Abstract
Although angiotensin II (Ang II) plays a key role in development of organ ischemia-reperfusion injury, it remains unclear whether it is involved in development of intestinal injury following trauma-hemorrhage (T-H). Studies have shown that 17β-estradiol (E2) administration following T-H improves small intestinal blood flow; however, it is unclear whether Ang II plays a role in this E2-mediated salutary effect. Male Sprague-Dawley rats underwent laparotomy and hemorrhagic shock (removal of 60% total blood volume, fluid resuscitation after 90 min). At onset of resuscitation, rats were treated with vehicle, E2, or E2 and estrogen receptor antagonist ICI 182,780 (ICI). A separate group of rats was treated with Ang II subtype I receptor (AT1R) antagonist losartan. At 24 h after T-H, plasma Ang II, IL-6, TNF-α, intercellular adhesion molecule (ICAM)-1, cytokine-induced neutrophil chemoattractant (CINC)-1 and CINC-3 levels, myeloperoxidase (MPO) activity, and AT1R expression were determined. T-H significantly increased plasma and intestinal Ang II, IL-6, TNF-α levels, intestinal ICAM-1, CINC-1, CINC-3 levels, MPO activity, and AT1R protein compared with shams. E2 treatment following T-H attenuated increased intestinal MPO activity, Ang II level, and AT1R protein expression. ICI administration abolished the salutary effects of E2. In contrast, losartan administration attenuated increased MPO activity without affecting Ang II and AT1R levels. Thus Ang II plays a role in producing small intestine inflammation following T-H, and the salutary effects of E2 on intestinal inflammation are mediated in part by Ang II and AT1R downregulation.
Keywords: shock, estrogen receptor antagonist, myeloperoxidase, chemokines, cytokines
the small intestine is one of the most susceptible organs to injury induced by trauma-hemorrhagic shock. Previous studies have shown that intestinal injury occurs during hemorrhagic shock and persists despite fluid resuscitation (15). Impaired intestinal perfusion during resuscitation results in persistent mucosal hypoxia and subsequent loss of mucosal integrity. This loss of gut mucosal integrity has been implicated in the pathogenesis of multiple organ dysfunction syndrome (32). A number of studies have demonstrated that the enhanced secretion of proinflammatory cytokines by mast cells, dendritic cells, and macrophages is an important factor in the initiation and perpetuation of intestinal inflammation (8). These proinflammatory cytokines recruit other immune cells including neutrophils, thereby increasing leukocyte trafficking and intestinal permeability (18, 30, 38, 41).
Previous studies have shown that 17β-estradiol (E2) administration following trauma-hemorrhage decreases plasma and intestinal endothelin (ET)-1 levels and improves small intestine blood perfusion under those conditions (2). E2 also reduces neutrophil accumulation in the gut via estrogen receptor (ER)-mediated process. In addition to reduction of neutrophil accumulation, E2 also upregulates heme oxygenase-1 expression and protects the organs against dysfunction and injury (36) in males following trauma-hemorrhage.
Angiotensin II (Ang II) is an important vasoconstrictor during hypovolemia and may contribute to shock-induced hypoxic/ischemic organ damage (27). Ang II also stimulates a wide variety of proinflammatory responses including increased leukocyte rolling and adhesion, production of oxidative stress, and induction of cysteine-x-cysteine chemokine expression (3, 28, 47). It has been shown that the Ang II subtype-1 receptor (AT1R) antagonist significantly inhibits the intestinal mucosal injury induced by ischemia-reperfusion in rats (40). The AT1R mediates many biological effects of the renin-angiotensin system (RAS), such as vasoconstriction, water and sodium retention, free radical release, and cell growth (9). Furthermore, AT1R antagonist prevents irreversible tissue injury and improves outcome from stroke in animal experiments (35). Recent studies have shown that E2 deficiency caused AT1R overexpression in vivo, leading to enhanced biological effects of the RAS. A recent in vitro study has shown that E2 downregulates AT1R mRNA expression and AT1R protein in vascular smooth muscle cells isolated from ovariectomized rats (21). Furthermore, E2 has been shown to inhibit Ang II-induced cell proliferation, ET-1 gene expression, reactive oxygen species (ROS) generation, extracellular signal-regulated kinase phosphorylation, and activator protein-1-mediated reporter activity in vascular smooth muscle cells (13).
Our previous studies have shown that treatment of animals with E2 following trauma-hemorrhage prevents intestinal tissue damage under those conditions (45). Nonetheless, it is unclear whether E2 protects against intestinal injury following trauma-hemorrhage via regulation of Ang II. Accordingly, we tested the hypothesis that the E2 modulates Ang II in the small intestine following trauma-hemorrhage and thus produces its salutary effects under those conditions.
MATERIALS AND METHODS
Animals.
Male adult (250–300 g) Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were used in this study. Rats were allowed to acclimatize in the animal facility for 1 wk before the experiments. All experiments were performed in adherence with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Experimental procedures.
