Abstract
Transepithelial Cl− transport in salivary gland ducts is a major component of the ion reabsorption process, the final stage of saliva production. It was previously demonstrated that a Cl− current with the biophysical properties of ClC-2 channels dominates the Cl− conductance of unstimulated granular duct cells in the mouse submandibular gland. This inward-rectifying Cl− current is activated by hyperpolarization and elevated intracellular Cl− concentration. Here we show that ClC-2 immunolocalized to the basolateral region of acinar and duct cells in mouse salivary glands, whereas its expression was most robust in granular and striated duct cells. Consistent with this observation, nearly 10-fold larger ClC-2-like currents were observed in granular duct cells than the acinar cells obtained from submandibular glands. The loss of inward-rectifying Cl− current in cells from Clcn2−/− mice confirmed the molecular identity of the channel responsible for these currents as ClC-2. Nevertheless, both in vivo and ex vivo fluid secretion assays failed to identify significant changes in the ion composition, osmolality, or salivary flow rate of Clcn2−/− mice. Additionally, neither a compensatory increase in Cftr Cl− channel protein expression nor in Cftr-like Cl− currents were detected in Clcn2 null mice, nor did it appear that ClC-2 was important for blood-organ barrier function. We conclude that ClC-2 is the inward-rectifying Cl− channel in duct cells, but its expression is not apparently required for the ion reabsorption or the barrier function of salivary ductal epithelium.
Keywords: inward-rectifying chloride current, barrier function, NaCl absorption
the clc family of Cl− channels and transporters (also known as CLCN) is comprised of nine members in mammals and can be grouped according to their mode of Cl− transport and cellular location (24). The ubiquitously expressed ClC-2 protein belongs to the group of Cl− channels that function mostly in the cytoplasmic membrane (24, 41, 46). Other members of this gene family act as intracellular Cl− channels or H+/Cl− exchangers (31). Analysis of genetic diseases in humans and of the phenotype of Clcn2 null mice suggests important physiological roles for ClC-2 in several different tissues. Human mutations in the gene encoding ClC-2 (CLCN2) have been linked to epilepsy (3, 18, 20), whereas Clcn2−/− mice develop severe degradation of the retina and the testis (6, 35). It has been hypothesized that disruption of ClC-2 expression impairs transepithelial transport of the blood-organ barrier, and this change in the environment of photoreceptors and spermatocytes leads to cell death and ultimately to blindness and infertility (6). Moreover, impairment of the blood-brain barrier has been observed in Clcn2−/− mice, although these mice do not appear to develop epilepsy (5). It has also been proposed that stimulation of ClC-2 channels can induce recovery of the epithelial barrier function of ischemia-injured ileum and colon (32, 33). The functional significance of ClC-2 for epithelial transport and barrier functions in most organs remains to be evaluated.
Three major pairs of glands (parotid, submandibular, and sublingual) produce saliva in response to autonomic stimulation (9, 30). The cells within the secretory endpieces are distinct in each gland type (in rodents: parotid-serous cells, submandibular-seromucous cells, and sublingual-mucous with serous demilune cells). Nevertheless, all salivary glands make saliva in two stages, both of which are Cl− dependent. Initially, transepithelial Cl− movement across acinar cells generates a plasma-like isotonic fluid, the so-called primary saliva. Cl− channels are the major anion efflux pathway in the apical membrane of acinar cells, whereas the basolateral Na+-coupled Cl− cotransporter Nkcc1 is the primary Cl− uptake mechanism (17). In an earlier study of mouse parotid acinar cells we characterized the properties of ClC-2-like currents (35), one of several types of Cl− currents in this cell type (2, 44). Functional analysis of gene-targeted Clcn2 null mice demonstrated that the ClC-2 channel does not appear to participate in the secretion of saliva (35). This result is consistent with the currently accepted secretory model whereby Ca2+-activated Cl− channels have a central role in this process (9, 29, 30).
Secondarily, salivary gland ducts reabsorb much of the Na+ and Cl− secreted by acinar cells, and because ducts are relatively impermeable to water, the final saliva is markedly hypotonic (9). NaCl reabsorption is most robust in the submandibular gland. The duct system in all salivary glands is composed of intercalated, striated, and excretory ducts, with granular duct cells being especially prominent in the submandibular glands of rodents. Although the ion transport machinery is not well understood in salivary ducts, Cl− channels are thought to be required for efficient NaCl reabsorption (9, 30). Indeed, several distinct Cl− currents have been identified in salivary duct cells that might support transepithelial Cl− transport. Among them is a cAMP-activated current generated by the Cftr Cl− channel located in the apical membrane of salivary gland duct cells (21, 45). Functional studies suggest that the Cftr channel very likely contributes to NaCl reabsorption across the apical membrane (21, 44, 45). In contrast, the basolateral Cl− efflux pathway is unknown but may involve Cl− channels. Other Cl− currents expressed in duct cells include inward-rectifying, Ca2+-activated, and volume-regulated currents, as well as currents similar to those associated with ClC-0 (22, 29, 44, 45). The molecular identities of Cl− channels involved in generating these latter Cl− currents remain unknown. Of particular interest are the inward-rectifying, ClC-2-like currents observed in rat (44) and mouse submandibular duct cells (27). Currents with similar properties to ClC-2 and localized to the basolateral membranes have been postulated to support NaCl reabsorption in the intestinal tract (7, 8, 36). The inward-rectifying current in salivary gland duct cells is activated by hyperpolarization and increased intracellular Cl− concentration ([Cl−]) (15, 27), in agreement with the requirements of a basolateral Cl− efflux pathway in a duct cell model (29). Nevertheless, neither the physiological importance of these ClC-2-like currents nor the molecular identity of the associated Cl− channel has been demonstrated in salivary gland ducts.
Here we build on our previous results in the mouse salivary gland (35) using immunolocalization, electrophysiology, and ex vivo and in vivo model systems to address the molecular basis of NaCl reabsorption in salivary gland duct cells. Comparison of wild-type and Clcn2−/− mice demonstrated that Clcn2 encodes for the inward-rectifying Cl− currents in the basolateral membrane of duct cells. Nevertheless, the Cl− currents generated by ClC-2 do not appear to play a major role in NaCl reabsorption or the barrier function of salivary gland ducts. Moreover, compensation for the loss of ClC-2 by increased Cftr expression did not occur in Clcn2 null mice.
MATERIALS AND METHODS
General procedures.
Clcn2 and Cftr null mice were generated as previously described (35). Clcn2−/− and Clcn2+/+ littermates 2–6 mo old were used. All experimental procedures were approved by the Animal Resources Committee of the University of Rochester. Mice were anesthetized with chloral hydrate (400 mg/kg ip), and depth of anesthesia was assessed by testing for their responsiveness to toe pinch; their respiratory rate, depth, and character; and their response to whisker manipulation. At the end of each procedure, the animal was rendered unconscious by exposure to carbon dioxide and the diaphragm and aorta were severed to ensure death. Sucrose was purchased from J. T. Baker (Philipsburg, NJ) and all other reagents were from Sigma-Aldrich (St. Louis, MO) except where indicated. Data are presented as means ± SE. Differences between means were determined by Student's t-test with P < 0.05 considered significant. A repeated-measures analysis was carried out using SAS 9.1 for the data shown in Fig. 3A. To increase robustness of the study findings, inference for the linear model was carried out using the estimating equations approach, which does not require the normal distribution assumption for the dependent variable. We used χ2 tests subsequent to the overall analysis for time, group, and time-by-group comparisons.
