Abstract
Helicobacter pylori infection of the gastric body induces transient hypochlorhydria and contributes to mucosal progression toward gastric carcinoma. Acid secretion is mediated by parietal cell H,K-ATPase, in which the catalytic α-subunit (HKα) promoter activity in transfected gastric epithelial [gastric adenocarcinoma (AGS)] cells is repressed by H. pylori through NF-κB p50 homodimer binding to the promoter. IL-1β, an acid secretory inhibitor whose mucosal level is increased by H. pylori, upregulates HKα promoter activity in AGS cells. Because IL-1β also activates NF-κB signaling, we investigated disparate HKα regulation by H. pylori and IL-1β, testing the hypothesis that IL-1β-induced HKα promoter activation is mediated by the transcription factor Sp1. DNase I footprinting revealed Sp1 binding to the HKα promoter at −56 to −39 bp. IL-1β stimulated the activity of three HKα promoter constructs containing NF-κB and Sp1 sites transfected into AGS cells and also stimulated a construct containing only an Sp1 site. This stimulation was abrogated by mutating the HKα promoter Sp1 binding site. Gelshift assays showed that IL-1β increased Sp1 but not p50 binding to cognate HKα probes and that Sp1 also interacts with an HKα NF-κB site when bound to its cognate HKα cis-response element. H. pylori did not augment Sp1 binding to an HKα Sp1 probe, and small interfering RNA-mediated knockdown of Sp1 expression abrogated IL-1β-induced HKα promoter stimulation. We conclude that IL-1β upregulates HKα gene transcription by inducing Sp1 binding to HKα Sp1 and NF-κB sites and that the H. pylori perturbation of HKα gene expression is independent of Sp1-mediated basal HKα transcription.
Keywords: adenosine 5′-triphosphatase, Helicobacter pylori, interleukin-1β
the gram-negative microaerophilic bacterium Helicobacter pylori is a class I carcinogen that colonizes the human gastric epithelium, causing gastritis and perturbing acid secretion and potentially triggering epithelial progression to carcinoma. In particular, H. pylori gastritis of the body of the stomach is strongly correlated with reduced acid secretion (10, 12). Both this hypochlorhydria and gastritis are associated with increased gastric epithelial cell proliferation, which in a significant subset (∼2%) of infected individuals may progress to distal gastric adenocarcinoma. The importance of hypochlorhydria in establishing gastric mucosal susceptibility to carcinoma was emphasized in a recent study of gastrin-deficient mice (38). In the absence of the potent acid secretagogue gastrin, G−/− mice were rendered hypochlorhydric, and their stomachs became chronically inflamed, progressing to atrophy of the fundic mucosa, intestinal metaplasia, dysplasia, and eventually antral carcinoma (38). This recapitulation of the phenotypic progression to gastric cancer (7), triggered not by H. pylori but by perturbation of gastric pH, provides the rationale for investigating the mechanisms whereby H. pylori inhibits acid secretion, thereby setting the stage for the pathological sequelae of infection.
Gastric acid secretion is mediated by K+-stimulated, proton-translocating H,K-ATPase, an integral heterodimeric protein of the apical membrane of gastric parietal cells. The enzyme consists of catalytic α-subunits (HKα; Mr, 94,000) and regulatory β-subunits (HKβ; Mr, 60,000–80,000), which acquire H+/K+ counter-transport activity when transferred from parietal cell tubulovesicular membranes to secretory canalicular membranes upon stimulation of acid secretion. HKα transcription is positively regulated by the gastric acid secretagogues histamine, gastrin, and acetyl choline (5). A number of transcription factors associated with HKα promoter activity have been identified. In rat gastric parietal cells, the zinc finger proteins GATA-GT1 and GATA-GT2 colocalize with HKα mRNA, suggesting a role for these transcription factors in the regulation of proton pump gene expression (21). In canine parietal cells, constitutive HKα transcriptional activity is regulated by Sp1 binding to the HKα promoter −54 to −45 bp upstream of the transcription initiation site (20), whereas an EGF-responsive factor binds to the promoter between −156 and −162 bp (16). More recently, our laboratory has shown that binding of an NF-κB p50 subunit homodimer to the −150 to −170 bp region of the human HKα promoter inhibits HKα transcriptional activity (25).
Studies of the H. pylori perturbation of host cell signaling mechanisms have revealed several pathways by which NF-κB may be activated. H. pylori strains with a cag pathogenicity island encoding a type IV secretion system (T4SS) inject cytotoxin-associated gene (CagA) oncoprotein into host cells, activating Ras-mediated mitogen-activated protein kinase (MAPK) signaling and the activation of the transcription factor NF-κB. Consequent upregulation of the interleukin (IL)-8 gene and increased epithelial cell secretion of chemotactic IL-8 recruits activated neutrophils and monocytes into the lamina propria where they secrete the proinflammatory cytokines IL-1β and TNF-α. In addition, T4SS-mediated delivery of peptidoglycan into host gastric adenocarcinoma (AGS) cells stimulates the intracellular receptor Nod1, leading to the direct activation of NF-κB (33). H. pylori lipopolysaccharide interaction with gastric epithelial cell Toll-like receptors 2 and 5 also culminates in NF-κB activation (29). In AGS cells, H. pylori-induced ERK1/2 signaling cascade activates NF-κB and also activates Sp1, leading to the activation of angiogenic growth factor VEGF-A (30).
IL-1β is an important factor in H. pylori-induced mucosal inflammation and perturbation of acid secretion. H. pylori-positive peptic ulcer disease patients showed a marked increase in IL-1β mRNA in fundic mucosa and increased gastric pH, which reverted to normal levels after H. pylori eradication (22, 35, 36). CagA-positive H. pylori strains are associated with higher mucosal IL-1β levels than CagA-negative strains (2). Both IL-1β and TNF-α activate NF-κB, leading to the upregulation of IL-8 and cyclooxygenase-2, and in the presence of H. pylori, certain IL-1β gene polymorphisms are associated with increased risk of gastric adenocarcinoma (11). IL-1β is also a potent inhibitor of gastric acid secretion (34) and thus may contribute to the transient hypochlorhydria associated with acute H. pylori infection. In some cell culture models, IL-1β inhibits activation of Sp1, leading to repression of promoter activities (4, 23), but in AGS cells IL-1β activates Sp1 phosphorylation through ERK1/2 pathways (9). We recently reported that in AGS cells IL-1β activates, rather than represses, phorbol ester-induced transcriptional activity of several human HKα promoter-reporter constructs through ERK1/2 signaling (24).
In the present study, we sought to clarify the mechanistic details of IL-1β-induced effects on HKα gene transcription. Although H. pylori and IL-1β are both inflammatory mediators and known activators of the NF-κB signaling cascade, H. pylori inoculation of AGS cells represses transfected HKα promoter activity (13, 25), whereas IL-1β activates HKα transcription (24). We resolved this apparent anomaly by focusing our investigation on Sp1 regulation of the transcriptional activity of the HKα promoter. Our data indicate that the IL-1β enhancement of HKα transcription is mediated by Sp1 transactivation of the HKα Sp1 cis-response element and that a proximal NF-κB site in the HKα promoter interacts with Sp1 and other nuclear factors, leading to enhanced HKα transcription.
MATERIALS AND METHODS
Cells, bacteria, and reagents.
Human AGS (CRL1739) and wild-type H. pylori (strain ATCC 49503) were from American Type Culture Collection (Manassas, VA). AGS cells were grown for ≤10 passages in Ham's F-12 containing L-glutamine (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA) at 37°C in a humidified incubator with 5% CO2-95% air. H. pylori cultures were grown on Brucella broth (Difco, Detroit, MI) plates containing 10% FBS and 2.4% agar (Brucella-agar plates) at 37°C using a microaerophilic gas pack system (BD Biosciences, Sparks, MD). Cultures were routinely urease tested during subculturing, and only positive cultures were used for cell infection. For AGS cell infection, H. pylori were harvested after 24 h of culture. Bacteria were resuspended in Ham's F-12 medium containing FBS and enumerated by absorbance at 600 nm (1 optical density at 600 nm = 2.4 × 108 bacteria/ml). Multiplicities of infection (MOI) were calculated based on AGS cell and bacterial cell counts. IL-1β was purchased from Chemicon (Billerica, MA). Restriction enzymes, the pGL2-basic vector transfection plasmid, 5× passive lysis buffer, and the luciferase assay substrate were obtained from Promega (Madison, WI). The transfection plasmid pMaxGFP was purchased from Amaxa (Gaithersburg, MD). All other reagents were of molecular biology grade with maximum possible purity.
