Abstract
β-Klotho, a newly described membrane protein, regulates bile acid synthesis. Fibroblast growth factor-15 (FGF-15) and FGF receptor-4 (FGFR4) knockout mice share a similar phenotype with β-Klotho-deficient mice. FGF-15 secretion by the intestine regulates hepatic bile acid biosynthesis. The effects of β-Klotho and FGF-15 on the ileal apical sodium bile transporter (ASBT) are unknown. β-Klotho siRNA treatment of the mouse colon cancer cell line, CT-26, and the human intrahepatic biliary epithelial cells (HIBEC) resulted in upregulation of endogenous ASBT expression that was associated with reduced expression of the farnesoid X receptor (FXR) and the short heterodimer partner (SHP). Silencing β-Klotho activated the ASBT promoter in CT-26, Mz-ChA-1 (human cholangiocarcinoma), and HIBEC cells. Site-directed mutagenesis of liver receptor homolog-1 (mouse) or retinoic acid receptor/retinoid X receptor (RAR/RXR) (human) cis-elements attenuated the basal activity of the ASBT promoter and abrogated its response to β-Klotho silencing. siSHP, siFXR, or dominant-negative FXR treatment also eliminated the β-Klotho response. FGF-15 secretion into cell culture media by CT-26 cells was diminished after siFGF-15 or siβ-Klotho treatment and enhanced by chenodeoxycholic acid. Exogenous FGF-19 repressed ASBT protein expression in mouse ileum, gallbladder, and in HIBEC and repressed ASBT promoter activity in Caco-2, HIBEC, and Mz-ChA-1 cells. Promoter repression was dependent on the expression of FGFR4. These results indicate that both β-Klotho and FGF-15/19 repress ASBT in enterocytes and cholangiocytes. These novel signaling pathways need to be considered in analyzing bile acid homeostasis.
Keywords: ileum, liver, regulation, gallbladder
bile acids are considered to be the main driving force for the generation of bile flow, and they facilitate absorption of dietary fat and fat-soluble vitamins (3). Bile acids may also act as signaling molecules by activating several nuclear receptors and regulating bile acid and cholesterol homeostasis. The apical sodium-dependent bile acid transporter (ASBT) primarily mediates lumenal ileal bile acid reabsorption. β-Klotho is a newly described protein that affects hepatic synthesis and excretion of bile acids (15). It is a membrane protein with extensive similarities to Klotho, which is involved in an antiaging phenotype (18). β-Klotho knockout mice have increased cholesterol 7-α hydroxylase (Cyp7a1) mRNA levels and markedly elevated fecal bile acid concentrations, suggesting that hepatic excretion of bile acids is increased. Bile acids act through negative feedback mechanisms to repress Cyp7a1 activity via activation of the nuclear bile acid receptor, farnesoid X receptor (FXR). Subsequent induction of the small heterodimer partner (SHP) by FXR inhibits the liver receptor homolog-1 (LRH-1) and leads to repression of Cyp7a1 promoter activity. Fibroblast growth factor-15 (FGF-15), shown to be expressed and induced by FXR in the small intestine, also represses hepatic bile acid synthesis through SHP (14). The effect of β-Klotho on ileal ASBT expression is not known. We hypothesized that β-Klotho also mediates suppression of ASBT through the FXR:SHP:LRH-1(mouse)/retinoic acid receptor-retinoid X receptor (RAR/RXR) (human) pathway in concert with FGF-15/19.
MATERIALS AND METHODS
Cell culture.
The mouse colon carcinoma cell line CT-26 (CRL-2638; American Type Culture Collection, Rockville, MD), human Caco-2 colon epithelial cells (HTB-37, American Type Culture Collection), human intrahepatic biliary epithelial cells (HIBEC; ScienCell, Carlsbad, CA), and human cholangiocarcinoma cell line Mz-ChA-1 (generously provided by Dr. Andrew Feranchak, University of Texas Southwestern, Dallas, TX) were utilized and grown in RPMI 1640 medium, DMEM, epithelial cell medium (ScienCell), and CMRL-1066 with 10 ml glutamine, respectively. All media were supplemented with 10% FCS unless specifically stated otherwise. When cells were treated with exogenous chenodeoxycholic acid (CDCA) (Sigma, St. Louis, MO) or recombinant human FGF-19 (R&D Systems, Minneapolis, MN), the cell medium was supplemented with 0.5% charcoal-treated FCS to minimize the effect of bile acids found in FCS. CDCA was chosen for these studies since it is a potent FXR agonist (7, 23, 26). All cell lines were cultured for 40 h at 37°C in 5% CO2 before harvest for reporter gene assays.
Plasmid constructs.
The following promoter reporter constructs were utilized in these studies to study the responsiveness of the ASBT gene: 1) pGL3-mASBT5′/P2, which contains nucleotides −378 to +118 of the mouse ASBT 5′ flanking region (5), 2) pGL3-mASBT5′/P2μ, which contains the same mouse ASBT 5′ flanking region with an inactivating mutation in the LRH-1 cis-element (5), 3) pGL3-hASBT, which contains nucleotides −337 to +297 of the human ASBT 5′ flanking region [generously provided by Dr. Paul Dawson, Wake Forest University School of Medicine, Winston-Salem, NC (24)], and 4) pGL3-hASBTμ, which contains the same human ASBT 5′ flanking region with an inactivating mutation in the RAR/RXR cis-element (24).
Northern blot analysis.
Total RNA was obtained using the TRIzol extraction method (GIBCO, Rockville, MD) as suggested by the manufacturer. For Northern blot analysis, RNA was isolated from CT-26 cells treated for 40 h with vehicle control (PBS, 1×), pRNAsiβ-Klotho, or pRNAsiScr, where siScr is scrambled antisense (sequences listed below). A sample (20 μg) of RNA was fractionated on 1% (wt/vol) agarose gels containing 2.2 M formaldehyde and transferred to Nytran (0.45 μm; Schleicher & Schuell, Keene, NH). A 0.6-kb β-Klotho cDNA fragment was synthesized by RT-PCR with the use of the mouse CT-26 cell RNA as template [oligonucleotide primers (synthesized at the Mount Sinai DNA Core Center, Mount Sinai School of Medicine, New York, NY) forward primer 5′-CCCACTACCAGTTTGCTCTGG-3′ and reverse primer 5′-GCGATGTATTCCTTCATAACCG-3′]. The synthesized 588-bp cDNA fragment corresponding to the coding sequence 1778-2366 of mouse β-Klotho mRNA (GenBank AF165170) was then subcloned into the vector pCR2.1-TOPO (Invitrogen, Carlsbad, CA). The RNA blots were hybridized with 32P-β-Klotho, ASBT, and SHP (5) cDNA probes, respectively. 28S rRNA was used as a loading control (4). Expression levels were quantified using a Typhoon 8600 PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Western blot analysis.
