Abstract
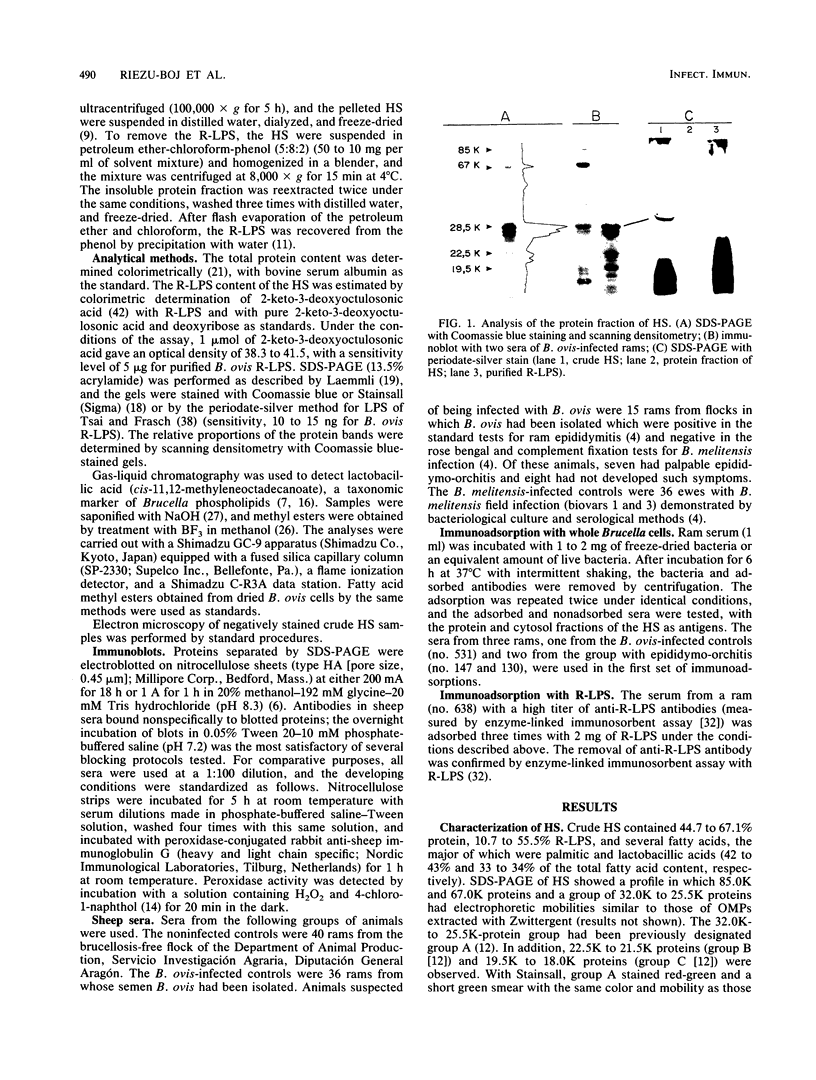
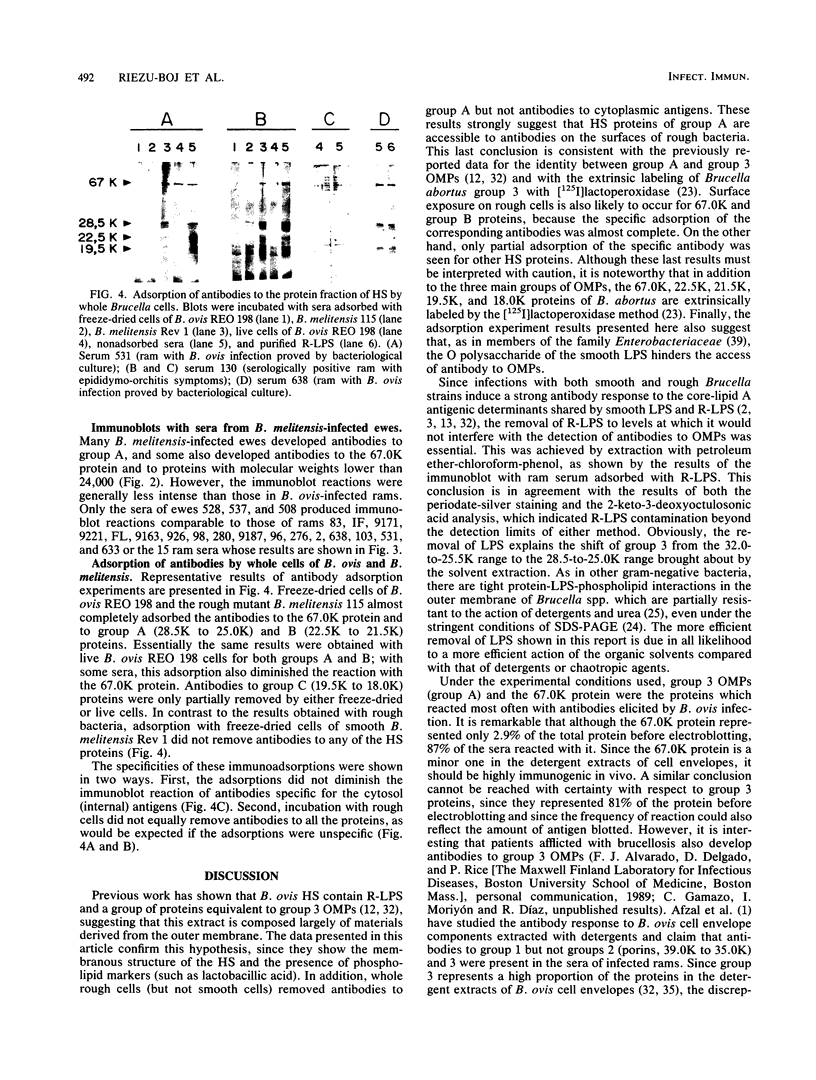
Hot saline extracts of Brucella ovis were composed of vesicles with outer membrane proteins (OMPs), lipopolysaccharide, and phospholipid as constituents. Extraction with petroleum ether-chloroform-phenol yielded a protein fraction free of detectable lipopolysaccharide, in which group 3 OMPs (28,500 apparent molecular weight [28.5K], 27.0K, and 25.5K) represented 81% of the total. Group 1 OMPs and 67.0K, 22.5K to 21.5K, and 19.5K to 18.0K proteins were also detected. Adsorption of immune sera with whole bacteria suggested that group 3 OMPs and 67.0K, 22.5K to 21.5K, and 19.5K to 18.0K proteins had antigenic determinants exposed on the surfaces of both B. ovis and rough B. melitensis cells but not on smooth B. melitensis cells. Antibodies to group 3 OMPs and the 67.0K protein in the sera of 93 and 87%, respectively, of B. ovis-infected rams were found by immunoblotting. Antibodies to other proteins were present in 67% of these animals. Compared with B. ovis-infected rams which had not developed lesions, rams with epididymo-orchitis had antibodies to a larger variety of proteins. Although ewes infected with B. melitensis also showed antibodies to OMPs, the immunoblot reactions were less intense.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afzal M., Brodie S. J., Tengerdy R. P., Squire P. G. Isolation and antigenic reactivity of Brucella ovis outer membrane proteins. J Clin Microbiol. 1987 Nov;25(11):2132–2135. doi: 10.1128/jcm.25.11.2132-2135.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal M., Tengerdy R. P., Squire P. G., Ellis R. P. Characterization of Brucella ovis lipopolysaccharide and its use for diagnosis of ram epididymitis by enzyme-linked immunosorbent assay. J Clin Microbiol. 1984 Dec;20(6):1159–1164. doi: 10.1128/jcm.20.6.1159-1164.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Urmeneta B., Moriyón I., Díaz R., Blasco J. M. Enzyme-linked immunosorbent assay with Brucella native hapten polysaccharide and smooth lipopolysaccharide. J Clin Microbiol. 1988 Dec;26(12):2642–2646. doi: 10.1128/jcm.26.12.2642-2646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Coloe P. J., Sinclair A. J., Slattery J. F., Burke D. Differentiation of Brucella ovis from Brucella abortus by gas-liquid chromatographic analysis of cellular fatty acids. J Clin Microbiol. 1984 Jun;19(6):896–898. doi: 10.1128/jcm.19.6.896-898.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubray G., Plommet M. Structure et constituants des Brucella. Caractérisation des fractions et propriétés biologiques. Dev Biol Stand. 1976;31:68–91. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gamazo C., Winter A. J., Moriyón I., Riezu-Boj J. I., Blasco J. M., Díaz R. Comparative analyses of proteins extracted by hot saline or released spontaneously into outer membrane blebs from field strains of Brucella ovis and Brucella melitensis. Infect Immun. 