HKα promoter-reporter plasmid constructs.
Genomic DNA representing a portion of the human HKα 5′-flanking region was provided by Dr. Gary Shull (University of Cincinnati). A 2,179-bp segment of this 5′-flanking region including 20 bp downstream of the transcription initiation site (HKα2179) was integrated into the luciferase reporter plasmid pGL2-basic vector as previously described (13). Truncated deletion constructs of the 5′-flanking region were generated by PCR amplification using the HKα2179 promoter-Luc reporter plasmid as a template, as previously described (24). An HKα206 construct containing a two-nucleotide mutation in the NF-κB site (HKα206ΔNF-κB1) was generated as described (25). An HKα206 construct containing a six-nucleotide mutation in the Sp1 site (HKα206ΔSp1) was generated using QuikChange Lightning Site-Directed Mutagenesis kit (Stratagene), following the manufacturer's protocol.
Transient transfection.
AGS cells (1.25 × 105 cells/well) were cultured overnight in 24-well cell culture plates, washed with phosphate-buffered saline (PBS), and then transfected with HKα promoter-Luc reporter constructs as described previously (24). After 24 h, the cells were incubated for a further 24 h with IL-1β (10 ng/ml) or H. pylori (24 h culture; MOI = 25) or F-12 medium alone (vehicle control) and then lysed for measurement of reporter activities. Luciferase relative light units were normalized to cotransfected pMaxGFP fluorescence and corrected by subtracting corresponding normalized promoterless pGL2-basic vector relative light units data. Data points are shown as means ± SD of three independent transfection experiments with each deletion construct.
Immunoblotting analysis.
Serum-deprived AGS cells (75–80% confluent) were preincubated as required with the ERK1/2 inhibitor PD-98059 (1 h) and then treated with IL-1β (10 ng/ml) for 4 h. The cells were washed and harvested in ice-cold PBS and centrifuged at 520 g for 5 min. The PBS was discarded, and the cells were dissolved in 1× Triton lysis buffer (Boston Bioproducts, Worcester, MA) containing 1× protease inhibitor cocktail (P8340; Sigma, St. Louis, MO) and 1× Halt phosphatase inhibitor cocktail (Thermo Scientific, Rockford, IL). The cells were lysed in a rotating shaker at 4°C for 15 min. Cell lysates (30–40 μg protein as measured with Bradford reagent) were boiled in equal volumes of SDS-PAGE sample buffer, resolved in 4–20% Tris-glycine precast gel, and transferred to polyvinylidene difluoride membrane (Millipore, Bedford, MA). The replicas were incubated for 1 h in 5% nonfat dry milk in Tris-buffered saline, 0.1% Tween 20 (TTBS), washed 3× in TTBS, and incubated overnight at 4°C with antibodies against Sp1, NF-κB p50, NF-κB p65, Ser337-phospho NF-κB p50 (Santa Cruz Biotechnology, Santa Cruz, CA), p44/42 MAP kinase, Thr202, and Tyr204-phospho p44/42-MAP kinase, Ser536-phospho NF-κB p65 (Cell Signaling Technology, Danvers, MA), Thr453-phospho Sp1 (Abcam, Cambridge, MA), or β-actin (A5441; Sigma). The replicas were washed 3× in TTBS and incubated at room temperature for 1 h with goat anti-mouse or goat anti-rabbit horseradish peroxidase-conjugated secondary antibodies, and chemiluminescent signals were detected using enhanced chemiluminescence substrate (Amersham Biosciences, Pittsburgh, PA).
DNAase I footprint assay.
A 297-bp footprint probe was generated by PCR using 32P-end-labeled primers and the cloned pGL2-basic vector-HKα206 deletion construct as substrate. The PCR product included 48 bp of vector sequence distally and 23 bp proximally. The concentration of the purified probe was measured using Quant-It PicoGreen dsDNA reagent. The 32P-labeled probe (2.8 fmol in 13 μl H2O) was combined with 25 μl binding buffer, containing (in mM) 50 Tris-HCl (pH 8.0), 100 KCl, 12.5 MgCl2, 1 EDTA, and 1 DTT and 20% glycerol, and with 12 μl Sp1 dilution buffer, containing 12 mM HEPES (pH 7.5), 50 mM KCl, 6 mM MgCl2, 5 μM ZnSO4, 2 mM DTT, 0.1% Nonidet P-40, and 50% glycerol, containing 1.92 μg recombinant human Sp1 (Promega). After 10 min incubation on ice, 50 μl Ca2+/Mg2+ solution containing (in mM) 5 CaCl2 and 10 MgCl2 was added, and incubation continued at 23°C for 1 min. RQ1 DNase (0.29 units; Promega) diluted in 3 μl Sp1 dilution buffer was added, and incubation was continued at 23°C for 1 min. The reaction was stopped by the addition of 90 μl stop solution containing 200 mM NaCl, 20 mM EDTA, 1% SDS, and 100 μg/ml yeast tRNA. Reactions were extracted with 200 μl phenol-chloroform-isoamyl alcohol (25:24:1) equilibrated with 10 mM Tris (pH 8.0), 1 mM EDTA, and 500 mM NaCl. DNA was ethanol precipitated from the aqueous phase, rinsed with 70% ethanol, and dissolved in sequencing gel-loading dye of 95% formamide, 10 mM EDTA (pH 11.0), and 0.25 mg/ml bromophenol blue. Sequencing reactions for the footprint probe were carried out using a SequiTherm Excel II sequencing kit (Epicentre Biotechnologies, Madison, WI) according to the manufacturer's instructions. DNAse I digests and sequencing reactions were electrophoresed on 8% acrylamide, 7 M urea gels in 1× Tris-borate-EDTA (TBE) buffer at 60 W for 2 h. The gels were dried and placed on X-ray film overnight at −80°C.
Electrophoretic mobility shift assay.
AGS cells (75–80% confluent) were serum deprived for 15–20 h, treated with IL-1β for 1–24 h, washed, and harvested in ice-cold PBS, and nuclear extract was prepared as described previously (25). Synthetic oligonucleotides (Table 1) were obtained from Sigma Genosys (St. Louis, MO). Single-stranded oligonucleotide pairs were dissolved in 1× Tris-EDTA buffer containing (in mM) 10 Tris (pH 8.0) and 1 EDTA and annealed in 1× Tris-EDTA buffer, 50 mM NaCl, by heating at 95°C for 4 min and then slowly cooled to room temperature. Annealed oligonucleotide probes (1 μg) were desalted and then phosphorylated with T4 polynucleotide kinase (New England Biolabs, Ipswich, MA) at 37°C for 45 min with γ-32PATP (Perkin Elmer, Wellesley, MA). Radiolabeled probes were isolated on a 2% agarose gel and purified using an Ultrafree-DA DNA purification kit (Millipore, Bedford, MA). Remaining impurities in the probe preparations were removed by buffer exchange using Microcon centrifugal filters (Millipore). Probe-specific activities [1–2 × 106 counts per minute (cpm)/ng] were calculated from 32P scintillation counting and Quant-It Picogreen dsDNA quantitation (Invitrogen). Nuclear extracts (8 μg protein) were incubated with 32P-labeled oligonucleotide probes (15,000 cpm) and poly(dI-dC) (0.3 μg) in binding buffer containing 10 mM Tris (pH 7.9), 100 mM KCl, 5 mM MgCl2, 1 mM EDTA, 10% glycerol, 1 mM DTT, and 700 μM PMSF at 4°C for 45 min. For supershift studies, antibodies (1 μg) were incubated with binding reactions overnight at 4°C for 45 min before the addition of radioactive probes. Binding reaction volumes were adjusted to 25 μl, resolved on 5% native polyacrylamide gels in 0.5× TBE (pH 8.0). Gels were dried and exposed for 24 h on phosphoimager screens.