Cytoplasmic (50 μg), nuclear (2.5 μg), and membrane (50 μg) proteins were assessed by Western blot analysis as previously described (6). For analysis of secreted proteins, media were isolated from five 100-mm plates containing 5 × 106 cells and 3 ml of the medium per plate. Medium was then collected and proteins >10 kDa were concentrated using a Centricon centrifugal filter device (Millipore, Bedford, MA). Crude plasma membranes were prepared from BALB/c mouse stomach, jejunum, proximal and distal ileum, and colon, using a modification of cation precipitation as previously described (28). For analysis of the effect of FGF-19 on mouse tissues, total cellular homogenates were used. Individual samples of ileum were used although the limited size of the gallbladder from mice necessitated pooling the samples. All antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) with the exceptions of the anti-actin antibodies (Sigma) and the previously described anti-ASBT antibodies (generous gift from P. Dawson, Wake Forest University School of Medicine) (9, 29). Histone H1 was used as a loading control for nuclear proteins, actin for cytoplasmic proteins, and Na+/K+-ATPase-α-1 for plasma membrane proteins. No loading control was known for the secreted proteins.
FGF-19 ELISA.
FGF-19 concentration in cell culture medium was determined by ELISA following the directions of the manufacturer (R&D Systems). Recombinant FGF-19 was used as a quantitative control.
Transient transfection and firefly luciferase assay.
The transient transfections of the CT-26, Caco-2, HIBEC, and Mz-ChA-1 cells were carried out as described previously (5). All transfections were performed in triplicate and replicated in three separate sets of experiments (i.e., 9 individual data points for each experimental transient transfection). Briefly, confluent cells were harvested and resuspended in 700 μl of PBS containing 3 μg of mouse or human ASBT 5′/luciferase hybrid constructs and 0.1 μg of quantitative control plasmid (pRL-TK) containing a thymidine kinase promoter-driven Renilla luciferase gene (Promega, Madison, WI). The cells were transfected by electroporation at 0.22 kV and 0.95 μF ×1,000 (Bio-Rad, Hercules, CA). After electroporation, the cells were cultured for 40 h at 37°C and in 5% CO2. Luciferase activities were measured by dual luciferase reporter assay system (Promega) according to the manufacturer's instructions, using a Turner 20/20n Luminometer (Turner BioSystems, Sunnyvale, CA) with a 10-s counting window. Results are expressed as relative light units (RLU), the ratio of firefly to Renilla luciferase activities.
To knock down the endogenous expression of mouse β-Klotho, human β-Klotho, human FGFR4, SHP, FGF-15, or FXR, cells were transfected with the following siRNA expression constructs or siRNA (for FGFR4): pRNA-mouse simβ-Klotho, 5′-UGCGCAAGGUCUCCGGUACUA-3′ (GenScript, Piscataway, NJ); pRNA-human sihβ-Klotho, 5′-CCGGCUAAGUGACAUCUACAA-3′ (GenScript); siFGFR4, 5′-CCUCGUUGAGUCUAGAUCUA-3′ + 5′-CCGUCAAGAUGCUCAAAGA-3′ + 5′GACACAAGAACAUCAUCAA-3′ (SC-35368, Santa Cruz Biotechnology); pRNA-siSHP, 5′-GAUUCUGCUGGAGGAGCCC-3′ (24) (Orbigen, San Diego, CA); pRNA-siFGF-15, 5′-CUGAUUCGCUACUCGGAGGAA (GenScript); and pRNA-siFXR, 5′UAGAUGCCAGGAGAAUACCAG-3′ (11) (GenScript). Additional cotransfections were performed with an FXR dominant-negative plasmid (FXRdn), pCMX-hFXR-w469A, which encodes a mutant FXR protein that binds to the FXR cis-element but does not activate transcription (2). One microgram of each siRNA was cotransfected with pRL-TK and either mouse or human ASBT promoter construct. For controls scrambled RNA antisense constructs were used (siScr).
Medium exchange.
CT-26 cells were treated for 20 h with vehicle, mouse siβ-Klotho, or siScr. After 20 h, the medium from the three different sets of cells was collected and transferred onto fresh CT-26 cells newly transfected with 3 μg of pGL3-mASBT5′/P2 and 0.1 μg of pRL-TK. Luciferase activity was assessed 20 h after medium exchange and transfection with the luciferase reporter constructs.
Immunodepletion of FGF-15.
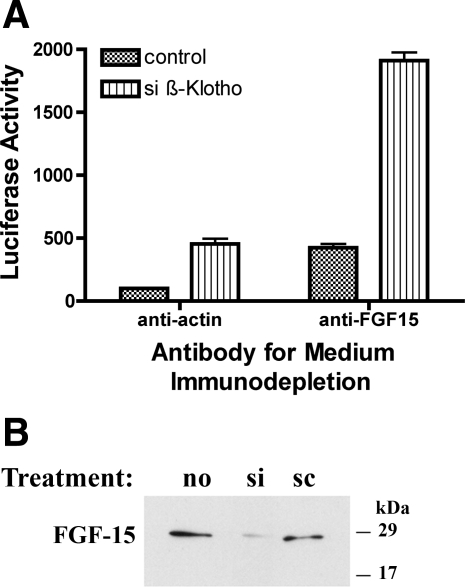
Nine sets of CT-26 cells were divided into three groups at time = 0. The first group was untransfected, the second group was transfected with mouse siβ-Klotho, and the third group was transfected with siScr. At time = 20 h, medium was collected and FGF-15 was removed by immunodepletion. Antiserum was added to the medium at a concentration of 5 μg/ml of medium. Two antisera treatments were utilized, anti-FGF-15 polyclonal antibody (Santa Cruz Biotechnology) and anti-actin antibody (Santa Cruz Biotechnology). A third group was treated with no antibody. The medium was then incubated with the antibody for 5 h at 4°C. Fifty microliters of protein A agarose (Roche Diagnostics, Indianapolis, IN) was added and incubated for 2 h at 4°C. After centrifugation to remove the protein A agarose:antibody:antigen complex, the remaining medium was used to culture new CT-26 cells transfected with 3 μg of pGL3-mASBT5′/P2 (mP2) and 0.1 μg of pRL-TK. Luciferase activity was measured at 20 h after the immunodepleted medium was added to the cells.