A nonheparinized rat trauma-hemorrhagic shock model was used in this study. Rats were fasted overnight before the experiment but allowed water ad libitum. Trauma-hemorrhage was induced as described previously (43). Briefly, the rats were anesthetized using 1.5% isoflurane (Attane; Minrad, Bethlehem, PA) inhalation and underwent a 5-cm midline laparotomy to induce soft-tissue trauma before the onset of hemorrhage. The abdomen was then closed in layers and catheters (polyethylene, PE-50 tubing; Becton-Dickinson, Franklin Lakes, NJ) were placed in both femoral arteries and the right femoral vein. The wounds were bathed with 1% lidocaine (Elkins-Sinn, Cherry Hill, NJ) throughout the surgical procedure to minimize postoperative pain. The rats were then allowed to awaken, after which they were rapidly bled to a mean arterial pressure (MAP) of 35–40 mmHg within 10 min. The time at which the rats could no longer maintain a MAP of 35–40 mmHg without fluid infusion was defined as maximum bleed-out volume. The rats were maintained at that MAP until 40% of the shed blood volume was returned in the form of Ringer's lactate and were then resuscitated with four times the volume of shed blood with Ringer's lactate over 60 min. Following resuscitation, the catheters were removed, the vessels were ligated, and skin incisions closed with sutures. The sham-operated animals underwent the same surgical procedure but were neither bled nor resuscitated. The animals were returned to their cages and allowed food and water ad libitum and euthanized at 24 h after resuscitation.
Chemicals and antibodies.
β-cyclodextrin and water-soluble E2 were purchased from Sigma Chemical (St. Louis, MO); ICI 182,780 (ICI) was purchased from Tocris Bioscience (New England, Ellisville, MO). Losartan was provided by Merck (Rathway, NJ) as a gift. All of the prepared agents were stored at −80°C until use. Anti-rat AT1R polyclonal and anti-rat GAPDH monoclonal antibodies were purchased from AbCam (Cambridge, MA).
Experimental groups.
Animals were randomly divided into eight groups: 1) sham + vehicle; 2) sham + losartan; 3) sham + E2; 4) sham + ICI + E2; 5) trauma-hemorrhage + vehicle; 6) trauma-hemorrhage + losartan; 7) trauma-hemorrhage + E2; and 8) trauma-hemorrhage + ICI + E2. Vehicle cyclodextrin (20 mg/kg) or E2 (1 mg/kg) was administered intravenously at the time of sham operation or at the beginning of resuscitation. The ER antagonist ICI (3 mg/kg) was given intraperitoneally at the time of sham operation or 30 min before E2 administration. AT1R antagonist losartan (30 mg/kg) was given intraperitoneally at the time of sham operation or at the beginning of resuscitation.
Preparation of blood samples.
The animals were anesthetized with isoflurane 24 h after sham operation or resuscitation in the trauma-hemorrhage groups, and blood was obtained via cardiac puncture using a syringe coated with heparin (Abraxis Pharmaceutical Production, Schaumburg, IL). Blood was centrifuged (2,500 g, 10 min, 4°C), and plasma was removed and stored at −80°C until analyzed.
Preparation of intestine samples.
Immediately after anesthetizing the rats and drawing blood, the intestine was exposed. An ∼15-cm-long proximal segment of jejunum was removed, flushed gently with saline, and snap frozen in liquid N2. The samples were stored at −80°C until analyzed.
Measurement of Ang II.
Levels of Ang II in plasma and small intestine were determined using enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions.
Western blot for AT1R expression.
Approximately 0.5-g small intestine specimens of full-wall thickness were collected at 24 h after resuscitation, snap frozen in liquid N2, and subsequently stored at −80°C. Specimens were thawed and homogenized on ice in 1 ml of lysis buffer containing 20 mM HEPES (pH 7.9), 1.5 mM MgCl2, 20 mM KCl, 0.2 mM EDTA, 2 mM Na3VO4, 10 mM NaF, 0.2 mM PMSF, 1% Triton X-100, 20% glycerol, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. The lysates were centrifuged at 10,000 g for 10 min and the supernatants stored at −80°C until use. Small intestine AT1R expression was determined by Western blot analysis. The protein concentrations were assayed (Bio-Rad Laboratories, Hercules, CA), and lysates were resolved by 12% SDS polyacrylamide gel electrophoresis according to the molecular weight and transferred to polyvinylidene fluoride membrane (Millipore, Bedford, MA). The Western blot transfers were immunoblotted with anti-rat AT1R, followed by the addition of horseradish peroxidase-conjugated secondary antibody for detection of bound antibody by enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ). Mouse monoclonal GAPDH antibody was used as the loading control (43).
Determination of IL-6, TNF, CINC-1, CINC-3 and ICAM-1 levels.
