Abstract
Vascular and associated ventricular stiffness is one of the hallmarks of the aging cardiovascular system. Both an increase in reactive oxygen species production and a decrease in nitric oxide (NO) bioavailability contribute to the endothelial dysfunction that underlies this vascular stiffness, independent of other age-related vascular pathologies such as atherosclerosis. The activation/upregulation of arginase appears to be an important contributor to age-related endothelial dysfunction by a mechanism that involves substrate (l-arginine) limitation for NO synthase (NOS) 3 and therefore NO synthesis. Not only does this lead to impaired NO production but also it contributes to the enhanced production of reactive oxygen species by NOS. Although arginase abundance is increased in vascular aging models, it appears that posttranslational modification by S-nitrosylation of the enzyme enhances its activity as well. The S-nitrosylation is mediated by the induction of NOS2 in the endothelium. Furthermore, arginase activation contributes to aging-related vascular changes by mechanisms that are not directly related to changes in NO signaling, including polyamine-dependent vascular smooth muscle proliferation and collagen synthesis. Taken together, arginase may represent an as yet elusive target for the modification of age-related vascular and ventricular stiffness contributing to cardiovascular morbidity and mortality.
Keywords: nitric oxide synthase 3, S-nitrosylation, vascular stiffness, l-arginine pools, nitric oxide synthase uncoupling
it is no coincidence that factors contributing to longevity are critical in the promotion of cardiovascular health. Whether they include genetic factors, such as klotho gene polymorphisms (2, 3), or healthy lifestyles, such as specific diet, exercise and stress reduction, the direct relationship between these factors and cardiovascular risk is now irrefutable. Cardiovascular disease is the leading cause of morbidity and mortality in industrialized countries, and is predicted to increase further in both industrialized and developing countries as population demographics change, even though effective treatments are available that target established cardiovascular risk factors. This apparent incongruity is due to the fact that age itself, even in the absence of established cardiovascular risk factors, is the most important predictor of cardiovascular disease because of age-related changes that occur in the cardiovascular system (52, 53, 65). Critically, although age itself is an important predictor of cardiovascular events, the vascular changes that accrue with age are highly variable. Hence, it is increasingly recognized that indices of vascular function are better predictors of future cardiovascular events than age itself (65).
The central aorta dilates with age (52), while the arterial wall thickness increases even in the absence of atherosclerotic disease (64) mainly as a result of intimal thickening (86). Pathologically, aging is associated with elastin depletion and fragmentation, and collagen deposition (68). These changes result in increased vascular stiffness (48) and central pressure augmentation (67), and they manifest themselves clinically as increased systolic and pulse pressures (35, 91). These vascular changes are important contributors to cardiac changes and vascular-ventricular coupling, and the dysregulation of vital organ blood flow that occurs with aging. Several studies across different populations have clearly demonstrated that increased vascular stiffness is an independent risk factor and predictor of cardiovascular morbidity and mortality (27, 37, 56, 79, 92).
At a cellular and molecular level, the mechanisms that initiate and propagate changes in the aging vasculature are currently the focus of increasing investigation. The histological changes that accrue in the vasculature over many years and decades are generally fixed and irreversible, and they are well recognized as significant contributors to increased cardiovascular morbidity and mortality. However, vascular properties, including vascular stiffness, are also modulated acutely, and the dynamic component of vascular stiffness particularly influences cardiovascular performance. Acutely, the endothelium also modulates vessel tone (dynamic tone). Endothelial function is now increasingly recognized as extending beyond that of modulating vasomotor tone, for example, via nitric oxide (NO) production (1). In addition and importantly, the endothelium is now recognized as an important modulator of chronic changes that accrue in the underlying vascular smooth muscle and extracellular matrix. Interestingly, despite the multitude of potential aging-related targets in general and in the vasculature in particular, for the most part, they all converge toward two signaling molecules, NO and reactive oxygen species (ROS) such as the superoxide anion (O2•−), which combines with NO to yield peroxynitrite and related biological oxidants. Here, we review nitroso-redox balance and imbalance in age-related endothelial dysfunction, and we examine the role that the manganese metalloenzyme arginase plays in modulating this balance.
NO IN THE AGING ENDOTHELIUM
The first seminal observation pointing to a role for arginase in age-related endothelial dysfunction accompanied the discovery that despite an increase in endothelial NO synthase 3 (NOS3) expression in old rat aorta, NO production and downstream signaling (cGMP) was decreased (14). Although a multitude of mechanisms might underlie this somewhat counterintuitive observation, the discovery that arginase (Arg) I is expressed not only in the liver (where it is a rate limiting enzyme in the urea cycle), but also in endothelial cells (99), suggested that this enzyme played a role in the impairment of NO signaling. It was reasoned that arginase, which shares l-arginine as a substrate with NO synthase, might compete for limited substrate and thereby regulate the activity of NOS3 in vascular endothelium. This idea was supported by studies of inflammatory cells (macrophages) (63), gastrointestinal smooth muscle tissue (6), and penile smooth muscle tissue (19) in which arginase inhibition enhanced NO production and in this way enhanced immune and erectile function. To understand how this process might occur in the endothelium, it is critical to understand not only the enzymology of both NOS3 and arginase but also to understand the compartmentalization of these enzymes within the endothelial cells in which they reside. Furthermore, it is critical to understand how intracellular pools of l-arginine are regulated and trafficked so that the mechanisms underlying arginase-NOS3 substrate competition become more apparent.
The NOS3 enzyme exists as a homodimer, and its activity is dependent on Ca2+-dependent calmodulin binding, requires protoporphyrin IX, tetrahydrobiopterin (BH4), FMN, and FAD as enzyme-bound cofactors, and a Zn2+ ion tetrahedrally ligated by two cysteine residues from each monomer. NOS is regulated at a transcriptional, posttranscriptional, translational, and posttranslational level (98). With regard to vascular aging, it is well established that there is a decrease in total NO bioavailability in aged vessels. Age-associated increase in the production of endothelial ROS produced by NADPH oxidase (28), xanthine oxidase (66), and mitochondrial enzymes (20, 83, 84), react with NO to form reactive nitrogen species, thereby quenching/diminishing bioavailable NO. Reduced NOS3 phosphorylation and activation in response to shear stress is another hallmark of the aging vascular phenotype (76). Interestingly, sirtuin (SIRT)-1 dependent deacetylation and activation of NOS3 might establish a direct causal connection between calorie restriction (leads to SIRT-1 activation) and NO production (57). Furthermore, cofactor depletion (particularly BH4) might lead to NOS3 uncoupling, which results in production of O2•− by NOS rather than NO (23). Finally, and from the perspective of the review, limitation of l-arginine substrate by arginase activation/upregulation leads to age-dependent impairment of NO production and might lead to NOS3 uncoupling. The phenomenon of l-arginine depletion or arginase activation leading to ROS generation has been described for NOS1 (93) and NOS2 (94) and recently in the context of diabetes-induced vascular disease (69) and atherogenesis for NOS3 (70).