FGF-19 dose response.
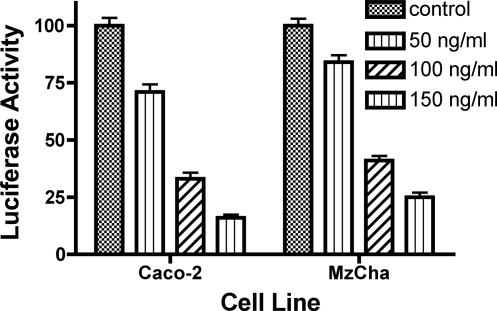
To examine the effect of FGF-19 (note FGF-15 is not commercially available) on mouse and human ASBT promoter activity, mouse pGL3-mASBT5′/P2 (mP2) and pGL3-hASBT reporter constructs, respectively, were transfected into CT-26 cells (mouse intestine), Caco-2 cells (human intestine), or Mz-ChA-1 cells (human cholangiocyte). Caco-2 and Mz-ChA-1 cells were treated for 40 h with increasing concentrations of recombinant FGF-19 (0, 50, 100, and 150 ng/ml) before measurement of dual luciferase activity. Similar transient transfection studies were performed in CT-26 cells treated with FGF-19 at a concentration of 150 ng/ml.
Mouse FGF-19 treatment.
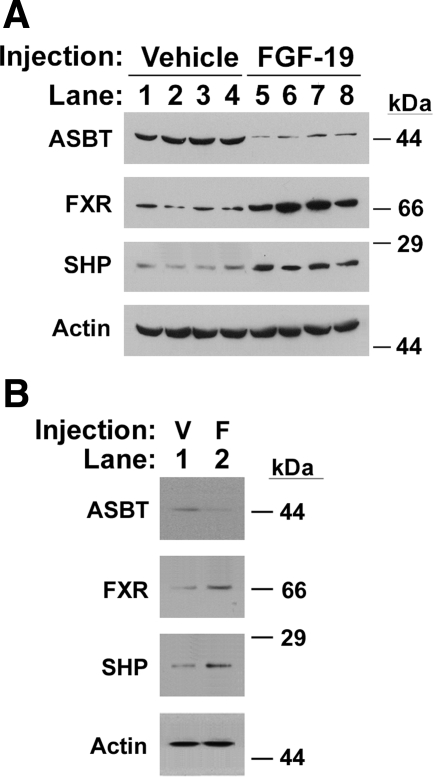
Four 12–18-wk-old C57BL/6 mice were given an intraperitoneal injection of 400 μg/kg of recombinant FGF-19 as previously described (8). Four other mice were injected with vehicle as a control. Eight hours after injection, mice were euthanized for tissue collection. Protein expression was assessed by Western blotting using tissue homogenates. Animal protocols were approved by the Animal Care and Use Committee of Children's Hospital of Pittsburgh of University of Pittsburgh Medical Center.
RESULTS
Silencing β-Klotho activates ASBT.
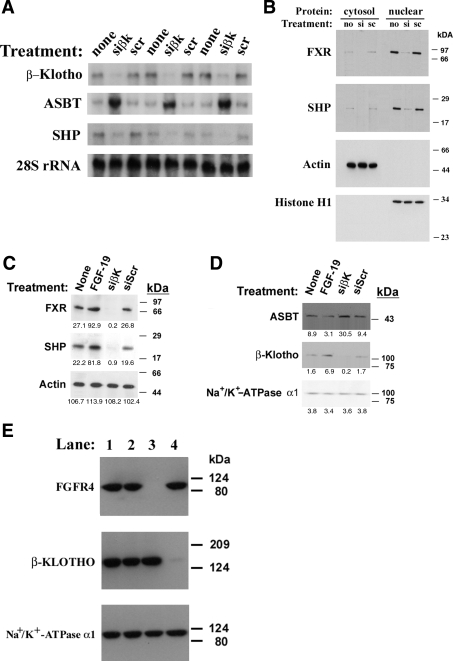
Northern blot analysis of RNA extracted from CT-26 cells demonstrated that siRNA silencing of β-Klotho was efficient (control, 41,700 ± 8,300 vs. siβ-Klotho, 3,000 ± 600 vs. siScr, 40,600 ± 4,500 phosphorimager units/μg of total RNA, n = 3, P < 0.001; Fig. 1A). β-Klotho knockdown resulted in a sixfold increase in steady-state mouse ASBT mRNA expression (control, 26,400 ± 6,300 vs. siβ-Klotho, 182,300 ± 21,700 vs. siScr, 27,000 ± 2,100 phosphorimager units/μg of total RNA, n = 3, P < 0.001) and a significant reduction in SHP mRNA levels (control, 26,400 ± 10,200 vs. siβ-Klotho, 7,900 ± 400 vs. siScr, 26,400 ± 9,800 phosphorimager units/μg of total RNA, n = 3, P < 0.05). Equivalent loading was demonstrated through analysis of 28S ribosomal RNA levels. The effects of β-Klotho silencing on protein expression in CT-26 (Fig. 1B) and Mz-ChA-1 (Supplemental Fig. 1) cells were then assessed by Western blotting. Supplemental information for this article is available at the American Journal of Physiology Gastrointestinal and Liver Physiology website. siRNA treatment led to a reduction in FXR and SHP protein expression. At baseline, the majority of both of these proteins was found within the nucleus. ASBT protein expression was not assessed because of its low level of expression in these cell lines. HIBEC were studied to examine the effects on ASBT protein expression. siβ-Klotho treatment of this primary cell line yielded similar reductions in FXR and SHP protein expression (Fig. 1C) that was accompanied by an increase in ASBT protein levels (Fig. 1D). The efficiency of both β-Klotho and FGFR4 silencing in Caco-2 cells was also demonstrated by Western analysis of cellular membranes (Fig. 1E).
