Abstract
To explore whether asthma and obesity share overlapping pathogenic features, we examined the impact of each alone, and in combination, on multiple aspects of lung function. We reasoned that if they influenced the lungs through similar mechanisms, the individual physiological manifestations in the comorbid state should interact in a complex fashion. If not, then the abnormalities should simply add. We measured specific conductance, spirometry, lung volumes, and airway responsiveness to adrenergic and cholinergic agonists in 52 normal, 53 asthmatic, 52 obese, and 53 asthmatic and obese patients using standard techniques. Six-minute walks were performed in subsets from each group. Asthma significantly lowered specific conductance and the spirometric variables while increasing airway reactivity and residual volume. Obesity also reduced the spirometric variables as well as total lung capacity and functional residual capacity. Residual volume, specific conductance, and airway responsivity were unaltered. With comorbidity, the disease-specific derangements added algebraically. Features that existed in isolation appeared unchanged in the combination, whereas shared ones either added or subtracted depending on the individual directional changes. Synergistic interactions were not observed. Body mass index weakly correlated with spirometry and lung volumes in asthma, but not with specific conductance or bronchial reactivity. Exercise performance did not aid in differentiation. Our findings indicate asthma and obesity appear to influence the respiratory system through different processes.
Keywords: comorbidity, pulmonary mechanics, spirometry, lung volumes, airway, reactivity
asthma and obesity are common problems that have undergone dramatic increases in prevalence over the last quarter of a century (11, 13, 28, 40). The effects of each condition on symptoms and lung function are well documented (7, 8, 21, 22, 23, 30, 32, 34, 42), but there is surprisingly little information about the global impact of the combination. Since some, but not all, population surveys indicate that obesity is associated with an increased incidence of asthma and heightened airway reactivity (10, 12, 17, 24, 34), it is widely assumed that the two illnesses share overlapping pathogenic features. Although this is an attractive concept, it remains unproven because of a lack of confirming physiological evidence (17, 41). Hence, it remains to be determined whether the epidemiological findings represent a cause-and-effect relationship or are only associations between common conditions that frequently coexist. Such information could be of importance in formulating appropriate therapies and guiding future research.
In the present study, we reasoned that it might be possible to begin to differentiate between the above contingencies by examining the impact of each condition alone and in combination on multiple elements of pulmonary function. We hypothesized that if asthma and obesity altered a particular test through similar mechanisms, and if the individual abnormalities were submaximal, there should be synergistic changes in the comorbid state that would correlate with the magnitude of adiposity (20, 29). Alternatively, if the combination represented the concurrence of two prevalent illnesses, the overall physiologic consequences would derive from an algebraic admixture of the individual conditions (20, 29). To test this possibility, we examined the individual components that comprise pulmonary mechanics, airway responsivity and exercise performance, to determine how the different abnormalities associated. Our observations form the basis of this report.
METHODS
Our data were obtained in a prospective, cross-sectional study involving normal subjects (N), nonobese asthmatics (A), obese nonasthmatics (O), and individuals with both conditions (OA). The normal volunteers were obtained from advertisements in the local community and hospital. Participants with the illnesses of interest were outpatients who were identified by chart review and recruited sequentially during visits to the asthma, pulmonary, and/or sleep clinics. The clinic patients were placed into one of the three study groups based on the existing diagnosis in the medical records. No attempt was made to include or exclude asthmatic or obese individuals with a predetermined level of severity or with a particular set of abnormalities. All diagnoses were established before enrollment by caregivers who were not involved in recruitment, data acquisition, or analysis. After joining the study, all subjects prospectively underwent new measures of body mass index (BMI) and detailed assessments of pulmonary function. These data were then used to phenotype members of each group. Weights were measured on a Tanita balance (BWR-627A), and heights were recorded in stocking feet. BMI was calculated as weight (kg)/height (m2) (14).