RECIPROCAL REGULATION OF NOS BY ARGINASE
Arginase converts l-arginine to l-ornithine and urea, and it is found in mammals as two distinct isoforms, Arg I and Arg II, both of which are widely expressed in many tissues, including the cardiovascular system. In extrahepatic tissues, arginase is thought to be involved in the biosynthesis of l-proline (which is a biosynthetic precursor of collagen) and glutamate, as well as polyamines such as spermine and spermidine; additionally, arginase is involved in the inflammatory and the immune response by competing with NOS for the common substrate, l-arginine. This would have important consequences in the cardiovascular system, where vascular tone and function depend on NO derived from NOS3 activity. In addition to the common substrate, NOS and arginase are functionally related in another manner: NG-hydroxyarginine, produced as an intermediate in the NOS reaction, is a modest inhibitor of arginase (30).
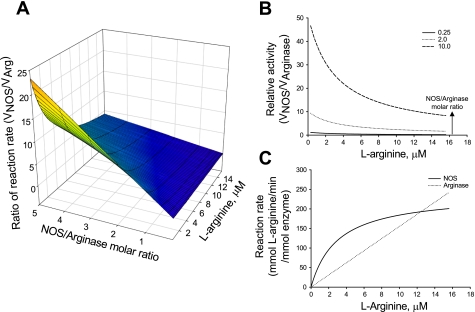
Direct comparison of the Km values could suggest that the high Km value of arginase (1–20 mM) compared with the low Km value of NOS (1–5 μM) should not allow for effective competition between these enzymes for their common l-arginine substrate. However, because these two enzymes are competing for a common substrate for catalysis, rather than two protein receptors competing for the binding of a common ligand, a better comparison of arginase and NOS should also include the rate of catalysis for each enzyme. Arginase has a Vmax of 1,400 μmol·min−1·mg−1, while NOS has a Vmax of 900 μmol·min−1·mg−1. In such a situation, a direct comparison of the Km values is not an appropriate measure of relative rates of reactions of two enzymes particularly when l-arginine concentration is much higher than Km of NOS (33). Assuming Michaelis-Menten kinetics and NOS activity independent of all other cofactors, it is possible to calculate the relative l-arginine consumption rates for NOS and arginase at different molar ratios of NOS/arginase and at different concentrations of l-arginine (Fig. 1). The following features of the NOS-arginase reciprocal regulation become clear: 1) NOS activity is higher at high NOS/arginase molar ratios and low l-arginine concentrations; 2) the two enzymes have comparable activities at lower NOS/arginase molar ratios; 3) the two enzymes have comparable activities at higher l-arginine concentrations, irrespective of the NOS/arginase molar ratio; and 4) arginase activity exceeds NOS activity at higher l-arginine concentrations at all NOS/arginase molar ratios and at all l-arginine concentrations at low NOS/arginase molar ratios. Indeed, it is evident that arginase is capable of competing for the l-arginine substrate. Furthermore, at any l-arginine concentration, arginase inhibition is, in essence, a shift toward higher NOS/arginase molar ratio, and hence it is a shift toward higher NOS/arginase relative activity.
Fig. 1.
Relative activity of nitric oxide synthase (NOS) to arginase (Arg) at different molar ratios of the enzymes demonstrates arginase can effectively compete with NOS for l-arginine substrate. A: surface plot of relative activity. B: parametric plot at increasing NOS/Arg molar ratios. C: specific activity of NOS and Arg demonstrate VNOS and VArg are comparable at higher l-arginine concentrations at all NOS/Arg molar ratios.
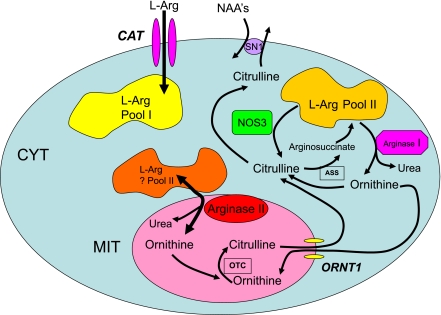
The concept that l-arginine pools are compartmentalized is emerging, although incompletely understood, helps in the understanding of the reciprocal regulation of NOS by arginase. A complete characterization of this phenomenon has been hindered by the ability to measure local cellular concentrations of l-arginine. However, data from Ellen Closs's group (17, 74) have demonstrated that endothelial cells contain three pools of l-arginine: The first, pool I, is regulated by the cationic transporter and can be depleted by the cationic amino acid l-lysine. In contrast, pool II is accessible to endothelial NOS but is not freely exchangeable with extracellular l-lysine (or l-arginine). In endothelial cells, there are two components of pool II: IIA and IIB. Pool IIA can be depleted by neutral amino acids and results from recycling of citrulline to arginine, with arginosuccinate synthetase (ASS) and arginosuccinate lyase (ASL) being critical enzymes in this process. Interestingly, given that this pool is an important source of l-arginine for NOS, recent data demonstrate that NOS3 is colocalized with ASS and ASL, suggesting that arginine-recycling enzymes, as one would predict, are close to and coupled with the enzyme that utilize the recycled l-arginine (75). How is it that the neutral amino acid exchanger can deplete a component of pool II (IIA)? It is proposed that the neutral amino acids, glutamine, histidine, and asparagine, transstimulate the exchanger with a resultant efflux of citrulline and a subsequent decrease in citrulline recycling (74). On the other hand, pool IIB results from protein breakdown and is not responsive to either cationic or neutral amino acids. Arginase, specifically Arg II in mitochondria, utilizes this pool. This arginase pool IIB is unaffected by extracellular l-arginine. However, this pool of l-arginine might be influenced by arginase and thus modulate the local concentrations of l-arginine available to NOS3. Furthermore, Topal et al. (82) recently demonstrated that depletion of freely exchangeable l-arginine pools using extracellular l-lysine did not modulate the influence of arginase on endothelial cell NO release. This suggests the presence of different l-arginine pools, at least one of which is accessible to NOS and arginase but is not exchangeable with extracellular l-arginine. Thus both enzymology and local substrate concentrations might explain the reciprocal regulation of NOS by arginase through substrate limitation. For a summary schematic outlining the different l-arginine pools, see Fig. 4.
ARGINASE STRUCTURE, FUNCTION, AND INHIBITOR DESIGN
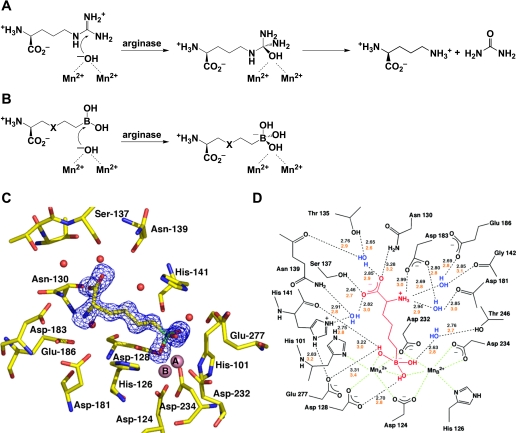
The X-ray crystal structure of rat Arg I provided the first view of the binuclear manganese cluster required for catalysis (47), and the subsequently determined structures of human Arg I (26) and human Arg II (12) revealed essentially identical metal clusters and active site architectures. In each arginase isozyme, the binuclear manganese cluster activates a bridging hydroxide ion for nucleophilic attack at the l-arginine guanidinium group to yield a tetrahedral intermediate, which then collapses to yield l-ornithine and urea (Fig. 2A).