Fig. 1.
A: Northern blot analysis of siβ-Klotho-treated CT-26 cells. mRNA levels were measured via phosphorimager analysis of a Northern blot of 20 μg of total RNA after siβ-Klotho antisense (siβK) treatment for 40 h in 3 separate cell groups. 3 separate groups treated with scrambled antisense (scr) served as a control for the antisense treatment, whereas 3 additional groups of controls (none) were not treated. There was a 6-fold increase in steady-state apical sodium bile transporter (ASBT) mRNA levels and 70% reduction in steady-state short heterodimer partner (SHP) mRNA levels in the siβK-treated cells relative to either of the controls. Similar RNA loading is demonstrated by equivalent signals for 28S RNA. B: Western blot analysis of siβ-Klotho-treated CT-26 cells. Cytoplasmic or nuclear extracts were prepared from untreated (no), siβ-Klotho (si)-, and scrambled antisense (sc)-treated cells. Farnesoid X receptor (FXR) and SHP proteins were predominantly found in the nuclear extracts, and siβ-Klotho treatment reduced the expression of both of these proteins. Equivalent loading of cytoplasmic and nuclear proteins is indicated by the signals for actin and Histone H1, respectively. C and D: Western blot analysis of cytoplasmic (1C) extracts and membrane proteins (1D) from fibroblast growth factor (FGF)-19- or siβ-Klotho-treated human intrahepatic biliary epithelial cells (HIBEC). HIBEC cells were treated with either FGF-19 or siβ-Klotho, and then cytoplasmic extracts or membrane proteins were isolated. FXR and SHP protein abundance was induced by FGF-19 and repressed after silencing β-Klotho. FGF-19 treatment led to a reduction in ASBT expression, whereas β-Klotho silencing increased ASBT levels. Equivalent cytoplasmic and membrane protein loading is seen by the signals for actin or Na+/K+-ATPase-α1, respectively. Densitometric quantification of signal intensity is displayed below each protein band. E: Western blot analysis of siβ-Klotho and siFGF receptor (siFGFR)4-treated Caco-2 cells. Crude plasma membranes were prepared from cells that were untreated (lane 1), treated with siScr (lane 2), siFGFR4 (lane 3), or siβ-Klotho (lane 4). Marked reduction of the appropriate target proteins can be seen (antibody utilized in left column). Equivalent loading is indicated by the signal for Na+/K+ ATPase-α1.
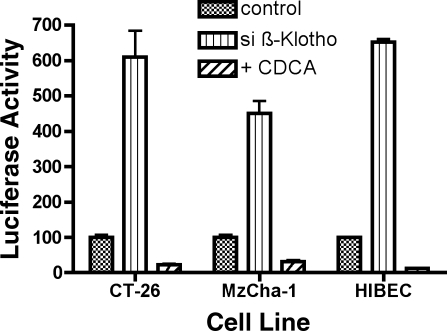
Promoter analysis was then undertaken to determine the mechanism of the β-Klotho effect. In CT-26 cells, β-Klotho silencing led to a sixfold increase in ASBT promoter activity (control, 100 ± 21 RLU vs. simβ-Klotho, 610 ± 221 RLU, P < 0.001) (Fig. 2). CDCA treatment of scrambled anti-sense-treated CT-26 cells or siβ-Klotho-treated CT-26 cells led to a similar degree of suppression of ASBT promoter activity, 77% suppression of activity in scrambled anti-sense-treated CT-26 cells, and 70% suppression in siβ-Klotho-treated cells (control + CDCA, 22 ± 4 RLU vs. simβ-Klotho + CDCA, 183 ± 61 RLU, P < 0.01).
Fig. 2.
Effect of β-Klotho silencing on ASBT promoter activity in CT-26, Mz-ChA-1, and HIBEC cells. In all 3 cell lines, ASBT promoter activity was reduced after treatment with chenodeoxycholic acid (CDCA), whereas siβ-Klotho treatment activated the ASBT promoter.
A similar promoter response was observed in the cholangiocyte-derived Mz-ChA-1 and HIBEC cells. Human ASBT promoter activity was increased fourfold after silencing of human β-Klotho activity (Mz-ChA-1: control, 100 ± 21 RLU vs. sihβ-Klotho, 450 ± 105 RLU, P < 0.001; HIBEC: control, 100 ± 4 RLU vs. sihβ-Klotho, 652 ± 25 RLU, P < 0.001) (Fig. 2). ASBT promoter activity was suppressed in these cell lines after addition of CDCA, indicating that bile acid-mediated suppression of ASBT also exists in Mz-ChA-1 cells and HIBEC (Mz-ChA-1: control, 100 ± 21 RLU vs. CDCA, 32 ± 12 RLU, P < 0.05; HIBEC: control, 100 ± 4 RLU vs. sihβ-Klotho, 12 ± 2 RLU, P < 0.001).
β-Klotho effect signals via FXR and SHP.
It has been suggested that β-Klotho acts via bile acid response pathways. Bile acid responsiveness of ASBT is regulated in part by FXR-dependent activation of the SHP. SHP then inactivates endogenous transcriptional activators of ASBT, e.g., LRH-1 in the mouse and RAR/RXR in humans (5, 24).
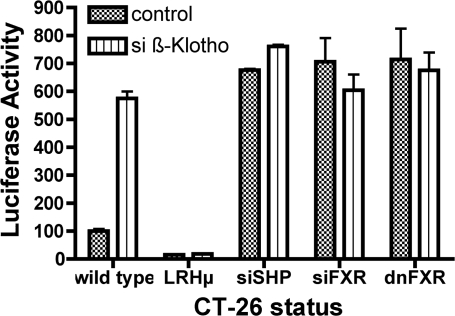
Signaling pathways were studied in CT-26, Mz-ChA-1, and HIBEC cells. Site-directed mutagenesis of the LRH-1 or RAR cis-elements respectively attenuated the basal activity of the ASBT promoter and abrogated its response to β-Klotho silencing (Fig. 3 and Supplemental Figs. 2 and 3). Silencing of SHP increased ASBT promoter activity, but there was no further activation after siβ-Klotho treatment. Transfection of either siFXR or FXRdn increased basal ASBT promoter activity and eliminated the activation by β-Klotho silencing. These studies confirm that the β-Klotho signals through the bile acid response pathways involving FXR and SHP.