The admission criteria consisted of physician-diagnosed asthma with and without obesity and obesity alone. Asthma was considered to be present if the medical records contained a diagnosis of such made by a pulmonary specialist based on a history of intermittent wheezing in combination with bronchodilator and/or methacholine responsiveness (27). Obesity was defined as a BMI ≥ 30 kg/m2 (14). Normality was defined as the absence of cardiac and pulmonary disease in a person with a BMI ≤ 29 kg/m2. The nonasthmatic obese subjects were also free of heart and lung disease and were enlisted from individuals being evaluated and/or treated for sleep disorder breathing. Enrollment in each group was sequential. To limit the study to the effects of asthma and obesity, we excluded individuals with a history of lung disease other than asthma, coronary artery disease, congestive heart failure, or cor pulmonale. Both the normal and overweight asthmatics discontinued use of bronchodilators for 12 h before the examination of lung function. None of the nonobese or obese asthmatics had recently experienced acute exacerbations, and all were stable at the time of study.
Airway resistance was measured in a constant volume plethysmograph by having the subjects pant at frequencies of 1–1.5 Hz with flows of 0.5–1.5 l/s (9, 16). Resistance was converted to its reciprocal, conductance, and expressed as a conductance-to-thoracic gas volume (TGV) ratio, termed specific conductance (sGaw) (9). Four to five measurements of each variable were averaged. The data were considered acceptable if the coefficients of variation were ≤5%.
Static lung volumes were also determined plethysmographically (37). Immediately following the measurement of TGV, the subjects exhaled completely and then inhaled fully in a single maneuver while flow was integrated (37). The expired volume was subtracted from TGV to obtain residual volume (RV), and the inspired vital capacity was added to RV to derive total lung capacity (TLC) (37). Thoracic gas volume was determined at functional residual capacity (FRC). Three to four complete maneuvers were performed. The FRC and the RV were reported from the trial with the largest TLC value. The coefficient of variation of the TGV maneuvers was <7%.
Maximum forced exhalations were performed in triplicate with a waterless spirometer (25). The curves with the largest 1-s forced expiratory volume (FEV1) and forced vital capacity (FVC) were analyzed. The mean forced expiratory flows between 25 and 75% of the FVC (FEF25-75) were taken from the exhalation with the largest sum of FEV1 and FVC (25). The static and dynamic lung volume data were expressed as a percentage of predicted normal using race-corrected regression equations (4, 19).
Adrenergic and cholinergic responsiveness were evaluated by employing consensus recommendations (3, 25). Bronchodilatation was assessed by recording the FEV1 before and 15 min after standard aerosols of albuterol (25). The magnitude of effect was expressed as a percentage change from the pretreatment value (%ΔFEV1). Airway reactivity was measured by inhaling increasing concentrations of methacholine from a DeVilbis nebulizer (Somerset, PA) with a breath-synchronized trigger (Rosenthal Dosimeter; PDS Instrumentation, Louisville, CO) (3). The initial concentration of 1 mg/ml was progressively raised until the FEV1 fell ≥20% from control or until an amount of 25 mg/ml was reached. The provocative concentrations (PC20meth) required to achieve this end point were determined by linear interpolation in the asthmatics and extrapolation in the nonasthmatic subjects. All of the normal and obese subjects received a maximum concentration of 25 mg/ml.
A subset from each group performed 6-min walks to assess the respiratory response to exercise. Selection was based entirely on interest and availability. Subjects moved at their own pace according to American Thoracic Society criteria (2). The intensity of dyspnea was measured before and immediately after exertion with a 10-point Borg scale (0, no symptoms; 10, severe). Arterial saturation was recorded continuously by pulse oximetry (Sp ). The Institutional Review Board for human investigations approved the study, and written informed consent was obtained.
). The Institutional Review Board for human investigations approved the study, and written informed consent was obtained.
Statistical comparisons were performed with one- and two-factor analyses of variance, univariate analysis of variance, χ2, regression analysis, and paired and unpaired t-tests. Post hoc analyses were made with Scheffé's tests with Bonferroni corrections. Two-tailed P values ≤0.05 were considered significant. The study was powered to detect ≥15% differences in the spirometric measurements between N and OA subjects with an α = 0.05 and a β = 0.20.