Immunohistochemistry.
Mice were anesthetized by intraperitoneal chloral hydrate injection (400 mg/kg) prior to arterial perfusion (Orion Sage Syringe Pump, Thermo Fisher Scientific, Waltham, MA) via the left ventricle, initially with PBS, followed by Bouin's fixative (75 ml of saturated picric acid, 25 ml of 40% formaldehyde, and 5 ml of glacial acetic acid) at room temperature. The salivary glands were removed and incubated 48 h in the same fixative solution and then stored in 70% ethanol at room temperature prior to being embedded in paraffin.
Sections of 5-μm thickness were dewaxed and hydrated before immunostaining. Pseudoperoxidase activity was eliminated by treatment with absolute methanol and 10% hydrogen peroxide. Before immunostaining, all tissues were microwaved with 50 mM Tris·HCl pH 10.0 for 5 min. The microwave's temperature-sensitive probe was used to clamp the temperature of the antigen retrieval buffer at 90°C using a maximum power of 1,150 W. Twenty minutes after treatment, sections were washed with three changes of 50 mM Tris·HCl pH 7.8 and then incubated overnight with various dilutions of an anti-peptide antibody directed to residues 888-906 of rat ClC-2 (1:100 to 1:500). This antibody recognizes the COOH-terminus epitope RSRHGLPREGTPSDSDDKC of rat ClC-2 that is identical to mouse ClC-2 (ACL-002, Alomone Labs, Jerusalem, Israel). Dilutions were performed in 50 mM Tris·HCl pH 7.8 containing 1% immunoglobulin-free bovine serum albumin. Bound immunoglobulins were detected with the LSAB+ biotin/streptavidin peroxidase kit (Dako, Carpinteria, CA) and peroxidase was visualized by incubation with 3,3′-diaminobenzidine (Dako) for 5 min. When immunostaining was complete, the sections were rinsed with distilled water and contrasted with Harris hematoxylin for 30 s. Finally, the sections were dehydrated in ethanol, cleared with xylene, and mounted by using Canada balsam.
Controls were carried out using tissues from Clcn2−/− mice prepared under identical conditions. Alternatively, primary antibody was omitted or replaced in the staining procedure with nonimmune serum of the same origin by use of tissue from wild-type animals.
Dispersion of salivary cells.
Single cells from mouse submandibular glands were prepared by Liberase digestion as previously reported for acinar (37) and for granular duct (34) cells.
Electrophysiological recordings.
Electrophysiological data were acquired via an Axopatch 200B amplifier and a Digidata 1320A digitizer (Axon Instruments, Foster City, CA). Pipettes from Corning 8161 patch glass (Warner Instruments, Hamden, CT) were pulled to give a resistance of 2–3 MΩ in the solutions described below. ClC-2-like currents were measured in whole-cell mode at room temperature as previously described (35). The external solution contained (in mM) 140 TEA-Cl, 0.5 CaCl2, 100 d-mannitol, 20 HEPES, pH 7.3; the pipette solution contained (in mM) 140 TEA-Cl, 20 EGTA, 20 HEPES, pH 7.3. The calculated liquid junction potential for these buffers is +1 mV; therefore, no correction was made. In some experiments, 0.3 mM CdCl2 or 0.5 mM DIDS was added.
To test for Ca2+-activated K+ channels the external solution contained (in mM) 150 Na-glutamate, 5 K-glutamate, 2 CaCl2, 2 MgCl2, 10 HEPES, pH 7.2; the pipette solution contained 135 K-glutamate, 5 EGTA, 10 HEPES, pH 7.2. The level of free [Ca2+] up to 1 μM in the pipette solution was obtained with CaCl2 following WEBMAXC calculation (http://www.stanford.edu/∼cpattonwebmaxcS.htm). We evaluated whether Ca2+-activated Cl− channels were present in granular duct cells using an external solution that contained 139 TEA-Cl, 20 HEPES, 0.5 CaCl2, 100 d-mannitol, and 0.3 mM CdCl2, pH 7.3; the intracellular solution contained 80 NMDG-glutamate, 50 NMDG-EGTA, 30 CaCl2 and 20 HEPES, pH 7.3. The level of free Ca2+ in the latter solution was estimated to be 250 nM. cAMP-activated Cl− currents were recorded with an external solution that contained 150 NaCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 20 sucrose, and 0.3 mM CdCl2, pH 7.4; the pipette solution contained 120 CsCl, 10 TEACl, 1 MgCl2, 0.5 EGTA, 1 ATP, 10 HEPES, pH 7.4. Current was activated by superfusion with 10 μM forskolin and 100 μM IBMX, and the current was effectively inhibited with 5 μM CFTR inhibitor CFTR(Inh)-172.
In vivo stimulated fluid secretion.
Mice were anesthetized by intraperitoneal injection of chloral hydrate (400 mg/kg body wt) prior to saliva collection as described (17). Secretion was stimulated by intraperitoneal injection of the cholinergic receptor agonist pilocarpine-HCl (10 mg/kg body wt). Gland-specific saliva was collected by isolating the ducts from the submandibular and parotid glands and inserting their distal ends into individual calibrated glass capillary tubes (Sigma-Aldrich). Body temperature was maintained at 37°C via a regulated blanket (Harvard Apparatus, Holliston, MA), and the trachea was incised to ensure a patent airway. The progression of saliva within the capillary tube was recorded at 5-min intervals. At the end of the saliva collection period, the glands were dissected, blotted dry, and weighed. Secreted saliva was expressed as microliters of saliva per minute.
Ex vivo perfused submandibular glands.
The mouse submandibular gland was perfused essentially as previously described (37). Briefly, mice were anesthetized with chloral hydrate (400 mg/kg intraperitoneal) and all branches of the common carotid artery were ligated with the exception of the submandibular artery. The gland was transferred to a perfusion chamber and perfused with a solution containing (in mM) 4.3 KCl, 125 NaCl, 25 NaHCO3, 5 glucose, 10 HEPES, 1 CaCl2, and 1 MgCl2 at 37°C and gassed with 95% O2-5% CO2. The gland was perfused at a flow rate of ∼0.8 ml/min via a peristaltic pump (Ismatec IPC, Glattbrugg, Switzerland). Saliva was collected from the perfused gland by inserting the main duct into a calibrated glass capillary tube. Salivation was stimulated by perfusion with the cholinergic receptor agonist carbachol (CCh, 0.1 μM) or with 0.1 μM CCh + the β-adrenergic receptor agonist isoproterenol (IPR, 5 μM). The saliva volume was recorded every minute and the flow rate expressed as microliters of saliva per minute. At the end of saliva collection, the glands were blotted dry and weighed.