Levels of cytokines (TNF and IL-6) in plasma and small intestine tissue were determined with cytometric bead array kits using flow cytometry according to the manufacturer's instructions (BD Biosciences, San Diego, CA). Intestinal cytokine-induced neutrophil chemoattractant (CINC)-1, CINC-3, and intercellular adhesion molecule (ICAM)-1 levels were determined using ELISA kits (R&D, Minneapolis, MN and Biosource, Camarillo, CA) according to the manufacturers’ instructions. Briefly, the samples were homogenized in 0.5 ml lysis buffer containing 50 mM HEPES, 10 mM sodium pyrophosphate, 1.5 mM MgCl2, 1 mM EDTA, 0.2 mM sodium orthovanadate, 0.15 M NaCl, 0.1 M NaF, 10% glycerol, 0.5% Triton X-100, and protease inhibitor cocktail (Sigma Chemical). The homogenates were centrifuged at 2,000 g for 20 min at 4°C, and the supernatant was assayed for IL-6, TNF, CINC-1, CINC-3, and ICAM-1 levels. An aliquot of the supernatant was used to determine protein concentration (Bio-Rad DC protein assay), which for these proinflammatory mediators, is expressed as pg/mg protein in each sample.
Measurement of MPO activity.
Small intestine myeloperoxidase (MPO) activity was measured in a 96-well plate as previously described (45). In brief, ∼0.2 g of small intestine tissue was suspended in 1 ml lysis buffer [0.5% hexadecyltrimethyl-ammonium bromide (HETAB) in 50 mM PBS, pH 6.0] and homogenized by sonication on ice for 30 s, twice, then centrifuged at 12,000 g for 10 min at 4°C. The supernatant was transferred into new tubes and aliquoted. The protein concentration of supernatant was measured by Power Wave (Bio-Tek, Winooski, VT). For standard, 5 U of MPO standard (Sigma-Aldrich) were dissolved in 1 ml of cold double-distilled H2O (20 mg O-dianisidine dihydrochloride in 1 ml water) diluted to 2.5 U, 1.25 U, 0.625 U, 0.3125 U, and 0 U. Then, 290 μl of 50 mM PBS, 3 μl of O-dianisidine dihydrochloride solution, and 3 μl of 20 mM H2O2 were added to each well; next, either 10 μl of standard or sample was added to each well. After 5 min, the reaction was stopped by the addition of 3 μl of 30% sodium azide. Light absorbance at 460 nm was read by Power Wave. MPO activity was determined by using the curve obtained from the standard MPO. All solutions including 0.5% HETAB, 20 mM H2O2, and O-dianisidine dihydrochloride were freshly prepared.
Statistical analysis.
Data are presented as means ± SE (n = 4–6 rats/group). Statistical differences among groups were determined by one-way ANOVA followed by Tukey's test. The differences were considered significant if the P value was < 0.05.
RESULTS
Ang II levels in plasma and small intestine.
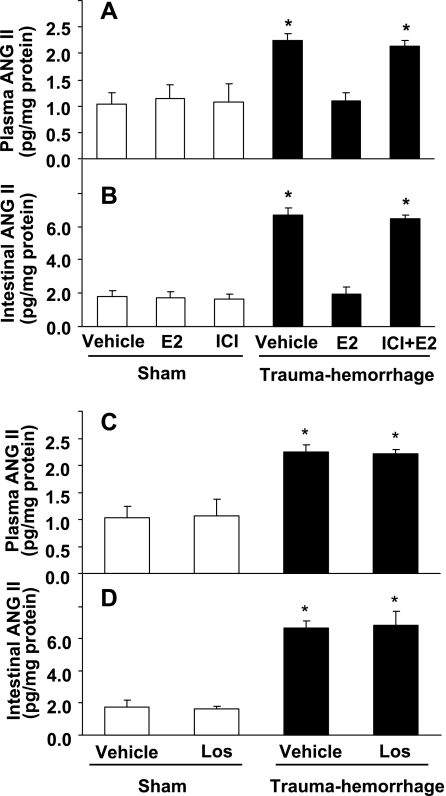
As shown in Fig. 1, the level of Ang II in plasma and small intestine increased significantly (P < 0.05) at 24 h following trauma-hemorrhage. Treatment of animals with E2 normalized Ang II levels in plasma and small intestine to sham levels. The salutary effect of E2 on Ang II levels in plasma and small intestine was abolished by ER antagonist ICI. In contrast, administration of AT1R antagonist losartan following trauma-hemorrhage did not produce any effect on Ang II levels in plasma or small intestine.
Fig. 1.
Angiotensin II (Ang II) level in plasma and small intestine in sham and trauma-hemorrhage animals treated with vehicle (Veh), 17β-estradiol (E2), and ICI 182,780 (ICI) + E2 (A and B), and Veh or Ang II subtype-I receptor (AT1R) antagonist losartan (Los) (C and D). Data are shown as means ± SE of 5 animals in each group. *P < 0.05 compared with sham or trauma-hemorrhage + E2.
Alteration of AT1R expression in small intestine following trauma-hemorrhage.