RESULTS
General.
Two hundred ten adults (52 N, 53 A, 52 O, and 53 OA) participated (Table 1). There were 52 men and 158 women with a mean age of 45.8 ± 14.5 yr. There were no significant between group differences among populations with regard to age (P = 0.16) or sex distribution (P = 0.71). The BMI of the N and A subjects averaged 24.5 ± 0.5 and 25.3 ± 0.5 kg/m2 (P = 0.98), whereas those of O and OA subjects were 42.1 ± 1.4 and 40.3 ± 1.1 kg/m2 (P = 0.49). There was a greater percentage of non-Caucasians in the obese compared with the nonobese groups (P > 0.001), but there were no differences between O and OA subjects (P = 0.77).
Table 1.
Demographic data
| Variable | N | A | O | OA | P Value |
|---|---|---|---|---|---|
| n | 52 | 53 | 52 | 53 | |
| Age, yr | 42.1±2.0 | 46.1±2.2 | 48.4±1.9 | 46.4±1.7 | 0.16 |
| Sex, M/F | 16/36 | 13/40 | 14/38 | 9/44 | 0.71 |
| BMI, kg/m2 | 24.5±0.5 | 25.3±0.5 | 42.1±1.3 | 40.3±1.1 | <0.001 |
| C/NC | 40/12 | 37/16 | 26/26 | 28/25 | 0.004 |
Data for age and body mass index (BMI) are means ± SE for n subjects in each of the following groups: N, normal; A, asthmatic; O, obese; OA, both obese and asthmatic. M, male; F, female; C, Caucasian; NC, non-Caucasian. P values are derived from between-group comparisons.
Specific conductance.
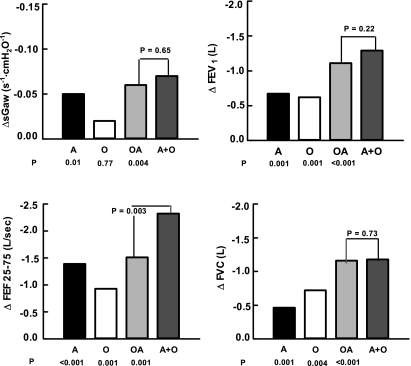
The average values for sGaw (Table 2) ranged between 0.22 ± 0.02 s−1·cmH2O−1 in the normal controls and 0.16 ± 0.01 s−1·cmH2O−1 in the OA subjects (P = 0.001). Asthma was associated with a 22.7% reduction from control (ΔsGaw = 0.05 s−1·cmH2O−1, P = 0.01) (Fig. 1). Obesity, per se, had no significant impact on this variable (ΔsGaw = 0.02 s−1·cmH2O−1, P = 0.77). In the comorbid state, sGaw was 0.06 s−1·cmH2O−1 lower than in the normal controls (P = 0.004). There was no statistical difference between the observed value for OA and the sum of the individual reductions in O and A subjects alone as shown by univariate analysis of variance (ΣΔO + ΔA = 0.07 s−1·cmH2O−1, P = 0.65 vs. OA) (Fig. 1).
Table 2.
Specific conductance and spirometry
| Variable | N | A | O | OA | P Value |
|---|---|---|---|---|---|
| sGaw, s−1·cmH2O−1 | 0.22±0.02 | 0.17±0.01 | 0.20±0.01 | 0.16±0.01 | 0.001 |
| FEV1, liters | 3.19±0.13 (97.4±2.4) | 2.52±0.12 (83.0±2.7) | 2.57±0.13 (83.8±2.7) | 2.08±0.09 (74.2±2.6) | <0.001 (<0.001) |
| FEF25-75, l/min | 3.33±0.19 (90.6±4.1) | 1.94±0.14 (58.1±3.2) | 2.40±0.16 (77.7±5.3) | 1.82±0.13 (60.6±3.9) | <0.001 (0.001) |
| FVC, liters | 3.98±0.15 (100.2±2.8) | 3.52±0.16 (91.2±2.5) | 3.26±0.15 (86.9±2.6) | 2.82±0.11 (81.1±2.5) | <0.001 (<0.001) |
Data are means ± SE. sGaw, specific airway conductance; FEV1, 1-s forced expiratory volume; FEF25-75, forced expiratory flow in the mid vital capacity; FVC, forced vital capacity. Percentages of predicted normal values are in parentheses. P values are derived from between-group comparisons.