To evaluate the permeability of the paracellular pathway, ex vivo submandibular glands were perfused with the above solution containing 9.7 mg/ml iohexol (Omnipaque, GE Healthcare, Amersham Division, Princeton, NJ) for 30 min to allow extracellular equilibration of iohexol. The ex vivo glands were then stimulated by the addition of 0.3 μM CCh + 5 μM IPR in the continued presence of iohexol. The concentration of iohexol in saliva was determined by HPLC analysis and expressed as micrograms per milliliter of saliva (39).
Western blot analysis.
Clcn2 and Cftr wild-type and null mice were rendered unconscious by exposure to CO2 prior to exsanguination and submandibular gland removal. Cftr glands were dispersed by collagenase digestion as before (17) whereas Clcn2 glands were finely minced. Cells were homogenized with a glass-Teflon tissue grinder (Wheaton Science Products; Millville, NJ) in ice-cold buffer containing 250 mM sucrose, 10 mM triethanolamine, leupeptin (1 μg/ml), phenylmethylsulfonyl fluoride (0.1 mg/ml), and 0.5% Triton X-100. An aliquot of 100 μl of cell lysate was stored at −80°C, and the remainder was pelleted at 4,000 g for 10 min at 4°C to remove unbroken cells and nuclei. The supernatant was centrifuged at 22,000 g for 20 min at 4°C, and the pellet was resuspended in the same buffer and then centrifuged at 46,000 g (Beckman SW28 rotor) for 30 min at 4°C. The resultant crude plasma membrane pellet was stored at −80°C for electrophoresis analysis.
Clcn2 and Cftr cell lysates and plasma membrane fractions from knockout mice (60 μg/lane) were heated at 55°C for 20 min prior to separation in a 10% SDS-PAGE Tris-glycine mini-gel (Bio-Rad; Hercules, CA). Protein was transferred overnight onto polyvinylidene difluoride membrane (Invitrogen, Carlsbad, CA) by using a transfer buffer containing 10 mM CAPS (3-[cyclohexylamino]-1-propanesulfonic acid), pH 11, in 10% methanol at 4°C. Membrane was blocked overnight at 4°C with 5% nonfat dry milk in 25 mM Tris·HCl pH 7.5, 150 mM NaCl (TBS), and then incubated with anti-Cftr antibody (Cell Signaling Technology, Danvers, MA) at a dilution of 1:1,000 in 2.5% nonfat dry milk solution at 4°C overnight. After being washed with TBS containing 0.05% Tween-20 (TBS-T), the membrane was incubated with horseradish peroxidase-conjugated goat-anti rabbit IgG secondary antibody (Pierce, Rockford, IL) at a dilution of 1:2,500 in TBS-T/2.5% nonfat dry milk for 1 h at room temperature. Labeled proteins were visualized by enhanced chemiluminescence (ECL detection kit, GE-Amersham Biosciences, Piscataway, NJ).
RESULTS
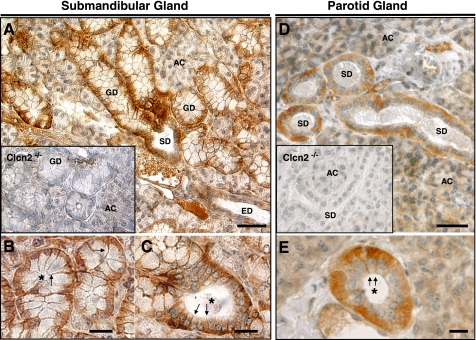
ClC-2 protein expression in salivary ducts. Immunohistochemistry was performed to evaluate the cellular and subcellular localization of ClC-2 protein in mouse submandibular and parotid glands. Submandibular salivary gland tissue from wild-type mice revealed ClC-2 staining in acinar cells and in all types of duct cells (Fig. 1A). Robust staining was observed in granular duct and striated duct cells, whereas staining in the acini and in excretory duct cells was less prominent. Tissue obtained from the Clcn2−/− mice revealed no significant immunostaining in duct cells and only weak background staining was detected in the acinar cells (Fig. 1A, inset).
Fig. 1.
ClC-2 distribution in mouse submandibular and parotid glands. A: immunoperoxidase labeling for ClC-2 in the submandibular gland of a Clcn2+/+ male mouse. AC, acinar cells; GD, granular duct; SD, striated duct; ED, excretory duct. Inset, submandibular gland section from a Clcn2−/− male mouse. B and C: higher magnifications showing basolateral staining in granular (B) and striated (C) duct cells. D: ClC-2 expression in the parotid glands of Clcn2+/+ and Clcn2−/− (inset) male mice. E: preferential basolateral staining in striated duct cells shown at higher magnification. Arrows in B, C, and E indicate basal and lateral membrane of duct cells. Asterisks show ductal lumens. Bars: A and D, 50 μm; B, C, and E, 20 μm. Essentially identical results were observed in female mice.
At a higher magnification the subcellular staining pattern could be clearly observed in the granular and striated duct cells (Fig. 1, B and C, respectively) of the submandibular gland. Most of the staining in both types of duct cells was localized to near the basal and lateral membranes (arrows), whereas the apical membranes had no detectable staining (ductal lumens are marked by asterisks).
Similar intense staining was observed in the striated duct cells of the parotid gland (Fig. 1D; parotid glands do not have granular ducts), whereas weak staining was detected in the acini and in excretory duct (not shown) cells. No specific immunostaining was detected in the parotid glands of Clcn2−/− mice (Fig. 1D, inset). Figure 1E shows a higher magnification image of a striated duct where immunostaining was localized to near the basal and lateral membranes (arrows).
Functional ClC-2 channels in granular duct and acinar cells.
Preparations enriched with either single granular duct or acinar cells were prepared from submandibular glands (34, 37). Consistent with previous reports, the submandibular gland preparations from both wild-type and Clcn2−/− male mice (Fig. 2A) contained relatively big cells, termed granular duct cells, marked by the presence of large granules that are easy to visually identify and differentiate from acinar cells (15, 43). Positive identification of individual striated and excretory duct cells was not possible. Preparations from female submandibular glands did not contain granular duct cells that we could positively identify (these cells and their granules are much smaller in females), and thus preparations from male glands were used for patch-clamp experiments. The morphological identification of the granular duct cells was further confirmed electrophysiologically by the absence of Ca2+-activated K+ and Cl− channels, which are hallmark currents of acinar cells (29). In whole-cell experiments, dialysis of granular duct cells with either 250 nM or 1 μM free Ca2+ did not activate either K+ or Cl− currents (n = 3 and 4, respectively, not shown).