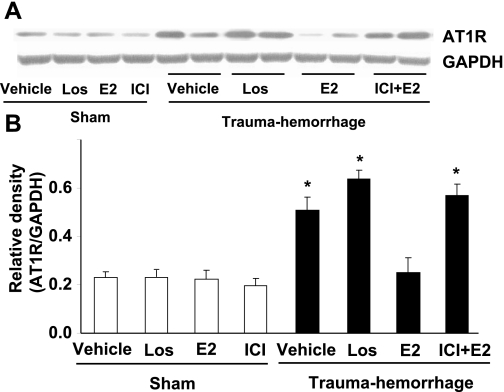
AT1R protein levels in small intestine increased significantly following trauma-hemorrhage (Fig. 2, P < 0.05). Treatment of animals with losartan did not influence intestinal AT1R expression following trauma-hemorrhage. In contrast, administration of E2 significantly decreased intestinal AT1R expression in trauma-hemorrhage animals compared with the vehicle-treated trauma-hemorrhage group (P < 0.05). The salutary effect of E2 on AT1R expression following trauma-hemorrhage was abolished by coadministration of ICI.
Fig. 2.
AT1R protein expression in small intestine from sham and trauma-hemorrhage animals treated with Veh, Los, E2, or ICI + E2. For equal protein loading, membranes were probed for GAPDH using mouse anti-GAPDH monoclonal antibody. Representative blot is shown in A. Blots were analyzed densitometrically; the densitometric values were pooled from 4 animals in each group and are shown in B. Data are expressed as means ± SE and compared by one-way ANOVA and Tukey's test: *P < 0.05 vs. sham or trauma-hemorrhage + E2.
Cytokine levels in plasma and small intestine.
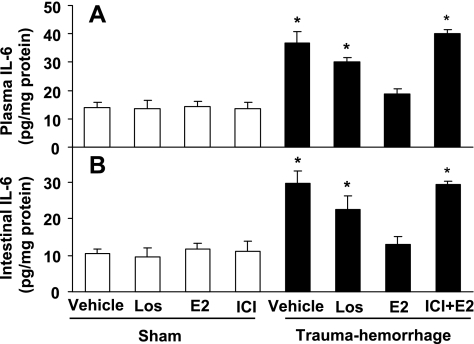
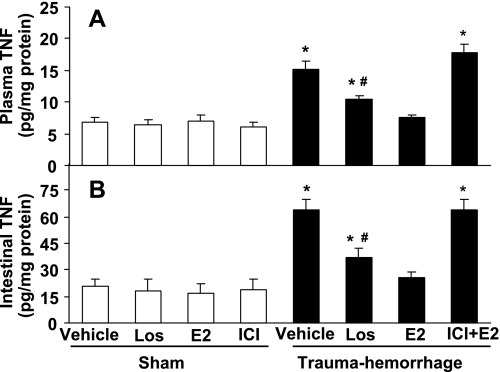
Trauma-hemorrhage led to a significant increase in systemic and small intestine IL-6 (Fig. 3) and TNF levels (Fig. 4) compared with shams. Treatment of animals with losartan lowered IL-6 and TNF levels following trauma-hemorrhage, but these levels remained significantly higher than shams (Figs. 3 and 4; P < 0.05). In contrast, administration of E2 following trauma-hemorrhage normalized the increase in systemic and small intestine IL-6 and TNF levels, which was abolished by coadministration of ICI (Figs. 3 and 4).
Fig. 3.
IL-6 level in plasma (A) and small intestine (B) in sham and trauma-hemorrhage animals treated with Veh, Los, E2, or ICI + E2. Data are shown as means ± SE of 5 animals in each group. *P < 0.05 vs. sham or trauma-hemorrhage + E2.
Fig. 4.
TNF level in plasma (A) and small intestine (B) in sham and trauma-hemorrhage animals treated with Veh, Los, E2, or ICI + E2. Data are shown as means ± SE of 5 animals in each group. *P < 0.05 vs. sham or trauma-hemorrhage + E2; #P < 0.05 vs. trauma-hemorrhage + Veh.
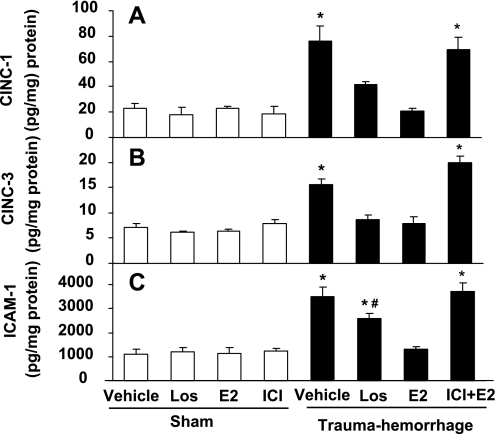
Intestinal CINC-1, CINC-3, and ICAM-1 levels.
Trauma-hemorrhage significantly increased intestinal CINC-1, CINC-3, and ICAM-1 levels (P < 0.05 compared with shams). Treatment of the animals with losartan normalized intestinal CINC-1 and CINC-3 levels and significantly lowered ICAM-1 levels following trauma-hemorrhage (Fig. 5). In contrast, administration of E2 following trauma-hemorrhage normalized CINC-1, CINC-3, and ICAM-1 levels (Fig. 5). Coadministration of ICI abolished the effect of E2 on intestinal CINC-1, CINC-3, and ICAM-1 levels in trauma-hemorrhage groups (Fig. 5).
Fig. 5.