Fig. 1.
Comparison of the differences from normal for specific conductance and the spirometric variables. Bars represent mean values. ΔsGaw, differences in specific airway conductance; ΔFEV1, differences in 1-s forced expiratory volume; ΔFEF25-75, differences in the forced expiratory flows in the mid vital capacity range; ΔFVC, differences in forced expiratory volumes; A, asthmatic subjects; O, obese subjects; OA, coexistence of obesity and asthma in subjects; O+A, the sum of the differences found with A and O subjects in isolation. P values below each bar represent comparisons with normal; whereas the P value in the graph is derived from comparisons of the OA and O+A values.
Spirometry.
The spirometric data are presented in Table 2 in both absolute and relative terms. The value for FEV1 ranged between a mean of 3.19 ± 0.13 liters in the N and 2.08 ± 0.09 liters in the OA subjects (P < 0.001). Asthma and obesity were associated with reductions of 0.67 (P = 0.001) and 0.62 liter (P = 0.001) from normal, respectively (Fig. 1). With OA subjects, there was a 1.11-liter decrement from control (P < 0.001). This value equaled the sum of both individual changes (ΣΔO + ΔA = −1.29 liters, P = 0.22 vs. OA) (Fig. 1).
The value for FEF25-75 varied from 3.33 ± 0.19 l/min in the N to 1.82 ± 0.13 l/min in the OA subjects (P < 0.001). Asthma was associated with a 1.39-liter decrease from normal (P < 0.001) (Fig. 1). The change with obesity was −0.93 liter (P = 0.001). The effect of comorbidity was a decrement of 1.51 liters (P < 0.001). This value was significantly less than the sum total of obesity and asthma in isolation (ΣΔO + ΔA = −2.32 liters, P = 0.003 vs. OA) (Fig. 1). Correcting the FEF25-75 value for the volume expelled by dividing by the FVC did not influence the findings (N, 0.75, A. 0.55, O. 0.72, and AO, 0.65 l/min) (ΣΔO + ΔA = 0.23 l/min, P = 0.001 vs. OA).
The FVC value fluctuated between 3.98 ± 0.15 liters in the N and 2.82 ± 0.11 liters in OA subjects (P < 0.001). Asthma and obesity were associated with individual reductions of 0.46 (P = 0.001) and 0.72 liter (P = 0.004) from control, respectively (Fig. 1). In OA subjects, the observed difference from N subjects was −1.16 liters (P < 0.001) and represented the sum of the effects of both conditions (ΣΔO + ΔA = −1.18 liters, P = 0.73 vs. OA) (Fig. 1).
Static lung volumes.
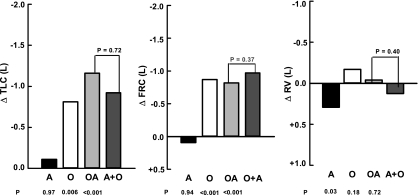
The lung volumes are presented in both absolute and relative terms in Table 3. The mean TLC ranged from 5.86 ± 0.17 liters in the N to 4.70 ± 0.15 liters in the OA subjects (P < 0.001). Asthma did not alter the TLC significantly (ΔTLC = −0.11 liter, P = 0.97) (Fig. 2). In contrast, obesity was associated with a 0.81-liter reduction (P = 0.006). With comorbidity, the observed difference from N subjects was −1.16 liters (P < 0.001). This value was statistically identical to the sum of obesity and asthma alone (ΣΔO + ΔA = −0.92 liter, P = 0.72 vs. OA) (Fig. 2).