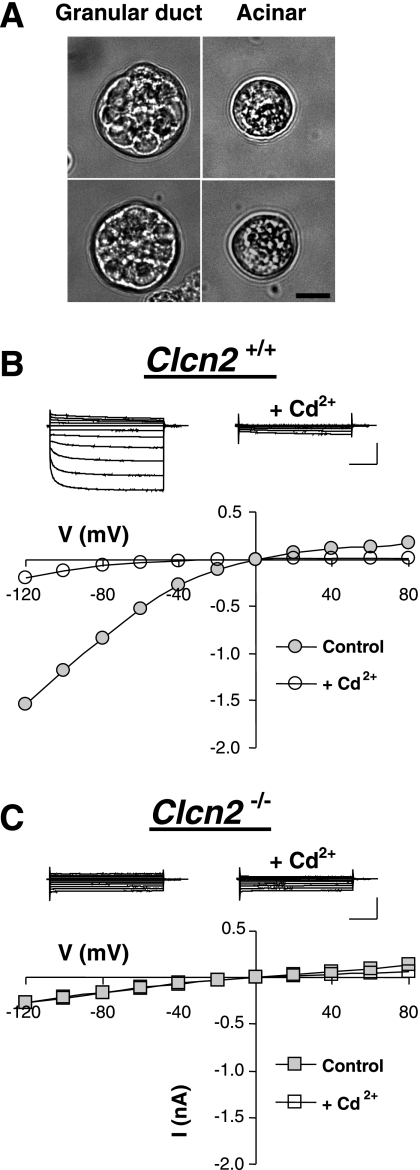
Fig. 2.
Functional expression of the ClC-2 channel in submandibular granular duct cells. A: differential interference contrast images of single granular duct and acinar cells freshly isolated from male mouse submandibular glands. Scale bar = 10 μm B: typical whole-cell Cl− currents recorded in a submandibular granular duct cell from a wild-type mouse. Top: raw currents elicited in response to 2-s voltage steps from −120 to +80 mV in 20-mV increments from 0-mV holding potential. The currents were recorded before (left) and after (right) superfusion with 0.3 mM Cd2+. Bottom: current (I)-voltage (V) relation determined at the end of the voltage steps of the currents shown above. C: same as in B but the cells were obtained from a Clcn2−/− animal. Calibration bars for current amplitude and time: 0.5 nA and 0.5 s, respectively.
Expression of ClC-2-like current was observed in all submandibular granular duct cells tested from wild-type mice (n = 8; average amplitude at −120 mV was −76 ± 15 pA/pF). Figure 2B shows representative traces and plots of their current-voltage relation (I-V) of the Cl− current recorded at high intracellular [Cl−]. This current had an electrophysiological footprint, i.e., time dependence and strong inward rectification of the steady-state current, consistent with the previously observed endogenous Cl− current in granular duct cells and with heterologously expressed ClC-2 channels (11, 15). As expected for ClC-2, the current was sensitive to Cd2+ but insensitive to 0.5 mM DIDS (95 ± 7% of the control current, n = 3, P = 0.893). Superfusion with 0.3 mM CdCl2 resulted in 80 ± 8% inhibition of the Cl− current measured at the end of a 2-s voltage pulse to −120 mV (from −76 ± 15 to −21 ± 12 pA/pF, n = 7, P = 0.005). The current resistant to CdCl2 did not display time or voltage dependence and was similar in magnitude to the current seen in Clcn2−/− mice (see below this section). Cd2+ block was reversible, suggesting that patched salivary gland cells remain intact under these conditions (not shown).
Submandibular acinar cells also expressed a ClC-2-like current, albeit of a significantly smaller amplitude than in granular duct cells (−9 ± 2 pA/pF at −120 mV, n = 6, not shown). As expected, these currents were insensitive to 0.5 mM DIDS (99 ± 9% of the control current, n = 3) but were strongly inhibited by 0.3 mM Cd2+ to −3 ± 1 pA/pF (n = 5). Comparison of the amplitude of the currents in acinar and duct cells suggests that duct cells express nearly 10-fold more ClC-2-like, Cd2+-sensitive current (−55 and −6 pA/pF for duct and acinar cells, respectively, at −120 mV) under identical experimental conditions.
To verify the molecular identity of the inward-rectifying Cl− current we analyzed the currents in cells obtained from Clcn2−/− mice. Figure 2C shows a representative current trace and I-V plot from a submandibular granular duct cell from a knockout mouse. The observed Cl− current was time- and voltage-independent, and the current density amplitude was similar to that found in wild-type cells after inhibition of the ClC-2-like current with Cd2+. The relatively small current in the Clcn2 null cells was insensitive to Cd2+: the current densities before and after addition of 0.3 mM CdCl2 were −24 ± 6 pA/pF (n = 11) and −25 ± 9 pA/pF (n = 7) at −120 mV, respectively.
ClC-2-like Cl− currents were also absent in all acinar cells obtained from Clcn2−/− mice (n = 7). The mean current density measured at −120 mV was −3 ± 1 pA/pF. In these experiments the average seal resistance was 3.3 ± 0.5 GΩ and the average cell capacitance was 15 ± 2 pF. Therefore, the measured current density in Clcn2−/− mice is equivalent to the expected background current from patch-clamp measurements with multigigaohm seals.
In vivo stimulated fluid secretion from submandibular glands.
The above results demonstrate that several types of salivary duct cells express ClC-2 protein (Fig. 1), and the ClC-2-like currents were confirmed to be due to ClC-2 channel expression in the granular duct cells of submandibular glands using Clcn2 knockout mice (compare Fig. 2, B vs. C). Consequently, because ClC-2-like currents in salivary gland duct cells were abolished in Clcn2−/− mice, we could evaluate the physiological importance of these Cl− channels for ductal NaCl reabsorption.
We demonstrated in our previous study that a null mutation of the Clcn2 gene did not impair the secretion of whole saliva (35). It is important to note that whole saliva is primarily composed of secretions from the three pairs of major glands, the morphology and functional properties of which are unique. Approximately 90% of whole saliva in mice is generated by the two largest of these paired glands, the parotid and submandibular. This earlier study did not evaluate whether the function of individual salivary glands were altered in Clcn2 null mice. Consequently, to determine whether Clcn2 gene ablation affects secretion of the submandibular and/or parotid glands, and to exclude possible compensation of one of the glands for the loss of secretion from the other gland, we collected in vivo ductal saliva from individual mouse submandibular and parotid glands. Figure 3 shows that the volume of fluid secreted by the submandibular (Fig. 3A) and parotid (Fig. 3B) glands of Clcn2 knockout and wild-type animals were not statistically different at individual time points (Student's t-test). To increase robustness of the study findings, inference for the repeated-measures analysis was also carried out for the submandibular data by the estimating equations approach, which does not require the normal distribution assumption for the dependent variable. Although the overall analysis of main effects and interaction failed to reveal significant differences, inspection of the curves led us to believe that differences between the wild-type and knockout mice, although not present at the earlier time points, may be present at the later end of the observation period. This was true, where tests at 10 and 15 min find the glands not significantly different, whereas at 20 min and later the differences were significant. These P values do not reflect a correction for multiple comparisons, although when using a Bonferroni adjustment comparisons at 25 and 30 min remain significantly different (P ≤ 0.01).
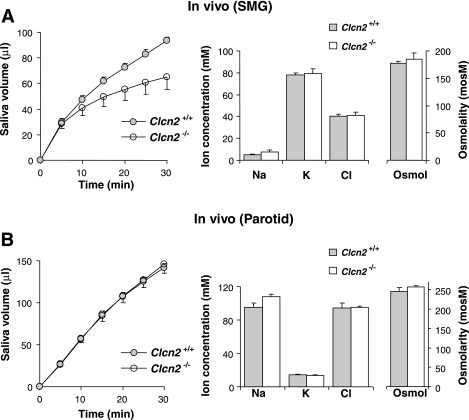
Fig. 3.
