nitric oxide (NO) synthase (NOS) has also not been focused on in this study. The family of NOS enzymes also produces a potent vasodilator, NO, that has been even more significantly linked with the development of PHT (38, 55). Data suggests that NOS and COX are significantly linked and both influence and compensate for one another (11, 38). Salvemini and coworkers (46) initially demonstrated that enhanced release of prostaglandins was nearly entirely driven by NO. Subsequently, additional mechanistic studies that have investigated how NO switches on/off the COX pathway have shown pathways through which NO modulates prostaglandin production. We did not study this NO/COX cross talk specifically but we did observe that endothelial NOS (eNOS) mRNA was significantly upregulated in both arteriolar and venous tissues of COX-1−/− mice but not COX-2−/− mice (data not shown). Moreover, preliminary studies investigating PGI2 in eNOS−/− mice show that plasma 6-keto-PGF1α was not increased following PVL (data not shown). Consequently, NO/COX cross talk is highly pertinent in both normal and disease pathophysiology and needs to be considered when considering the potential of PGI2 inhibition in any PHT treatment paradigm.
In conclusion, PGI2 is very important to the development of systemic hyperemia and elevated portal pressure in the PVL model of prehepatic PHT. Moreover, PGI2 biosynthesis is not COX-1 or COX-2 dependent. These findings direct further research toward other aspects of PHT vasculopathy. Targeting of PGI2 directly via either its synthesis or signaling might be an improvement but may have the same caveats as COX-2 inhibitors and may also affect renal function. Inhibition of PGI2 should not interfere with the gastric protective PGE2 but cardiovascular complication risk may increase. Recent studies have shown that increased PGI2 in the rat PVL model corresponds with a decreased in vivo platelet activity resulting in reduced laser-induced thrombus formation (13). Although this protects against cardiovascular accidents it exacerbates variceal bleeding. Therefore, not only would PGI2 inhibition reduce splanchnic hyperemia, it would also reduce bleeding complications associated with PHT. Any increased cardiovascular risk may be reduced by utilizing a multitarget approach. Targeted inhibition of both thromboxane and PGI2 synthesis would be one option. In addition to PGIS inhibitors thromboxane synthase inhibitors have been identified (U44069) as have IP antagonists (8, 21). Alternatively, given the resistance of COX heterodimers to isoform selective inhibitors and the discovery of COX-1 inhibitors that do not induce gastrointestinal complications, it may be possible to reduce systemic thromboxane via COX-1 inhibition without disturbing PGE2 gastrointestinal protection. However, evidence in this article and others suggests that COX-1 inhibitors are not neutral under pathological conditions (1). Nevertheless, given the renewed interest in COX and prostanoids in disease pathology, additional studies to better understand the role of PGI2 in PHT are warranted and relevant to develop new treatments to reduce PHT and its associated mortality and morbidity. In particular, a better understanding of PGI2, trigger, signaling, and NO cross talk within PHT models is needed and may bear fruit for novel targets for clinical therapy.
GRANTS
The research was funded by a grant awarded to J. V. Sitzmann through the National Institute of Diabetes and Digestive and Kidney Diseases (08RO1DK47067).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akahoshi T, Tanigawa T, Sarfeh IJ, Chiou SK, Hashizume M, Maehara Y, Jones MK. Selective cyclooxygenase (COX) inhibition causes damage to portal hypertensive gastric mucosa: roles of nitric oxide and NF-kappaB. FASEB J 19: 1163–1165, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Arzilli F, Giovannetti R, Lenzi M, Salvetti A. Acute hemodynamic (systemic and renal) and humoral effects of three increasing doses of iloprost in essential hypertensives. Am J Hypertens 2: 856–860, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Belton O, Byrne D, Kearney D, Leahy A, Fitzgerald DJ. Cyclooxygenase-1 and -2-dependent prostacyclin formation in patients with atherosclerosis. Circulation 102: 840–845, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bosch J, Garcia-Pagan JC. Prevention of variceal rebleeding. Lancet 361: 952–954, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Buchanan FG, Chang W, Sheng H, Shao J, Morrow JD, DuBois RN. Up-regulation of the enzymes involved in prostacyclin synthesis via Ras induces vascular endothelial growth factor. Gastroenterology 127: 1391–1400, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Cao H, Xu J, Liu H, Meng FB, Qiu JF, Wu ZY. Influence of nitric oxide synthase and cyclooxygenase blockade on expression of cyclooxygenase and hemodynamics in rats with portal hypertension. Hepatobiliary Pancreat Dis Int 5: 564–569, 2006. [PubMed] [Google Scholar]