Intestinal cytokine-induced neutrophil chemoattractant (CINC)-1 (A), CINC-3 (B), intercellular adhesion molecule (ICAM)-1 (C) levels in rats after sham operation or trauma-hemorrhage and resuscitation receiving Veh, Los, E2, or ICI + E2. Data are shown as means ± SE of five rats in each group. *P < 0.05 vs. sham or trauma-hemorrhage + E2; #P < 0.05 vs. trauma-hemorrhage + Veh.
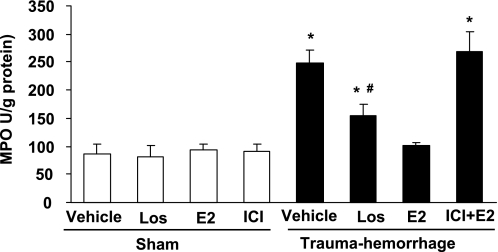
Intestinal MPO activity following trauma-hemorrhage.
Neutrophil accumulation was evaluated by the measurement of tissue-associated MPO activity in small intestine tissue homogenates. As indicated in Fig. 6, small intestine MPO activity significantly increased following trauma-hemorrhage (P < 0.05). Losartan administration significantly reduced the increase of MPO in small intestine (P < 0.05) following trauma-hemorrhage; however, the MPO activity remained higher than shams and E2-treated trauma-hemorrhage animals (P < 0.05). Administration of E2 significantly attenuated the increase in small intestine MPO activity after trauma-hemorrhage, which was abolished by coadministration of ICI.
Fig. 6.
Intestinal myeloperoxidase (MPO) activity in animals at 24 h after sham operation or resuscitation following trauma hemorrhage. Animals were treated with Veh, Los, E2, or ICI + E2. Data represent the means ± SE of 5 animals in each group, compared by one-way ANOVA and Tukey's test. *P < 0.05 compared with sham-operated rats or trauma-hemorrhage + E2; #P < 0.05 compared with trauma-hemorrhage + Veh.
DISCUSSION
The gut is considered a critical organ in the development of the delayed organ dysfunction in patients suffering from traumatic injuries and severe blood loss (11, 19). Gut ischemia is frequently encountered in trauma, shock, cardiovascular surgery, and organ transplantation (4). Splanchnic hypoperfusion is a characteristic feature in the cardiovascular response to hemorrhagic shock (24) and can cause hypoxia of the intestinal mucosa and subsequent increase in intestinal permeability (7). Our present studies indicate that plasma Ang II, IL-6, and TNF levels and intestinal Ang II, IL-6, TNF, CINC-1, CINC-3, and ICAM-1 levels markedly increased at 24 h following trauma-hemorrhage. This was accompanied with an increase in small intestine MPO activity. Administration of losartan at the beginning of resuscitation attenuated the increase in those inflammatory markers but did not affect the trauma-hemorrhage-induced increase in AT1R expression in the intestine. In contrast, administration of a single dose of E2 at the beginning of resuscitation normalized all the above parameters to levels observed in sham animals. Administration of E2 also prevented the trauma-hemorrhage-induced increase in intestinal AT1R expression. The salutary effects of E2 were receptor mediated since administration of ICI along with E2 following trauma-hemorrhage abolished the salutary effects of E2 on the above parameters (46). These studies collectively suggest that the salutary effects of E2 on intestinal injury following trauma-hemorrhage are mediated via downregulation of AT1R.
Ang II is an important vasoconstrictor during hypovolemia and thus may contribute to shock-induced ischemic organ damage (27). The overall function of the RAS is to maintain extracellular fluid and electrolyte homeostasis, as well as to regulate vascular tone and blood pressure (3, 23). Ang II also stimulates a wide variety of proinflammatory responses such as increased induction of proinflammatory chemokines and cytokines (25, 28, 47), expression of adhesion molecules (26), activation of NF-κB (31), and production of oxidative stress (10). Moreover, Ang II increases the level of hypoxia-inducible factor-1α expression via a ROS-dependent activation of the phosphatidylinositol 3-kinase pathway (29). Ultimately, Ang II causes vascular injury and organ damage. In fact, several lines of evidence have implicated that inhibition of Ang II may protect against ischemia-reperfusion-induced tissue injury in the heart, brain, liver, kidney, and small intestine (1, 5, 17, 42). Previous studies have shown that the increase in Ang II is more prominent in the splanchnic circulation than in the systemic circulation during major aortic surgery and hypovolemia (44). This further suggests that Ang II plays a more critical role in the gastrointestinal system than in other organ systems in critical situations. Moreover, it has been shown that the splanchnic vasculature has extraordinarily high concentrations of Ang II receptors (27). The highest angiotensin-converting enzyme (ACE) mRNA, ACE protein, and activity within the intestine have been found in the brush-border membrane fraction in the proximal to mid region of the rat small intestine (6). Functional AT1Rs have been found in the rat ileum and duodenum (34). Other studies have shown that the AT1R antagonist inhibited the development of intestinal mucosal injury induced by ischemia-reperfusion in rats (40). Furthermore, ACE-I inhibition, which decreases the production of angiotensin II, has been shown to decrease postischemic injury (14, 26a, 28). AT1R blockade reduces cerebral ischemia-reperfusion injury in part by attenuating inflammatory processes (35). Studies using in vitro techniques have also shown that E2 downregulated AT1R mRNA expression and AT1R protein in vascular smooth muscle cells isolated from ovariectomized rats (21) and inhibited Ang II-induced cell proliferation and ET-1 gene expression studies (13).
