Abstract
Curcumin (diferulolylmethane) demonstrates profound anti-inflammatory effects in intestinal epithelial cells (IEC) and in immune cells in vitro and exhibits a protective role in rodent models of chemically induced colitis, with its presumed primary mechanism of action via inhibition of NF-κB. Although it has been demonstrated effective in reducing relapse rate in ulcerative colitis patients, curcumin's effectiveness in Crohn's disease (CD) or in Th-1/Th-17 mediated immune models of CD has not been evaluated. Therefore, we investigated the effects of dietary curcumin (0.1–1%) on the development of colitis, immune activation, and in vivo NF-κB activity in germ-free IL-10−/− or IL-10−/−;NF-κBEGFP mice colonized with specific pathogen-free microflora. Proximal and distal colon morphology showed a mild protective effect of curcumin only at 0.1%. Colonic IFN-γ and IL-12/23p40 mRNA expression followed similar pattern (∼50% inhibition at 0.1%). Secretion of IL-12/23p40 and IFN-γ by colonic explants and mesenteric lymph node cells was elevated in IL-10−/− mice and was not decreased by dietary curcumin. Surprisingly, activation of NF-κB in IL-10−/− mice (phospho-NF-κBp65) or in IL-10−/−;NF-κBEGFP mice (whole organ or confocal imaging) was not noticeably inhibited by curcumin. Furthermore, we demonstrate that IL-10 and curcumin act synergistically to downregulate NF-κB activity in IEC and IL-12/23p40 production by splenocytes and dendritic cells. In conclusion, curcumin demonstrates limited effectiveness on Th-1 mediated colitis in IL-10−/− mice, with moderately improved colonic morphology, but with no significant effect on pathogenic T cell responses and in situ NF-κB activity. In vitro studies suggest that the protective effects of curcumin are IL-10 dependent.
Keywords: inflammatory bowel disease, Crohn's disease, NF-κB
crohn's disease (CD), one of the two most common types of inflammatory bowel diseases (IBD), is a spontaneously relapsing, immunologically mediated disorder of the gastrointestinal tract that is characterized by intestinal inflammation and mucosal damage. Both environmental factors and genetic predispositions have been implicated in the pathogenesis of CD. However, the precise cause remains poorly understood. Current research suggests that an inappropriate and persistent immune response against commensal intestinal bacterial flora plays a pivotal role in the pathogenesis of chronic IBD (36).
Balance between an adequate immune response against invasive pathogens and a controlled immune response against commensal microbiota is essential for maintaining intestinal integrity. Mucosal T cells orchestrate the immune response and maintain intestinal homeostasis. Intestinal inflammation is perpetuated by the release of proinflammatory cytokines and other soluble mediators, which determine the orientation of the immune response toward Th-1, Th-17, or Th-2 pathway. CD is primarily Th-1- and Th-17-mediated inflammation (14, 32) with increased LPS-mediated IL-12 production, which in turn elicits secretion of IFN-γ and IL-2 by Th-1 cells. This is associated with increased production of IL-23 (37), a cytokine required for maintenance of Th-17 cell lineage, differentiating under control of IL-6 and TGF-β (45). IL-21 has also been implicated in the pathogenesis of CD and is thought to participate in generation and maintenance of both Th-1 and Th-17 mucosal immune responses (13). Effector Th-1 cells with cytotoxic functions have been found in the intestinal lamina propria of patients with CD (29).
Nuclear factor-κB (NF-κB) is a dominant regulator of expression of numerous genes, many of which play critical roles in initiation and perpetuation of inflammation, as well as in programmed cell death (apoptosis). Excessive or inappropriate activation of mucosal NF-κB has been described both in ulcerative colitis (UC) and CD patients (33, 38), as well as in animal models of IBD, thus becoming an attractive target for potential therapeutic intervention. Despite the known role of NF-κB in blocking apoptosis in the intestinal epithelial cells (7, 12) and potential detrimental effects of NF-κB blockade (11), specific inhibition of NF-κB by p65 antisense oligonucleotides in mouse models of colitis is beneficial (27, 30, 31, 44). In addition, drugs used as a first-line therapy of IBD such as glucocorticoids or aminosalicylates all modulate NF-κB activation (3, 22).
Curcumin is another example of a nonspecific inhibitor of NF-κB with potent anti-inflammatory effects. Curcumin is the active component of turmeric, the dried rhizome of Curcuma longa Linn. Its effects on the immune responses (both innate and adaptive) have generated considerable attention in the past decade (17). Numerous in vitro studies on biological activity of curcumin demonstrate a very wide range of effects with its presumed primary, though not only, mechanism of action being inhibition of NF-κB (17). These effects have been reported in many cell types, including intestinal epithelial cells (20), macrophages, dendritic cells, B cells, and T cells (17). The potential usefulness of curcumin in the treatment of IBD was suggested by studies in chemically induced rodent models of colitis (19, 35, 46, 48, 49, 51, 52). Curcumin has also been demonstrated as an effective agent in maintenance therapy of UC (15), and encouraging, though not conclusive, data were obtained in a small clinical trial in CD patients (16). Our initial observations on the efficacy of curcumin in TNBS-induced mouse colitis indicated that dietary curcumin significantly increased survival, prevented weight loss, and normalized disease indexes in BALB/c mice, which exhibit a mixed Th1-Th2 response, but not in Th-1-driven colitis in NKT-deficient SJL/J mice (6). These observations suggest that curcumin's potency may be limited in Th-1-mediated experimental colitis and in CD. Since curcumin has not been previously tested in more chronic immune-mediated models of IBD, we tested its efficacy in an established animal model of CD offered by IL-10−/− mice, in which IL-10 deficiency allows for the development of unrestrained activation of a Th-1-mediated immune response (50). This model represents a conjunction of several key factors implied in the pathogenesis of IBD, which include genetic predisposition and loss of immune tolerance to colonic commensal bacteria. Importantly, IL-10−/− mice demonstrate persistent activation of NF-κB (as judged by phospho-RelA expression) in colonic epithelial cells after monoassociation of germ-free mice with Enterococcus faecalis, thus making it a particularly attractive model to test curcumin efficacy in CD (34).
Here, we describe that in IL-10−/− mouse model of colitis induced by colonization of germ-free mice with specific pathogen-free microbiota, dietary curcumin has mild effects on mucosal damage, paradoxically limited to the lowest of the three curcumin concentrations tested (0.1%). All tested concentrations of dietary curcumin failed to inhibit bacterial antigen-stimulated pathogenic T cell responses and did not attenuate NF-κB activation in IL-10−/−;NF-κBEGFP transgenic mice. In vitro analysis demonstrated that IL-10 and curcumin might be acting synergistically to downregulate NF-κB activity and IL-12/23p40 production. The lack of efficacy of curcumin in Th-1/Th-17-mediated colitis in IL-10-deficient mice and reciprocal dose dependency may need to be taken into consideration in planning further clinical trials with CD patients.
MATERIALS AND METHODS
Curcumin.
Curcumin, 98.05% pure and free of contaminating curcuminoids (demethoxy-curcumin and bis-demethoxy-curcumin), was obtained from ChromaDex (Irvine, CA) and tested for its efficacy in vitro with LPS-treated RAW 264.7 mouse macrophages. Curcumin was incorporated into NIH-31 modified open formula at 0.1, 0.5, and 1% and pelleted by Harlan Teklad (Madison, WI). HPLC analysis indicated no significant loss of curcumin during the incorporation and pelleting process. In a control experiment, 129Sv/Ev mice received control and curcumin-supplemented diets for 5 days to monitor food consumption. At the end of the 5-day period, animals were euthanized; colonic content was collected, weighed, and resuspended in HPLC-grade methanol at 1:2 ratio (wt/vol). Samples were sonicated with a probe sonicator and allowed to rotate at room temperature for 20 min. After two centrifugations (12,500 g, 10 min), the supernatants were collected and analyzed for curcumin concentration by HPLC. The analyses were performed by GAAS (Tucson, AZ) with Agilent 1200RR HPLC instrument (Agilent Technologies, Santa Clara, CA) and Chemstation for LC 3D software (Agilent).
Experimental animals.