- 7.Cheng Y, Austin SC, Rocca B, Koller BH, Coffman TM, Grosser T, Lawson JA, FitzGerald GA. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science 296: 539–541, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Clark RD, Jahangir A, Severance D, Salazar R, Chang T, Chang D, Jett MF, Smith S, Bley K. Discovery and SAR development of 2-(phenylamino) imidazolines as prostacyclin receptor antagonists [corrected]. Bioorg Med Chem Lett 14: 1053–1056, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Couzin J Drug safety. FDA panel urges caution on many anti-inflammatory drugs. Science 307: 1183–1185, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Cullen L, Kelly L, Connor SO, Fitzgerald DJ. Selective cyclooxygenase-2 inhibition by nimesulide in man. J Pharmacol Exp Ther 287: 578–582, 1998. [PubMed] [Google Scholar]
- 11.Cuzzocrea S, Salvemini D. Molecular mechanisms involved in the reciprocal regulation of cyclooxygenase and nitric oxide synthase enzymes. Kidney Int 71: 290–297, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Egan KM, Lawson JA, Fries S, Koller B, Rader DJ, Smyth EM, Fitzgerald GA. COX-2-derived prostacyclin confers atheroprotection on female mice. Science 306: 1954–1957, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Eizayaga FX, Aguejouf O, Desplat V, Belon P, Doutremepuich C. Modifications produced by selective inhibitors of cyclooxygenase and ultra low dose aspirin on platelet activity in portal hypertension. World J Gastroenterol 13: 5065–5070, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez M, Garcia-Pagan JC, Casadevall M, Mourelle MI, Pique JM, Bosch J, Rodes J. Acute and chronic cyclooxygenase blockage in portal-hypertensive rats: influence in nitric oxide biosynthesis. Gastroenterology 110: 1529–1535, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Fries S, Grosser T, Price TS, Lawson JA, Kapoor S, DeMarco S, Pletcher MT, Wiltshire T, FitzGerald GA. Marked interindividual variability in the response to selective inhibitors of cyclooxygenase-2. Gastroenterology 130: 55–64, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Fujita T, Fuke Y, Satomura A, Hidaka M, Ohsawa I, Endo M, Komatsu K, Ohi H. PGl2 analogue mitigates the progression rate of renal dysfunction improving renal blood flow without glomerular hyperfiltration in patients with chronic renal insufficiency. Prostaglandins Leukot Essent Fatty Acids 65: 223–227, 2001. [DOI] [PubMed] [Google Scholar]
- 17.García Rodríguez LA, Barreales Tolosa L. Risk of upper gastrointestinal complications among users of traditional NSAIDs and COXIBs in the general population. Gastroenterology 132: 498–506, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 46: 922–938, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Gretzer B, Maricic N, Respondek M, Schuligoi R, Peskar BM. Effects of specific inhibition of cyclo-oxygenase-1 and cyclo-oxygenase-2 in the rat stomach with normal mucosa and after acid challenge. Br J Pharmacol 132: 1565–1573, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest 116: 4–15, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hecker M, Ullrich V. On the mechanism of prostacyclin and thromboxane A2 biosynthesis. J Biol Chem 264: 141–150, 1989. [PubMed] [Google Scholar]