In vivo stimulated fluid secretion from submandibular and parotid glands. A, left: amount of saliva secreted over 30 min by submandibular glands (SMG) from wild-type (•) and Clcn2 null (○) mice in response to 10 mg/kg body wt of pilocarpine (n = 6 and 6, respectively). Middle and right: measured ion concentrations (sodium, potassium, and chloride) and osmolality of submandibular saliva from wild-type (shaded bars) mice and from mice with the Clcn2 channel gene ablated (open bars). There were no significant differences in the volumes or ion compositions of the saliva secreted from wild-type and Clcn2 null mice, although the volume of saliva tended to be less in Clcn2 null submandibular glands, the decrease approached significance after 30 min of collection (P > 0.06). This trend decreased when secretion volume was normalized to the gland weight (wild-type, 80 ± 5 and Clcn2 null, 71 ± 10 μl/100 mg gland weight in 30 min; P > 0.60). B: same as described for A, but data were for saliva collected from parotid glands (wild-type and Clcn2 null mice; n = 4 and 7, respectively). There were no significant differences.
Thus the results in left panels of Fig. 3 demonstrate that ClC-2 channels do not play a major role in the fluid secretion mechanism of either submandibular or parotid acinar cells. Furthermore, we found no effect of Clcn2 ablation on the ion composition or the osmolality of secreted saliva (right panels of Fig. 3), suggesting that the ClC-2 channel does not appear to be required for NaCl reabsorption by the salivary ducts in either gland. However, we have shown previously that in vivo saliva production in response to systemic administration of a broad-range cholinergic secretagogue (pilocarpine) may in some cases not faithfully reflect perturbations of the saliva production machinery (37). To circumvent this concern, we used an ex vivo perfused submandibular gland preparation in the following section.
Ex vivo stimulated fluid secretion from perfused submandibular glands.
To directly test the function of the ClC-2 channel for NaCl reabsorption, an ex vivo perfused submandibular gland preparation was used that not only eliminates circulating factors and central neural inputs that complicate the interpretation of in vivo experiments but also permits control of the ion composition and agonist concentration of the perfusate. The ex vivo preparation was found to be a sensitive and robust model system that produces results consistent with data obtained by other functional methods (37).
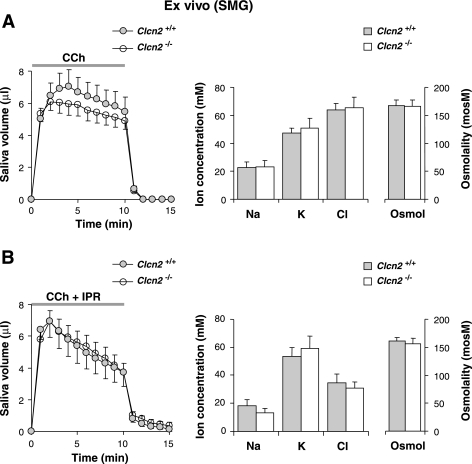
Figure 4 illustrates the saliva secretion rate and ion composition in response to perfusion of the ex vivo submandibular gland with agonists under two different conditions. Figure 4A shows results from experiments with the muscarinic agonist CCh, whereas Fig. 4B is from experiments using carbachol with IPR to stimulate β-adrenergic receptors and raise the intracellular cAMP content of duct cells (12, 16) and thus further enhance Cl− reabsorption through the activation of Cftr (25, 38). As expected, the addition of IPR enhanced Cl− uptake from saliva by ∼50% in wild-type glands, and to a comparable magnitude in Clcn2 null glands. None of the saliva parameters that we monitored (flow rate, ion concentration, or osmolality) were significantly affected by ablation of the ClC-2 channel with either type of agonist stimulation. Thus ClC-2 does not appear to be required for fluid secretion or Cl− reabsorption by salivary ducts.
Fig. 4.
Muscarinic-stimulated saliva secretion from ex vivo submandibular glands. A, left: saliva secretion from ex vivo submandibular glands from wild-type or Clcn2 null mice; the artery and main excretory duct were cannulated. Secretion was stimulated by vascular perfusion with carbachol (CCh, 0.1 μM; wild-type and Clcn2 null glands, n = 8 and 9, respectively). Middle and right: ion composition and the osmolality of submandibular saliva from wild-type (shaded bars) and Clcn2 null (open bars) mice. B: same as in A except that secretion was stimulated with carbachol (0.1 μM CCh) + isoproterenol (IPR, 5 μM). There were no significant differences (wild-type and Clcn2 null mice, n = 9 and 8, respectively).
Cftr channels in submandibular cells.
An alternate possibility for the apparent lack of effect of the Clcn2 null mutation on Cl− reabsorption is that another Cl− channel(s) may have been upregulated to compensate for the loss of ClC-2 in duct cells. Duct cells express Cftr, a cAMP-activated Cl− channel involved in NaCl absorption and fluid secretion. Cftr has been localized to the apical membrane of salivary gland duct cells. It is assumed that Cftr acts as the apical Cl− uptake pathway for transductal Cl− absorption (13, 45). Thus, if compensatory mechanisms are activated in the Clcn2 null gland, it might be predicted that both the apical (Cftr) and an alternative basolateral Cl− flux pathway are upregulated to compensate for loss of the basolateral ClC-2 Cl− channel.
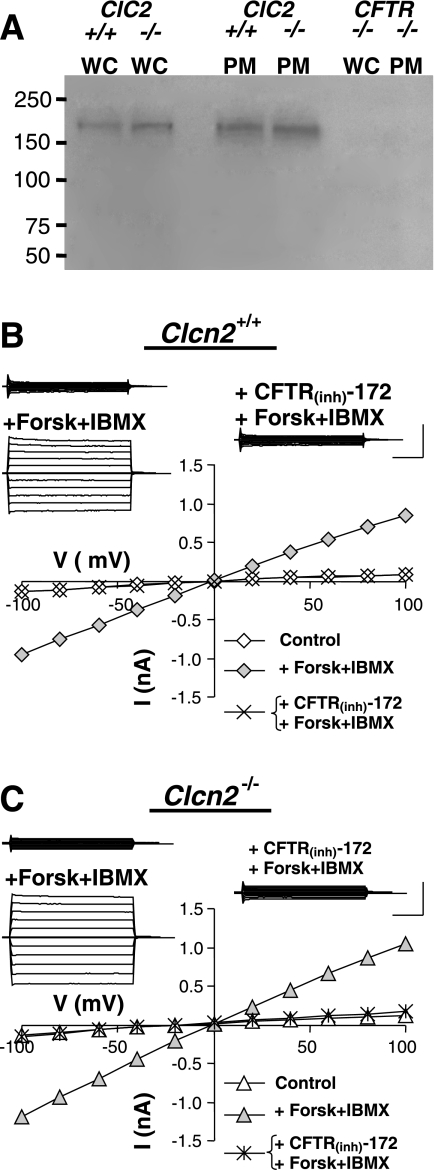
To test this possibility we compared the level of Cftr expression in the submandibular salivary glands of wild-type and Clcn2 null mice. Figure 5A shows that there was no apparent change in the levels of Cftr protein expression in whole-cell lysates (WC) or in the plasma membranes (PM) of Clcn2 null glands. Note also that there was no detectable protein signal in Cftr null mice (far right lanes), verifying the specificity of the antibody. Incubation of duct cells with a Cftr activation cocktail containing forskolin and IBMX to increase the intracellular cAMP level readily upregulated Cl− current with characteristic properties of Cftr, i.e., time- and voltage-independence and sensitivity to the Cftr-specific inhibitor CFTR(Inh)-172 (Fig. 5B). Moreover, there was no significant change in the kinetic properties of the cAMP-activated Cl− current in Clcn2 null mice (Fig. 5C). The magnitudes of the current at +100 mV in the granular duct cells of the submandibular glands of Clcn2 null mice were comparable to those found in wild-type cells (Clcn2 wild-type, 63.4 ± 12.6 pA/pF, n = 5; Clcn2 null, 83.8 ± 26.9, n = 10, P > 0.6). Together, these results suggest that an increase in Cftr expression did not appear to compensate for the loss ClC-2 expression in duct cells. Cftr-like currents were not detected in acinar cells from wild-type or Clcn2 null mice (not shown).