Fig. 2.
A: arginase catalyzes the nucleophilic attack of a metal-bridging hydroxide ion at the guanidinium group of l-arginine to yield l-ornithine and urea. B: boronic acid analogues of l-arginine, 2(S)-amino-6-hexanoic acid (ABH) (X = CH2) and S-(2-boronoethyl)-l-cysteine (BEC) (X = S), undergo nucleophilic attack by the metal-bridging hydroxide ion in the arginase active site to yield tight-binding boronate anions. C: electron density map of ABH bound to human arginase I, confirming the binding of the tetrahedral boronate anion. [From Di Costanzo et al. (26).] D: scheme showing enzyme-inhibitor hydrogen bond (black dashed lines) and metal coordination interactions (green dashed lines) observed in arginase I-ABH complexes. [From Cox et al. (19) and Di Costanzo et al. (26).] On average, shorter enzyme-inhibitor hydrogen bond interactions are observed in the human arginase I-ABH complex (black numbers) compared with the rat arginase I-ABH complex (orange numbers). This structural feature may account for the higher affinity of ABH toward human arginase I (Kd = 5 nM) compared with rat arginase I (Kd = 110 nM).
Structural studies of complexed arginase with boronic acid analogs of l-arginine provide important mechanistic inferences on the catalytic mechanism (16). Both (2)S-amino-6-hexanoic acid (ABH) and S-(2-boronoethyl)-l-cysteine (BEC) contain trigonal planar boronic acid moieties in place of the trigonal planar guanidinium group of l-arginine (Fig. 2B) (5, 49). On binding to the active site of arginase, the electron-deficient boron atoms of ABH and BEC undergo nucleophilic attack by the metal-bridging hydroxide ion to yield a tetrahedral boronate anion, just as a nucleophilic attack of hydroxide ions at the guanidinium group of l-arginine yields a tetrahedral intermediate. This binding mode is confirmed in X-ray crystallographic structure determinations of enzyme-inhibitor complexes (12, 19, 49); to illustrate, the structure of the human Arg I-ABH complex (Kd = 5 nM) is shown in Fig. 2C, and a schematic showing enzyme-inhibitor interactions is presented in Fig. 2D.
Notably, other amino acids bearing side chains that mimic the tetrahedral intermediate in the arginase mechanism, such as an aldehyde that binds as a tetrahedral gem-diol (73), or a tetrahedral sulfonamide that binds as the ionized sulfonamidate anion (13), bind 50,000 times more weakly than amino acids bearing boronic acid side chains such as ABH or BEC. Thus the chemical properties of the boronic acid side chains of ABH and BEC are optimally matched to the chemical reactivity of the metal-bridging hydroxide ion in the arginase active site.
Interestingly, another class of arginase inhibitors consists of amino acids bearing N-hydroxyguanidinium side chains, such as N-hydroxy-l-arginine (NOHA; Ki = 10 μM) (11). Although NOHA is a modest inhibitor of arginase, its shorter analog N-hydroxy-nor-l-arginine (nor-NOHA) is more potent, with Ki = 500 nM (80). The crystal structures of the complexes between rat Arg I and NOHA or nor-NOHA reveal that the N-hydroxy group of each inhibitor displaces the metal-bridging hydroxide ion of the native enzyme (18). Although the N-hydroxyguanidinium amino acids exhibit slightly weaker affinities in binding to arginase compared with the boronic amino acids, both classes of inhibitors serve as useful tools for probing the chemical biology of arginase and arginase/NOS competition for the l-arginine substrate in various disease states.
IMPLICATIONS FOR ARGINASE/NOS REGULATION
Age-related vascular changes have been investigated in humans and in a number of animal species. In general, it is believed that the relative contributions of dysregulated mechanisms to vascular pathobiology in aging may be species-dependent. Moreover, the contribution of vascular control mechanisms, both in health and in aging, and in disease conditions, is influenced by the specific regional vascular bed and the specific vessel type/size within a vascular bed that is being investigated. While arginase is increasingly recognized as an important mechanisms regulating vasomotor tone, its relative contribution may vary depending on the vascular bed and the vessel size and type. For example, in aging rats, arginase inhibition did not decrease blood flow in muscle resistance arterioles (23). Moreover, BH4 increased brachial artery flow-mediated dilatation in sedentary elderly individuals compared with that observed in young humans, suggesting that arginase may play little or no role (34), although redundancy of mechanisms cannot be ruled out, nor an interaction between BH4 and arginase. A further example of the differential influence of species and vessel type is the observation that BH4 was observed to be decreased in old rat skeletal vessels (23), but it was not increased in the aorta of old mice (10). Notwithstanding, the influence of species, vascular bed and vessel size, and unresolved questions regarding the theoretical underpinnings of the reciprocal regulation of NOS by arginase, there is now sufficient evidence in multiple organ systems and in multiple pathophysiological scenarios to indicate that reciprocal regulation of NOS by arginase is an important mechanism underlying vasomotor regulation, especially in aging and disease states. The process of reciprocal regulation of NOS by arginase has in fact been demonstrated in the majority of cell types/organs in which NO is an important signaling molecule, including the cardiac myocyte (78), penis (8), airway (58), skin [where it is an important modulator of skin blood flow (40, 41)], inflammatory mediator cells such as macrophages (63) and importantly, the endothelium (7). Furthermore, upregulation of arginase activity has been demonstrated to contribute to the vasoregulatory dysfunction associated with systemic (25, 39, 46, 99) and pulmonary hypertension (43, 61, 96), diabetes and erectile dysfunction (8, 9), impaired bronchodilatory function in asthma (62), and vascular dysfunction in aging (7, 71, 90). These advances have in large measure been a function of the design and synthesis of specific arginase inhibitors such as ABH, BEC, and nor-NOHA.
Our laboratory's initial observations in an aging rat model suggested that Arg I was the predominant but not exclusive isoform upregulated in rat aorta (7). Furthermore, the cellular source of the upregulated enzyme was confined predominantly but not exclusively to the endothelium. Further studies revealed that upregulated Arg I was indeed confined to the endothelium and that knockdown of Arg I with specific oligonucleotides could restore NO production and endothelial-dependent vasorelaxation in old rat aorta in organ chamber experiments toward that of young rats (90).