- 22.Hou MC, Cahill PA, Zhang S, Wang YN, Hendrickson RJ, Redmond EM, Sitzmann JV. Enhanced cyclooxygenase-1 expression within the superior mesenteric artery of portal hypertensive rats: role in the hyperdynamic circulation. Hepatology 27: 20–27, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Houchen CW, Stenson WF, Cohn SM. Disruption of cyclooxygenase-1 gene results in an impaired response to radiation injury. Am J Physiol Gastrointest Liver Physiol 279: G858–G865, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Kargman S, Charleson S, Cartwright M, Frank J, Riendeau D, Mancini J, Evans J, O'Neill G. Characterization of prostaglandin G/H synthase 1 and 2 in rat, dog, monkey, and human gastrointestinal tracts. Gastroenterology 111: 445–454, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi T, Tahara Y, Matsumoto M, Iguchi M, Sano H, Murayama T, Arai H, Oida H, Yurugi-Kobayashi T, Yamashita JK, Katagiri H, Majima M, Yokode M, Kita T, Narumiya S. Roles of thromboxane A(2) and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J Clin Invest 114: 784–794, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komhoff M, Lesener B, Nakao K, Seyberth HW, Nusing RM. Localization of the prostacyclin receptor in human kidney. Kidney Int 54: 1899–1908, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep 56: 2008, 1–120. [PubMed] [Google Scholar]
- 28.Laleman W, Nevens F. Cirrhotic portal hypertension: current and future medical therapy for primary and secondary prevention of variceal bleeding. Minerva Med 97: 325–345, 2006. [PubMed] [Google Scholar]
- 29.Lanas A, Baron JA, Sandler RS, Horgan K, Bolognese J, Oxenius B, Quan H, Watson D, Cook TJ, Schoen R, Burke C, Loftus S, Niv Y, Ridell R, Morton D, Bresalier R. Peptic ulcer and bleeding events associated with rofecoxib in a 3-year colorectal adenoma chemoprevention trial. Gastroenterology 132: 490–497, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, Mahler JF, Lee CA, Goulding EH, Kluckman KD, Kim HS, Smithies O. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell 83: 483–492, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Lee CH, Chawla A, Urbiztondo N, Liao D, Boisvert WA, Evans RM, Curtiss LK. Transcriptional repression of atherogenic inflammation: modulation by PPARdelta. Science 302: 453–457, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Lim H, Dey SK. A novel pathway of prostacyclin signaling-hanging out with nuclear receptors. Endocrinology 143: 3207–3210, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Loftin CD, Trivedi DB, Tiano HF, Clark JA, Lee CA, Epstein JA, Morham SG, Breyer MD, Nguyen M, Hawkins BM, Goulet JL, Smithies O, Koller BH, Langenbach R. Failure of ductus arteriosus closure and remodeling in neonatal mice deficient in cyclooxygenase-1 and cyclooxygenase-2. Proc Natl Acad Sci USA 98: 1059–1064, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luketic VA, Sanyal AJ. Esophageal varices. I. Clinical presentation, medical therapy, and endoscopic therapy. Gastroenterol Clin North Am 29: 337–385, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Maclouf J, Folco G, Patrono C. Eicosanoids and iso-eicosanoids: constitutive, inducible and transcellular biosynthesis in vascular disease. Thromb Haemost 79: 691–705, 1998. [PubMed] [Google Scholar]
- 36.Masferrer JL, Zweifel BS, Colburn SM, Ornberg RL, Salvemini D, Isakson P, Seibert K. The role of cyclooxygenase-2 in inflammation. Am J Ther 2: 607–610, 1995. [DOI] [PubMed] [Google Scholar]
- 37.McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci USA 96: 272–277, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev 57: 217–252, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, Mahler JF, Kluckman KD, Ledford A, Lee CA, Smithies O. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell 83: 473–482, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Oida H, Namba T, Sugimoto Y, Ushikubi F, Ohishi H, Ichikawa A, Narumiya S. In situ hybridization studies of prostacyclin receptor mRNA expression in various mouse organs. Br J Pharmacol 116: 2828–2837, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouellet M, Percival MD. Effect of inhibitor time-dependency on selectivity towards cyclooxygenase isoforms. Biochem J 306: 247–251, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pakrasi PL, Jain AK. Evaluation of cyclooxygenase 2 derived endogenous prostacyclin in mouse preimplantation embryo development in vitro. Life Sci 80: 1503–1507, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Potenza MA, Botrugno OA, De Salvia MA, Lerro G, Nacci C, Marasciulo FL, Andriantsitohaina R, Mitolo-Chieppa D. Endothelial COX-1 and -2 differentially affect reactivity of MVB in portal hypertensive rats. Am J Physiol Gastrointest Liver Physiol 283: G587–G594, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Pratico D, Tillmann C, Zhang ZB, Li H, FitzGerald GA. Acceleration of atherogenesis by COX-1-dependent prostanoid formation in low density lipoprotein receptor knockout mice. Proc Natl Acad Sci USA 98: 3358–3363, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riutta A, Nurmi E, Weber C, Hansson G, Vapaatalo H, Mucha I. Selective solid-phase extraction of urinary 2,3-dinor-6-ketoprostaglandin F1 alpha for determination with radioimmunoassay. Anal Biochem 220: 351–359, 1994. [DOI] [PubMed] [Google Scholar]