Table 1.
Synthetic oligonucleotide sequences of electrophoretic mobility shift assay probes
| Probe | Sequence |
|---|---|
| HKαSp1F | 5′-CGATCTGCTCCACCCCACCGCCC-3′ |
| HKαSp1R | 5′-CGAGGGCGGTGGGGTGGAGCAGA-3′ |
| HKαCMSp1F | 5′-CGATCTGCTCCACCCCATTATTT-3′ |
| HKαCMSp1R | 5′-CGAAAATAATGGGGTGGAGCAGA-3′ |
| HKαT18F | 5′-CGAGACATGGGGGGATCTGGGCA-3′ |
| HKαT18R | 5′-CGATGCCCAGATCCCCCCATGTC-3′ |
| Consensus NF-κBF | 5′-CGAAGTTGAGGGGACTTTCCCAGGC-3′ |
| Consensus NF-κBR | 5′-CGAGCCTGGGAAAGTCCCCTCAACT-3′ |
HKα, H,K-ATPase α-subunit.
Small interfering RNA transfection.
Four different small interfering RNAs (siRNAs) against human Sp1 (J-026959-05-08), and nontargeting siRNA (D-001810-03) were acquired from Dharmacon RNA Technology (Lafayette, CO). AGS cells (105; 75–85% confluent) were incubated with Dharmafect transfection reagent 1 (T-2001-07), and either pooled or individual siRNAs (100 nM) were solubilized according to the manufacturer's protocol. Sp1 mRNA content of the cells was measured by real time RT-PCR using β2-microglobin as the internal control, and the protein levels of expressed Sp1 were estimated by the immunoblotting of whole AGS cell extracts using β-actin as the internal control. To study the effect of siRNA on HKα promoter activity, AGS cells were transiently transfected with HKα206 for 24 h. Transfected cells were then treated with or without IL-1β for 24 h, and the transcriptional activities of the HKα206 deletion construct were measured as described earlier.
Real time RT-PCR.
AGS cells were grown to 75–80% confluence in 24-well cell culture plates and then serum deprived for 15–20 h. Total RNA was isolated using STAT-60 reagent (Tel Test, Friendswood, TX) and reverse-transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer's protocol. Measurement of AGS cell Sp1 mRNA was carried out by real-time RT-PCR using an iCycler iQ with iQ SYBR Green Supermix (Bio-Rad) and Sp1 primer mix (PPH01482A) from SuperArray Bioscience (Frederick, MD). The primer sequences for β2-microglobin used as internal control were as follows: 5′-AGATGAGTATGCCTGCCGTGTG-3′ (forward) and 5′-TCAAACCTCCATGATGCTGCTTAC-3′ (reverse).
Statistical analysis.
The levels of significance of the differences between control and treatment groups were assessed by two-way ANOVA using the Bonferroni posttest method, as implemented in the statistical software package GraphPad PRISM version 4. Statistical significance was ascribed to P values of <0.05.
RESULTS
Basal HKα promoter activity is increased by IL-1β and repressed by H. pylori.
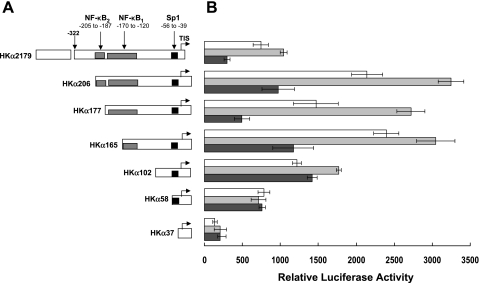
We have previously reported that IL-1β upregulates the phorbol ester-induced activity of HKα promoter-reporter constructs that are otherwise repressed by H. pylori (24). To determine the effect of IL-1β on constitutive HKα promoter activity, AGS cells were independently transiently transfected with a panel of seven HKα promoter-Luc reporter deletion constructs, which also shows the relative positions of Sp1 and NF-κB binding sites on the HKα promoter (Fig. 1A). The cells were treated for 24 h with IL-1β (10 ng/ml), H. pylori (MOI = 25), or medium alone (vehicle control), and the resulting IL-1β-dependent, H. pylori-dependent, and basal (no treatment) HKα activities were measured by luminometry and normalized to the fluorometric expression levels of cotransfected green fluorescent protein reporter plasmids. As shown in Fig. 1B, the transcriptional activity of the longest HKα promoter-reporter construct (HKα2179) was increased ∼40% by IL-1β and decreased ∼60% by H. pylori. IL-1β significantly increased the activity of deletion constructs HKα206, HKα177, HKα165, and HKα102 compared with HKα2179, whereas deletion constructs smaller than HKα102 showed no significant activation by IL-1β. In contrast, H. pylori significantly repressed the transcriptional activity of all HKα deletion constructs except HKα102, HKα58, and HKα37. The data indicate that cis-response elements in HKα 5′-flanking regions downstream of −206 bp are responsible for both the IL-1β-induced activation and H. pylori-induced repression of the promoter. Interestingly, deletion of the proximal NF-κB1 site caused a 36% decrease in IL-1β-stimulated activity and completely abrogated H. pylori repression of HKα activity without significantly affecting basal HKα activity (compare HKα177 and HKα102 activities in Fig. 1B). These data suggest that the NF-κB1 and Sp1 binding sites contribute to both basal and IL-1β-stimulated HKα transcriptional activity. The persistence of IL-1β-stimulated HKα activity after deletion of the NF-κB1 binding site (HKα102) suggests the involvement of an Sp1 binding site; however, deletion of 44 bp upstream of the Sp1 site (HKα58) abrogated IL-1β stimulation, suggesting that additional sequence-dependent interactions participate in IL-1β stimulation. The significantly increased basal and IL-1β-dependent activities of HKα206 compared with HKα2179 are also consistent with the presence of hitherto unidentified repressor sites upstream of −205 bp in the HKα promoter.
Fig. 1.
A: schematic depiction of 7 progressively truncated human catalytic α-subunit of H,K-ATPase (HKα) 5′-flanking sequence deletion constructs. Numbering represents base pair position with respect to transcription initiation site (TIS) shown as bent arrows. HKα2179 shows 5′-terminus of homology domain I at −322 bp. NF-κB1, NF-κB2, and Sp1 represent the 2 NF-κB and 1 Sp1 binding regions in HKα promoter. B: HKα promoter activity is increased by IL-1β and decreased by Helicobacter pylori. Human gastric adenocarcinoma (AGS) cells were independently transfected with HKα deletion-Luc constructs followed by incubation with H. pylori [25 multiplicity of infection (MOI); 6 h] or IL-1β (10 ng/ml; 24 h). HKα promoter activity was expressed in terms of relative luciferase activity. White bar, control AGS cells; light gray bar, IL-1β-treated AGS cells; dark gray bar, H. pylori-treated AGS cells. Data are shown as means ± SD of 3 separate experiments. For HKα206, HKα177, HKα165, and HKα102 deletion constructs, IL-1β-induced promoter activity differed significantly from control (P < 0.001). For shorter HKα58 and HKα37 deletion constructs, IL-1β-induced and control promoter activity did not differ significantly. For HKα2179, HKα206, HKα177, and HKα165 deletion constructs, H. pylori-induced promoter activity differed significantly from control (P < 0.001). For HKα102, HKα58, and HKα37 deletion constructs, IL-1β-induced and basal promoter activity did not differ significantly.
IL-1β activates both NF-κB and Sp1 through ERK1/2 signaling.