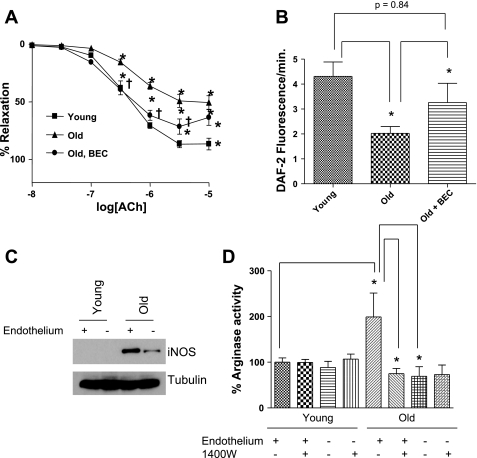
The mechanisms underlying increases in arginase expression and/or activity have not been extensively investigated. Studies in rat aortic vascular smooth muscle demonstrated that interleukin-4 and -13 induced upregulation of Arg I was mediated by the JAK/STAT6 pathway and associated with increased DNA synthesis and cell proliferation (88). In the porcine coronary arterioles, oxidant stress induced by hydrogen peroxide impairs endothelial-dependent dilation. This effect was associated with increased Arg I DNA and protein expression, and it was associated with increased arginase protein synthesis (81). The specific mechanisms underlying posttranslational modification and increased arginase activity have also not been clearly defined. Given the interaction between NOS and arginase signaling, we hypothesized that S-nitrosylation of Arg I might be an important posttranslational modification mechanism that regulates its activity. The protein sequence for human Arg I contains three cysteines, C45, C168, and C303, with C303 being very close to residue 308 and the COOH terminal S-shaped tail of arginase. The latter mediates 54% of the intersubunit interactions to form the active arginase homotrimer (47, 54, 59). While the monomeric forms of arginase are active, they display only 15–25% activity compared with the trimer (54). We therefore studied S-nitrosylation of each cysteine residue of human Arg I, as well as the effect of S-nitrosylation on enzyme activity and the stability of the Arg I trimer in vitro and ex vivo. Finally, we examined whether altered arginase nitrosylation could contribute to the pathobiology of vascular aging by limiting NO bioavailability, thereby contributing to impaired endothelial function. Aging is well recognized to be associated with an increase in oxidative stress and NOS2 expression (14, 21). Thus we determined whether NOS2 plays a role in arginase upregulation and vascular dysfunction in aging. We have demonstrated that Arg I is activated by nitrosylation of C303; that this activation results from increased stabilization of the arginase trimer; and that nitrosylation is NOS2 dependent. Moreover, this nitrosylation and upregulation of arginase contributes to the endothelial dysfunction in aged vessels (Fig. 3A). Arginase inhibition significantly improves but does not completely reverse age-related endothelial dysfunction. This suggests, not surprisingly, that other mechanisms, both endothelial dependent and independent (e.g., advanced glycation end products) contribute to the aged phenotype. Improved endothelial-dependent relaxation following arginase inhibition is associated with increased NO production (Fig. 3B). NOS2 was not found in younger vessels but was clearly demonstrated in both the endothelial and nonendothelial components of old vessels (Fig. 3C). Increased arginase activity was clearly demonstrated in the endothelial component of old vessels only (Fig. 3D). Importantly, this increased arginase activity was completely reversed by N-[3-aminomethylbenzyl]acetamidine (1400W), a specific NOS2 inhibitor (Fig. 3D).
Fig. 3.
NOS2 S-nitrosylates arginase and regulates its activity in aging endothelium. A: arginase inhibition with BEC leads to improved endothelium-dependent vasodilation in old rat tail artery. acetylcholine, ACh concentration. B: arginase inhibition also restores NO production in old rat aorta as measured by diaminofluorescin (DAF) fluorescence C: NOS2 is expressed in old rat aorta, including importantly in the endothelium. iNOS, inducible NOS. D: arginase activity is significantly higher in old rat aorta than young (n = 4) and largely confined to the endothelium, because the endothelium-denuded rings show markedly reduced activity. Treatment of old vessels with the NOS2 inhibitor N-[3-aminomethylbenzyl]acetamidine (1400W) for 16 h leads to significant reduction (*P < 0.05) in arginase activity in old but not in young rats. D: endothelium-denuded rings are unaffected by 1400W. [From Santhanam et al. (71).]
So what is the connection between arginase activation, NOS2 induction and aging? It is well established that NOS2 is coinduced in response to inflammatory stimuli mediated by a variety of cytokines (51, 60, 63) in rat and mouse models. While there is limited evidence for NOS2 expression in cultured endothelial cells treated with proinflammatory cytokines and lipopolysaccharide ,x studies have demonstrated the expression of NOS2 in cultured human umbilical venous endothelial cells under various conditions, including vasoactive stimuli (72), infection with Pseudomonas aeruginosa (4), and upregulation of tissue factor by anti-phospholipid antibodies (85). The expression of NOS2 in brain endothelial cells has been shown during embryonic and postnatal development (36), after ischemic injury (44), and Alzheimer's disease (29). While a recent study shows expression of NOS2 in endothelial cells overlaying stage 3 human atherosclerotic plaque (97), alterations in the expression of NOS2 in aging human aortic endothelial cells is yet to be examined. Furthermore, a recent study has supported the role that arginase plays in mediating loss of NO and the pathogenesis of endothelial dysfunction in human blood vessels exposed to proinflammatory conditions, specifically inflammatory bowel disease (42). This, in combination with the enhanced expression of proinflammatory markers and cytokines, as well as their receptors, suggests that the contribution of arginase to endothelial dysfunction may be a function of the degree of the vascular inflammatory response to aging. While the rat is a well-accepted model of cardiovascular aging, there is now evidence that upregulation of arginase in humans might contribute to age-related vascular endothelial function (40, 41). This evidence arises from studies in human skin. Reflex cutaneous vasodilation in response to heat is mediated in part by NO signaling, which is significantly attenuated in aging. Interestingly, arginase inhibition in combination with the antioxidant ascorbate or l-arginine significantly restores the reflex responsiveness toward the young phenotype, supporting a role for arginase in endothelial dysfunction associated with aging. Because ascorbate reduces nitrosothiols, it is possible that the effect observed might be a function not only of arginase inhibition but also of a reduction of the NOS2-dependent S-nitrosylation-mediated activation of arginase by nitrosothiol reduction.
The idea that arginase upregulation in aging compromises endothelial function is not limited to the systemic vasculature. Bivalaqua et al. (8) have recently demonstrated that Arg I is upregulated in the corporal tissue of aging mice and rats. Furthermore, both systemic and cavernosal inhibition of arginase, with the specific inhibitor ABH, results in markedly improved erectile function in response to electrical stimulation of the penile nerve. Given these observations regarding restoration of endothelial NO production and endothelial function in multiple beds, it seems likely that arginase represents an important therapeutic target for age-related endothelial dysfunction in which nitroso-redox balance is disturbed. This is relevant to all tissues in which the endothelium plays a critical physiological role, including blood vessels and erectile tissue. While the inhibition of arginase represents a potential risk for suppressing the urea cycle, this remains theoretical because the concentration of enzyme in the liver is 100–1,000 times that present in the endothelium, and it is unlikely to be suppressed by clinically relevant doses of inhibitor. The possibility of inhibition of white blood cell arginase and its effect in immunity also remain theoretical and awaits the results of clinical trials.