- 46.Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci USA 90: 7240–7244, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanyal AJ, Bosch J, Blei A, Arroyo V. Portal hypertension and its complications. Gastroenterology 134: 1715–1728, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Seibert K, Masferrer J, Zhang Y, Gregory S, Olson G, Hauser S, Leahy K, Perkins W, Isakson P. Mediation of inflammation by cyclooxygenase-2. Agents Actions Suppl 46: 41–50, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Sitzmann JV, Campbell K, Wu Y, St Clair C. Prostacyclin production in acute, chronic, and long-term experimental portal hypertension. Surgery 115: 290–294, 1994. [PubMed] [Google Scholar]
- 50.Smith CJ, Zhang Y, Koboldt CM, Muhammad J, Zweifel BS, Shaffer A, Talley JJ, Masferrer JL, Seibert K, Isakson PC. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc Natl Acad Sci USA 95: 13313–13318, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith WL, Dewitt DL. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol 62: 167–215, 1996. [DOI] [PubMed] [Google Scholar]
- 52.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 69: 145–182, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Sznaidman ML, Haffner CD, Maloney PR, Fivush A, Chao E, Goreham D, Sierra ML, LeGrumelec C, Xu HE, Montana VG, Lambert MH, Willson TM, Oliver WR Jr, Sternbach DD. Novel selective small molecule agonists for peroxisome proliferator-activated receptor delta (PPARdelta)—synthesis and biological activity. Bioorg Med Chem Lett 13: 1517–1521, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Tan NS, Michalik L, Noy N, Yasmin R, Pacot C, Heim M, Fluhmann B, Desvergne B, Wahli W. Critical roles of PPAR beta/delta in keratinocyte response to inflammation. Genes Dev 15: 3263–3277, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Theodorakis NG, Wang YN, Skill NJ, Metz MA, Cahill PA, Redmond EM, Sitzmann JV. The role of nitric oxide synthase isoforms in extrahepatic portal hypertension: studies in gene-knockout mice. Gastroenterology 124: 1500–1508, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Tsugawa K, Hashizume M, Migou S, Kishihara F, Kawanaka H, Tomikawa M, Sugimachi K. A selective cyclo-oxygenase-2 inhibitor, NS-398, may improve portal hypertension without inducing gastric mucosal injury. J Gastroenterol Hepatol 14: 642–651, 1999. [DOI] [PubMed] [Google Scholar]
- 57.Vorobioff J, Bredfeldt JE, Groszmann RJ. Hyperdynamic circulation in portal-hypertensive rat model: a primary factor for maintenance of chronic portal hypertension. Am J Physiol Gastrointest Liver Physiol 244: G52–G57, 1983. [DOI] [PubMed] [Google Scholar]
- 58.Wallace JL, McKnight W, Reuter BK, Vergnolle N. NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology 119: 706–714, 2000. [DOI] [PubMed] [Google Scholar]
- 59.Wallace JL, Tigley AW. Review article: new insights into prostaglandins and mucosal defence. Aliment Pharmacol Ther 9: 227–235, 1995. [DOI] [PubMed] [Google Scholar]
- 60.Wort SJ, Mitchell JA, Woods M, Evans TW, Warner TD. The prostacyclin-mimetic cicaprost inhibits endogenous endothelin-1 release from human pulmonary artery smooth muscle cells. J Cardiovasc Pharmacol 36: S410–S413, 2000. [DOI] [PubMed] [Google Scholar]
- 61.Wu ZY, Chen XS, Qiu JF, Cao H. Role of PGI2 in the formation and maintenance of hyperdynamic circulatory state of portal hypertensive rats. World J Gastroenterol 11: 752–755, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yokoyama C, Yabuki T, Shimonishi M, Wada M, Hatae T, Ohkawara S, Takeda J, Kinoshita T, Okabe M, Tanabe T. Prostacyclin-deficient mice develop ischemic renal disorders, including nephrosclerosis and renal infarction. Circulation 106: 2397–2403, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Yu Y, Fan J, Chen XS, Wang D, Klein-Szanto AJ, Campbell RL, FitzGerald GA, Funk CD. Genetic model of selective COX2 inhibition reveals novel heterodimer signaling. Nat Med 12: 699–704, 2006. [DOI] [PubMed] [Google Scholar]