Table 3.
Lung volumes
| Variable | N | A | O | OA | P Value |
|---|---|---|---|---|---|
| TLC, liters | 5.86±0.17 (101.1±2.0) | 5.75±0.19 (102.4±1.7) | 5.05±0.18 (88.6±1.8) | 4.70±0.15 (89.3±2.3) | <0.001 (<0.001) |
| FRC, liters | 3.06±0.10 (101.1±2.7) | 3.16±0.12 (101.4±3.1) | 2.29±0.10 (83.8±4.6) | 2.34±0.10 (90.6±4.4) | <0.001 (<0.002) |
| RV, liters | 2.00±0.10 (111.7±4.2) | 2.30±0.10 (124.0±4.1) | 1.83±0.09 (95.4±3.4) | 1.96±0.10 (111.11±4.9) | 0.003 (<0.001) |
Data are means ± SE. TLC, total lung capacity; FRC, functional residual capacity; RV, residual volume. Percentages of predicted normal values are in parentheses. P values are derived from between-group comparison.
Fig. 2.
Comparisons of differences from normal for lung volumes. The format is identical to Fig. 1. ΔTLC, differences in total lung capacity; ΔFRC, differences in functional residual capacity; ΔRV, differences in residual volume.
FRC varied from 3.16 ± 0.12 liters in A to 2.29 ± 0.10 liters in O subjects (P < 0.001). With asthma, the FRC rose insignificantly (ΔFRC = +0.10 liter, P = 0.94) (Fig. 2). Obesity produced a 0.77-liter reduction (P < 0.001). The FRC in OA subjects was 0.72 liter smaller than in N subjects (P < 0.001) and statistically equaled the algebraic sum of the findings with obesity and asthma (ΣΔO + ΔA = −0.67 liter, P = 0.98 vs. OA) (Fig. 2).
The RV ranged from 2.30 ± 0.10 liters in A to 1.83 ± 0.09 liters in O subjects (P = 0.003). Asthma and obesity had opposite effects. Asthma was associated with mild hyperinflation (ΔRV = +0.30 liter, P = 0.03), whereas obesity caused a small decrement (ΔRV = −0.17 liter, P = 0.18). Comorbidity represented the algebraic combination of the two events (ΔRV = −0.04 liter). In this case, the RV fell between the changes with obesity and asthma (ΣΔO + ΔA = +0.13 liter, P = 0.40 vs. OA) and was similar to the value in the controls (P = 0.98) (Fig. 2).
Bronchial responsiveness.
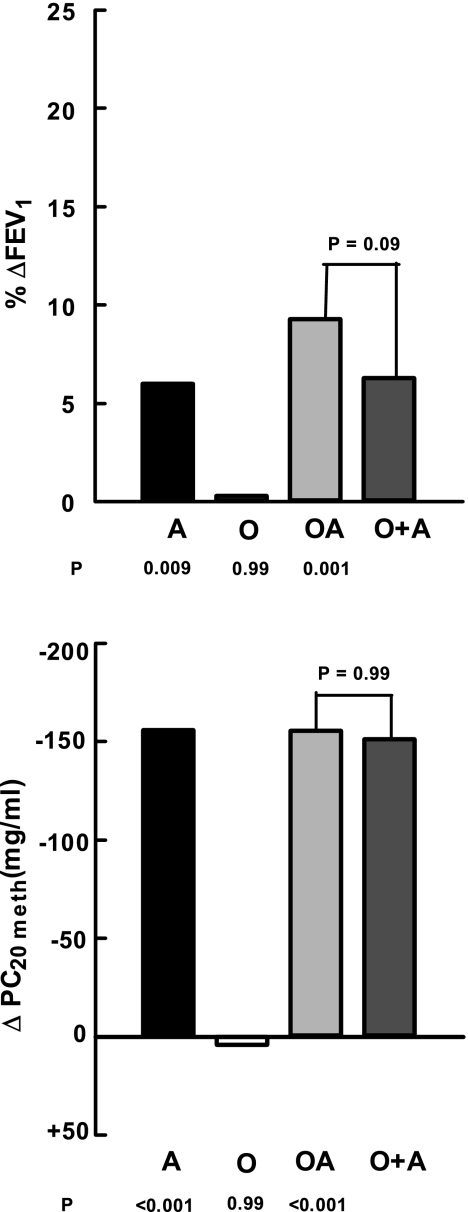