SPATIAL CONFINEMENT OF NO SIGNALING, ARGINASE, AND l-ARGININE POOLS
Nitrosylation of Arg I is NOS2 dependent and leads to its activation. The modification promotes l-arginine depletion and reduced NOS3 activity, contributing to endothelial dysfunction associated with aging. However, several key features of this regulatory mechanism remain poorly understood. For example, how does the S-nitrosylation by NOS2 occur in a specific and spatially confined manner? S-nitrosylation of Arg I by NOS2 should cause increased competition with NOS2 for l-arginine, leading to reduced NOS2 activity, and therefore should be a self-limiting step. How then does the balance of nitrosylated Arg I and NOS2 shift in the aging endothelium? Is this caused by translocation of Arg I away from NOS2? Furthermore, based on the spatial confinement of NO signaling, how does cytosolic Arg I constrain the activity of caveolin-bound NOS3? We proposed that this specificity is determined by NOS2 interacting with Arg I, allowing the nitrosylation to occur. The hypothesis is based on the observations of the Snyder group (50), who have demonstrated that NOS2 binds to and activates cyclooxygenase 2, a proinflammatory protein. Furthermore, another recently published manuscript describes the interaction of NOS2 with phospholipase A2, a critical enzyme in the synthesis of proinflammatory prostaglandins (95). Thus NOS2 seems to activate proinflammatory mediations. In as yet unpublished experiments, we have demonstrated a direct interaction between NOS2 and Arg I based on coimmunoprecipitation experiments. The exact sites of this interaction remain to be determined. So what of the mechanism underlying Arg I-mediated constraint of NOS3 in aging and inflammation? Recent data in red blood cells suggest that Arg I has a binding partner, flotillin (Flot)-1, which promotes translocation of Arg I to the cell membrane (45). Flot-1 is a scaffolding protein and is an integral component of caveolae and lipid rafts (15, 87). Both Flot-1 and Flot-2 are expressed in endothelial cells (15, 77) and could represent a mechanism for the trafficking of Arg I from cytosolic loci to caveolae/lipid rafts in endothelial cells, thus placing Arg I in close proximity to NOS3. The translocation of proteins from one cellular domain to another as a result of S-nitrosylation has been previously demonstrated. For example, S-nitrosylation of GAPDH triggers apoptosis by promoting its binding to Siah1, which translocates to the nucleus (38). In unpublished data, we demonstrated a direct interaction between Arg I and flotillin. We have demonstrated that Arg I interacts with flotillin and in this way might traffic Arg I into a domain in which it constrains NOS3 activity by substrate l-arginine depletion.
NON-NO-DEPENDENT EFFECTS OF ARGINASE ACTIVATION/UPREGULATION AND THE POTENTIAL CONSEQUENCES FOR VASCULAR AGING
While the focus of this review has been on the reciprocal regulation of NOS by arginase as a result of substrate limitation, the effects of arginase activation/upregulation might extend well beyond this effect (Fig. 4). Ornithine, the product of the conversion of l-arginine to urea is the precursor for the synthesis of the primary amino acid, proline, and the polyamine-derived amino acids, spermidine and spermine, that could be important in altering vascular properties (30). Proline, a primary amino acid that is critical in the synthesis of structural proteins and collagen in particular, is produced by the conversion of ornithine by ornithine aminotransferase. Furthermore, the overexpression of Arg I or Arg II in endothelial cells is associated with increased production of proline (55). In addition, the predicted production of collagen in response to stimuli such as tumor growth factor-β and cyclic strain are dependent on proline formation (31, 32). Ornithine decarboxylase converts l-ornithine to the polyamine putrescince with the subsequent synthesis of spermidine and spermine. These polyamines are critical in vascular smooth muscle proliferation, with overexpression enhancing and pharmacological inhibition preventing this proliferative effect (89). Although the effects of arginase on these parameters have not been directly measured in models of aging, it is conceivable that arginase activation might contribute to age-dependent vascular stiffening and pathology through vascular smooth muscle proliferation, as well as collagen deposition.
Fig. 4.
l-Arginine (l-Arg) pools in the context of the metabolic network of l-arginine in cytosol and mitochondria, including NO synthesis and arginase activity. The concept of specific subcellular l-arginine pools is complex and is dependent not only on the knowledge of the specific membrane cationic transporters but also on an understanding of the networks for l-arginine metabolism in both the cytoplasmic and mitochondrial compartments as well as its coupling to urea synthesis. The picture that seems to be emerging is summarized in the above figure. Pool I is freely exchangeable with extracellular l-arginine and is regulated by the cationic transporter (CAT). This pool can be depleted by exchanging the pool with the cationic amino acid lysine. Pool II, on the other hand, is not exchangeable with extracellular l-arginine and cannot be depleted by extracellular lysine. Part of pool II, pool IIA, appears to be a function of the recycling of citrulline to arginine with arginosuccinate synthetase (ASS) and arginosuccinate lyase (ASL) being critical enzymes. Ornithine is converted to citrulline by ornithine transcarbamylase (OTC). Finally, the remaining component of pool II, pool IIB (not shown) cannot be depleted by the neutral amino acids such as histidine (perhaps by transtimulation/exchange and depletion of recycling citrulline) through the system N, neutral amino acid (NAA), transporter (SN1) and is likely provided by protein breakdown. ORTN1, ornithine/citrulline exchanger (transporter); CYT, cytoplasm; MIT, mitochondria.
IN SUMMARY
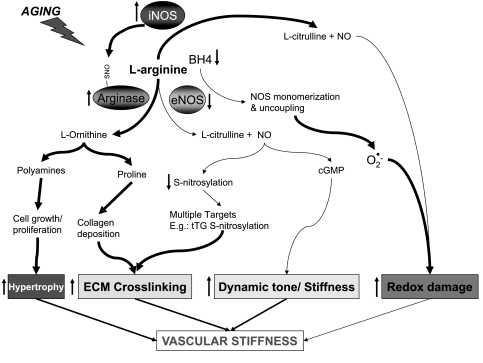
Figure 5 summarizes the potential mechanisms by which arginase might be upregulated and how this may contribute to age related vascular stiffness. The activation of arginase appears to be an important contributor to age-related endothelial dysfunction by a mechanism that involves substrate limitation for NOS3. Not only does this lead to impaired NO production but also it might contribute to the enhanced production of reactive oxygen species by NOS. While arginase abundance might be increased in vascular aging models, it appears that posttranslational modification by S-nitrosylation of the enzyme enhances its activity as well. The S-nitrosylation is mediated by the induction of NOS2 in the endothelium. What remains to be understood involves the mechanism by which NOS2 is induced. Does it involve the activation of receptors for advanced glycation end products as a result of age-dependent accumulation of endothelial advanced glycation end products, or is it dependent on the activations of toll receptors involved in innate immunity and inflammatory responses? Also, arginase activation might contribute to aging-related vascular changes by mechanisms that are not directly related to changes in NO signaling, including polyamine-dependent vascular smooth muscle proliferation and collagen synthesis. Taken together, arginase may represent an as yet elusive target for the modification of age-related vascular and ventricular stiffness contributing to cardiovascular morbidity and mortality.