Specific pathogen-free (SPF) wild-type (WT) 129/SvEv mice and germ-free IL-10−/− mice on the same genetic background were obtained from the National Gnotobiotic Rodent Resource Center at the University of North Carolina, Chapel Hill. Germ-free IL-10−/− mice were transferred to the SPF facility and kept in sterile cages 2 days prior to being colonized with SPF fecal bacteria. Treatment with control or curcumin supplemented diets (0.1, 0.5, 1%, color coded) was initiated at the time of transfer and continued until euthanasia (14 days postcolonization). The 14 days of bacteria colonization allowed for development of mild to moderate colitis in SPF-associated previously germ-free IL-10−/− mice (39) (see results). Germ-free NF-κBEGFP transgenic (TG) mice expressing the enhanced green fluorescent protein (EGFP) under the transcriptional control of NF-κB cis elements on WT or IL-10−/− background (23) were similarly SPF associated, fed control diet or diet supplemented with 1% curcumin, and euthanized 28 days after colonization. Additional WT 129/SvEv mice and IL-10−/− mice (originally transferred from SPF facility at University of North Carolina) were obtained from a conventional animal facility at the University of Arizona Health Sciences Center. Sentinel mice were routinely monitored and determined as free from common murine pathogens (MHV, MPV, MVM, TMEV, Mycoplasma pulmonis, Sendai, EDIM, MNV, ecto- and endoparasites). Animal use protocols were approved by the Institutional Animal Care and Use Committee, North Carolina State University and by the University of Arizona Animal Care and Use Committee.
Histology and scoring.
Proximal and distal colons from WT and IL-10−/− mice were harvested and fixed in 10% neutral buffered formalin (Fisher Scientific, Tustin, CA). Fixed tissues were then embedded in paraffin, and 5-μm-thick tissue cuts were stained with hematoxylin and eosin (H&E) for light microscopic examination. Sections were graded by a veterinary pathologist blinded to the study design according to previously published criteria (24).
Immunohistochemistry.
Sections of proximal and distal colon were prepared as above. After deparaffinization and rehydration, antigen retrieval was performed by heating slides in citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0). After washing in PBS, residual endogenous peroxidase activity was quenched by incubation in 3% H2O2 in water for 10 min. Slides were then incubated for 1 h in normal goat serum blocking buffer (KPL, Gaithersburg, MD). Next, sections were incubated with a primary antibody against phospho-NF-κB p65 (RelA) (Ser276; 1/50, Cell Signaling Technology, Danvers, MA) in 1 × PBS with 1% BSA overnight at 4°C. After three washes in PBS, slides were incubated with biotinylated secondary antibody and streptavidin-peroxidase complex according to the manufacturer's recommendation (KPL). Slides were then incubated with 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA) and mounted with Vectamount medium (Vector Laboratories). Slide examination was performed independently in a blind manner by two experienced scientists using a Zeiss Axioplan microscope (Carl Zeiss MicroImaging, Thornwood, NY). Images were captured with Nikon Digital Sight DS-Fi1 camera and NIS-Element software (Nikon Instruments, Melville, NY). Staining was scored by counting the number of phospho-RelA positive cells per high-power field (×200 magnification). A total of five fields in each section were analyzed for all experimental animals.
EGFP imaging (whole organ and confocal analysis).
SPF-associated IL-10−/−;NF-κBEGFP and control IL-10wt/wt;NF-κBEGFP mice were euthanized, and the intestine dissected and immediately imaged via a charge-coupled device camera in a light-tight imaging box with a dual-filtered light source and emission filters specific for EGFP (LT-99D2 Illumatools, Lightools Research, Encinitas, CA). Identical exposure times were used to capture images within each experiment. For confocal microscopy on living tissues, colonic segments were placed on the stage of a Leica SP2 Upright Laser Scanning Confocal Microscope without further processing or fixation. EGFP was excited at 495-nm wavelength, and images were acquired using detection filters specific for the EGFP emission spectrum. Images were analyzed with the Leica SP2 Laser Scanning Confocal Imaging Software (Leica).
Real-time RT-PCR.
Real-time RT-PCR was used to evaluate colonic expression of IFN-γ and IL-12/23p40 mRNA. Total RNA was isolated from mouse colon by use of TRIzol reagent (Invitrogen, Carlsbad, CA) and its integrity was confirmed by denaturing agarose gel electrophoresis and calculated densitometric 28S/18S ratio. Total RNA (250 ng) was reverse-transcribed by using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Subsequently, 20 μl of the PCR reactions were set up in 96-well plates containing 10 μl of 2 × IQ Supermix (Bio-Rad), 1 μl TaqMan primer/probe set (ABI, Foster City, CA), 2 μl of the cDNA synthesis reaction (10% of RT reaction), and 7 μl of nuclease-free water. Reactions were run and analyzed on a Bio-Rad iCycler iQ real-time PCR detection system. Cycling parameters were determined and resulting data were analyzed by the comparative CT method as means of relative quantification, normalized to an endogenous reference (TATA Box Bonding Protein, TBP) and relative to a calibrator (normalized CT value obtained from control mice) and expressed as 2−ΔΔCT (Applied Biosystems User Bulletin no. 2: Rev. B “Relative Quantification of Gene Expression”).
Colonic explant culture.
Colon fragments were prepared as described previously (39). Briefly, colon segments were flushed with phosphate-buffered saline (PBS) to remove fecal contents, opened lengthwise, and shaken vigorously for 30 min in PBS. Tissue was then apportioned to wells (50–100 mg of tissue per well) of a 24-well tissue culture plate (Corning Costar, Lowell, MA) and cultured in 1 ml of complete RPMI 1640 medium containing 5% heat-inactivated fetal bovine serum, penicillin, streptomycin, and amphotericin B (all from Invitrogen). Tissues were incubated at 37°C for 18 h, and supernatants were collected and stored at −80°C until being assayed.
MLN cell preparation and stimulation with cecal antigen.
Cecal bacterial lysates were obtained by collecting cecal content in RPMI medium to a ratio of 2 ml/g, and 100 mM of MgCl2 and DNAse (100 μg/ml) was added to the RPMI medium (1:4 ratio) and homogenized with glass beads (0.1 mm) on a mini bead beater. After centrifugation, supernatants were collected and tested for protein concentration. Mesenteric lymph nodes (MLN) were removed and single cell suspensions were prepared by gentle teasing. Cells were washed and resuspended in complete RPMI 1640 medium supplemented with 5% heat-inactivated fetal calf serum (Irvine Scientific, Santa Ana, CA); 2 mM l-glutamine, 1 mM sodium pyruvate, 0.05 mM 2-mercaptoethanol, 50 ml of gentamicin (Sigma, St. Louis, MO); and penicillin (100 U/ml), streptomycin (100 ml), and amphotericin B (0.25 ml) (Invitrogen). MLN cells were cultured at 106 cells/ml in 96-well flat-bottom plates (Corning Costar, Cambridge, MA) and stimulated with cecal antigen extract at a concentration of 10 μg/ml for 72 h. Cell culture supernatant was collected and stored at −80°C until being assayed.
Cell culture.
Mode-K cells, murine intestinal epithelial cells, were cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 mM sodium pyruvate, and nonessential amino acids. Cells were cultured at 37°C in a 95% air-5% CO2 atmosphere and passaged at 80% confluency. Media and other reagents used for cell culture were purchased from Invitrogen.
Mode K cell transfection and treatment.
NF-κB-inducible reporter plasmid (pNiFty2-Luc) was purchased from InvivoGen (San Diego, CA). The transfection was performed using TransIT-LT1 reagent (Mirus, Madison, WI) according to the manufacturer's protocol. Stably transfected cells were cultured in the presence of Zeocin at 150 μg/ml (Invitrogen). Cells were seeded in 24-well plates and were treated with recombinant murine IL-10 (Peprotech, Rocky Hill, NJ), recombinant murine IL-1β (Peprotech), or curcumin individually or in combination. After cell lysis, reporter gene assay was performed according to manufacturer's instruction (Luciferase Reporter assay system; Promega, Madison, WI). Luciferase activity was measured with a tube luminometer (Femtomaster FB12, Berthold Detection System, Pforzheim, Germany) and expressed as normalized luciferase activity relative to protein concentration (BCA assay; Pierce, Rockford, IL).
Crude membrane preparation and cell surface protein extraction.
Crude membranes were prepared from Mode-K according to Tse et al. (47). Cell surface biotinylation and protein extraction were performed by use of the cell surface protein isolation kit (Pierce) according to the manufacturer's instructions.
Splenocyte preparation and treatment.
Spleens were removed from wild-type or IL-10−/− mice housed in conventional facility and single cell suspensions were prepared by gentle teasing. Cells were washed and resuspended in complete medium (RPMI 1640, Invitrogen) supplemented with 10% heat-inactivated fetal calf serum (Gemini Bio-Products, West Sacramento, CA), 2 mM l-glutamine, and penicillin (100 U/ml)-streptomycin (100 μg/ml) (Invitrogen). Red blood cells were lysed in a hypotonic buffer (BD Biosciences, San Jose, CA) and washed twice in complete medium. Cells were cultured at a concentration of 106 cells/ml in 96-well flat-bottom plates (BD Bioscience). Splenocytes were stimulated in vitro with LPS (Lipopolysaccharide from Escherichia coli O55:B5, Calbiochem, Gibbstown, NJ) at the concentration of 10 ng/ml and treated with increasing concentration of curcumin (from 0.5 to 10 μM) for 24 h. Cell culture supernatant was collected and stored at −80°C until being assayed.