Fig. 3.
The role of the FXR:SHP cascade in mediating the effect of β-Klotho on ASBT. The effect of siβ-Klotho on the ASBT promoter in CT-26 cells is abrogated when the liver receptor homolog (LRH)-1 cis-element is mutated (LRHμ) and when the effects of SHP and FXR are obviated by siRNA (siSHP or siFXR) or by a dominant-negative FXR construct (dnFXR).
β-Klotho effect is mediated by secreted factors.
Studies were undertaken to determine whether the effects of β-Klotho could be mediated by secreted factors. Transfer of the media from siβ-Klotho-treated CT-26 cells to untreated CT-26 cells yielded the same effect on ASBT promoter activity (control medium, 100 ± 12 RLU vs. siβ-Klotho medium, 361 ± 89 RLU vs. siScr medium, 106 ± 18 RLU, P < 0.0001).
CT-26 and Caco-2 cells secrete FGF-15/19.
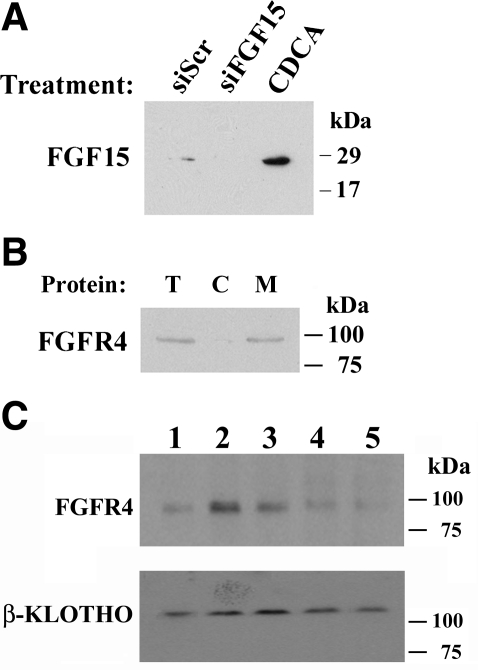
FGF-15/19 is secreted by ileal enterocytes and mediates effects on bile acid biosynthesis in hepatocytes. Therefore, experimental analyses of the role of FGF-15/19 in mediating the β-Klotho effect were undertaken. Western blot analysis of concentrated media showed that CT-26 cells secrete FGF-15, which is silenced by siFGF-15 and activated by the bile acid, CDCA (Fig. 4A). Quantitation of FGF-19 secretion was performed in Caco-2 cells since the commercially available ELISA kit measures FGF-19 and quantitative controls for FGF-19 exist. CDCA treatment led to a sixfold increase in FGF-19 secretion (control, 42 ± 8 pg/ml vs. CDCA, 257 ± 32 pg/ml, P < .001, n = 9 for each measurement). FGFR4, a membrane protein that potentially mediates responses to FGF-15, is expressed in CT-26 cells and is enriched in membrane fractions from those cells (Fig. 4B). Western analysis of plasma membranes from mouse intestine revealed that both β-Klotho and FGFR4 can be detected in mouse ileum (Fig. 4C).
Fig. 4.
Western blot analysis of CT-26 cells and mouse intestinal membrane proteins. A: concentrated extracellular media from CT-26 cells contains FGF-15. The amount of FGF-15 is increased after CDCA treatment and is decreased after siFGF-15 treatment. B: FGFR4, which mediates the effect of FGF-15, is present in total (T) and cellular membrane (M) proteins but not cytoplasmic (C) proteins. C: β-Klotho and FGFR4 are detectable in mouse plasma membrane proteins derived from various segments of the longitudinal axis of the intestine (lane 1, stomach; lane 2, jejunum; lane 3, proximal ileum; lane 4, distal ileum; and lane 5, colon). A loading control was not utilized as a specific protein that was equivalently expressed in the membranes from these tissues was not known.
FGF-15/19 represses ASBT promoter activity and protein expression.
The effect of FGF-15/19 on ASBT is unknown. Addition of exogenous FGF-19 to Caco-2, Mz-ChA-1, or HIBEC cells significantly decreased human ASBT promoter activity in a dose-dependent fashion using concentrations of FGF-19 ranging from 50 to 150 ng/ml (Fig. 5). Repression of ASBT promoter activity was also observed in CT-26 treated with 150 ng/ml of FGF-19 (CT-26: control, 100 ± 12 RLU; FGF-19, 19 ± 8, P < 0.0001, P < 0.0001). FGF-19 treatment of HIBEC yielded a reduction in ASBT protein expression (Fig. 1D). Intraperitoneal administration of FGF-19 to C57BL/6 mice led to a reduction in ASBT protein expression in ileum and gallbladder (Fig. 6, A and B). Both FXR and SHP protein abundances were induced in the ileum and gallbladder after FGF-19 treatment (Fig. 6, A and B). Silencing of FGF-15 in CT-26 cells increased ASBT promoter activity, [wild-type (wt): control, 100 ± 21 RLU; siFGF-15, 387 ± 65 RLU; P < 0.0001]. Repression of ASBT promoter activity by FGF-19 could be abrogated in its entirety by silencing of FGFR4 in Caco-2 cells (control, 100 ± 14 RLU; FGF-19, 21 ± 6, siFGFR4, 927 ± 54; FGF-19 + siFGFR4, 934 ± 70; P > 0.05 for siFGFR4 vs. siFGFR4 + FGF-19). ASBT promoter activity was increased after silencing either FGF or its receptor.
Fig. 5.
Effect of FGF-19 on ASBT. Dose-dependent repression of ASBT promoter activity by FGF-19 in Caco-2 and Mz-ChA-1 cells. The human ASBT promoter was transfected into Caco-2 and Mz-ChA-1 cells and treated for 40 h with increasing concentrations of human recombinant FGF-19. A dose-dependent reduction in promoter activity was observed.
Fig. 6.