1989 May;57(5):1419–1426. doi: 10.1128/iai.57.5.1419-1426.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Miguel M. J., Moriyón I., Alonso-Urmeneta B., Riezu-Boj J. I., Díaz R. Serological response to the outer membrane lipoprotein in animal brucellosis. Infect Immun. 1988 Mar;56(3):716–718. doi: 10.1128/iai.56.3.716-718.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hughes K. L., Claxton P. D. Brucella ovis infection. I. An evaluation of microbiological, serological and clinical methods of diagnosis in the ram. Aust Vet J. 1968 Feb;44(2):41–47. doi: 10.1111/j.1751-0813.1968.tb04951.x. [DOI] [PubMed] [Google Scholar]
- Jantzen E., Knudsen E., Winsnes R. Fatty acid analysis for differentiation or Bordetella and Brucella species. Acta Pathol Microbiol Immunol Scand B. 1982 Oct;90(5):353–359. doi: 10.1111/j.1699-0463.1982.tb00131.x. [DOI] [PubMed] [Google Scholar]
- King L. E., Jr, Morrison M. The visualization of human erythrocyte membrane proteins and glycoproteins in SDS polyacrylamide gels employing a single staining procedure. Anal Biochem. 1976 Mar;71(1):223–230. doi: 10.1016/0003-2697(76)90031-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Limet J., Plommet A. M., Dubray G., Plommet M. Immunity conferred upon mice by anti-LPS monoclonal antibodies in murine brucellosis. Ann Inst Pasteur Immunol. 1987 May-Jun;138(3):417–424. doi: 10.1016/s0769-2625(87)80052-1. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Montaraz J. A., Winter A. J., Hunter D. M., Sowa B. A., Wu A. M., Adams L. G. Protection against Brucella abortus in mice with O-polysaccharide-specific monoclonal antibodies. Infect Immun. 1986 Mar;51(3):961–963. doi: 10.1128/iai.51.3.961-963.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyon I., Berman D. T. Effects of nonionic, ionic, and dipolar ionic detergents and EDTA on the Brucella cell envelope. J Bacteriol. 1982 Nov;152(2):822–828. doi: 10.1128/jb.152.2.822-828.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyón I., Gamazo C., Díaz R. Properties of the outer membrane of Brucella. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):89–91. doi: 10.1016/0769-2609(87)90082-2. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Lambert M. A., Merwin W. H. Comparison of rapid methods for analysis of bacterial fatty acids. Appl Microbiol. 1974 Jul;28(1):80–85. doi: 10.1128/am.28.1.80-85.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers D. M., Jones L. M., Varela-Diaz V. M. Studies of antigens for complement fixation and gel diffusion tests in the diagnosis of infections caused by Brucella ovis and other Brucella. Appl Microbiol. 1972 May;23(5):894–902. doi: 10.1128/am.23.5.894-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers D. M., Varela-Diaz V. M. Serodiagnosis of ram epididymitis by counterimmunoelectrophoresis, using Brucella ovis surface R antigen. J Clin Microbiol. 1979 Oct;10(4):451–453. doi: 10.1128/jcm.10.4.451-453.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon P. Resistance against a subcutaneous Brucella challenge of mice immunized with living or dead Brucella or by transfer of immune serum. Ann Immunol (Paris) 1977 Nov-Dec;128(6):1025–1037. [PubMed] [Google Scholar]