DC2.4 dendritic cell treatment.
A bone marrow-derived, GM-CSF-differentiated, and myc- and raf-transduced murine dendritic cell line DC2.4 was kindly provided by Dr. Kenneth L. Rock (University of Massachusetts Medical School, Worcester, MA) (42). Cells were cultured in RPMI supplemented with 10% heat-inactivated fetal calf serum (Gemini Bio-Products), 100 U/ml penicillin, 100 μg/ml streptomycin, and nonessential amino acids (Invitrogen). Cells were cultured at 37°C in a 95% air-5% CO2 atmosphere and passaged at 60–70% confluency. Dendritic cells were treated with recombinant murine IL-10 (Peprotech) for 1 h and with curcumin for 10 min prior to stimulation by LPS (Calbiochem) at 100 ng/ml. After 24 h, cell culture supernatant was collected and stored at −80°C.
Cytokine assays.
IL-12/23p40 and IFN-γ concentrations in the supernatant of colonic explant and mesenteric lymph nodes cultures were measured by enzyme-linked immunosorbent assay (ELISA; eBioscience, San Diego, CA) according to the manufacturer's protocols.
Statistical analysis.
Statistical significance was determined by the ANOVA followed by Fisher paired least-significant difference (PLSD) post hoc test with StatView software package v.4.53 (SAS Institute, Cary, NC). Data are expressed as means ± SE.
RESULTS
Food consumption and colonic curcumin concentration in mice fed experimental diets.
Mice fed curcumin-supplemented diets exhibited slightly lower daily food intake compared with mice fed control diet (12, 14, and 18% decrease for 0.1, 0.5, and 1% curcumin diets, respectively). HPLC analysis in the colonic content indicated that significant amounts of unmetabolized and undegraded curcumin reached colonic lumen. Measured curcumin concentrations were 1.54 ± 0.2 mM (for 0.1% dietary curcumin), 5.23 + 0.44 mM (for 0.5%), and 7.54 + 0.3 mM (for 1.0%). No detectable amounts of curcumin were documented for the control diet.
Curcumin has a moderate protective effect on colonic morphology only at 0.1%.
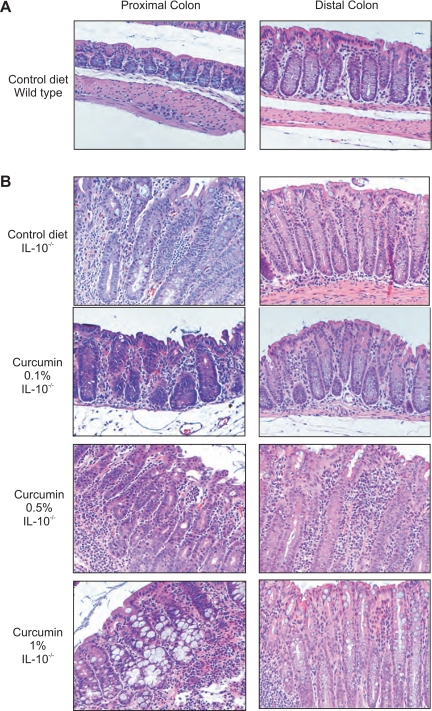
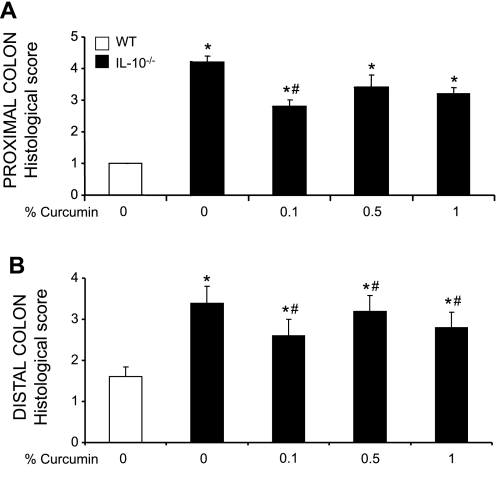
Germ-free IL-10−/− mice were transferred to SPF conditions and simultaneously started on control or curcumin-supplemented diets (0.1, 0.5, or 1%). Two days later they were colonized with SPF flora and euthanized 14 days postcolonization. Genetically matched WT mice fed control diet maintained in SPF conditions were used as baseline controls. Morphology of the proximal and distal colon was analyzed. Both proximal and distal colon of the control WT mice demonstrated a very mild lymphocytic infiltration, typical for the “physiological inflammation” observed in murine colon under normal conditions (Fig. 1A). Consistent with previously published data (4), histological analysis of the colon of IL-10−/− mice showed a characteristic pattern of moderate inflammation associated with crypt hyperplasia, lymphocytic and neutrophilic infiltrations, and occasionally mucosal ulceration (Fig. 1B). Feeding diet supplemented with curcumin at 0.1% significantly reduced the signs of inflammation, reducing crypt hyperplasia and lymphocytic and neutrophilic infiltration. At 0.5 and 1%, dietary curcumin did not improve the histological signs of colonic inflammation (Fig. 1B). These microscopic observations were further confirmed by histological scoring performed by an unbiased pathologist (Fig. 2).
Fig. 1.
Histology of the proximal and distal colon or wild type (WT) and IL-10−/− mice fed control or curcumin-supplemented diets. A: hematoxylin and eosin (H&E) staining of proximal and distal colon from WT mice fed control diet. B: H&E staining of proximal and distal colon of IL-10−/− mice fed control diet or diets supplemented with increasing concentration of curcumin 0.1, 0.5, and 1%. Magnifications ×200.
Fig. 2.
Histological scoring of the proximal (A) and distal colon (B) performed by a veterinary pathologist blinded to the study design according to previously published criteria (24). Values represent the mean of 5 WT animals and 7 IL-10−/− animals per group of treatment. Statistical analysis was performed with ANOVA followed by Fisher paired least-significant difference (PLSD) post hoc test. *Statistically significant differences (P ≤ 0.05) between control (WT, no curcumin; open bars) and respective dietary group of IL-10−/− mice. #Statistical difference between IL-10−/− mice on control diet and IL-10−/− mice on respective curcumin-supplemented diets.
Colonic expression of IFN-γ and IL-12/23p40 mRNA is decreased by 0.1% curcumin, but ex vivo cytokine secretion by colonic explants remains unaffected.
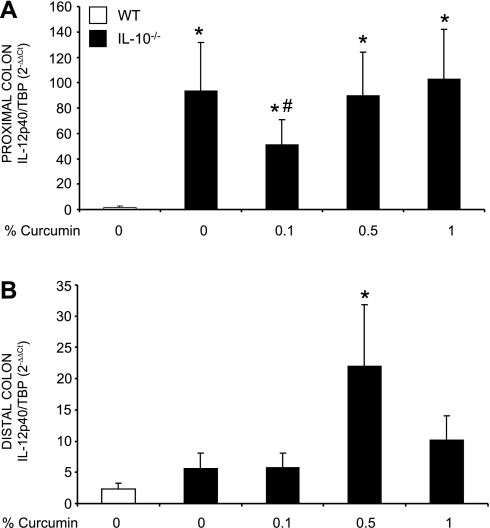
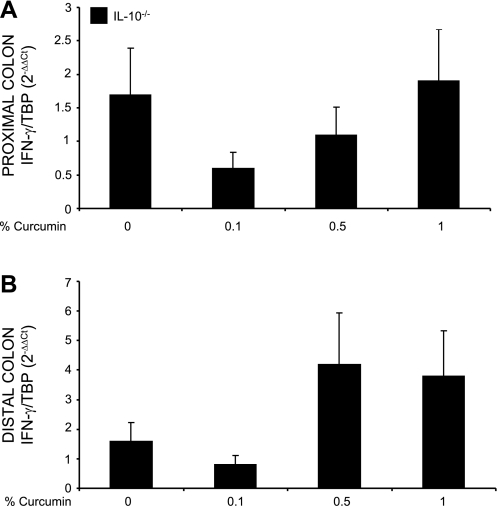
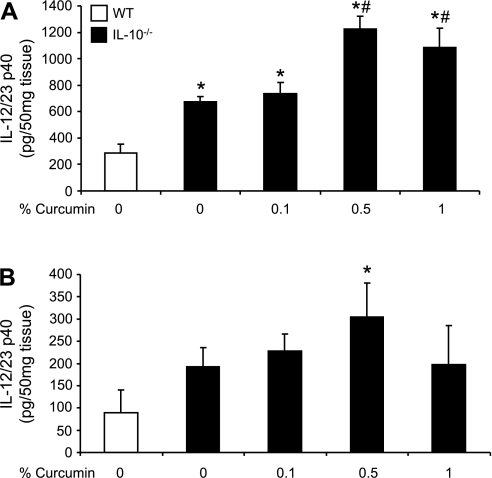
We investigated whether the expression of proinflammatory cytokines corresponded with the histological observations. IL-12/23p40 and IFN-γ were chosen as key cytokines implicated in the Th-1 pathway. We first evaluated the effect of dietary curcumin on the colonic expression of IL-12/23p40 and IFN-γ mRNA by real-time RT-PCR. The effects of dietary curcumin on steady-state mRNA expression of the two cytokines paralleled those observed by colonic histology. Curcumin at 0.1% significantly reduced IL-12/23p40 expression only in the proximal segment (Fig. 3), whereas IFN-γ gene expression was reduced by the lowest dietary concentration of curcumin in both proximal (Fig. 4A) as well as in the distal colon (Fig. 4B). However, higher doses of curcumin (0.5 or 1%) had no significant effect on transcriptional activation of IL-12/23p40 and IFN-γ genes in SPF-colonized IL-10−/− mice. Intriguingly, when cytokine secretion was evaluated by ELISA in colonic explant culture supernatant (without curcumin in the medium), elevated production of IL-12/23p40 (Fig. 5A) and IFN-γ (Fig. 5B) observed in IL-10−/− mice on control diet was not affected by dietary curcumin. In fact, colonic tissues obtained from IL-10−/− mice fed higher concentrations of curcumin (0.5 and 1%) produced significantly more IL-12/23p40 and IFN-γ than tissues from IL-10−/− mice fed control diet when cultured without the continuous presence of curcumin in the medium (Fig. 5).