Our laboratory has previously shown that H. pylori upregulation of NF-κB is mediated by the MAPK ERK 1/2 (25). To identify signaling pathways and transcription factors putatively involved in the IL-1β upregulation of proximal HKα promoter deletion constructs, AGS cells were treated with IL-1β (10 ng/ml; 4 h) in the presence or absence of the ERK1/2 inhibitor PD-98059 (50 μM; added 1 h before IL-1β treatment). The cellular content of phosphorylated and total ERK1/2, NF-κB p50, and Sp1 was determined by immunoblot analysis of the cell lysates. As shown in Fig. 2, IL-1β upregulates the phosphorylation of ERK1/2, Sp1, and NF-κB p50, while their total amounts remain constant. The preincubation of cells with PD-98059 abrogated IL-1β-mediated increases in ERK1/2, Sp1, and NF-κB p50 phosphorylation. The data indicated that ERK1/2 signaling to both NF-κB and Sp1 provides a basis for IL-1β perturbation of basal HKα promoter activity mechanistically comparable with H. pylori-induced NF-κB p50 homodimer repression of the HKα promoter (25). In addition, the data also indicate that IL-1β may induce changes in the transactivating capacity of Sp1 and NF-κB p50 subunits by regulating their posttranslational phosphorylation status.
Fig. 2.
IL-1β activates both Sp1 and NF-κB in AGS cells. AGS cells were treated with IL-1β (10 ng/ml) for 4 h, and cellular content of ERK1/2, Sp1, and NF-κB p50 subunits was assayed by immunoblotting using antibodies recognizing phosphorylated (p) and nonphophosphorylated forms of ERK1/2, Sp1, and NF-κB p50 subunit. Left: content of p-ERK1/2, Sp1, and NF-κB p50 in untreated, IL-1β-treated, and PD-98059 + IL-1β-treated AGS cells. Right: cellular content of total ERK1/2, Sp1, and NF-κB p50 subunits, acting as normalization control for left. Representative data are from 3 independent sets of experiments. Con, control.
Sp1 binding region is located upstream of TATA box in HKα promoter.
Given that IL-1β activated Sp1 in AGS cells, we sought to identify HKα promoter nucleotides serving as an Sp1 binding site. In silico analysis (www.genomatrix.de/online_help/help_matinspector/matinspector_help.html) identified several putative Sp1 binding regions within a 200-bp domain 5′ of the HKα transcription initiation site (analysis not shown). As shown in Fig. 3, DNase I footprint analysis of a 218-bp HKα promoter substrate incubated with recombinant human Sp1 protein revealed single complementary footprints on both the forward and reverse strands of the substrate DNA (compare lanes 1 and 2 for the forward strand footprint and lanes 3 and 4 for the reverse strand footprint). Comparison of these footprints with the sequencing lanes (G, A, T, and C) defined −56 bp to −39 bp on the HKα promoter as the Sp1 binding site.
Fig. 3.
Sp1 binding region is located upstream of TATA box region in HKα promoter. Representative DNA sequencing gel electropherogram showing DNase I footprinting of the HKα 5′-flanking sequence with recombinant human Sp1 protein is shown. Protected and hypersensitive regions are identified by base pair numbering with respect to transcription initiation site. DNase I digestion products in the absence of recombinant Sp1 are shown in lanes 1 and 3, and digestion products in the presence of recombinant Sp1 are shown in lanes 2 and 4. Lanes G, A, T, and C represent the sequencing lanes.
Binding of Sp1 to the HKα promoter is increased by IL-1β and not H. pylori.
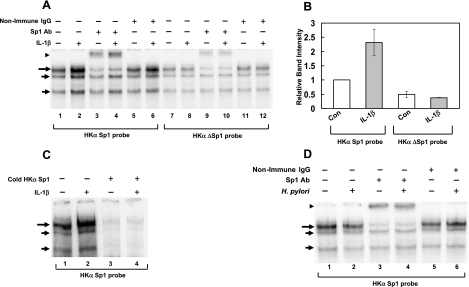
Although IL-1β clearly increases the level of phosphorylated Sp1 and phosphorylated NF-κB subunits in AGS cells as shown in Fig. 2, we were particularly interested in the IL-1β modulation of Sp1 and NF-κB binding to HKα cis-reponse elements. We have previously reported that NF-κB p50 homodimer binding to the HKα promoter downregulates HKα transcriptional activity (25) and that IL-1β upregulates HKα activity (24). To delineate more precisely IL-1β-induced effects of NF-κB and Sp1 binding to HKα promoter, we turned to EMSA using nuclear extracts of AGS cells treated from 1 to 24 h with or without IL-1β (10 ng/ml) and 32P-labeled HKα promoter sequences as DNA probes [T18 for NF-κB1 (25) and the Sp1 footprint region defined in Fig. 3 for Sp1]. When incubated with the Sp1 probe, AGS cell nuclear extracts treated with IL-1β for 4 h showed the enhancement of three DNA-protein complexes (Fig. 4A; compare lanes 2 and 3, long and short arrows). The slowest migrating band (long arrow) was significantly supershifted by the addition of Sp1 antibody but not by the addition of nonimmune rabbit IgG (Fig. 4A, arrowhead; compare lanes 4 and 5 with lanes 6 and 7). Nuclear extracts of AGS cells treated with IL-1β for 12 h also showed a significant enhancement of the Sp1-specific DNA; protein complex but 1, 2, or 24 h IL-1β treatments did not (data not shown). The insignificant supershifting of the two faster migrating bands (short arrows) by Sp1 antibody suggests that other AGS cell nuclear factors in addition to Sp1 interact with the HKα Sp1 binding sequence.
Fig. 4.
IL-1β but not H. pylori increases binding of Sp1 to the HKα promoter. AGS cells were treated with IL-1β (10 ng/ml; A–C) or with H. pylori (25 MOI) for 4 h (D). Interactions of AGS cell nuclear extract proteins with 32P-labeled HKα-specific Sp1 oligonucleotide probes were assessed by EMSA and supershift analysis with Sp1 antibody (Ab) or nonimmune rabbit IgG. A: EMSA and supershift analysis with 32P-labeled HKα Sp1 probe or HKαΔSp1 probe (6 nucleotide mutation in the core Sp1 binding region), IL-1β-treated AGS cells, and Sp1-specific antibody or nonimmune rabbit IgG. B: densitometric quantitation of DNA-protein complexes from lanes 1, 2, 7, and 8 in A. Data are shown as means ± SD of 3 independent experiments. C: EMSA with 32P-labeled HKα Sp1probe (lanes 1 and 2) and with 200× molar excess of cold (nonradioactive) HKα Sp1 probe (lanes 3 and 4). D: EMSA and supershift analysis with HKα Sp1 probe and H. pylori-treated AGS cells and Sp1-specific antibody. Gels are representative of 3 replicates for A and 2 replicates for experiments shown in C and D. Long and short arrows indicate DNA-protein complexes; arrowheads indicate supershifted complexes.
We sought to identify the site of Sp1 binding to the HKα promoter by EMSA supershift assays using an HKα DNA probe (ΔSp1) mutated at six nucleotides corresponding to an Sp1 binding motif encompassed by the Sp1 footprint shown in Fig. 3 (−43 to −38 bp). As shown in Fig. 4A, nuclear extracts of untreated and treated (IL-1β; 10 ng/ml; 4 h) AGS cells yielded mobility-shifted bands of comparable signal intensity (compare lanes 7 and 8, long arrow). Sp1 antibody supershifted these ΔSp1 DNA-protein complexes (lanes 9 and 10, arrowhead), but nonimmune rabbit IgG did not (lanes 11 and 12). These data contrast with the IL-1β-induced enhancement of Sp1 binding to a wild-type HKα Sp1 probe (Fig. 4A, lane 2), indicating that HKα promoter sequence −43 bp to −38 bp forms the Sp1 core binding region and is essential for IL-1β-induced enhanced binding of Sp1 to the HKα promoter. Densitometric quantitation of IL-1β-mediated changes in band intensity of DNA-Sp1 complexes is shown in Fig. 4B, and the specificity of the HKα Sp1 probe was demonstrated by cold competition (Fig. 4C). The fact that DNA-Sp1 complexes persist despite the mutation of six nucleotides suggests that residues flanking the mutated domain may show Sp1 binding affinity but cannot accommodate a functionally competent Sp1 complex formed by IL-1β-mediated signaling.