Effect of intraperitoneal FGF-19 on mouse ASBT. A: Ileum. FGF-19 treatment (lanes 1 to 4) relative to vehicle injection as a control (lanes 5 to 8) led to an induction of FXR and SHP protein expression and a repression of ASBT expression. Equivalent loading is demonstrated by the signal for actin. B: Gallbladder. 4 separate gallbladder samples were pooled with vehicle (V) and FGF-19 treatment (F). FXR and SHP protein expression was induced, whereas ASBT protein expression was inhibited. Equivalent loading is demonstrated by the signal for actin.
Combinatorial effects of β-Klotho and FGF-15.
It was hypothesized that the effects of β-Klotho were linked to FGF-15/19. Combinatorial depletion studies were performed to assess this hypothesis. Theoretically, if these two pathways were completely overlapping or sequential, combined inhibition would not be additive as observed above in the effects of siFXR or siSHP on the effect of siβ-Klotho.
Supporting this hypothesis was the finding that there was no combinatorial activation of ASBT after siβ-Klotho and siFGF-15 treatment in CT-26 cells [(wt) control, 100 ± 21 RLU; siβ-Klotho, 543 ± 61 RLU; P < 0.001; siFGF-15, 387 ± 65 RLU; P < 0.0001; siFGF-15 + siβ-Klotho, 614 ± 14 RLU; P > 0.05 for comparison of combination vs. β-Klotho alone]. Similar findings were observed when FGFR4 was silenced in Caco-2 cells (control, 100 ± 14 RLU; siβ-Klotho, 650 ± 37 RLU; siFGFR4, 927 ± 54; siFGFR4 + siβ-Klotho, 1,111 ± 118). There was minimal activation of the ASBT promoter when β-Klotho silencing was added to FGFR4 silencing.
In contrast, immunodepletion of FGF-15 from the media of β-Klotho-silenced cells produced a multiplicative increase in ASBT promoter activity [control (anti-actin antibody), 100 ± 12 RLU; simβ-Klotho (anti-actin antibody), 454 ± 146 RLU, n = 12, P < 0.001; siScr (anti-actin antibody), 89 ± 24 RLU, n = 12, P > 0.05; control (FGF-15 antibody), 424 ± 104 RLU, n = 12, P < 0.001; simβ-Klotho (FGF-15 antibody), 1,911 ± 223 RLU, n = 12, P < 0.001; siScr (FGF-15 Antibody), 360 ± 55 RLU, n = 12, P < 0.001] (Fig. 7A). siβ-Klotho treatment led to a reduction in secretion of FGF-15 (Fig. 7B).
Fig. 7.
A: effects of immunodepletion of FGF-15 in β-Klotho-silenced CT-26 cells. Anti-actin antibody treatment of the media from CT-26 cells has no effect on the action of siβ-Klotho (left two bars). In contrast, immunodepletion of FGF-15 from the media of siβ-Klotho-treated cells (right two bars) yields a multiplicative increase in ASBT promoter activity relative to scrambled antisense-treated controls (control). B: effect of siβ-Klotho on secreted FGF-15. FGF-15 secretion by CT-26 cells is reduced after siβ-Klotho treatment.
DISCUSSION
β-Klotho plays an active role in regulating hepatic gene expression (15). Impaired negative feedback suppression of bile acid biosynthesis was observed in β-Klotho-deficient mice. Knockout mice had marked increases in Cyp7a1 mRNA and indirect evidence of increased hepatic bile acid excretion. The focus of the present work was to investigate whether β-Klotho impacts intestinal and biliary gene regulation (Fig. 8). The intestine is not only the site of bile acid absorption but also plays a important role in negative feedback of bile acid synthesis in the liver (14, 25). Interestingly, despite increased synthesis and excretion of bile acids by the liver, ileal expression of ASBT was not reduced in β-Klotho-deficient mice (15). Ileal lipid binding protein was increased in the β-Klotho knockout mice, confirming the increased bile acid flux through the ileum. The lack of response by ASBT is contrary to prior studies that suggest that mouse ASBT is under negative feedback regulation by bile acids (5). Transgenic mice that overexpress Abcb11 (bile salt export pump) have enhanced hepatic excretion of bile acids, which is accompanied by the expected downregulation of ASBT (10). The absence of downregulation of ASBT in the β-Klotho knockout mouse model suggested abnormal regulation of ASBT and a potential direct effect of β-Klotho on intestinal gene expression.
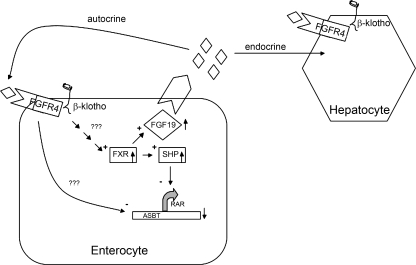
Fig. 8.
Model for the effect of β-Klotho and FGF-19 in enterocytes. β-Klotho is found on the cellular membrane and signals by unknown pathways to activate the FXR. FXR, in turn, enhances the expression of the SHP, which, in turn, inactivates the retinoic acid receptor (RAR), yielding a net result of diminished expression of the ASBT. FXR also enhances the expression of FGF-19 (◊); FGF-19 is secreted by the enterocyte and has paracrine effects on hepatocytes and/or cholangiocytes (not shown), whereas there are local autocrine effects on enterocytes. FGF-19 binding to the FGFR4 also leads to inhibition of ASBT expression by presently unidentified mechanisms.
β-Klotho is predominantly expressed in the liver, pancreas, and adipose tissue with lower levels observed in the gut epithelium (16). Our studies confirm that β-Klotho is expressed in the mouse small intestine. Cell line-based studies suggest that β-Klotho is functionally active in both enterocytes and cholangiocytes. siRNA-mediated knockdown leads to activation of the ASBT promoter in mouse- and human-derived cells. Basal activity of β-Klotho suppresses ASBT expression, similar to what is observed for Cyp7a in the liver. In the β-Klotho knockout, mouse basal activity of ASBT is upregulated, which we hypothesize is then repressed to the level of the wild-type animal by the enhanced enterocytic flux of bile acids resulting from the increased synthesis and excretion of bile acids by the liver, the net result being one of no change in ASBT expression in the intestine. The functional significance of β-Klotho in the cholangiocyte is unclear although the β-Klotho-deficient mice were resistant to gallstone formation (15).