Fig. 5.
Functional expression of Cftr channel protein and cAMP-activated current in submandibular granular duct cells. A: Western blot analysis demonstrating the expression level of Cftr channel protein in the submandibular glands of Clcn2 wild-type (+/+), Clcn2 null (−/−), and Cftr null (−/−) mice. Lanes were loaded with either whole-cell lysate (WC) or plasma membrane fraction (PM). The approximate molecular weight of Cftr is 168 kDa. B: a representative cAMP-activated, Cftr-like current recorded in the presence of 0.3 mM Cd2+ to inhibit the inward-rectifying current. Insets: raw currents recorded in response to 2-s voltage steps from −100 to +100 mV in 20 mV increments from 0 mV holding potential. The currents were recorded before (top left) and after addition of a Cftr activation cocktail (+Forsk+IBMX), and after superfusion with CFTR inhibitor CFTR(Inh)-172 in the continued presence of the Cftr activation cocktail (right). Main panel: current-voltage relation constructed from the raw currents. C: same as in B but the cells were obtained from a Clcn2−/− animal. Calibration bars for current amplitude and time: 0.5 nA and 0.5 s, respectively.
Tight junction permeability in submandibular glands.
Finally, it has been hypothesized that ClC-2 regulates the transepithelial transport and barrier properties of different organs (6, 35). Here we tested whether paracellular permeability was altered in Clcn2 null mice using iohexol as an index of tight junction permeability in the ex vivo submandibular gland. Iohexol is a nonionic contrast agent with a molecular weight of 821 Da used as an extracellular indicator to monitor glomerular filtration rate (1, 42) and for detecting epithelial barrier defects in the intestines (1). Iohexol inclusion in the perfusate did not alter the stimulated flow rate or the ion composition of the collected saliva (not shown). Moreover, there was no change in the iohexol concentration in the saliva collected from the submandibular glands of wild-type and Clcn2 null mice (Clcn2 wild-type, n = 7, 122.8 ± 40.7 μg iohexol/ml of saliva; Clcn2 null, n = 10, 115.8 ± 39.9 μg iohexol/ml of saliva, P > 0.9). These results indicate that the paracellular permeability was apparently not altered in Clcn2 null mice.
DISCUSSION
Transepithelial Cl− movement drives both fluid secretion and NaCl reabsorption. The role of the ClC-2 Cl− channel in these two separate functions of epithelia remains controversial. The direction of Cl− movement determines whether an epithelial cell is secretory or absorptive, which is dictated by the basolateral and apical locations of the various Cl− transport pathways in the plasma membrane. Thus, to understand the function of the ClC-2 Cl− channel in epithelia, its subcellular localization has to be determined. In secretory epithelia, the basolateral Na+/K+/2Cl− cotransporter is the primary Cl− uptake mechanism (17, 19), whereas an apical Cl− channel acts as the efflux pathway. Therefore, if ClC-2 Cl− channels are located in the apical membrane of secretory cells, then activation of the inward-rectifying Cl− current generated by this channel would lead to transepithelial Cl− movement and fluid secretion. In agreement with this secretion model, ClC-2 has been localized to the apical membrane of human intestinal cells (10, 28), mouse lung epithelia (4), and the parietal cells of rabbit stomach (40). However, our results using Clcn2 null mice demonstrate that the ClC-2 channel was diffusely expressed in acinar cells and did not appear to specifically target to the apical membrane. Consistent with this observation, functional studies with the Clcn2 knockout mice showed that only subtle changes in salivary gland fluid secretion was observed in either the parotid or submandibular gland (Figs. 3 and 4). Thus the ClC-2 Cl− channel does not appear to be targeted to the apical membrane of secretory cells in salivary glands, and disruption of the Clcn2 gene indicated that it is not likely to be involved in fluid secretion.
In contrast to reports in different cell types (4, 40), ClC-2 was localized to the basolateral membrane of surface colonic cells and villus duodenal enterocytes (36), results that were confirmed by using Clcn2 null mice (36). Basolateral localization in these cells suggests that ClC-2 channel may be involved in NaCl absorption by the gastrointestinal tract. Taking into consideration the limits of immunolocalization methods, ClC-2 appears to be targeted to the basolateral membranes in the duct cells of mouse submandibular and parotid glands. This raised the possibility that ClC-2 regulates NaCl absorption in salivary glands, acting as the basolateral efflux pathway. Examination of the biophysical and pharmacological footprint of the Cl− current in the submandibular granular duct cells of wild-type mice revealed that it was comparable to that described for the ClC-2 channel (24, 41). Moreover, this current was absent in granular duct cells from Clcn2−/− mice, which agreed with the disappearance of ClC-2 immunostaining in the Clcn2 null mice. It should be noted that the magnitude of the ClC-2-like current in granular duct cells was more that nine times larger than that found in acinar cells. This observation is consistent with the much more intense immunostaining of the granular duct cells and with ClC-2 playing an important functional role in duct cells.