Given the IL-1β activation of Sp1 and ensuing upregulation of HKα promoter activity, we sought to establish whether H. pylori also modulated Sp1 activity while exerting its NF-κB-mediated repression of HKα promoter activity. Using EMSA and supershift analysis, we assessed the effect of H. pylori on Sp1 binding to the HKα Sp1 footprint region. In the absence of H. pylori, significant binding of Sp1 to HKα promoter, confirmed by Sp1 antibody supershift, was observed (Fig. 4D, compare lanes 1 and 3). No significant change in Sp1 binding to the HKα Sp1 probe was observed in the presence of H. pylori (25 MOI; 4 h; compare lanes 1 and 2, long arrow), and the Sp1 supershifts were comparable with or without H. pylori (compare lanes 3 and 4, arrowhead). The significant attenuation of the slowest migrating band (long arrow) by Sp1 antibody identifies this band as the DNA-Sp1 complex; the two faster migrating bands are complexes of two other nuclear factors with the Sp1 DNA probe. These data confirm our previous result that Sp1 binds to HKα promoter in absence of any external stimulus and also show that H. pylori infection does not modulate Sp1 binding to the HKα promoter region.
Sp1 interacts with NF-κB binding site of HKα promoter.
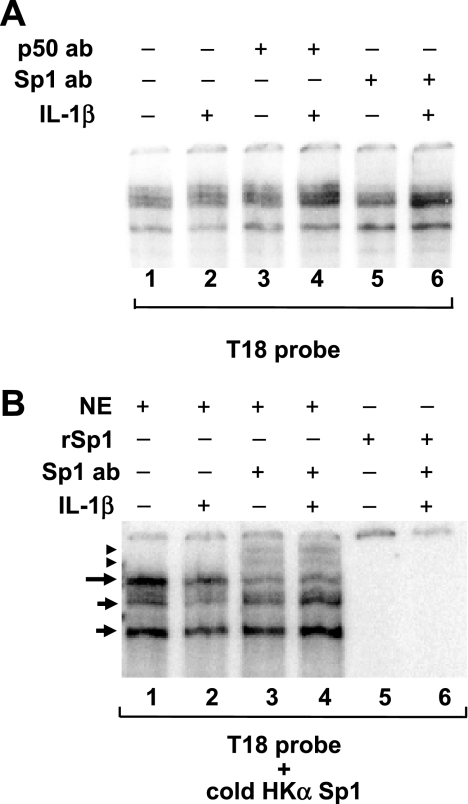
Because our HKα promoter deletion analysis (Fig. 1) had indicated that the NF-κB1 binding site contributes to both basal and IL-1β-stimulated HKα activity, we sought to assess the effects of IL-1β on transcription factor interactions with the HKα NF-κB1 binding site, hypothesizing that Sp1 may exhibit promiscuous binding to an NF-κB site. Nuclear extracts of AGS cells were assayed by EMSA using the T18 HKα promoter DNA probe containing the NF-κB1 binding site (25). As shown in Fig. 5A, IL-1β treatment of AGS cells caused no change in DNA-protein complex band intensities (compare lanes 1 and 2), and neither NF-κB p50 subunit antibody (lanes 3 and 4) nor Sp1 antibody (lanes 5 and 6) produced a supershift of any of these complexes. The data indicate that IL-1β does not modulate nuclear factor interactions with the HKα NF-κB1 binding site and that Sp1 is not a constituent of the DNA-protein complexes formed by the T18 probe of the HKα NF-κB1 binding site. Reasoning that Sp1 may interact with an NF-κB site if bound simultaneously to the Sp1 binding site, we incubated nuclear extracts of AGS cells with a radioactive NF-κB1 T18 probe and a cold (nonradioactive) HKα Sp1 probe. As shown in Fig. 5B, the resulting EMSA banding distributions differed from those acquired with the T18 probe alone (long and short arrows; lanes 1 and 2). Significantly, Sp1 antibody now supershifted the slowest migrating (long arrow) DNA-protein complex, but the supershifted bands (arrowhead; lanes 3 and 4) were unaffected by IL-1β treatment. Coincubation of the T18 probe and cold HKα Sp1 probe with recombinant human Sp1, rather than with AGS cell nuclear extracts, yielded no mobility-shifted bands and no Sp1 supershift (lanes 5 and 6, respectively). Taken together, these data indicate that, in AGS cells, basal and IL-1β-mediated HKα activation involves the interaction of Sp1 and other essential AGS nuclear protein(s) with both the HKα Sp1 and HKα NF-κB1 binding sites.
Fig. 5.
IL-1β modulates Sp1 binding to NF-κB binding region in HKα promoter. A: interactions of AGS cell nuclear extract (NE) with and without IL-1β treatment (10 ng/ml) with 32P-labeled HKα-specific NF-κB1 oligonucleotide probe (T18; Ref. 25) by EMSA and supershift analysis with NF-κB p50 antibody (lanes 3 and 4) or Sp1 antibody (lanes 5 and 6). B: interactions of AGS nuclear extracts (lanes 1-4) or recombinant (r) human Sp1 protein (lanes 5 and 6) with an equimolar mixture of 32P-labeled NF-κB1 T18 probe and cold (nonradioactive) HKα Sp1 probe. Long and short arrows indicate DNA-protein complexes; arrowheads indicate supershifted complexes. Note the supershifts induced by addition of Sp1 antibody in lanes 3 and 4 (change in long arrow band to arrowheads). The gel is a representative EMSA gel from 2 independent experiments.
IL-1β-induced increase in HKα promoter activity is primarily regulated by Sp1.
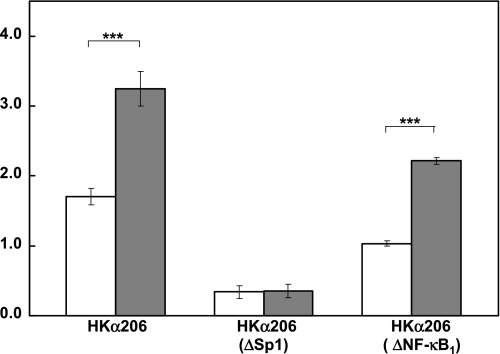
We next investigated the functional consequences of the IL-1β-mediated increased Sp1 binding to a cognate HKα promoter cis-response element. AGS cells were transfected with wild-type HKα206 deletion construct, ΔNF-κB HKα206 with two mutated nucleotides in the NF-κB1 binding region as described previously (25) or ΔSp1 HKα206 bearing a six-nucleotide mutation in the Sp1 cis-response element. Cells were treated with IL-1β (10 ng/ml; 24 h), and the activity of the transfected promoter-reporter constructs was measured by luminometry. When compared with wild-type HKα206, the activity of the ΔSp1 HKα206 construct was reduced by 81% and that of the ΔNF-κB1 HKα206 construct was reduced by 40% (Fig. 6). These data show that both Sp1 and NF-κB1 cis-response elements confer HKα transcriptional activity. An essential requirement for this activity is an intact Sp1 cis-response element, in the absence of which an intact NF-κB1 cis-response element confers neither basal nor IL-1β-stimulated HKα transcriptional activity. IL-1β caused a twofold increase in wild-type HKα206 promoter activity, had no effect on the significantly reduced activity of the ΔSp1 HKα206 construct, and caused a twofold increase in activity of the ΔNF-κB1 HKα206 construct (Fig. 6). These data confirm the functional participation of Sp1 binding in IL-1β-induced HKα promoter upregulation and additionally emphasize a requirement for a functional NF-κB1 binding site on the HKα promoter for maximal IL-1β stimulation of HKα promoter activity.
Fig. 6.