ASBT is under negative feedback regulation by bile acids in mouse and human ileum (5, 24). A complex regulatory loop involves bile acid-mediated activation of the FXR, which induces expression of the SHP. SHP then binds to and inactivates LRH-1 in mice or RAR/RXR in humans. LRH-1 and RAR/RXR activate the transcription of ASBT; thus SHP represses ASBT gene transcription. Bile acid-mediated induction of SHP was reduced in β-Klotho knockout mice (15).
The present study indicates that β-Klotho plays an important role in modulation of the LRH-1 or RAR/RXR pathway via FXR and thus SHP. The mechanisms by which cell surface expression of β-Klotho influences FXR expression/activity are unknown at present (Fig. 8). Ablation of the LRH-1 or RAR/RXR cis-acting element in the ASBT promoter by site-directed mutagenesis resulted in a loss of response to β-Klotho knockdown. Furthermore, silencing of SHP also abrogated the response of ASBT to β-Klotho. Finally, loss of FXR through siRNA or dominant-negative constructs interfered with the response of ASBT to β-Klotho silencing.
ASBT is clearly expressed in cholangiocytes and is presumably involved in reclamation of bile acids although the physiological consequences of bile acid transport in cholangiocytes are unknown (21, 30). Bile acid responsiveness of ASBT in cholangiocytes is controversial since inconsistent regulation of ASBT activity by bile acids has been observed. Kip et. al. (17) noted a decrease in ASBT expression after 1% taurocholate feeding, whereas Alpini et. al. (1) observed upregulation of ASBT in large cholangiocytes after taurocholate feeding in rats. Since there are many changes in bile acid transport and homeostasis that occur in multiple organs, it can be difficult to assess a single system in vivo. As such, these investigations utilize a reductionist approach using cell line studies as a method to more clearly delineate the mechanisms at a cellular level. The present study reveals negative feedback regulation of ASBT by bile acids in a cholangiocyte-derived cell line and in nontransformed isolated HIBEC.
Klotho family proteins regulate FGF signaling potentially by functioning as a cofactor with FGFR for activation (20). In the mouse, FGF-15 is secreted by the intestine in response to bile acids and regulates bile acid biosynthesis in the liver, whereas FGF-19 is the homolog that suppresses bile acid synthesis in human hepatocytes (13, 14). β-Klotho-deficient mice, FGF-15 knockout mice, and FGFR4 knockout mice all share similar phenotypes manifested by an increase in synthesis and excretion of bile acids with concomitant activation of Cyp7a1 gene expression (15). Recent studies imply that β-Klotho may function as a cofactor for FGF-19 binding to its cognate receptor, FGFR4. β-Klotho may be involved in promoting binding affinity by interacting with ligand and receptor (12). In the present study, transferability of activation of ASBT in β-Klotho-deficient cells via cell culture medium exchange suggests a mechanism involving a secreted component by β-Klotho. It is hypothesized that FGF-15 in mice and FGF-19 in humans in coordination with β-Klotho could be responsible for the bile acid response, which is medium transferable.
Inagaki et. al. (14) proposed that FGF-15 functions as an enterohepatic hormone in the small intestine, which represses Cypa7a1 via activation of SHP in vivo. FGF-15 is produced in the ileum and induced by bile acids through FXR (14). In this study, loss of FGF-15 activity increased ASBT activity. Addition of recombinant FGF-19, which in contrast to FGF-15 is commercially available in a stable protein form, to the cell lines revealed a novel finding of dose-dependent repression of ASBT promoter activity in mouse and human enterocytes. Intraperitoneal administration of FGF-19 to mice led to a marked induction of SHP expression and a commensurate repression in ASBT expression in ileum and gallbladder. The effect on the ASBT promoter was dependent upon the expression of FGFR4, suggesting that this is a receptor for FGF-15/19. Thus, in the ileum, FGF-15 may have local autocrine effects similar to the described paracrine effects on the liver (Fig. 8). Recent experiments have shown that induction of FGF-15 by bile acids stimulates gallbladder refilling (8). Our studies indicate that FGF-19 regulates a cholangiocyte cell line and gallbladder, implying the involvement of FGFs in mediation of bile acid metabolism in the gallbladder and biliary tree.
The interrelationship between FGF-15/19 and β-Klotho was examined by combinatorial inactivation of their respective functions. If the pathways were completely overlapping, then one would expect that there would be no additional effect of combinatorial inactivation compared with either inactivation alone. This response is observed when one examines the relationship between β-Klotho and FXR or SHP. Combined siRNA-mediated inactivation of both β-Klotho and FXR or SHP yields no augmentation of the effect of either inactivation of either element alone. siRNA-mediated knockdown of both β-Klotho and FGF-15 did not enhance the effect of either element, suggesting that the pathways are intimately linked. In contrast, immunodepletion of FGF-15 from the medium of β-Klotho-silenced cells significantly increased ASBT promoter activity comparatively, suggesting a divergence of these pathways. One potential explanation for this phenomenon is that the FGF-15 antibody immunoprecipitates an additional protein that also influences ASBT expression. This additional protein may be bound by FGF-15 but would not be silenced with the siRNA constructs utilized in these studies. Our finding that the concentration of FGF-19 in the media of Caco-2 cells is below the concentration needed for repression of ASBT promoter activity potentially supports the hypothesis that there are other proteins secreted by Caco-2 cells that inhibit the ASBT promoter. Thus these combinatorial studies are not completely definitive as to the interaction between β-Klotho and FGF-15/19. Physical interaction between β-Klotho and FGFR4 may mediate the complex pathways influenced by both FGF-15/19 and β-Klotho (19, 22). Clearly, further studies along these lines are necessary to unravel the potential complex interactions between β-Klotho and FGF-15/19.