In the currently accepted saliva production model, the duct system modifies the concentration of electrolytes of the isotonic plasmalike primary saliva secreted by acinar cells. Salivary duct epithelium reabsorbs most of the Na+ and Cl− secreted by acinar cells, and because the duct is relatively water impermeable the final saliva is hypotonic. The transepithelial flux of Cl− is supported by distinct transport pathways in the apical and basolateral membranes of the duct cells. The main route for Cl− absorption across the lumen appears to be through Cftr, an apical cAMP-activated Cl− channel. Indeed, we found that increasing the intracellular cAMP content both activated Cftr-like currents in the duct cells (Fig. 5, A and B) and enhanced Cl− uptake (compare Fig. 4, A to B). In contrast, the mechanism by which Cl− efflux is mediated across the basolateral membrane is unknown. The Cftr-mediated Cl− uptake (apical Cl− absorption) requires an inwardly oriented electrochemical gradient for Cl−; i.e., a relatively depolarized membrane potential [positive to the Cl− equilibrium potential (ECl), probably because of Na+ influx via the epithelial Na+ channel ENaC] and low intracellular [Cl−]. These conditions are inconsistent with passive basolateral Cl− efflux. Therefore, a push-pull model has been proposed where the elevation of intracellular [Cl−] and membrane depolarization during NaCl absorption are temporally segregated from basolateral efflux of intracellular Cl− when the cell hyperpolarizes (negative to ECl), likely because of gating of a K+ channel (14). Activation by high intracellular [Cl−] and hyperpolarization are properties of the ClC-2 channel. Taken together with its basolateral localization and high expression in duct cells, the ClC-2 channel is thus a good candidate for the basolateral Cl− efflux pathway. Yet the in vivo and ex vivo assays suggested that ClC-2 does not play a major role in NaCl reabsorption; i.e., the ion composition and the osmolality of saliva secreted by submandibular and parotid glands were virtually identical in wild-type and Clcn2−/− littermate mice. Alternatively, Cftr expression may have been upregulated to compensate for the loss of ClC-2. However, there was no significant change in the magnitude of the cAMP-activated Cl− current in Clcn2−/− mice (Fig. 5C) or in the amount of Cftr protein expression (Fig. 5A), suggesting that an increase in Cftr expression did not compensate for the loss of ClC-2 expression in duct cells. Another possibility is that the Cl− secretion mechanism in the basolateral membrane may contain redundant systems, as shown to be the case with two Ca2+-activated K+ channels in acinar cells (37). This other Cl− transporter might compensate for the loss of ClC-2 and, inversely, ClC-2 may become essential when this alternative Cl− pathway is dysfunctional. In fact, other types of Cl− channels and transporters have been detected in salivary gland duct cells (26, 44), although the subcellular distributions of these alternate pathways are unknown.
What then is the function of the ClC-2 channel in salivary gland duct cells? ClC-2 is important in transepithelial ion transport across the retinal pigmented epithelium and Sertoli cells in the testis (6, 23, 35), and stimulation of ClC-2 channels can induce recovery of epithelial barrier function of ischemia-injured ileum and colon (32, 33). Although the mechanism of the ClC-2-dependent effect on the blood-organ barrier has not been identified, expression of ClC-2 might be important for maintenance and regulation of the permeability of salivary ductal epithelium. Salivary gland ducts are relatively water impermeable. Thus NaCl absorption produces final saliva that is hypotonic, usually less than 200 mosM. However, the lack of the effect of ClC-2 ablation on saliva osmolality and flow rate (the osmolality of saliva is flow rate dependent) indicates that the barrier function of the salivary duct epithelium remains unperturbed. Moreover, we did not detect a change in the permeability of the paracellular pathway to iohexol, a tracer used to monitor renal clearance and defects in the intestinal transepithelial barrier (1, 39, 42). Nevertheless, ClC-2 is highly expressed in duct cells. Consequently, it seems likely that ClC-2 has some important physiological role in salivary glands that is apparently compensated for or masked in the Clcn2 knockout mouse. One avenue of research to address this issue is to identify other Cl− transport pathways present in the ducts. For example, an additional basolateral Cl− transport pathway and/or a paracellular Cl− permeability pathway may have been upregulated to compensate for the loss of ClC-2 expression. Future studies are required to address the lack of supporting evidence of such pathways in salivary gland ducts.
GRANTS
This work was supported in part by National Institute of Dental Research (NIDR) Grants DE09692 and DE08921 (to J. E. Melvin). M. Gonzalez-Begne was supported by NIDR Training Grant T32 DE07202.
Acknowledgments
We thank Laurie Koek, Mark Wagner, and Jennifer Scantlin for technical assistance. We are also grateful to Ted Begenisich for discussions and critical reading of the manuscript and Christopher Beck and Susan Messing for biostatistics analyses.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Andersen R, Stordahl A, Aase S, Laerum F. Intestinal permeability of x-ray contrast media iodixanol and iohexol during bacterial overgrowth of small intestines in rats. Dig Dis Sci 46: 208–213, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Arreola J, Park K, Melvin JE, Begenisich T. Three distinct chloride channels control anion movements in rat parotid acinar cells. J Physiol 490: 351–362, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertelli M, Cecchin S, Lapucci C, de Gemmis P, Danieli D, d'Amore ES, Buttolo L, Giunta F, Mortini P, Pandolfo M. Quantification of chloride channel 2 (CLCN2) gene isoforms in normal versus lesion- and epilepsy-associated brain tissue. Biochim Biophys Acta 1772: 15–20, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Blaisdell CJ, Edmonds RD, Wang XT, Guggino S, Zeitlin PL. pH-regulated chloride secretion in fetal lung epithelia. Am J Physiol Lung Cell Mol Physiol 278: L1248–L1255, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Blanz J, Schweizer M, Auberson M, Maier H, Muenscher A, Hubner CA, Jentsch TJ. Leukoencephalopathy upon disruption of the chloride channel ClC-2. J Neurosci 27: 6581–6589, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosl MR, Stein V, Hubner C, Zdebik AA, Jordt SE, Mukhopadhyay AK, Davidoff MS, Holstein AF, Jentsch TJ. Male germ cells and photoreceptors, both dependent on close cell-cell interactions, degenerate upon ClC-2 Cl− channel disruption. EMBO J 20: 1289–1299, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catalan M, Cornejo I, Figueroa CD, Niemeyer MI, Sepulveda FV, Cid LP. ClC-2 in guinea pig colon: mRNA, immunolabeling, and functional evidence for surface epithelium localization. Am J Physiol Gastrointest Liver Physiol 283: G1004–G1013, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Catalan M, Niemeyer MI, Cid LP, Sepulveda FV. Basolateral ClC-2 chloride channels in surface colon epithelium: regulation by a direct effect of intracellular chloride. Gastroenterology 126: 1104–1114, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Cook DI, Van Lennep EW, Roberts ML, Young JA. Secretion by the Major Salivary Glands. In: Physiology of the Gastrointestinal Tract (3rd ed.), edited by Johnson LR. New York: Raven, 1994, p. 1061–1117.