Fig. 5.
Increased arginase activity contributes to vascular stiffness during aging via several mechanisms. BH4, tetrahydrobiopterin; eNOS, endothelial NOS; ECM, endothelial cell membrane; tT6, tissue transglutamase.
GRANTS
This work is supported in part by National Institutes of Health Grants R01 AG-021523 (to D. E. Berkowitz) and R01 GM-49758 (to D. W. Christianson).
REFERENCES
- 1.Abdu TA, Elhadd T, Pfeifer M, Clayton RN. Endothelial dysfunction in endocrine disease. Trends Endocrinol Metab 12: 257–265, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Arking DE, Becker DM, Yanek LR, Fallin D, Judge DP, Moy TF, Becker LC, Dietz HC. KLOTHO allele status and the risk of early-onset occult coronary artery disease. Am J Hum Genet 72: 1154–1161, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arking DE, Krebsova A, Macek M Sr, Macek M Jr, Arking A, Mian IS, Fried L, Hamosh A, Dey S, McIntosh I, Dietz HC. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci USA 99: 856–861, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assis MC, Freitas C, Saliba AM, APDAC, Simao TA, Albano RM, Plotkowski MC. Up-regulation of Fas expression by Pseudomonas aeruginosa-infected endothelial cells depends on modulation of iNOS and enhanced production of NO induced by bacterial type III secreted proteins. Int J Mol Med 18: 355–363, 2006. [PubMed] [Google Scholar]
- 5.Baggio R, Elbaum D, Kanyo ZF, Carroll PJ, Cavalli RC, Ash DE, Christianson DW. Inhibition of Mn2+ 2-arginase by borate leads to the design of a transition state analogue inhibitor, 2 (s)-amino-6-boronohexanoic acid. J Am Chem Soc 119: 8107–8108, 1997. [Google Scholar]
- 6.Baggio R, Emig FA, Christianson DW, Ash DE, Chakder S, Rattan S. Biochemical and functional profile of a newly developed potent and isozyme-selective arginase inhibitor. J Pharmacol Exp Ther 290: 1409–1416, 1999. [PubMed] [Google Scholar]
- 7.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 108: 2000–2006, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Bivalacqua TJ, Burnett AL, Hellstrom WJ, Champion HC. Overexpression of arginase in the aged mouse penis impairs erectile function and decreases eNOS activity: influence of in vivo gene therapy of anti-arginase. Am J Physiol Heart Circ Physiol 292: H1340–H1351, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Bivalacqua TJ, Hellstrom WJ, Kadowitz PJ, Champion HC. Increased expression of arginase II in human diabetic corpus cavernosum: in diabetic-associated erectile dysfunction. Biochem Biophys Res Commun 283: 923–927, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K, Katusic ZS. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol 287: H2448–H2453, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Boucher JL, Custot J, Vadon S, Delaforge M, Lepoivre M, Tenu JP, Yapo A, Mansuy D. N omega-hydroxyl-L-arginine, an intermediate in the l-arginine to nitric oxide pathway, is a strong inhibitor of liver and macrophage arginase. Biochem Biophys Res Commun 203: 1614–1621, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Cama E, Colleluori DM, Emig FA, Shin H, Kim SW, Kim NN, Traish AM, Ash DE, Christianson DW. Human arginase II: crystal structure and physiological role in male and female sexual arousal. Biochemistry 42: 8445–8451, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Cama E, Shin H, Christianson DW. Design of amino acid sulfonamides as transition-state analogue inhibitors of arginase. J Am Chem Soc 125: 13052–13057, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Cernadas MR, Sanchez de Miguel L, Garcia-Duran M, Gonzalez-Fernandez F, Millas I, Monton M, Rodrigo J, Rico L, Fernandez P, de Frutos T, Rodriguez-Feo JA, Guerra J, Caramelo C, Casado S, Lopez F. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res 83: 279–286, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Chatenay-Rivauday C, Cakar ZP, Jeno P, Kuzmenko ES, Fiedler K. Caveolae: biochemical analysis. Mol Biol Rep 31: 67–84, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Christianson DW Arginase: structure, mechanism, and physiological role in male and female sexual arousal. Acc Chem Res 38: 191–201, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Closs EI, Scheld JS, Sharafi M, Forstermann U. Substrate supply for nitric-oxide synthase in macrophages and endothelial cells: role of cationic amino acid transporters. Mol Pharmacol 57: 68–74, 2000. [PubMed] [Google Scholar]
- 18.Cox JD, Cama E, Colleluori DM, Pethe S, Boucher JL, Mansuy D, Ash DE, Christianson DW. Mechanistic and metabolic inferences from the binding of substrate analogues and products to arginase. Biochemistry 40: 2689–2701, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Cox JD, Kim NN, Traish AM, Christianson DW. Arginase-boronic acid complex highlights a physiological role in erectile function. Nat Struct Biol 6: 1043–1047, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Csiszar A, Labinskyy N, Orosz Z, Ungvari Z. Altered mitochondrial energy metabolism may play a role in vascular aging. Med Hypotheses 67: 904–908, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Daghigh F, Fukuto JM, Ash DE. Inhibition of rat liver arginase by an intermediate in NO biosynthesis, NG-hydroxy-l-arginine: implications for the regulation of nitric oxide biosynthesis by arginase. Biochem Biophys Res Commun 202: 174–180, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol 586: 1161–1168, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demougeot C, Prigent-Tessier A, Bagnost T, Andre C, Guillaume Y, Bouhaddi M, Marie C, Berthelot A. Time course of vascular arginase expression and activity in spontaneously hypertensive rats. Life Sci 80: 1128–1134, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Di Costanzo L, Sabio G, Mora A, Rodriguez PC, Ochoa AC, Centeno F, Christianson DW. Crystal structure of human arginase I at 1.29-A resolution and exploration of inhibition in the immune response. Proc Natl Acad Sci USA 102: 13058–13063, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dolan E, Thijs L, Li Y, Atkins N, McCormack P, McClory S, O'Brien E, Staessen JA, Stanton AV. Ambulatory arterial stiffness index as a predictor of cardiovascular mortality in the Dublin Outcome Study. Hypertension 47: 365–370, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Dorheim MA, Tracey WR, Pollock JS, Grammas P. Nitric oxide synthase activity is elevated in brain microvessels in Alzheimer's disease. Biochem Biophys Res Commun 205: 659–665, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol 34: 906–911, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durante W, Liao L, Reyna SV, Peyton KJ, Schafer AI. Physiological cyclic stretch directs l-arginine transport and metabolism to collagen synthesis in vascular smooth muscle. FASEB J 14: 