In the present study, intestinal injury was assessed by the measurement of the small intestinal MPO activity, a marker of neutrophil content. Neutrophils are the principal cells involved in host defense against acute bacterial and fungal infections (16). Neutrophil movement and migration are mediated by multiple adhesion molecules on the neutrophils, endothelial cell surfaces, and chemotactic factors. ICAM-1 is an important mediator in the firm adhesion of neutrophils to the vascular endothelium and is upregulated strongly following trauma-hemorrhagic shock (22). With regard to chemokines, rat CINC-1 and CINC-3 are members of the IL-8 family and are potent chemotactic factors for neutrophils (20, 37). Chemotaxis of neutrophils is an important, functional response to chemokines and is a key event in the recruitment of neutrophils during inflammation. In addition, it has been reported that pretreatment with anti-CINC-1 monoclonal antibodies attenuates reperfusion injury in the small intestine in association with a reduction of TNF-α and MPO content, and thereby prolongs survival (39). Our previous studies indicate that CINC-1 and macrophage inflammatory protein-2 levels correlated with intestinal MPO activity following trauma-hemorrhage (33). The present study is consistent with these findings and indicates that administration of the AT1R antagonist decreases small intestinal CINC-1, CINC-3, and ICAM-1 levels and MPO activity. These results indicate that AT1R is involved in the development of trauma-hemorrhage-induced intestinal inflammation.
There is increasing evidence indicating a role for E2 in the modulation of proinflammatory mediator ICAM-1 expression and chemokine production in shock states (12). Previous studies have shown that E2 administration following trauma-hemorrhage improves cardiac function and decreases intestinal neutrophil infiltration. It is therefore possible that the salutary effects of E2 on small intestine following trauma-hemorrhage are a result of the systemically improved cardiac function by E2. Our present results showed that administration of E2 normalized the trauma-hemorrhage-induced increase in intestinal CINC-1, CINC-3, and ICAM-1 levels and inhibited the increase in intestinal inflammation (45). Moreover, the results also showed that the small intestinal Ang II level and AT1R expression increased in trauma-hemorrhage animals. Administration of AT1R antagonist losartan decreased intestinal TNF and ICAM-1 levels and MPO activity without changing Ang II level and AT1R expression, thus indicating that Ang II and AT1R are involved in the intestinal inflammation following trauma-hemorrhage. Since a group with administration of losartan and E2 was not included in this study, it remains unclear whether the combination of these two agents has additional salutary effects compared with either of these agents alone. Nonetheless, since administration of E2 normalized plasma and intestinal Ang II levels, downregulated intestinal AT1R expression, and prevented small intestinal inflammation following trauma-hemorrhage, it is unlikely that the combination of these two agents would have produced additional salutary effects. Furthermore, ER antagonist ICI 182,780 abolished the effect of E2 on Ang II and AT1R. Moreover, although the effects of E2 on intestinal structure and function (histology, absorption, and permeability) were examined in this study, it appears that the salutary effects of E2 on intestine are likely mediated in part due to downregulation of Ang II and AT1R and a decrease of intestinal MPO activity. It could, however, be argued that we should have also measured mRNA levels of cytokines and chemokines since they are secretory factors and may not be appropriately detected by ELISA. Since RNA was not extracted from intestine in this study, we cannot correlate plasma results of chemokines and cytokines exclusively with the intestine in this study.
In conclusion, our data demonstrated that modulation of Ang II and AT1R expression plays an important role in the salutary effects of E2 on small intestine injury following trauma-hemorrhage.
GRANTS
This work was supported by National Institute of Health/National Institute of General Medical Sciences Grant R37 GM39519.