- Plommet M., Plommet A. M. Immune serum-mediated effects on brucellosis evolution in mice. Infect Immun. 1983 Jul;41(1):97–105. doi: 10.1128/iai.41.1.97-105.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezu-Boj J. I., Moriyón I., Blasco J. M., Marín C. M., Diaz R. Comparison of lipopolysaccharide and outer membrane protein-lipopolysaccharide extracts in an enzyme-linked immunosorbent assay for the diagnosis of Brucella ovis infection. J Clin Microbiol. 1986 May;23(5):938–942. doi: 10.1128/jcm.23.5.938-942.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris D. R., Hamel K. L., Long D. L. Comparison of an enzyme-linked immunospecific assay (ELISA) with the cold complement fixation test for the serodiagnosis of Brucella ovis infection. N Z Vet J. 1984 Jan-Feb;32(1-2):18–20. doi: 10.1080/00480169.1984.35048. [DOI] [PubMed] [Google Scholar]
- SULITZEANU D., JONES L., STABLEFORTH A. W. Protective action of monospecific anti-Brucella sera in mice. Nature. 1955 Jun 11;175(4467):1040–1041. doi: 10.1038/1751040a0. [DOI] [PubMed] [Google Scholar]
- Santos J. M., Verstreate D. R., Perera V. Y., Winter A. J. Outer membrane proteins from rough strains of four Brucella species. Infect Immun. 1984 Oct;46(1):188–194. doi: 10.1128/iai.46.1.188-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer T. L., Burgess G. W. Enzyme-linked immunosorbent assay for Brucella ovis specific antibody in ram sera. Res Vet Sci. 1984 Mar;36(2):194–198. [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Walker R. L., LeaMaster B. R., Stellflug J. N., Biberstein E. L. Use of enzyme-linked immunosorbent assay for detection of antibodies to Brucella ovis in sheep: field trial. Am J Vet Res. 1985 Aug;46(8):1642–1646. [PubMed] [Google Scholar]
- Webb R. F., Quinn C. A., Cockram F. A., Husband A. J. Evaluation of procedures for the diagnosis of Brucella ovis infection in rams. Aust Vet J. 1980 Apr;56(4):172–175. doi: 10.1111/j.1751-0813.1980.tb05673.x. [DOI] [PubMed] [Google Scholar]
- Winter A. J., Rowe G. E., Duncan J. R., Eis M. J., Widom J., Ganem B., Morein B. Effectiveness of natural and synthetic complexes of porin and O polysaccharide as vaccines against Brucella abortus in mice. Infect Immun. 1988 Nov;56(11):2808–2817. doi: 10.1128/iai.56.11.2808-2817.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ley P., Kuipers O., Tommassen J., Lugtenberg B. O-antigenic chains of lipopolysaccharide prevent binding of antibody molecules to an outer membrane pore protein in Enterobacteriaceae. Microb Pathog. 1986 Feb;1(1):43–49. doi: 10.1016/0882-4010(86)90030-6. [DOI] [PubMed] [Google Scholar]