IL-1β-induced increase in HKα promoter activity is regulated by Sp1 and not NF-κB. AGS cells were transiently transfected with either wild-type HKα206 or double-mutated HKα206 (HKα NF-κB1) or Sp1 core-mutated HKα206 (HKαΔSp1) and incubated with (gray bars) or without (white bars) IL-1β (10 ng/ml) for 24 h. HKα206 promoter activity was measured as normalized relative light units. Data are shown as means ± SD of 3 independent experiments. ***P < 0.001.
Sp1 downregulation abrogates IL-1β-induced increase in HKα promoter activity.
Further confirmation that Sp1 mediates IL-1β-induced HKα promoter activation was obtained by transfecting AGS cells with Sp1-specific siRNA and subsequently measuring transfected HKα promoter activity in response to IL-1β treatment. Sp1 mRNA levels in AGS cells 48 h after transfection with a pool of four different Sp1 siRNAs, or with one of three individual siRNA members of the pool, were <35% of the levels measured in AGS cells transfected with either nontargeting siRNA (nt siRNA) or transfection reagent alone (control), as measured by RT-PCR (Fig. 7A). Of the three individual siRNAs, siRNA 3 showed maximum knockdown of Sp1 mRNA (>90%). Sp1 protein levels 72 h after transfection with Sp1 siRNA 3 were similarly downregulated as measured by Sp1-specific antibody immunoblotting of AGS cell lysates (Fig. 7B). AGS cell transfections with the other two Sp1-specific siRNAs caused comparable downregulation of Sp1 protein expression (data not shown). Finally, AGS cells were transfected with Sp1 siRNA 3 for 48 h and then transfected with the HKα206 deletion construct for 24 h. The cells were then incubated with IL-1β (10 ng/ml; 24 h). As shown in Fig. 7C, IL-1β treatment failed to elicit the activation of the HKα construct. Interestingly, the basal activity of HKα206 deletion construct was reduced by 22% in Sp1-specific siRNA-transfected AGS cells compared with non-siRNA-transfected AGS cells, a finding consistent with significantly reduced levels of cellular Sp1 being sufficient to promote HKα transcriptional activity and with the possibility that cis-activation sites other than Sp1 sites may contribute to HKα basal transcription.
Fig. 7.
Sp1 downregulation abrogates IL-1β-induced increase in HKα promoter activity. A: AGS cells were transfected with Sp1-specific small interfering RNA (siRNA) for 48 h, and the cellular content of Sp1 in presence and absence of siRNA was measured by real-time PCR using β2-microglobin as the internal control. Control, mock-transfected AGS cells; siRNA pool, AGS cells transfected with a mixture of 4 different siRNAs; siRNA 1, siRNA 2, and siRNA 3, AGS cells transfected with individual siRNAs; nontargeting (nt) siRNA, AGS cells transfected with nt siRNA as a negative control. Control and nt siRNA-transfected cells were harvested at the same time point as siRNA-treated cells. Data are shown as means ± SD of 3 independent experiments (***P < 0.001 compared with control). B: AGS cells were transfected with Sp1-specific siRNA 3, and the cellular content of Sp1 protein was measured after 72 h by immunoblotting using β-actin as the internal control. Control, mock-transfected AGS cells; siRNA, AGS cells transfected with siRNA 3 for 72 h. C: AGS cells were transfected with Sp1-specific siRNA 3 for 48 h, transfected with HKα206 for 24 h, and then treated with IL-1β for 24 h. HKα206 promoter activity was expressed as normalized relative light units (RLU) of luciferase activity. Data are shown as means ± SD of 3 independent experiments. ***P < 0.001.
DISCUSSION
The present study explored the mechanistic basis of IL-1β perturbation of HKα promoter activity at the level of Sp1 and NF-κB transcription factor interactions with the promoter. Our recent report of IL-1β activation, and not inhibition, of human HKα promoter-Luc reporter constructs transfected into AGS cells (24) suggests that IL-1β inhibits acid secretion at a posttranslational rather than transcriptional level. As a potent inhibitor of gastric acid secretion, IL-1β probably contributes to H. pylori-induced hypochlorhydria through impairment of inositol (1,4,5)-triphosphate-dependent increase in intracellular Ca2+ concentration (26) and consequent failure of parietal cell tubulovesicular fusion and H,K-ATPase activation. Evidence that H. pylori interferes with the normal regulation of HKα transcription has accrued from studies examining the effects of H. pylori in the HKα promoter-transfected AGS cell model (13, 25), and we have recently reported that the mechanistic basis of HKα downregulation by H. pylori is ERK1/2-mediated NF-κB p50 homodimer binding to an NF-κB1 cis-response element at −170 to −150 bp on the HKα 5′-flanking sequence (25).
We find in the present study that although IL-1β promotes Sp1 binding to a defined HKα cis-response element, the consequent activation of HKα promoter activity is mediated by Sp1 interaction with both the Sp1 and NF-κB binding sites. These findings are based on several lines of experimental evidence. First, deletion analysis of a 2,179-bp human HKα 5′-flanking sequence showed that IL-1β treatment of transfected AGS cells significantly upregulated the activity of deletion constructs encompassing only NF-κB and Sp1 binding sites, and this activation was sustained though less pronounced with progressive deletion of the NF-κB sites; H. pylori treatment of the same constructs inhibited their transcriptional activity. Second, IL-1β increased the expression of phospho-ERK, phospho-Sp1, and phospho-p50 NF-κB subunits without affecting expression of total ERK1/2, Sp1 and NF-κB p50. Third, DNase I footprints of recombinant Sp1 subunit on a 206-bp length of HKα promoter showed the presence of an Sp1 binding site from −56 bp to −39 bp. Fourth, IL-1β increased binding of Sp1 to the proximal HKα promoter, whereas NF-κB p50 interaction with NF-κB1 site was not detected; in contrast, H. pylori had no effect on Sp1 binding to the promoter. Fifth, Sp1 was detected as a constituent of the NF-κB1 DNA-protein complex by the coincubation of HKα Sp1 and NF-κB binding site probes with AGS cell nuclear extracts. Sixth, the core mutation of the HKα Sp1 binding site abrogated IL-1β-induced activation of the HKα promoter, whereas mutation of two nucleotides in the HKα NF-κB binding site did not. Finally, the IL-1β-induced activation of HKα promoter was prevented by siRNA knockdown of Sp1.
These results illuminate aspects of the regulation of normal HKα expression and the mechanisms of H. pylori and IL-1β perturbation of this regulation. In isolated canine parietal cells transfected with HKα promoter deletion constructs, basal transcriptional activity of H,K-ATPase α-subunit promoter was shown to be conferred by Sp1 binding to −54 to −45 bp of the promoter (20). The human HKα Sp1 site we identified is 80% homologous to the canine HKα Sp1 binding sequence, and its 5′ addition to the proximal 37 bp of an HKα promoter construct confers a 5.5-fold increase in basal HKα transcriptional activity in transfected AGS cells. Further additions of HKα sequence confer progressively greater basal HKα activity, culminating in the 15-fold activation exerted by a 206-bp HKα promoter construct. Since 5′ extension of the HKα promoter construct from −102 bp to −206 bp adds two NF-κB1 binding sites, the resulting enhancement of HKα basal transcriptional activity may be attributed to cooperative binding of nuclear factors to both the Sp1 site and at least one of the NF-κB sites. This was confirmed by deletion of two nucleotides in the core recognition motif of the proximal NF-κB1 sequence, which reduced HKα basal activity by 40% (Fig. 6). We detected no NF-κB p50 subunit in control AGS cell nuclear extracts, consistent with the insignificant activation of NF-κB in resting gastric epithelial cells in vivo in the absence of proinflammatory stimuli. We propose therefore that the cooperative participation of the HKα NF-κB1 binding site in basal HKα transcriptional activity reflects the interaction of Sp1 with this site. Functional interaction of Sp1 with NF-κB sites has been reported in HeLa cells transfected with P-selectin promoter-reporter constructs and treated with TNF-α (15). Sp1 and NF-κB sites also cooperate in mediating basal and interferon γ-stimulated CD98 transcription in transiently transfected Caco-BBE epithelial cells (37).