In conclusion, these studies are the first to indicate that the previously described regulation of β-Klotho and FGF-15/19 in the liver is also present in intestinal and bile duct cells. These findings are consistent with our interpretation of the findings in the β-Klotho knockout mouse. β-Klotho expression suppresses basal ASBT activity through the LRH-1 cis-element, presumably via FXR and SHP. The exact signaling pathways between β-Klotho and FXR are not known, and it is not known whether there is a specific ligand for β-Klotho. We also hypothesize that FGF-15 is involved in an enterocytic autocrine signaling pathway potentially mediated by the interactions of β-Klotho with FGFR4. This pathway may be operant in the Ost-α−/− mouse where ASBT expression is reduced in the setting of enhanced ileal FGF-15 synthesis (27). Future studies will be required to fully understand the signal transduction pathways influenced by β-Klotho and FGF-15/19.
GRANTS
This work was supported in part by a grant from the National Institutes of Health, DK 54165 (to B. L. Shneider).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alpini G, Ueno Y, Glaser SS, Marzioni M, Phinizy JL, Francis H, Lesage G. Bile acid feeding increased proliferative activity and apical bile acid transporter expression in both small and large rat cholangiocytes. Hepatology 34: 868–876, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf D, Suchy F. Human bile salt export pump promoter is transactivated by farnesoid X receptor/bile acid receptor. J Biol Chem 276: 28857–28865, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Arrese M, Trauner M. Molecular aspects of bile formation and cholestasis. Trends Mol Med 9: 558–564, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Barbu V, Dautry F. Northern blot normalization with a 28S rRNA oligonucleotide probe. Nucleic Acids Res 17: 7115, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen F, Ma L, Dawson PA, Sinal CJ, Sehayek E, Gonzalez FJ, Breslow J, Ananthanarayanan M, Shneider BL. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J Biol Chem 278: 19909–19916, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Chen F, Ma L, Sartor B, Li F, Xiong H, Sun A, BS. Inflammatory-mediated repression of the rat ileal sodium-dependent bile acid transporter by c-fos nuclear translocation. Gastroenterology 123: 2005–2016, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Chiang JY, Kimmel R, Weinberger C, Stroup D. Farnesoid X receptor responds to bile acids and represses cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J Biol Chem 275: 10918–10924, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Choi M, Moschetta A, Bookout AL, Peng L, Umetani M, Holmstrom SR, Suino-Powell K, Xu HE, Richardson JA, Gerard RD, Mangelsdorf DJ, Kliewer SA. Identification of a hormonal basis for gallbladder filling. Nat Med 12: 1253–1255, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Christie DM, Dawson PA, Thevananther S, Shneider BL. Comparative analysis of the ontogeny of a sodium-dependent bile acid transporter in rat kidney and ileum. Am J Physiol Gastrointest Liver Physiol 271: G377–G385, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Figge A, Lammert F, Paigen B, Henkel A, Matern S, Korstanje R, Shneider BL, Chen F, Stoltenberg E, Spatz K, Hoda F, Cohen DE, Green RM. Hepatic overexpression of murine Abcb11 increases hepatobiliary lipid secretion and reduces hepatic steatosis. J Biol Chem 279: 2790–2799, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Frankenberg T, Rao A, Chen F, Haywood J, Shneider BL, Dawson PA. Regulation of the mouse organic solute transporter α-β, Ostα-Ostβ, by bile acids. Am J Physiol Gastrointest Liver Physiol 290: G912–G922, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, Xu C, Neubert TA, Zhang F, Linhardt RJ, Yu X, White KE, Inagaki T, Kliewer SA, Yamamoto M, Kurosu H, Ogawa Y, Kuro OM, Lanske B, Razzaque MS, Mohammadi M. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol 27: 3417–3428, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, Donahee M, Wang DY, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev 17: 1581–1591, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2: 217–225, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Ito S, Fujimori T, Furuya A, Satoh J, Nabeshima Y, Nabeshima Y. Impaired negative feedback suppression of bile acid synthesis in mice lacking betaKlotho. J Clin Invest 115: 2202–2208, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito S, Kinoshita S, Shiraishi N, Nakagawa S, Sekine S, Fujimori T, Nabeshima YI. Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech Dev 98: 115–119, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Kip NS, Lazaridis KN, Masyuk AI, Splinter PL, Huebert RC, LaRusso NF. Differential expression of cholangiocyte and ileal bile acid transporters following bile acid supplementation and depletion. World J Gastroenterol 10: 1440–1446, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 282: 26687–26695, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281: 6120–6123, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazaridis KN, Pham L, Tietz P, Marinelli RA, deGroen PC, Levine S, Dawson PA, LaRusso NF. Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J Clin Invest 100: 2714–2721, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin BC, Wang M, Blackmore C, Desnoyers LR. Liver-specific activities of FGF19 require Klotho beta. J Biol Chem 282: 27277–27284, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Makishima M, Okamoto A, Repa J, Tu H, Learned R, Luk A, Hull M, Lustig K, Mangelsdorf D, Shan B. Identification of a nuclear receptor for bile acids. Science 284: 1362–1365, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Neimark E, Chen F, Li X, Shneider BL. Bile acid-induced negative feedback regulation of the human ileal bile acid transporter. Hepatology 40: 149–156, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Pandak WM, Li YC, Chiang JY, Studer EJ, Gurley EC, Heuman DM, Vlahcevic ZR, Hylemon PB. Regulation of cholesterol 7 alpha-hydroxylase mRNA and transcriptional activity by taurocholate and cholesterol in the chronic biliary diverted rat. J Biol Chem 266: 3416–3421, 1991. [PubMed] [Google Scholar]
- 26.Parks D, Blanchard S, Bledsoe R, Chandra G, Consler T, Kliewer S, Stimmel J, Wilson T, Zavacki A, Moore D, Lehmann J. Bile acids: natural ligands for an orphan nuclear receptor. Science 284: 1365–1368, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Rao A, Haywood J, Craddock AL, Belinsky MG, Kruh GD, Dawson PA. The organic solute transporter alpha-beta, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci USA 105: 3891–3896, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shneider B, Setchell KDR, Crossman M. Fetal and perinatal expression of ileal and renal sodium-dependent bile acid transport in the rat. Pediatr Res 42: 189–194, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Wong MH, Oelkers P, Dawson PA. Identification of a mutation in the ileal sodium-dependent bile acid transporter gene that abolishes transport activity. J Biol Chem 270: 27228–27234, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Xia X, Francis H, Glaser S, Alpini G, LeSage G. Bile acid interactions with cholangiocytes. World J Gastroenterol 12: 3553–3563, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]