- 10.Cuppoletti J, Malinowska DH, Tewari KP, Li QJ, Sherry AM, Patchen ML, Ueno R. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol 287: C1173–C1183, 2004. [DOI] [PubMed] [Google Scholar]
- 11.de Santiago JA, Nehrke K, Arreola J. Quantitative analysis of the voltage-dependent gating of mouse parotid ClC-2 chloride channel. J Gen Physiol 126: 591–603, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dehaye JP, Turner RJ. Isolation and characterization of rat submandibular intralobular ducts. Am J Physiol Cell Physiol 261: C490–C496, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Dinudom A, Komwatana P, Young JA, Cook DI. A forskolin-activated Cl− current in mouse mandibular duct cells. Am J Physiol Gastrointest Liver Physiol 268: G806–G812, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Dinudom A, Young JA, Cook DI. Ion channels in the basolateral membrane of intralobular duct cells of mouse mandibular glands. Pflügers Arch 428: 202–208, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Dinudom A, Young JA, Cook DI. Na+ and Cl− conductances are controlled by cytosolic Cl− concentration in the intralobular duct cells of mouse mandibular glands. J Membr Biol 135: 289–295, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Evans RL, Lau KR, Case RM. Structural and functional characterization of striated ducts isolated from the rabbit mandibular salivary gland. Exp Physiol 78: 49–64, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Evans RL, Park K, Turner RJ, Watson GE, Nguyen HV, Dennett MR, Hand AR, Flagella M, Shull GE, Melvin JE. Severe impairment of salivation in Na+/K+/2Cl− cotransporter (NKCC1)-deficient mice. J Biol Chem 275: 26720–26726, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Everett K, Chioza B, Aicardi J, Aschauer H, Brouwer O, Callenbach P, Covanis A, Dooley J, Dulac O, Durner M, Eeg-Olofsson O, Feucht M, Friis M, Guerrini R, Heils A, Kjeldsen M, Nabbout R, Sander T, Wirrell E, McKeigue P, Robinson R, Taske N, Gardiner M. Linkage and mutational analysis of CLCN2 in childhood absence epilepsy. Epilepsy Res 75: 145–153, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, Lorenz JN, Yamoah EN, Cardell EL, Shull GE. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem 274: 26946–26955, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Haug K, Warnstedt M, Alekov AK, Sander T, Ramirez A, Poser B, Maljevic S, Hebeisen S, Kubisch C, Rebstock J, Horvath S, Hallmann K, Dullinger JS, Rau B, Haverkamp F, Beyenburg S, Schulz H, Janz D, Giese B, Muller-Newen G, Propping P, Elger CE, Fahlke C, Lerche H, Heils A. Mutations in CLCN2 encoding a voltage-gated chloride channel are associated with idiopathic generalized epilepsies. Nat Genet 33: 527–532, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Ishibashi K, Okamura K, Yamazaki J. Involvement of apical P2Y2 receptor-regulated CFTR activity in muscarinic stimulation of Cl− reabsorption in rat submandibular gland. Am J Physiol Regul Integr Comp Physiol 294: R1729–R1736, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Ishibashi K, Yamazaki J, Okamura K, Teng Y, Kitamura K, Abe K. Roles of CLCA and CFTR in electrolyte re-absorption from rat saliva. J Dent Res 85: 1101–1105, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Jentsch TJ, Poet M, Fuhrmann JC, Zdebik AA. Physiological functions of CLC Cl− channels gleaned from human genetic disease and mouse models. Annu Rev Physiol 67: 779–807, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev 82: 503–568, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Jirakulsomchok D, Schneyer CA. Effects of adrenergic agonists on electrolyte transport in perfused salivary duct of rat. J Auton Nerv Syst 11: 233–241, 1984. [DOI] [PubMed] [Google Scholar]
- 26.Ko SB, Luo X, Hager H, Rojek A, Choi JY, Licht C, Suzuki M, Muallem S, Nielsen S, Ishibashi K. AE4 is a DIDS-sensitive Cl−/HCO3− exchanger in the basolateral membrane of the renal CCD and the SMG duct. Am J Physiol Cell Physiol 283: C1206–C1218, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Komwatana P, Dinudom A, Young JA, Cook DI. Characterization of the Cl− conductance in the granular duct cells of mouse mandibular glands. Pflügers Arch 428: 641–647, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Lipecka J, Bali M, Thomas A, Fanen P, Edelman A, Fritsch J. Distribution of ClC-2 chloride channel in rat and human epithelial tissues. Am J Physiol Cell Physiol 282: C805–C816, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Melvin JE Chloride channels and salivary gland function. Crit Rev Oral Biol Med 10: 199–209, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 67: 445–469, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Miller C ClC chloride channels viewed through a transporter lens. Nature 440: 484–489, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Moeser AJ, Haskell MM, Shifflett DE, Little D, Schultz BD, Blikslager AT. ClC-2 chloride secretion mediates prostaglandin-induced recovery of barrier function in ischemia-injured porcine ileum. Gastroenterology 127: 802–815, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Moeser AJ, Nighot PK, Engelke KJ, Ueno R, Blikslager AT. Recovery of mucosal barrier function in ischemic porcine ileum and colon is stimulated by a novel agonist of the ClC-2 chloride channel, lubiprostone. Am J Physiol Gastrointest Liver Physiol 292: G647–G656, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Nakamoto T, Romanenko VG, Takahashi A, Begenisich T, Melvin JE. Apical maxi-K (KCa1.1) channels mediate K+ secretion by the mouse submandibular exocrine gland. Am J Physiol Cell Physiol 294: C810–C819, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nehrke K, Arreola J, Nguyen HV, Pilato J, Richardson L, Okunade G, Baggs R, Shull GE, Melvin JE. Loss of hyperpolarization-activated Cl− current in salivary acinar cells from Clcn2 knockout mice. J Biol Chem 277: 23604–23611, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Pena-Munzenmayer G, Catalan M, Cornejo I, Figueroa CD, Melvin JE, Niemeyer MI, Cid LP, Sepulveda FV. Basolateral localization of native ClC-2 chloride channels in absorptive intestinal epithelial cells and basolateral sorting encoded by a CBS-2 domain di-leucine motif. J Cell Sci 118: 4243–4252, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Romanenko VG, Nakamoto T, Srivastava A, Begenisich T, Melvin JE. Regulation of membrane potential and fluid secretion by Ca2+-activated K+ channels in mouse submandibular glands. J Physiol 581: 801–817, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneyer LH, Thavornthon T. Isoproterenol-induced stimulation of sodium absorption in perfused salivary duct. Am J Physiol 224: 136–139, 1973. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz GJ, Furth S, Cole SR, Warady B, Munoz A. Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int 69: 2070–2077, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Sherry AM, Malinowska DH, Morris RE, Ciraolo GM, Cuppoletti J. Localization of ClC-2 Cl− channels in rabbit gastric mucosa. Am J Physiol Cell Physiol 280: C1599–C1606, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Thiemann A, Grunder S, Pusch M, Jentsch TJ. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature 356: 57–60, 1992. [DOI] [PubMed] [Google Scholar]
- 42.Work DF, Schwartz GJ. Estimating and measuring glomerular filtration rate in children. Curr Opin Nephrol Hypertens 17: 320–325, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Young JA, Van Lennep EW. The Morphology of Salivary Glands. London: Academic, 1978.
- 44.Zeng W, Lee MG, Muallem S. Membrane-specific regulation of Cl− channels by purinergic receptors in rat submandibular gland acinar and duct cells. J Biol Chem 272: 32956–32965, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Zeng W, Lee MG, Yan M, Diaz J, Benjamin I, Marino CR, Kopito R, Freedman S, Cotton C, Muallem S, Thomas P. Immuno and functional characterization of CFTR in submandibular and pancreatic acinar and duct cells. Am J Physiol Cell Physiol 273: C442–C455, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Zifarelli G, Pusch M. CLC chloride channels and transporters: a biophysical and physiological perspective. Rev Physiol Biochem Pharmacol 158: 23–76, 2007. [DOI] [PubMed] [Google Scholar]