Fig. 3.
Real-time PCR analysis of IL-12/23p40 mRNA expression in the proximal (A) and distal colon (B). Data were normalized to TATA-box binding protein (TBP) mRNA as an internal control and calculated by the ΔΔCT method (see materials and methods) with WT mice fed control diet (open bars) used as calibrator. Statistical analysis was performed with ANOVA followed by Fisher PLSD post hoc test. *Statistically significant differences (P ≤ 0.05) between control (WT, no curcumin; open bars) and respective dietary group of IL-10−/− mice. #Statistical difference between IL-10−/− mice on control diet and IL-10−/− mice on respective curcumin-supplemented diets.
Fig. 4.
Real-time PCR analysis of IFN-γ mRNA expression in the proximal (A) and distal colon (B). Data were normalized to TBP mRNA as an internal control and calculated by the ΔΔCT method (see materials and methods). Since expression of IFN-γ in the colon of WT mice was extremely low to nondetectable, IL-10−/− mice fed control diet (0% curcumin) were used as calibrator. Statistical analysis was performed with ANOVA followed by Fisher PLSD post hoc test. No statistically significant differences (P ≤ 0.05) were seen between IL-10−/− mice on control diet and respective group of IL-10−/− mice fed curcumin-supplemented diets (ANOVA P = 0.24 and P = 0.06 for proximal and distal colon, respectively).
Fig. 5.
Secretion of IL-12/23p40 (A) and IFN-γ (B) by cultured colonic explants obtained from wild-type (WT) or IL-10−/− mice fed control or curcumin-supplemented diets. Colonic tissues were cultured for 18 h, and concentration of the 2 cytokines accumulated in the medium was measured by ELISA. Values represent mean concentrations ± SE expressed pg/ml per 0.05 g of tissue. Duplicate measurements were performed with tissues obtained from each individual animal. Statistical analysis was performed with ANOVA followed by Fisher PLSD post hoc test. *Statistically significant differences (P ≤ 0.05) between control (WT, no curcumin; open bars) and respective dietary group of IL-10−/− mice. #Statistical difference between IL-10−/− mice on control diet and IL-10−/− mice on respective curcumin-supplemented diets.
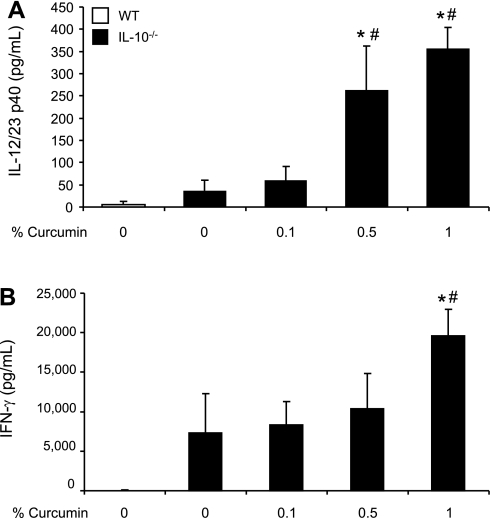
Cecal antigen-stimulated secretion of IL-12/23p40 and IFN-γ by MLN cells is moderately increased in colitic IL-10−/− mice and is not inhibited by dietary curcumin.
To further investigate our observations, we asked whether Th-1-related cytokines produced by immune cells selected from secondary draining lymphoid organs were affected by dietary curcumin. MLN-derived immune cells were cultured for 72 h under the stimulation with cecal bacterial antigens prepared from cecum of SPF mice from the same mouse facility. By ELISA measurements, IFN-γ and IL-12/23p40 concentrations were increased in IL-10−/− mice compared with control WT mice, although without reaching statistical significance (Fig. 6). Dietary supplementation with curcumin did not result in inhibition of cytokine secretion. On the contrary, similar to the results obtained with colonic explants, MLN cells isolated from mice fed higher concentrations of curcumin (0.5 or 1%) secreted more IFN-γ and IL-12/23p40 upon stimulation with cecal antigen extract (Fig. 6). These findings suggest that whereas continuous presence of curcumin in the diet moderately inhibits colonic expression of IFN-γ and IL-12/23p40, activation of the immune responses remains unaffected or is restored in ex vivo assays.
Fig. 6.
Secretion of IL-12/23p40 (A) and IFN-γ (B) by mesenteric lymph node (MLN) cells obtained from WT or IL-10−/− mice fed control or curcumin-supplemented diets and stimulated with cecal antigen extract. MLN cells were stimulated by 10 μg/ml of cecal antigen [purified from wild-type, specific pathogen-free (SPF) mice from the same facility] for 72 h of culture. Duplicate ELISA measurements were performed for each animal. Statistical analysis was performed with ANOVA followed by Fisher PLSD post hoc test. *Statistically significant differences (P ≤ 0.05) between control (WT, no curcumin; open bars) and respective dietary group of IL-10−/− mice. #Statistical difference between IL-10−/− mice on control diet and IL-10−/− mice on respective curcumin-supplemented diets.
Dietary curcumin has limited effect on the activation of NF-κB in IL-10−/− mice.