Acknowledgments
The authors thank Ms. Bobbi Smith for skill and assistance in preparing this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anthuber M, Farkas S, Rihl M, Menger MD, Schildberg FW, Jauch KW, Messmer K. Angiotensin-converting enzyme inhibition by enalapril: a novel approach to reduce ischemia/reperfusion damage after experimental liver transplantation. Hepatology 25: 648–651, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Ba ZF, Shimizu T, Szalay L, Bland KI, Chaudry IH. Gender differences in small intestinal perfusion following trauma hemorrhage: the role of endothelin-1. Am J Physiol Gastrointest Liver Physiol 288: G860–G865, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Cheng ZJ, Vapaatalo H, Mervaala E. Angiotensin II and vascular inflammation. Med Sci Monit 11: RA194–RA205, 2005. [PubMed] [Google Scholar]
- 4.Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology 94: 1133–1138, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Engelhorn T, Goerike S, Doerfler A, Okorn C, Forsting M, Heusch G, Schulz R. The angiotensin II type 1-receptor blocker candesartan increases cerebral blood flow, reduces infarct size, and improves neurologic outcome after transient cerebral ischemia in rats. J Cereb Blood Flow Metab 24: 467–474, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Erickson RH, Yoon BC, Koh DY, Kim DH, Kim YS. Dietary induction of angiotensin-converting enzyme in proximal and distal rat small intestine. Am J Physiol Gastrointest Liver Physiol 281: G1221–G1227, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Fink MP Gastrointestinal mucosal injury in experimental models of shock, trauma, and sepsis. Crit Care Med 19: 627–641, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Fiocchi C Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 115: 182–205, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Griendling KK, Murphy TJ, Alexander RW. Molecular biology of the renin-angiotensin system. Circulation 87: 1816–1828, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Harrison DG, Cai H, Landmesser U, Griendling KK. Interactions of angiotensin II with NAD(P)H oxidase, oxidant stress and cardiovascular disease. J Renin Angiotensin Aldosterone Syst 4: 51–61, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann M, Montgomery A, Jonsson K, Haglund U. Tissue oxygenation in hemorrhagic shock measured as transcutaneous oxygen tension, subcutaneous oxygen tension, and gastrointestinal intramucosal pH in pigs. Crit Care Med 19: 205–210, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Homma H, Hoy E, Xu DZ, Lu Q, Feinman R, Deitch EA. The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am J Physiol Gastrointest Liver Physiol 288: G466–G472, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Hong HJ, Liu JC, Chan P, Juan SH, Loh SH, Lin JG, Cheng TH. 17beta-estradiol downregulates angiotensin-II-induced endothelin-1 gene expression in rat aortic smooth muscle cells. J Biomed Sci 11: 27–36, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Konstam MA Administration of captopril following MI reduced the incidence of ischemia-related events. Evid Based Cardiovasc Med 2: 21, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Kuebler JF, Toth B, Yokoyama Y, Bland KI, Rue LW 3rd, Chaudry IH. Alpha1-acid-glycoprotein protects against trauma-hemorrhagic shock. J Surg Res 119: 21–28, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Malech HL, Gallin JI. Current concepts: immunology. Neutrophils in human diseases. N Engl J Med 317: 687–694, 1987. [DOI] [PubMed] [Google Scholar]
- 17.Masuko H, Jin MB, Horiuchi H, Suzuki T, Taniguchi M, Shimamura T, Fukai M, Magata S, Ogata K, Ishikawa H, Fujita M, Nagashima K, Furukawa H, Todo S. Protective effect of angiotensin II type I receptor antagonist, CV-11974, on ischemia and reperfusion injury of the liver. Transplantation 71: 1034–1039, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Meldrum KK, Meldrum DR, Meng X, Ao L, Harken AH. TNF-alpha-dependent bilateral renal injury is induced by unilateral renal ischemia-reperfusion. Am J Physiol Heart Circ Physiol 282: H540–H546, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Moore FA The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg 178: 449–453, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa H, Komorita N, Shibata F, Ikesue A, Konishi K, Fujioka M, Kato H. Identification of cytokine-induced neutrophil chemoattractants (CINC), rat GRO/CINC-2 alpha and CINC-2 beta, produced by granulation tissue in culture: purification, complete amino acid sequences and characterization. Biochem J 301: 545–550, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nickenig G, Strehlow K, Wassmann S, Baumer AT, Albory K, Sauer H, Bohm M. Differential effects of estrogen and progesterone on AT(1) receptor gene expression in vascular smooth muscle cells. Circulation 102: 1828–1833, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Olanders K, Sun Z, Borjesson A, Dib M, Andersson E, Lasson A, Ohlsson T, Andersson R. The effect of intestinal ischemia and reperfusion injury on ICAM-1 expression, endothelial barrier function, neutrophil tissue influx, and protease inhibitor levels in rats. Shock 18: 86–92, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Padmanabhan N, Padmanabhan S, Connell JM. Genetic basis of cardiovascular disease—the renin-angiotensin-aldosterone system as a paradigm. J Renin Angiotensin Aldosterone Syst 1: 316–324, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Peitzman AB, Billiar TR, Harbrecht BG, Kelly E, Udekwu AO, Simmons RL. Hemorrhagic shock. Curr Probl Surg 32: 925–1002, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Piqueras L, Kubes P, Alvarez A, O'Connor E, Issekutz AC, Esplugues JV, Sanz MJ. Angiotensin II induces leukocyte-endothelial cell interactions in vivo via AT(1) and AT(2) receptor-mediated P-selectin upregulation. Circulation 102: 2118–2123, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol 20: 645–651, 2000. [DOI] [PubMed] [Google Scholar]