1775–1783, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Durante W, Liao L, Reyna SV, Peyton KJ, Schafer AI. Transforming growth factor-beta (1) stimulates l-arginine transport and metabolism in vascular smooth muscle cells: role in polyamine and collagen synthesis. Circulation 103: 1121–1127, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Eisenthal R, Danson MJ, Hough DW. Catalytic efficiency and kcat/KM: a useful comparator? Trends Biotechnol 25: 247–249, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568: 1057–1065, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franklin SS, Gustin Wt Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 96: 308–315, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Galea E, Reddi J, Feinstein DL. Differential suppression of glial nitric oxide synthase induction by structurally related tyrosine kinase inhibitors. Neurosci Lett 200: 195–198, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Hansen TW, Staessen JA, Zhang H, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Cardiovascular outcome in relation to progression to hypertension in the Copenhagen MONICA cohort. Am J Hypertens 20: 483–491, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol 7: 665–674, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Holowatz LA, Kenney WL. Upregulation of arginase activity contributes to attenuated reflex cutaneous vasodilatation in hypertensive humans. J Physiol 581: 863–872, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holowatz LA, Thompson CS, Kenney WL. Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. Am J Physiol Heart Circ Physiol 291: H2965–H2970, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Holowatz LA, Thompson CS, Kenney WL. l-Arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin. J Physiol 574: 573–581, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horowitz S, Binion DG, Nelson VM, Kanaa Y, Javadi P, Lazarova Z, Andrekopoulos C, Kalyanaraman B, Otterson MF, Rafiee P. Increased arginase activity and endothelial dysfunction in human inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 292: G1323–G1336, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, Noguchi CT, Gladwin MT. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood 109: 3088–3098, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iadecola C, Ross ME. Molecular pathology of cerebral ischemia: delayed gene expression and strategies for neuroprotection. Ann NY Acad Sci 835: 203–217, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Jiang M, Ding Y, Su Y, Hu X, Li J, Zhang Z. Arginase-flotillin interaction brings arginase to red blood cell membrane. FEBS Lett 580: 6561–6564, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Johnson FK, Johnson RA, Peyton KJ, Durante W. Arginase inhibition restores arteriolar endothelial function in Dahl rats with salt-induced hypertension. Am J Physiol Regul Integr Comp Physiol 288: R1057–R1062, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Kanyo ZF, Scolnick LR, Ash DE, Christianson DW. Structure of a unique binuclear manganese cluster in arginase. Nature 383: 554–557, 1996. [DOI] [PubMed] [Google Scholar]
- 48.Kass DA Age-related changes in ventricular-arterial coupling: pathophysiologic implications. Heart Fail Rev 7: 51–62, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Kim NN, Cox JD, Baggio RF, Emig FA, Mistry SK, Harper SL, Speicher DW, Morris SM Jr, Ash DE, Traish A, Christianson DW. Probing erectile function: S-(2-boronoethyl)-l-cysteine binds to arginase as a transition state analogue and enhances smooth muscle relaxation in human penile corpus cavernosum Biochemistry 40: 2678–2688, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science 310: 1966–1970, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Koga T, Koshiyama Y, Gotoh T, Yonemura N, Hirata A, Tanihara H, Negi A, Mori M. Coinduction of nitric oxide synthase and arginine metabolic enzymes in endotoxin-induced uveitis rats. Exp Eye Res 75: 659–667, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Lakatta EG Cardiovascular regulatory mechanisms in advanced age. Physiol Rev 73: 413–467, 1993. [DOI] [PubMed] [Google Scholar]
- 53.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Lavulo LT, Emig FA, Ash DE. Functional consequences of the G235R mutation in liver arginase leading to hyperargininemia. Arch Biochem Biophys 399: 49–55, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Li H, Meininger CJ, Hawker JR Jr, Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM Jr, Wu G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab 280: E75–E82, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 113: 657–663, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104: 14855–14860, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meurs H, Maarsingh H, Zaagsma J. Arginase and asthma: novel insights into nitric oxide homeostasis and airway hyperresponsiveness. Trends Pharmacol Sci 24: 450–455, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Mora A, del Ara Rangel M, Fuentes JM, Soler G, and Centeno F. Implications of the S-shaped domain in the quaternary structure of human arginase. Biochim Biophys Acta 1476: 181–190, 2000. [DOI] [PubMed] [Google Scholar]
- 60.Mori M, Gotoh T. Regulation of nitric oxide production by arginine metabolic enzymes. Biochem Biophys Res Commun 275: 715–719, 2000. [DOI] [PubMed] [Google Scholar]
- 61.Morris CR New strategies for the treatment of pulmonary hypertension in sickle cell disease: the rationale for arginine therapy. Treat Respir Med 5: 31–45, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM Jr. Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med 170: 148–153, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Morris SM, Kepka-Lenhart D, Chen LC. Differential regulation of arginases and inducible nitric oxide synthase in murine macrophage cells. Am J Physiol Endocrinol Metab 275: E740–E747, 1998. [DOI] [PubMed] [Google Scholar]
- 64.Nagai Y, Metter EJ, Fleg JL. Increased carotid artery intimal-medial thickness: risk factor for exercise-induced myocardial ischemia in asymptomatic older individuals. Vasc Med 4: 181–186, 1999. [DOI] [PubMed] [Google Scholar]
- 65.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension 46: 454–462, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Newaz MA, Yousefipour Z, Oyekan A. Oxidative stress-associated vascular aging is xanthine oxidase-dependent but not NAD (P)H oxidase-dependent. J Cardiovasc Pharmacol 48: 88–94, 2006. [DOI] [PubMed] [Google Scholar]
- 67.Nichols WW Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens 18: 3S–10S, 2005. [DOI] [PubMed] [Google Scholar]
- 68.O'Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension 45: 652–658, 2005. [DOI] [PubMed] [Google Scholar]