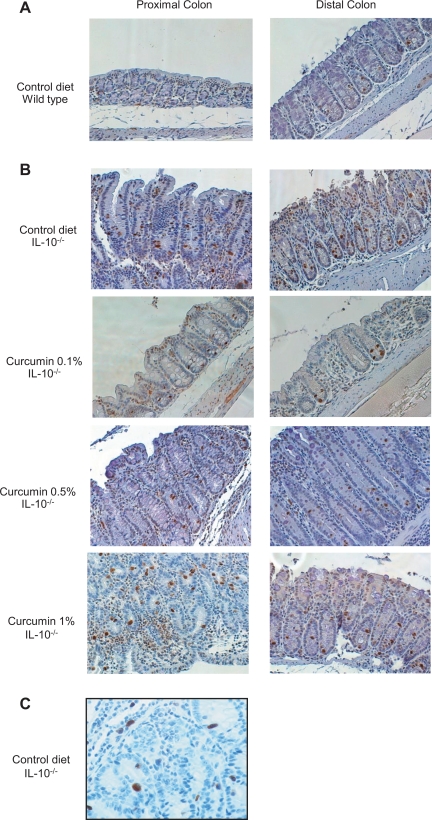
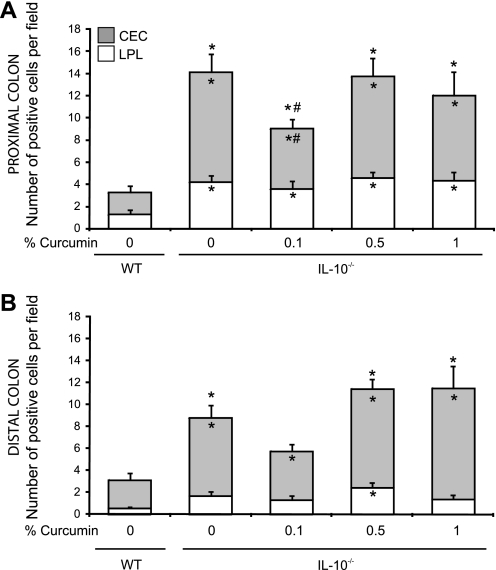
On the basis of a large number of in vitro studies (20, 21), as well as several in vivo observations (10, 18, 35, 46), curcumin has been considered as a nonspecific inhibitor of NF-κB. It is believed to exert its effects largely through inhibiting NF-κB-dependent transcriptional activation of proinflammatory factors. Considering the limited effects of dietary curcumin on colitis in IL-10−/− mice, particularly the lack of inhibitory effects on proinflammatory cytokines production by colonic explants and by MLN cells, we investigated the effect of curcumin on in vivo NF-κB activation in the colon of control and SPF-associated IL-10−/− mice. We took two complementary approaches, different from the typically employed gel mobility shift assays reported by others (18, 35, 48) or the less quantitative total p65 staining (46). Considering the crucial role of the p65 subunit in NF-κB signaling pathway, we first investigated its activation by phosphorylation at Ser276 by immunohistochemistry. As anticipated, phosphorylation of p65 was significantly enhanced in both the proximal and distal colon of IL-10−/− mice compared with wild-type mice (Fig. 7), with both epithelial and infiltrating immune cells positively stained (Figs. 7C and 8). At this relatively early stage of colitis (14 days postcolonization), NF-κB activation was seen predominantly in colonic epithelial cells, although the number of pSer276-p65 positive cells in the lamina propria was also significantly elevated (Fig. 8). Consistent with decreased expression of IL-12/23p40 and IFN-γ, 0.1% of dietary curcumin reduced the number of phospho-p65-positive colonic epithelial cells, especially in the proximal colonic segments (Fig. 8, A and B). However, higher concentrations of dietary curcumin (0.5 and 1%) had no effect (Fig. 7 and 8). Moreover, neither concentration of curcumin affected NF-κB activation in the subepithelial compartment (depicted as open bars in Fig. 8).
Fig. 7.
Immunohistochemical analysis of the effects of dietary curcumin on NF-κB activation (phospho-Ser276p65) in the proximal and distal colon of IL-10−/− mice in situ. Colonic tissues from wild-type mice fed control diet (A) and IL-10−/− fed control or curcumin-supplemented diets (B) were collected 14 days postcolonization. Brown staining indicates cells positive for phospho-Ser276p65 subunit of NF-κB. Magnification ×200. C: proximal colonic tissue from colitic IL-10−/− mouse on control diet demonstrating phospho-Ser276p65 positive nuclear brown staining in both epithelial cells and immune cells of the lamina propria (magnification ×400).
Fig. 8.
Scoring summary of phospho-Ser276p65-positive cells in the proximal (A) and distal colon (B) of WT or IL-10−/− mice fed control or curcumin-supplemented diets. Staining was scored by counting the number of phospho-Ser276p65-positive cells; colonic epithelial cells (CEC; open bars) and lamina propria (LPL; shaded bars) per high-power field of vision (magnification ×200). In total 5 randomly selected fields per section were analyzed for each mouse. Statistical analysis was performed with ANOVA followed by Fisher PLSD post hoc test. *Statistically significant differences (P ≤ 0.05) between control (WT, no curcumin) and respective dietary group of IL-10−/− mice. #Statistical difference between IL-10−/− mice on control diet and IL-10−/− mice on respective curcumin-supplemented diets. Differences are indicated for total number of phospho-Ser276p65 positive cells (CEC+LPL; outside the bars), in CEC (inside shaded bars), and in LPL (inside open bars).
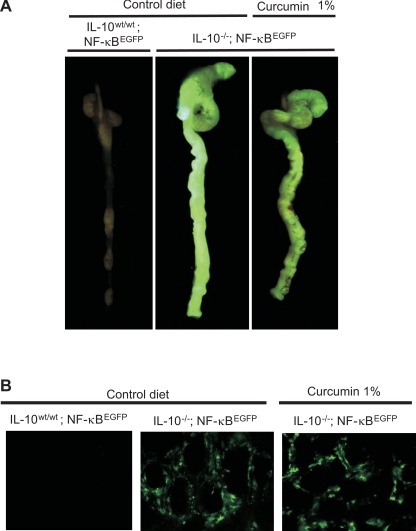
To study activation of NF-κB in a more functional setting, and at a later time postcolonization (28 days), when primarily lamina propria cells exhibit enhanced NF-κB activity (23) we employed TG mice expressing EGFP from an NF-κB-inducible promoter. NF-κBEGFP TG mice were maintained germ-free on wild-type or IL-10−/− background, thus allowing us to study the activation of NF-κB in the context of bacterially induced chronic inflammation and spontaneous colitis by monitoring EGFP-related fluorescence in situ rather than by in vitro analyses. Following transfer to SPF conditions, macroscopic analysis of EGFP fluorescence in the intact colon demonstrated that NF-κB activation was significantly increased in IL-10−/− compare to WT mice (Fig. 9A). Consistent with phospho-p65 staining, histological analysis and cytokine production, in the whole organ fluorescence analysis, dietary curcumin at 1% did not demonstrate a significant or easily noticeable attenuation of the EGFP expression and by the same token did not affect NF-κB-mediated gene transcription. In a more unequivocal fashion, confocal microscopy on whole colonic tissues indicated no effect of dietary curcumin on NF-κB activity in the lamina propria compartment (Fig. 9B).
Fig. 9.
Evaluation of enhanced green fluorescent protein (EGFP) fluorescence in the colon of IL-10wt/wt/NF-κBEGFP and IL-10−/−/NF-κBEGFP mice fed control or 1% curcumin-supplemented diet. Germ-free mice were colonized with SPF flora and euthanized 28 days postcolonization. A: colon and cecum were resected and EGFP fluorescence was imaged as described in materials and methods. B: confocal imaging of colonic mucosa immediately after dissection was performed as described in materials and methods, with predominantly interstitial cells being EGFP positive and thus likely representing LPL mononuclear cells.
IL-10 potentiates the effect of curcumin to downregulate IL-12/23p40 production by splenocytes.
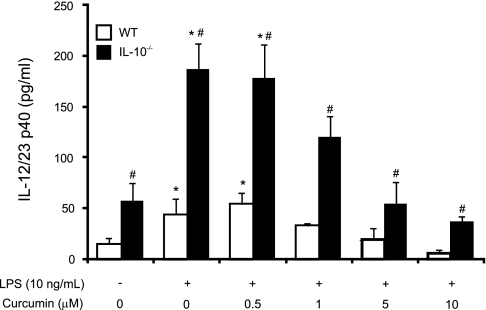
To explain the limited protective potential of curcumin in IL-10−/− mice, we further investigated the effect of curcumin on the production of IL-12/23p40 by LPS-stimulated splenocytes from WT or IL-10−/− mice. WT and IL-10−/− splenocytes were pretreated with curcumin (0.5–10 μM) for 2 h prior to 24 h stimulation by LPS (10 ng/ml). IL-12/23p40 production was assayed by ELISA. As anticipated, splenocytes from IL-10-deficient mice exhibited enhanced secretion of IL-12/23p40 protein under both unstimulated and LPS-induced conditions (Fig. 10). Curcumin inhibited the production of IL-12/23p40 in a dose-dependent manner in both WT and IL-10−/− splenocytes, although in the case of IL-10−/− cells, curcumin was unable to reduce the cytokine secretion to the basal levels observed in wild-type, unstimulated cells (Fig. 10). These results were suggestive that in the IL-10−/− mice, the limited potency of curcumin can be partially explained by the cooperative anti-inflammatory effects of the compound with IL-10.
Fig. 10.
Inhibition of IL-12/23p40 secretion in LPS-stimulated splenocytes from WT or IL-10−/− mice by curcumin. Splenocytes from WT and IL-10−/− mice were treated with curcumin (0.5 to 10 μM) for 2 h prior to LPS stimulation (10 ng/ml) for 24 h. IL-12/23p40 concentration in the culture supernatant was determined by ELISA. *Statistically significant differences (P ≤ 0.05) between control cells (no LPS, no curcumin) and LPS or LPS/curcumin-treated cells (within respective genotype). #Statistical difference in IL-12/23p40 secretion between wild-type and IL-10 deficient splenocytes (within respective treatment group).
Curcumin and IL-10 act synergistically to inhibit NF-κB activity in intestinal epithelial cells.