The same nuclear factor binding sites were implicated in IL-1β activation of HKα promoter constructs. IL-1β caused the 26-fold activation of the 206-bp HKα construct compared with the 37-bp construct. Although the progressive 5′ extension of the HKα promoter included both a partial (HKα165) and a complete (HKα177) NF-κB1 binding site, NF-κB subunits themselves play no role in IL-1β-induced HKα activation as shown by the failure of a mutated NF-κB1 binding site to abrogate this activation. As appears to be the case in the regulation of basal HKα transcription, Sp1 binding to both Sp1 and NF-κB cis-response elements may underlie IL-1β-mediated upregulation of HKα transcription. Sp1 binding to the HKα NF-κB1 site would prevent the interaction of NF-κB p50 homodimers with that site and preempt consequent repression of HKα promoter activity (25). With the use of a consensus NF-κB probe, the significant activation of both NF-κB p50 and p65 subunits was reported in AGS cells exposed to 25 ng/ml IL-1β for 2 h (17), presumably allowing the formation of p50/p65 heterodimers and p50/p50 homodimers. In the present study, using a HKα-specific NF-κB1 probe (T18), we detected no binding of p50 subunits in nuclear extracts of AGS cells exposed to 10 ng/ml IL-1β for 4 h, and so the failure of IL-1β to repress HKα transcription is consistent with the inability of NF-κB p50 homodimers to bind to the NF-κB1 site due to site occupancy by Sp1.
The functional significance in a physiological setting (i.e., in vivo gastric mucosa) of nuclear factor interactions with the HKα promoter described in this article cannot be readily assessed. AGS cells are useful in studies of H. pylori pathophysiology because acquisition and maintenance of human gastric parietal cells in culture in a functionally competent state for longer than 3 or 4 days is extremely difficult. As transformed dedifferentiated cells originating in a gastric adenocarcinoma (1), AGS cells differ in many respects from terminally differentiated gastric parietal cells, not least in their lack of proton pump expression. Accordingly, H. pylori or IL-1β-driven interactions of AGS cell transcription factors with transfected proton pump promoter constructs may not faithfully recapitulate IL-1 receptor-mediated and H. pylori-mediated perturbations of parietal cell intracellular signaling. The net impact on parietal cell HKα transcription of the integrated contributions of both perturbations is presumably modulated by numerous other considerations. These include relative concentrations, phosphorylation status, and access of transcription factors and related accessory proteins to promoter DNA, appropriate assembly of transcriptional complexes on the promoter, acetylation status of nucleosomal histones, etc., all conditions that are unique to the parietal cell and are necessarily different in the AGS cell model used in this study. However, reported parallels between AGS cells and parietal cells include the presence of functional histamine H2 receptors (13), EGF receptors (18), components of proinflammatory signaling pathways (19), IL-8 secretory capacity (8, 28), and sensitivity to H. pylori and IL-1β (17). That Sp1 confers basal transcriptional activity on transfected HKα promoter-reporter constructs both in AGS cells (this article) and in primary canine parietal cells (20) lends further credence to AGS cells as an experimental surrogate for human parietal cells.
Our demonstration that IL-1β enhances Sp1 binding to the HKα promoter and that the consequent activation of the promoter is further enhanced by the presence of the NF-κB1 cis-response element, without detectable increased binding of NF-κB p50 subunit, suggests that IL-1β does not exert an acid inhibitory effect at the level of HKα transcription. Our previous report that NF-κB p50 homodimer binding to the HKα promoter NF-κB1 site is responsible for H. pylori-mediated HKα gene repression (25) in AGS cells suggests that the same mechanism plays a role in H. pylori-mediated clinical hypochlorhydria. Interestingly, EGF activation of HKα gene transcription in canine parietal cells (16) has been attributed to the activation of the Akt signal transduction pathway (32) and binding of cis-regulatory protein to a canine HKα cis-response element between −162 and −156 bp (5′-GACATGG-3′) (16) that exhibits 100% sequence homology with the human HKα NF-κB1 site mediating the H. pylori repression of HKα promoter activity (25). We have also reported the EGF activation of human HKα full-length promoter-Luc construct in AGS cells, although its mechanistic basis was not explored (13). EGF is known to activate NF-κB in nonsmall cell lung carcinoma cell lines (27), in breast cancer cells (3), in A-431 carcinoma cells and mouse embryo fibroblasts (31), and in human proximal tubule cells (14). Accordingly, we hypothesize that the EGF activation of NF-κB in gastric epithelial cells favors the assembly of NF-κB p50/p65 heterodimers that activate HKα gene expression, in contrast with the H. pylori induction of NF-κB p50/p50 homodimers that repress HKα gene expression. Also of interest is the observation that EGF activates gastrin promoter constructs transfected into AGS cells by inducing the phosphorylation of Sp1 (6), and so cooperative interactions between Sp1 and NF-κB and their cognate cis-response elements may have significant regulatory consequences in EGF-stimulated signaling in gastric epithelial cells.
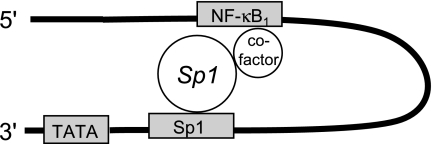
In conclusion, we have found that IL-1β upregulates the activity of human H,K-ATPase α-subunit proximal promoter sequences through Sp1 transactivation of a cis-response element located between −56 bp to −39 bp upstream of the HKα transcription start site that also confers basal HKα transcriptional activity. Our data are consistent with the model of Sp1 interaction with the HKα promoter shown in Fig. 8, whereby Sp1 confers basal and IL-1β-mediated HKα promoter activity by direct binding to the HKα Sp1 cis-response element and indirect binding to the HKα NF-κB1 binding site via the concurrent recruitment of nuclear cofactor(s). NF-κB subunits do not participate in this transactivation complex. IL-1β augments Sp1 binding to the HKα Sp1 cis-response element through the activation of an ERK1/2 signaling pathway, enhancing HKα promoter activity. Sp1 transactivation of HKα promoter is not perturbed by H. pylori, which represses HKα transcription by the mobilization of NF-κB p50 homodimers. These findings provide a mechanistic understanding of the differential effects of H. pylori and IL-1β on H,K-ATPase α-subunit gene expression and uncover a novel mechanism of IL-1β-induced HKα promoter regulation.
Fig. 8.
Schematic diagram of proposed interaction of Sp1 transcription factor with Sp1 and NF-κB binding sites on the proximal HKα promoter. The proximal 5′-flanking sequence of the HKα promoter is represented by the black hairpin; gray boxes represent transcription factor binding sites, and white circles represent Sp1 protein and putative cofactor(s) required for Sp1-mediated coupling of the Sp1 and NF-κB binding sites.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-064371 (to A. J. Smolka).