- 69.Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res 102: 95–102, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, Lim HK, Sohi J, Santhanam L, Soucy K, Tuday E, Baraban E, Ilies M, Gerstenblith G, Nyhan D, Shoukas A, Christianson DW, Alp NJ, Champion HC, Huso D, Berkowitz DE. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res 102: 923–932, 2008. [DOI] [PubMed] [Google Scholar]
- 71.Santhanam L, Lim HK, Lim HK, Miriel V, Brown T, Patel M, Balanson S, Ryoo S, Anderson M, Irani K, Khanday F, Di Costanzo L, Nyhan D, Hare JM, Christianson DW, Rivers R, Shoukas A, Berkowitz DE. Inducible NO synthase dependent S-nitrosylation and activation of arginase1 contribute to age-related endothelial dysfunction. Circ Res 101: 692–702, 2007. [DOI] [PubMed] [Google Scholar]
- 72.Schena M, Mulatero P, Schiavone D, Mengozzi G, Tesio L, Chiandussi L, Veglio F. Vasoactive hormones induce nitric oxide synthase mRNA expression and nitric oxide production in human endothelial cells and monocytes. Am J Hypertens 12: 388–397, 1999. [PubMed] [Google Scholar]
- 73.Shin H, Cama E, Christianson DW. Design of amino acid aldehydes as transition-state analogue inhibitors of arginase. J Am Chem Soc 126: 10278–10284, 2004. [DOI] [PubMed] [Google Scholar]
- 74.Simon A, Plies L, Habermeier A, Martine U, Reining M, Closs EI. Role of neutral amino acid transport and protein breakdown for substrate supply of nitric oxide synthase in human endothelial cells. Circ Res 93: 813–820, 2003. [DOI] [PubMed] [Google Scholar]
- 75.Solomonson LP, Flam BR, Pendleton LC, Goodwin BL, Eichler DC. The caveolar nitric oxide synthase/arginine regeneration system for NO production in endothelial cells. J Exp Biol 206: 2083–2087, 2003. [DOI] [PubMed] [Google Scholar]
- 76.Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA, Berkowitz DE. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol 101: 1751–1759, 2006. [DOI] [PubMed] [Google Scholar]
- 77.Sprenger RR, Speijer D, Back JW, De Koster CG, Pannekoek H, Horrevoets AJ. Comparative proteomics of human endothelial cell caveolae and rafts using two-dimensional gel electrophoresis and mass spectrometry. Electrophoresis 25: 156–172, 2004. [DOI] [PubMed] [Google Scholar]
- 78.Steppan J, Ryoo S, Schuleri KH, Gregg C, Hasan RK, White AR, Bugaj LJ, Khan M, Santhanam L, Nyhan D, Shoukas AA, Hare JM, Berkowitz DE. Arginase modulates myocardial contractility by a nitric oxide synthase 1-dependent mechanism. Proc Natl Acad Sci USA 103: 4759–4764, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 111: 3384–3390, 2005. [DOI] [PubMed] [Google Scholar]
- 80.Tenu JP, Lepoivre M, Moali C, Brollo M, Mansuy D, Boucher JL. Effects of the new arginase inhibitor N (omega)-hydroxy-nor-l-arginine on NO synthase activity in murine macrophages. Nitric Oxide 3: 427–438, 1999. [DOI] [PubMed] [Google Scholar]
- 81.Thengchaisri N, Hein TW, Wang W, Xu X, Li Z, Fossum TW, Kuo L. Upregulation of arginase by H2O2 impairs endothelium-dependent nitric oxide-mediated dilation of coronary arterioles. Arterioscler Thromb Vasc Biol 26: 2035–2042, 2006. [DOI] [PubMed] [Google Scholar]
- 82.Topal JL, Brunet A, Walch L, Boucher JL, David-Dufilho M. Mitochondrial arginase II modulates nitric-oxide synthesis through nonfreely exchangeable l-arginine pools in human endothelial Cells. J Pharmacol Exp Ther 318: 1368–1374, 2006. [DOI] [PubMed] [Google Scholar]
- 83.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007. [DOI] [PubMed] [Google Scholar]
- 84.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res 102: 519–528, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vega-Ostertag M, Casper K, Swerlick R, Ferrara D, Harris EN, Pierangeli SS. Involvement of p38 MAPK in the up-regulation of tissue factor on endothelial cells by antiphospholipid antibodies. Arthritis Rheum 52: 1545–1554, 2005. [DOI] [PubMed] [Google Scholar]
- 86.Virmani R, Avolio AP, Mergner WJ, Robinowitz M, Herderick EE, Cornhill JF, Guo SY, Liu TH, Ou DY, O'Rourke M. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol 139: 1119–1129, 1991. [PMC free article] [PubMed] [Google Scholar]
- 87.Volonte D, Galbiati F, Li S, Nishiyama K, Okamoto T, Lisanti MP. Flotillins/cavatellins are differentially expressed in cells and tissues and form a hetero-oligomeric complex with caveolins in vivo. Characterization and epitope-mapping of a novel flotillin-1 monoclonal antibody probe. J Biol Chem 274: 12702–12709, 1999. [DOI] [PubMed] [Google Scholar]
- 88.Wei LH, Jacobs AT, Morris SM Jr, Ignarro LJ. IL-4 and IL-13 upregulate arginase I expression by cAMP and JAK/STAT6 pathways in vascular smooth muscle cells. Am J Physiol Cell Physiol 279: C248–C256, 2000. [DOI] [PubMed] [Google Scholar]
- 89.Wei LH, Wu G, Morris SM Jr, Ignarro LJ. Elevated arginase I expression in rat aortic smooth muscle cells increases cell proliferation. Proc Natl Acad Sci USA 98: 9260–9264, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.White AR, Ryoo S, Li D, Champion HC, Steppan J, Wang D, Nyhan D, Shoukas AA, Hare JM, Berkowitz DE. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension 47: 245–251, 2006. [DOI] [PubMed] [Google Scholar]
- 91.Wilkinson IB, Franklin SS, Hall IR, Tyrrell S, Cockcroft JR. Pressure amplification explains why pulse pressure is unrelated to risk in young subjects. Hypertension 38: 1461–1466, 2001. [DOI] [PubMed] [Google Scholar]
- 92.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 113: 1213–1225, 2006. [DOI] [PubMed] [Google Scholar]
- 93.Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci USA 93: 6770–6774, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci USA 94: 6954–6958, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu L, Han C, Lim K, Wu T. Activation of cytosolic phospholipase A2alpha through nitric oxide-induced S-nitrosylation: involvement of inducible nitric oxide synthase and cyclooxygenase-2. J Biol Chem 283: 3077–3087, 2008. [DOI] [PubMed] [Google Scholar]
- 96.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurum SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 18: 1746–1748, 2004. [DOI] [PubMed] [Google Scholar]
- 97.Yang Y, Yang Y, Xu Y, Lick SD, Awasthi YC, Boor PJ. Endothelial glutathione-S-transferase A4-4 protects against oxidative stress and modulates iNOS expression through NF-kappaB translocation. Toxicol Appl Pharmacol 230: 187–196, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yetik-Anacak G, Catravas JD. Nitric oxide and the endothelium: history and impact on cardiovascular disease. Vascul Pharmacol 45: 268–276, 2006. [DOI] [PubMed] [Google Scholar]
- 99.Zhang C, Hein TW, Wang W, Chang CI, Kuo L. Constitutive expression of arginase in microvascular endothelial cells counteracts nitric oxide-mediated vasodilatory function. FASEB J 15: 1264–1266, 2001. [DOI] [PubMed] [Google Scholar]