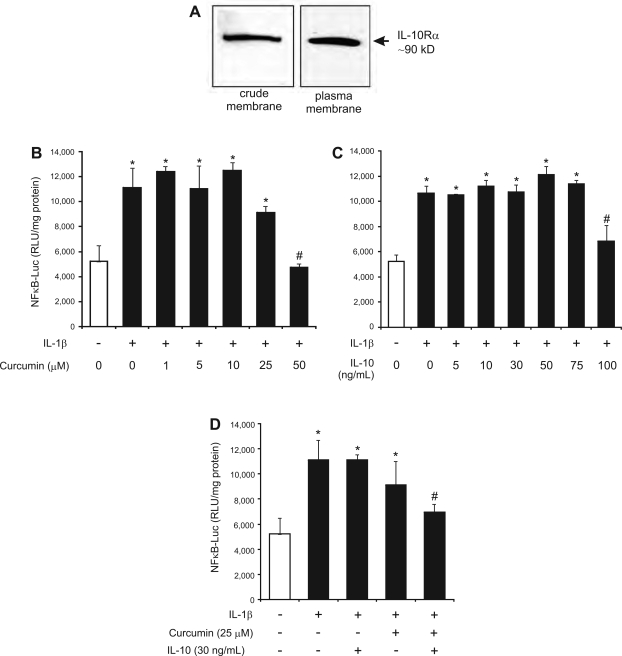
In support of the above hypothesis, two earlier reports indicated that curcumin increases expression of IL-10 in chemically induced colitis in rats (18, 52). Moreover, Al-Ashy et al. (1) recently described IL-10-mediated inhibition of IL-1β-induced NF-κB activity and COX-2 expression in Mode-K mouse intestinal epithelial cells. In macrophages, Zhou et al. (53) described IL-10 inhibition of LPS-stimulated IL-12p40 transcription at the chromatin structure level. We therefore hypothesized that curcumin may exert its anti-inflammatory effects partially through an IL-10-dependent pathway. To test for the possible synergistic activities of curcumin and IL-10 in inhibiting NF-κB activation, we utilized Mode-K cells stably transfected with a NF-κB inducible reporter plasmid (pNiFty2-Luc). First, we verified that IL-10 receptor (IL-10Rα), a ∼90-kDa protein, was expressed in Mode-K cells and present at the cytoplasmic membrane (Fig. 11A). Mode-K cells were then stably transfected with an NF-κB inducible reporter plasmid (pNiFty2-Luc). We determined suboptimal concentrations of curcumin or IL-10 administered individually by testing the effects of increasing concentrations of these two factors on NF-κB activity induced by IL-1β. IL-1β was recently described as a potent inducing factor of NF-κB in Mode-K cells (1). Cells were pretreated for 1 h with IL-10 (5–100 ng/ml) or for 10 min with curcumin (1–50 μM) prior to 6 h treatment with IL-1β (10 ng/ml). In our experimental settings, 25 μM of curcumin or 30 ng/ml of IL-10 alone were not sufficient to significantly inhibit NF-κB and luciferase activity (Fig. 11, B and C). However, when cells were pretreated with curcumin and IL-10 in combination (25 μM and 30 ng/ml, respectively) we observed a significant inhibition of NF-κB activity, which was reduced to the baseline level (Fig. 11D). This novel observation may partially account for limited effects of dietary curcumin on NF-κB activation in IL-10-deficient mice.
Fig. 11.
Synergistic inhibition of IL-1β-induced NF-κB activity by IL-10 and curcumin in stably transfected Mode-K cells. Mode-K cells transfected with NF-κB-inducible reporter plasmid (pNiFty2-Luc) were determined to express detectable amounts of IL-10 receptor as judged by Western blotting with crude cell membranes and on the cell surface as analyzed by cell surface biotinylation (A). Cells were pretreated with or without increasing concentration of curcumin or murine IL-10 and then treated with IL-1β (10 ng/ml) for 6 h. Curcumin remained ineffective in concentrations 1–25 μM (B) whereas IL-10 significantly inhibited NF-κB activity only at 100 ng/ml (C). Combination of curcumin and IL-10 in suboptimal concentrations (25 μM and 30 ng/ml, respectively) resulted in significant inhibition of NF-κB (D). Relative luminescence unit (RLU) corresponding to the luciferase activity was normalized to protein concentration. Three independent experiments were performed, each in triplicate. Statistical analysis was performed with ANOVA followed by Fisher PLSD post hoc test. *Statistically significant differences (P ≤ 0.05) between control cells (open bars) and IL-1β-treated cells (solid bars). #Statistical difference between IL-1β-treated cells and cells cotreated with IL-1β and curcumin and/or IL-10.
Curcumin and IL-10 act synergistically to inhibit IL-12/23p40 production by dendritic cells.
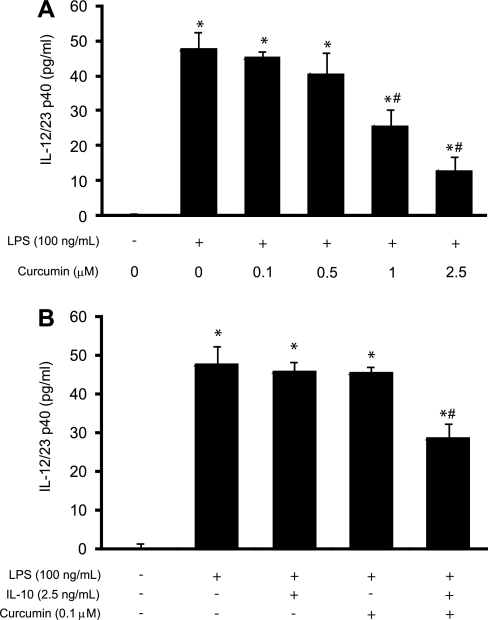
Dendritic cells are the most potent antigen-presenting cell at the interface between innate and adaptive immune responses. They play an active role in the maintenance of gut homeostasis and T cell differentiation. Because they are one of the main producers of proinflammatory cytokines, the study of the synergistic effect between IL-10 and curcumin was of particular interest. The in vitro effects of micromolar concentrations of curcumin on antigen presenting cells have been recently reported (25). The demonstrated effective concentrations leading to inhibition of NF-κB activation and impaired induction of Th1 responses were 10–25 μM (25), concentrations that have not been reported as achievable in peripheral circulation after oral administration of curcumin (41). However, we have detected millimolar concentrations of curcumin in the colonic content, and even with poor bioavailability and rapid metabolism by the epithelial cells, concentrations of curcumin in the lamina propria may be significantly higher than that in the peripheral circulation. We investigated the effects of submicromolar concentrations of curcumin and IL-10 treatment on IL-12/23p40 production by LPS-stimulated DC2.4 cells, a murine dendritic cell line. The expression of IL-10 receptor in DC2.4 cells was confirmed in a way analogous to that described for Mode-K cells (data not shown). The cells were pretreated with IL-10 in a suboptimal concentration (2.5 ng/ml) determined in a separate series of experiments (data not shown) and with curcumin (0.1, 0.5, and 1 μM) for 1 h and 10 min, respectively, prior to 24-h stimulation with 100 ng/ml of LPS. Curcumin alone induced a downregulation of IL-12/23p40 production in LPS-treated DC cells in a dose-dependent response (Fig. 12A). IL-10 (2.5 ng/ml) and curcumin (0.1 μM) used independently did not significantly reduce the production of IL-12/23p40 (Fig. 12B), but when used in combination they significantly inhibited IL-12/23p40 production (Fig. 12B). Collectively, obtained data strongly suggest that curcumin and IL-10 act synergistically to reduce the production of proinflammatory cytokines and immune activation, a result that may also explain the limited potential of curcumin in the IL-10-deficient mice.
Fig. 12.
Synergistic inhibition of LPS-induced IL-12/23p40 production by IL-10 and submicromolar concentrations of curcumin in dendritic cells in vitro. Dose response to increasing concentrations of curcumin in DC2.4 cells stimulated with LPS (100 ng/ml) for 24 h (A). Combination of curcumin and IL-10 in suboptimal concentrations (0.1 μM and 2.5 ng/ml, respectively) resulted in significant inhibition of IL-12/23p40 production in LPS-stimulated DC2.4 cells (B). Two independent experiments were performed, each in triplicate. Statistical analysis was performed with ANOVA followed by Fisher PLSD post hoc test. *Statistically significant differences (P ≤ 0.05) between control cells (no LPS, no curcumin) and treated cells. #Statistical difference between LPS-treated cells and cells cotreated with curcumin, IL-10, or IL-10 and curcumin.