Acknowledgments
We thank Dr. Gary Shull (University of Cincinnati) for the generous provision of a plasmid containing 2,199 bp of human gastric H,K-ATPase α-subunit 5′-flanking DNA and Dr. Richard M. Peek, Jr. (Vanderbilt University School of Medicine), for critical reading of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barranco SC, Townsend CM Jr, Casartelli C, Macik BG, Burger NL, Boerwinkle WR, Gourley WK. Establishment and characterization of an in vitro model system for human adenocarcinoma of the stomach. Cancer Res 43: 1703–1709, 1983. [PubMed] [Google Scholar]
- 2.Basso D, Scrigner M, Toma A, Navaglia F, Di Mario F, Rugge M, Plebani M. Helicobacter pylori infection enhances mucosal interleukin-1 beta, interleukin-6, and the soluble receptor of interleukin-2. Int J Clin Lab Res 26: 207–210, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Biswas DK, Cruz AP, Gansberger E, Pardee AB. Epidermal growth factor-induced nuclear factor kappa B activation: a major pathway of cell-cycle progression in estrogen-receptor negative breast cancer cells. Proc Natl Acad Sci USA 97: 8542–8547, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brasse-Lagnel C, Lavoinne A, Loeber D, Fairand A, Bole-Feysot C, Deniel N, Husson A. Glutamine and interleukin-1β interact at the level of Sp1 and nuclear factor-κB to regulate argininosuccinate synthetase gene expression. FEBS J 274: 5250–5262, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Campbell VW, Yamada T. Acid secretagogue-induced stimulation of gastric parietal cell gene expression. J Biol Chem 264: 11381–11386, 1989. [PubMed] [Google Scholar]
- 6.Chupreta S, Du M, Todisco A, Merchant JL. EGF stimulates gastrin promoter through activation of Sp1 kinase activity. Am J Physiol Cell Physiol 278: C697–C708, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Correa P Human gastric carcinogenesis: a multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 52: 6735–6740, 1992. [PubMed] [Google Scholar]
- 8.Crabtree JE, Farmery SM, Lindley IJ, Figura N, Peichl P, Tompkins DS. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol 47: 945–950, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cramer T, Juttner S, Plath T, Mergler S, Seufferlein T, Wang TC, Merchant J, Hocker M. Gastrin transactivates the chromogranin A gene through MEK-1/ERK- and PKC-dependent phosphorylation of Sp1 and CREB. Cell Signal 20: 60–72, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Derakhshan MH, El-Omar E, Oien K, Gillen D, Fyfe V, Crabtree JE, McColl KE. Gastric histology, serological markers and age as predictors of gastric acid secretion in patients infected with Helicobacter pylori. J Clin Pathol 59: 1293–1299, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JF Jr, Rabkin CS. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404: 398–402, 2000. [DOI] [PubMed] [Google Scholar]
- 12.El-Omar EM, Oien K, El-Nujumi A, Gillen D, Wirz A, Dahill S, Williams C, Ardill JE, McColl KE. Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology 113: 15–24, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Gooz M, Hammond CE, Larsen K, Mukhin YV, Smolka AJ. Inhibition of human gastric H+-K+-ATPase α-subunit gene expression by Helicobacter pylori. Am J Physiol Gastrointest Liver Physiol 278: G981–G991, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Haussler U, von Wichert G, Schmid RM, Keller F, Schneider G. Epidermal growth factor activates nuclear factor-κB in human proximal tubule cells. Am J Physiol Renal Physiol 289: F808–F815, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Hirano F, Tanaka H, Hirano Y, Hiramoto M, Handa H, Makino I, Scheidereit C. Functional interference of Sp1 and NF-kappaB through the same DNA binding site. Mol Cell Biol 18: 1266–1274, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaise M, Muraoka A, Yamada J, Yamada T. Epidermal growth factor induces H+,K+-ATPase alpha-subunit gene expression through an element homologous to the 3′ half-site of the c-fos serum response element. J Biol Chem 270: 18637–18642, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Keates S, Hitti YS, Upton M, Kelly CP. Helicobacter pylori infection activates NF-kappa B in gastric epithelial cells. Gastroenterology 113: 1099–1109, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Keates S, Sougioultzis S, Keates AC, Zhao D, Peek RM Jr, Shaw LM, Kelly CP. cag+ Helicobacter pylori induce transactivation of the epidermal growth factor receptor in AGS gastric epithelial cells. J Biol Chem 276: 48127–48134, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Maeda S, Otsuka M, Hirata Y, Mitsuno Y, Yoshida H, Shiratori Y, Masuho Y, Muramatsu Ma Seki N, Omata M. cDNA microarray analysis of helicobacter pylori-mediated alteration of gene expression in gastric cancer cells. Biochem Biophys Res Commun 284: 443–449, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Muraoka A, Kaise M, Guo YJ, Yamada J, Song I, DelValle J, Todisco A, Yamada T. Canine H+-K+-ATPase α-subunit gene promoter: studies with canine parietal cells in primary culture. Am J Physiol Gastrointest Liver Physiol 271: G1104–G1113, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Mushiake S, Etani Y, Shimada S, Tohyama M, Hasebe M, Futai M, Maeda M. Genes for members of the GATA-binding protein family (GATA-GT1 and GATA-GT2) together with H+/K+-ATPase are specifically transcribed in gastric parietal cells. FEBS Lett 340: 117–120, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Peek RM, Miller GG, Tham KT, Perez-Perez GI, Zhao X, Atherton JC, Blaser MJ. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest 73: 760–770, 1995. [PubMed] [Google Scholar]
- 23.Piera-Velazquez S, Hawkins DF, Whitecavage MK, Colter DC, Stokes DG, Jimenez SA. Regulation of the human SOX9 promoter by Sp1 and CREB. Exp Cell Res 313: 1069–1079, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saha A, Hammond CE, Gooz M, Smolka AJ. IL-1β modulation of H,K-ATPase α-subunit gene transcription in Helicobacter pylori infection. Am J Physiol Gastrointest Liver Physiol 292: G1055–G1061, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Saha A, Hammond CE, Trojanowska M, Smolka AJ. Helicobacter pylori-induced H,K-ATPase α-subunit gene repression is mediated by NF-κB p50 homodimer promoter binding. Am J Physiol Gastrointest Liver Physiol 294: G795–G807, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Schepp W, Dehne K, Herrmuth H, Pfeffer K, Prinz C. Identification and functional importance of IL-1 receptors on rat parietal cells. Am J Physiol Gastrointest Liver Physiol 275: G1094–G1105, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Sethi G, Ahn KS, Chaturvedi MM, Aggarwal BB. Epidermal growth factor (EGF) activates nuclear factor-kappaB through IkappaBalpha kinase-independent but EGF receptor-kinase dependent tyrosine 42 phosphorylation of IkappaBalpha. Oncogene 26: 7324–7332, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Sharma SA, Tummuru MK, Miller GG, Blaser MJ. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun 63: 1681–1687, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith MF, Mitchell A, Li G, Ding S, Fitzmaurice AM, Ryan K, Crowe S, Goldberg JB. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-κB activation and chemokine expression by epithelial cells. J Biol Chem 278: 32552–32560, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Strowski MZ, Cramer T, Schafer G, Juttner S, Walduck A, Schipani E, Kemmner W, Wessler S, Wunder C, Weber M, Meyer TF, Wiedenmann B, Jons T, Naumann M, Hocker M. Helicobacter pylori stimulates host vascular endothelial growth factor-A (vegf-A) gene expression via MEK/ERK-dependent activation of Sp1 and Sp3. FASEB J 18: 218–220, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Sun L, Carpenter G. Epidermal growth factor activation of NF-kappaB is mediated through IkappaBalpha degradation and intracellular free calcium. Oncogene 16: 2095–2102, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Todisco A, Pausawasdi N, Ramamoorthy S, Del Valle J, Van Dyke RW, Askari FK. Functional role of protein kinase B/Akt in gastric acid secretion. J Biol Chem 276: 46436–46444, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Memet S, Huerre MR, Coyle AJ, DiStefano PS, Sansonetti PJ, Labigne A, Bertin J, Philpott DJ, Ferrero RL. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol 5: 1166–1174, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Wallace JL, Cucala M, Mugridge K, Parente L. Secretagogue-specific effects of interleukin-1 on gastric acid secretion. Am J Physiol Gastrointest Liver Physiol 261: G559–G564, 1991. [DOI] [PubMed] [Google Scholar]
- 35.Wang M, Furuta T, Takashima M, Futami H, Shirai N, Hanai H, Kaneko E. Relation between interleukin-1beta messenger RNA in gastric fundic mucosa and gastric juice pH in patients infected with Helicobacter pylori. J Gastroenterol 34, Suppl 11: 10–17, 1999. [PubMed] [Google Scholar]
- 36.Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut 41: 442–451, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan Y, Dalmasso G, Sitaraman S, Merlin D. Characterization of the human intestinal CD98 promoter and its regulation by interferon-γ. Am J Physiol Gastrointest Liver Physiol 292: G535–G545, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Zavros Y, Eaton KA, Kang W, Rathinavelu S, Katukuri V, Kao JY, Samuelson LC, Merchant JL. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene 24: 2354–2366, 2005. [DOI] [PubMed] [Google Scholar]