- 26a.Przyklenk K, Kloner RA. “Cardioprotection” by ACE-inhibitors in acute myocardial ischemia and infarction? (Review). Basic Res Cardiol 88, Suppl 1: 139–154, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Reilly PM, Bulkley GB. Vasoactive mediators and splanchnic perfusion. Crit Care Med 21: S55–S68, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Riaz AA, Wang Y, Schramm R, Sato T, Menger MD, Jeppsson B, Thorlacius H. Role of angiotensin II in ischemia/reperfusion-induced leukocyte-endothelium interactions in the colon. FASEB J 18: 881–883, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Richard DE, Berra E, Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1alpha in vascular smooth muscle cells. J Biol Chem 275: 26765–26771, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez P, Heyman M, Candalh C, Blaton MA, Bouchaud C. Tumour necrosis factor-alpha induces morphological and functional alterations of intestinal HT29 cl.19A cell monolayers. Cytokine 7: 441–448, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Ortega M, Lorenzo O, Ruperez M, Blanco J, Egido J. Systemic infusion of angiotensin II into normal rats activates nuclear factor-kappaB and AP-1 in the kidney: role of AT(1) and AT(2) receptors. Am J Pathol 158: 1743–1756, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell DH, Barreto JC, Klemm K, Miller TA. Hemorrhagic shock increases gut macromolecular permeability in the rat. Shock 4: 50–55, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Samy TS, Schwacha MG, Cioffi WG, Bland KI, Chaudry IH. Androgen and estrogen receptors in splenic T lymphocytes: effects of flutamide and trauma-hemorrhage. Shock 14: 465–470, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Schinke M, Doods HN, Ganten D, Wienen W, Entzeroth M. Characterization of rat intestinal angiotensin II receptors. Eur J Pharmacol 204: 165–170, 1991. [DOI] [PubMed] [Google Scholar]
- 35.Schulz R, Heusch G. Angiotensin II type 1 receptors in cerebral ischaemia-reperfusion: initiation of inflammation. J Hypertens Suppl 24: S123–S129, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Schunkert H, Danser AH, Hense HW, Derkx FH, Kurzinger S, Riegger GA. Effects of estrogen replacement therapy on the renin-angiotensin system in postmenopausal women. Circulation 95: 39–45, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Shibata F, Konishi K, Kato H, Komorita N, al Mokdad M, Fujioka M, Nakagawa H. Recombinant production and biological properties of rat cytokine-induced neutrophil chemoattractants, GRO/CINC-2 alpha, CINC-2 beta and CINC-3. Eur J Biochem 231: 306–311, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Singh G, Chaudry KI, Morrison MH, Chaudry IH. Tumor necrosis factor depresses gut absorptive function. Circ Shock 39: 279–284, 1993. [PubMed] [Google Scholar]
- 39.Souza DG, Bertini R, Vieira AT, Cunha FQ, Poole S, Allegretti M, Colotta F, Teixeira MM. Repertaxin, a novel inhibitor of rat CXCR2 function, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. Br J Pharmacol 143: 132–142, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takagi T, Yoshida N, Isozaki Y, Shimozawa M, Katada K, Manabe H, Hanada O, Kokura S, Ichikawa H, Naito Y, Okanoue T, Yoshikawa T. CV-11974, angiotensin II type I receptor antagonist, protects against ischemia-reperfusion injury of the small intestine in rats. Eur J Pharmacol 535: 283–290, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Vaday GG, Franitza S, Schor H, Hecht I, Brill A, Cahalon L, Hershkoviz R, Lider O. Combinatorial signals by inflammatory cytokines and chemokines mediate leukocyte interactions with extracellular matrix. J Leukoc Biol 69: 885–892, 2001. [PubMed] [Google Scholar]
- 42.Xu Y, Kumar D, Dyck JR, Ford WR, Clanachan AS, Lopaschuk GD, Jugdutt BI. AT(1) and AT(2) receptor expression and blockade after acute ischemia-reperfusion in isolated working rat hearts. Am J Physiol Heart Circ Physiol 282: H1206–H1215, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Yang S, Zheng R, Hu S, Ma Y, Choudhry MA, Messina JL, Rue LW 3rd, Bland KI, Chaudry IH. Mechanism of cardiac depression after trauma-hemorrhage: increased cardiomyocyte IL-6 and effect of sex steroids on IL-6 regulation and cardiac function. Am J Physiol Heart Circ Physiol 287: H2183–H2191, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Yilmaz EN, Vahl AC, van Rij GL, Vink GQ, Brom HL, Rauwerda JA. The effect of inhibition of renin-angiotensin system by valsartan during hypovolemic shock and low flow sigmoideal ischaemia in pigs. Cardiovasc Surg 11: 45–51, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Yu HP, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Mechanism of the nongenomic effects of estrogen on intestinal myeloperoxidase activity following trauma-hemorrhage: up-regulation of the PI-3K/Akt pathway. J Leukoc Biol 82: 774–780, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Yu HP, Chaudry IH. The role of estrogen and receptor agonists in maintaining organ function following trauma-hemorrhage. Shock. In press. [DOI] [PMC free article] [PubMed]
- 47.Yusof M, Kamada K, Gaskin FS, Korthuis RJ. Angiotensin II mediates postischemic leukocyte-endothelial interactions: role of calcitonin gene-related peptide. Am J Physiol Heart Circ Physiol 292: H3032–H3037, 2007. [DOI] [PubMed] [Google Scholar]