DISCUSSION
The dried rhizome of a perennial herb Curcuma longa Linn., also called turmeric, has been used in Asian medicine for centuries. Curcumin has been identified as the most active constituent of turmeric and is also defined as an anti-inflammatory, antioxidant, proapoptotic, antiproliferative, and anti-infectious agent. The anti-inflammatory activity of curcumin has been investigated in various in vitro and in vivo studies (40). Curcumin was shown to be effective in acute as well as chronic models of inflammation, by enhancing phagocytic activity of macrophages as well as NK cell functions and by inhibiting lymphocyte proliferation and the immunostimulatory function of dendritic cells (5, 17, 25). In addition, curcumin has been described to regulate the activity of several enzymes via direct interaction (e.g., COX2, 5LOX, iNOS) and to modulate gene transcription through the inhibition of transcription factors (e.g., NF-κB) and related signaling pathways (20, 40). In vitro, curcumin was shown to block cytokine-induced and NF-κB-mediated activation of proinflammatory cytokines such as IL-12 and IFN-γ (20).
On the basis of these encouraging observations, curcumin has been extensively tested for efficacy in prevention or treatment of active disease in multiple models of rodent colitis. With the abundance of data from chemically induced colitis (TNBS, DNB, dextran sulfate sodium), models relying on initial mucosal injury, there have been no studies demonstrating effectiveness of curcumin in immune-mediated animal models of IBD. Whereas curcumin was shown as an effective supportive therapy reducing the relapse rate in UC patients, its efficacy in CD or in animals models more pertinent to CD has not been evaluated. Our recently published data with TNBS colitis in BALB/c and SJL/J mice demonstrated (6) no protective effects of dietary curcumin in NKT-cell deficient SJL/J mice, thus suggesting possible limited effects in a Th-1-skewed colonic inflammation. In the present study, we provide another evidence of the limited potency of curcumin in the setting of Th-1/Th-17-mediated colitis developed by IL-10 knockout mice. In IL-10−/− mice, curcumin partially improved colonic morphology in both distal and proximal colon at the dose of 0.1%. Higher concentrations of curcumin did not display any protective effect and did not reduce histological markers of inflammation: crypt hyperplasia, lymphocytic and neutrophilic infiltration, and mucosal ulceration. To further characterize the effect of curcumin in IL-10−/− mice, we evaluated the production of two major proinflammatory cytokines implicated in the colonic inflammation in IL-10-deficient mice: IL-12/23p40 and IFN-γ. Curcumin showed only a very mild inhibitory effect on IL-12/23p40 and IFN-γ mRNA expression and protein concentrations in the proximal and distal colon. However, IL-12/23p40 and IFN-γ secretion was not affected by curcumin in MLN cell culture stimulated with commensal bacterial antigens. We further focused on the effect of curcumin on the activation of NF-κB in vivo. This key transcription factor has been well known to be the major target of curcumin in various in vitro cellular models. Dietary curcumin had limited effects on NF-κB p65 activation (Ser276 phosphorylation) in the colon of IL-10−/− mice, observed only at the lowest concentration of the compound (0.1%) and limited to the colonic epithelial cells. Using a unique model to study NF-κB activation in situ, we further looked at the activation of NF-κB in transgenic IL-10−/−/NF-κBEGFP mice. In this model, 1% of dietary curcumin failed to noticeably inhibit the activation of NF-κB. In vitro, curcumin inhibited the production of IL-12/23p40 in LPS-stimulated splenocytes from WT and IL-10−/− mice, although it was unable to reduce IL-12/23p40 secretion to levels observed in unstimulated WT cells. Moreover, when used in combination, submicromolar concentration of curcumin (100 nM) acted synergistically with IL-10 to inhibit the production of IL-12/23p40 in murine dendritic cells. Similar synergism was demonstrated in IL-1β-induced NF-κB activity in intestinal epithelial Mode-K cells. These observations suggest that this potential synergism between the two factors is partially responsible for limited efficacy of curcumin in IL-10−/− mice. The IL-10-dependent effects of low concentrations of curcumin on immune cells may be of particular relevance, since in human patients receiving 4, 6, and 8 g of curcumin the average peaks of serum curcumin concentrations after ingestion were 0.51, 0.63, and 1.77 μM, respectively (8).
Limited bioavailability of orally administered curcumin and its rapid metabolism (41) may translate into insufficient concentration of the compound in the lamina propria and secondary lymphoid tissues. Although very difficult to address experimentally, this could explain the limited effects of curcumin in an IBD model initiated by primary immune dysregulation and loss of immune tolerance rather than by epithelial injury. The limited effects of the compound in the IL-10 deficiency model may be related to the effects of luminal curcumin on epithelial cells rather than on the hyperactive T cells and may point to epithelial cells as a primary target of curcumin. Indeed, curcumin has been demonstrated to inhibit TNF-α- or IL-1β-mediated increased epithelial tight junction permeability (2, 28), consistent with inhibition of NF-κB signaling by higher concentrations of the drug (20). Whereas continuous presence of curcumin (at 0.1% in diet) in the colonic lumen appeared beneficial to both mucosal morphology and cytokine gene expression, removal of the anti-inflammatory pressure of the compound in ex vivo studies (colonic explant culture or MLN stimulation) restored immune reactivity and cytokine production. Similarly, in the clinical trial with UC patients, withdrawal of curcumin resulted in increased relapse rate to levels observed in the placebo group (15). It is, therefore, possible that the potential pharmacological application of curcumin in IBD patients may be limited to prevention or maintenance of remission with continuous and regular oral intake of curcumin.
In addition, the limited effects of curcumin in IL-10−/− mice could be related to the lack of induction of endogenous immunomodulatory IL-10. Our in vitro data suggest an interplay between curcumin and IL-10 in downregulating NF-κB activity and proinflammatory cytokine production. This finding has been recently lent support by an observation that curcumin (50 μM) enhances basal and LPS-induced production of IL-10 by mouse bone marrow derived dendritic cells, which gain the ability to direct Treg cell differentiation (Ref. 9 and Y. Cong, personal communication). Whereas IL-10 knockout mice provide an excellent model to study CD pathogenesis, particularly its microbial aspects, human genetic variations known to date as associated with CD do not include the IL-10 locus and serum IL-10 concentrations are increased in the acute phase of CD (26). Therefore, the data presented here should not be considered as definitive evidence against the use of curcumin in CD patients.
It is of importance to notice an inverse dose-effect relationship of curcumin in IL-10 deficiency colitis. Although not entirely unexpected, the limited protective effects of curcumin in Th-1 mediated colitis were somewhat surprising. Lower (0.1%) dose of curcumin provided modest protection in IL-10−/− mice from developing colitis, coinciding with decreased activation of NF-κB in the colonic epithelial cells, whereas higher dietary concentrations of the compound were not only ineffective but appeared to exacerbate cytokine release from colonic explants and MLN cells. Similarly, higher concentrations of curcumin were without effect on NF-κB activation in vivo. A similar reciprocal dose-effect relationship was not described in chemically induced models of colitis. Curcumin is generally well tolerated by human patients and doses up to 8,000 mg daily for 3 mo did not result in discernible systemic toxicities except mild nausea and diarrhea (8). Because of its poor bioavailability it may be tempting to use high daily doses of curcumin in clinical trials with IBD patients. In a recent study with UC patients, curcumin was used at a dose of 2 g/day to maintain remission (15). On the other hand, our recent microarray analysis of colonic gene expression profile were indicative of some degree of ER stress (6). It is, therefore, not inconceivable that higher concentrations of dietary curcumin, in the absence of cytoprotective effects of IL-10 (43), may attenuate mucosal healing and exacerbate the immune response. Our results indicate that higher concentrations of dietary curcumin do not always correlate with highest efficacy in vivo and may in fact be detrimental. Therefore, in the design of future clinical trials, a broad spectrum of doses that include low concentrations of curcumin should be considered to determine optimal benefits of the compound.
GRANTS
This investigation was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants 5R01DK067286 (to P. R. Kiela), 5RO1DK53347 (to R. B. Sartor), and 5R01DK047700 (to C. Jobin).
Acknowledgments
We thank Aniko Solyom of the GAAS for technical expertise in HPLC analysis of curcumin concentration.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Al-Ashy R, Chakroun I, El-Sabban ME, Homaidan FR. The role of NF-kappaB in mediating the anti-inflammatory effects of IL-10 in intestinal epithelial cells. Cytokine 36: 1–8, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol 178: 4641–4649, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ardite E, Panes J, Miranda M, Salas A, Elizalde JI, Sans M, Arce Y, Bordas JM, Fernandez-Checa JC, Pique JM. Effects of steroid treatment on activation of nuclear factor kappaB in patients with inflammatory bowel disease. Br J Pharmacol 124: 431–433, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest 98: 1010–1020, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhaumik S, Jyothi MD, Khar A. Differential modulation of nitric oxide production by curcumin in host macrophages and NK cells. FEBS Lett 483: 78–82, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Billerey-Larmonier C, Uno JK, Larmonier N, Midura AJ, Timmermann B, Ghishan FK, Kiela PR. Protective effects of dietary curcumin in mouse model of chemically induced colitis are strain dependent. Inflamm Bowel Dis 14: 780–793, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med 9: 575–581, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 21: 2895–2900, 2001. [PubMed] [Google Scholar]
- 9.Cong Y, Elson CO. Dietary component, curcumin, modulates dendritic cells to differentiate naive CD4+CD25- T cells into intestine-protective regulatory T cells through retinoic acid (RA) production (Abstract). Gastroenterology 134: A-518, 2008. [Google Scholar]
- 10.Deguchi Y, Andoh A, Inatomi O, Yagi Y, Bamba S, Araki Y, Hata K, Tsujikawa T, Fujiyama Y. Curcumin prevents the development of dextran sulfate sodium (DSS)-induced experimental colitis. Dig Dis Sci 52: 2993–2998, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Egan LJ, de Lecea A, Lehrman ED, Myhre GM, Eckmann L, Kagnoff MF. Nuclear factor-kappa B activation promotes restitution of wounded intestinal epithelial monolayers. Am J Physiol Cell Physiol 285: C1028–C1035, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Egan LJ, Eckmann L, Greten FR, Chae S, Li ZW, Myhre GM, Robine S, Karin M, Kagnoff MF. IkappaB-kinasebeta-dependent NF-kappaB activation provides radioprotection to the intestinal epithelium. Proc Natl Acad Sci USA 101: 2452–2457, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fina D, Caruso R, Pallone F, Monteleone G. Interleukin-21 (IL-21) controls inflammatory pathways in the gut. Endocr Metab Immune Disord Drug Targets 7: 288–291, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol 157: 1261–1270, 1996. [PubMed] [Google Scholar]
- 15.Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, Tsujikawa T, Fujiyama Y, Mitsuyama K, Sata M, Yamada M, Iwaoka Y, Kanke K, Hiraishi H, Hirayama K, Arai H, Yoshii S, Uchijima M, Nagata T, Koide Y. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol 4: 1502–1506, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci 50: 2191–2193, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Jagetia GC, Aggarwal BB. “Spicing up” of the immune system by curcumin. J Clin Immunol 27: 19–35, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Jian YT, Mai GF, Wang JD, Zhang YL, Luo RC, Fang YX. Preventive and therapeutic effects of NF-kappaB inhibitor curcumin in rats colitis induced by trinitrobenzene sulfonic acid. World J Gastroenterol 11: 1747–1752, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang H, Deng CS, Zhang M, Xia J. Curcumin-attenuated trinitrobenzene sulphonic acid induces chronic colitis by inhibiting expression of cyclooxygenase-2. World J Gastroenterol 12: 3848–3853, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, Sartor RB. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol 163: 3474–3483, 1999. [PubMed] [Google Scholar]
- 21.Jobin C, Sartor RB. The IκB/NF-κB system: a key determinant of mucosal inflammation and protection. Am J Physiol Cell Physiol 278: C451–C462, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser GC, Yan F, Polk DB. Mesalamine blocks tumor necrosis factor growth inhibition and nuclear factor kappaB activation in mouse colonocytes. Gastroenterology 116: 602–609, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karrasch T, Kim JS, Muhlbauer M, Magness ST, Jobin C. Gnotobiotic IL10−/−;NFkappa B(EGFP) mice reveal the critical role of TLR/NF-kappa B signaling in commensal bacteria-induced colitis. J Immunol 178: 6522–6532, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Kiela PR, Midura AJ, Kuscuoglu N, Jolad SD, Solyom AM, Besselsen DG, Timmermann BN, Ghishan FK. Effects of Boswellia serrata in mouse models of chemically induced colitis. Am J Physiol Gastrointest Liver Physiol 288: G798–G808, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Kim GY, Kim KH, Lee SH, Yoon MS, Lee HJ, Moon DO, Lee CM, Ahn SC, Park YC, Park YM. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kappa B as potential targets. J Immunol 174: 8116–8124, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Kucharzik T, Stoll R, Lugering N, Domschke W. Circulating antiinflammatory cytokine IL-10 in patients with inflammatory bowel disease (IBD). Clin Exp Immunol 100: 452–456, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrance IC, Wu F, Leite AZ, Willis J, West GA, Fiocchi C, Chakravarti S. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-kappa B. Gastroenterology 125: 1750–1761, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-α-induced increase in intestinal epithelial tight junction permeability requires NF-κB activation. Am J Physiol Gastrointest Liver Physiol 286: G367–G376, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Mariani P, Bachetoni A, D'Alessandro M, Lomanto D, Mazzocchi P, Speranza V. Effector Th-1 cells with cytotoxic function in the intestinal lamina propria of patients with Crohn's disease. Dig Dis Sci 45: 2029–2035, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Murano M, Maemura K, Hirata I, Toshina K, Nishikawa T, Hamamoto N, Sasaki S, Saitoh O, Katsu K. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol 120: 51–58, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neurath MF, Pettersson S, Meyer zum Buschenfelde KH, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med 2: 998–1004, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Parronchi P, Romagnani P, Annunziato F, Sampognaro S, Becchio A, Giannarini L, Maggi E, Pupilli C, Tonelli F, Romagnani S. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn's disease. Am J Pathol 150: 823–832, 1997. [PMC free article] [PubMed] [Google Scholar]
- 33.Rogler G, Brand K, Vogl D, Page S, Hofmeister R, Andus T, Knuechel R, Baeuerle PA, Scholmerich J, Gross V. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 115: 357–369, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz PA, Shkoda A, Kim SC, Sartor RB, Haller D. IL-10 gene-deficient mice lack TGF-beta/Smad signaling and fail to inhibit proinflammatory gene expression in intestinal epithelial cells after the colonization with colitogenic Enterococcus faecalis. J Immunol 174: 2990–2999, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Salh B, Assi K, Templeman V, Parhar K, Owen D, Gomez-Munoz A, Jacobson K. Curcumin attenuates DNB-induced murine colitis. Am J Physiol Gastrointest Liver Physiol 285: G235–G243, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Sartor RB Microbial influences in inflammatory bowel diseases. Gastroenterology 134: 577–594, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt C, Giese T, Ludwig B, Mueller-Molaian I, Marth T, Zeuzem S, Meuer SC, Stallmach A. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn's disease but not in ulcerative colitis. Inflamm Bowel Dis 11: 16–23, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut 42: 477–484, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 66: 5224–5231, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer 41: 1955–1968, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamics of curcumin. Adv Exp Med Biol 595: 453–470, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol 158: 2723–2730, 1997. [PubMed] [Google Scholar]
- 43.Shkoda A, Ruiz PA, Daniel H, Kim SC, Rogler G, Sartor RB, Haller D. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology 132: 190–207, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Spiik AK, Ridderstad A, Axelsson LG, Midtvedt T, Bjork L, Pettersson S. Abrogated lymphocyte infiltration and lowered CD14 in dextran sulfate induced colitis in mice treated with p65 antisense oligonucleotides. Int J Colorectal Dis 17: 223–232, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol 19: 281–286, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Sugimoto K, Hanai H, Tozawa K, Aoshi T, Uchijima M, Nagata T, Koide Y. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology 123: 1912–1922, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Tse CM, Levine SA, Yun CH, Khurana S, Donowitz M. Na+/H+ exchanger-2 is an O-linked but not an N-linked sialoglycoprotein. Biochemistry 33: 12954–12961, 1994. [DOI] [PubMed] [Google Scholar]
- 48.Ukil A, Maity S, Karmakar S, Datta N, Vedasiromoni JR, Das PK. Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br J Pharmacol 139: 209–218, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Venkataranganna MV, Rafiq MD, Gopumadhavan S, Peer G, Babu UV, Mitra SK. NCB-02 (standardized Curcumin preparation) protects dinitrochlorobenzene- induced colitis through down-regulation of NFkappa-B and iNOS. World J Gastroenterol 13: 1103–1107, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Delivery Res 59: 1073–1083, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Zhang M, Deng C, Zheng J, Xia J, Sheng D. Curcumin inhibits trinitrobenzene sulphonic acid-induced colitis in rats by activation of peroxisome proliferator-activated receptor gamma. Int Immunopharmacol 6: 1233–1242, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Zhang M, Deng CS, Zheng JJ, Xia J. Curcumin regulated shift from Th1 to Th2 in trinitrobenzene sulphonic acid-induced chronic colitis. Acta Pharmacol Sin 27: 1071–1077, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Zhou L, Nazarian AA, Smale ST. Interleukin-10 inhibits interleukin-12 p40 gene transcription by targeting a late event in the activation pathway. Mol Cell Biol 24: 2385–2396, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]