The bronchodilator response to albuterol varied from a 2.3 ± 0.07% increase in FEV1 over baseline in the N subjects to an 11.6 ± 1.3% improvement in OA subjects (P < 0.001) (Table 4). Albuterol had no effect in N subjects (P = 0.35) but produced significant improvements in FEV1 in A subjects (P < 0.001). The differences between the N and A groups equaled 6.0% (P = 0.009). The obese subjects did not have a bronchodilator response (%ΔFEV1 vs. baseline = 2.6 ± 0.06%, P = 0.72; ΔFEV1 O vs. N = 0.3%, P = 0.99). In the combination, the FEV1 rose 11.6 ± 1.3% over pretreatment baseline (P = 0.001), a difference of 9.3% from N subjects (P = 0.001). The latter was statistically equivalent to the sum of the individual elements (ΣΔO + ΔA = 6.3%, P = 0.09 vs. OA) (Fig. 3).
Table 4.
Bronchial responsiveness
| Variable | N | A | O | OA | P Value |
|---|---|---|---|---|---|
| ΔFEV1, % | 2.3±0.7 | 8.3±1.1 | 2.6±0.6 | 11.6±1.3 | <0.001 |
| PC20meth, mg/ml | 160.8±22.3 | 4.7±0.6 | 165.6±31.7 | 5.4±0.7 | <0.001 |
Data are means ± SE. ΔFEV1, percentage improvement in FEV1 following albuterol; PC20meth, provocative concentration of methacholine required to reduce the FEV1 20% from control. P values are derived from between-group comparisons.
Fig. 3.
Comparison of differences from normal in the measures of airway responsiveness. The format is identical to Fig. 1. %ΔFEV1, %increase in 1-s forced expiratory volume over baseline following a standard dose of albuterol; ΔPC20meth, differences in the provocative concentration of methacholine required to reduce the FEV1 20% from baseline.
The PC20meth ranged from 160.8 ± 22.3 mg/ml in N to 4.7 ± 0.6 mg/ml in A subjects (P < 0.001). The extrapolated threshold dose of methacholine in the normal subjects was 160.8 ± 22.3 mg/ml. The PC20meth in A subjects was significantly smaller at 4.7 ± 0.6 mg/ml (P < 0.001). The between-group difference was 156.1 mg/ml (Fig. 3). The methacholine responsiveness in O subjects mirrored that in N subjects (165.6 vs. 160.8 mg/ml, P = 0.99). The PC20meth in the combined illnesses was 5.4 ± 0.7 mg/ml. This value equaled that in A subjects (P = 0.92). It was 155.4 mg/ml less than control (P < 0.001) and represented the algebraic sum of the individual components (ΣΔO +ΔA = 151.3 mg/ml, P = 0.99 vs. OA) (Fig. 3).
BMI vs. lung function.
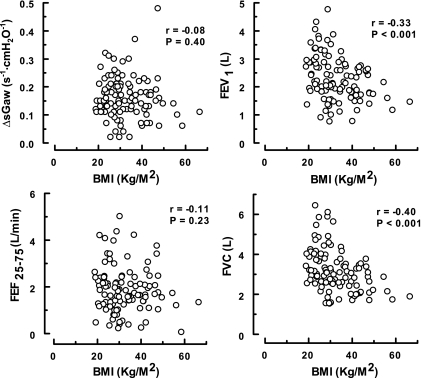
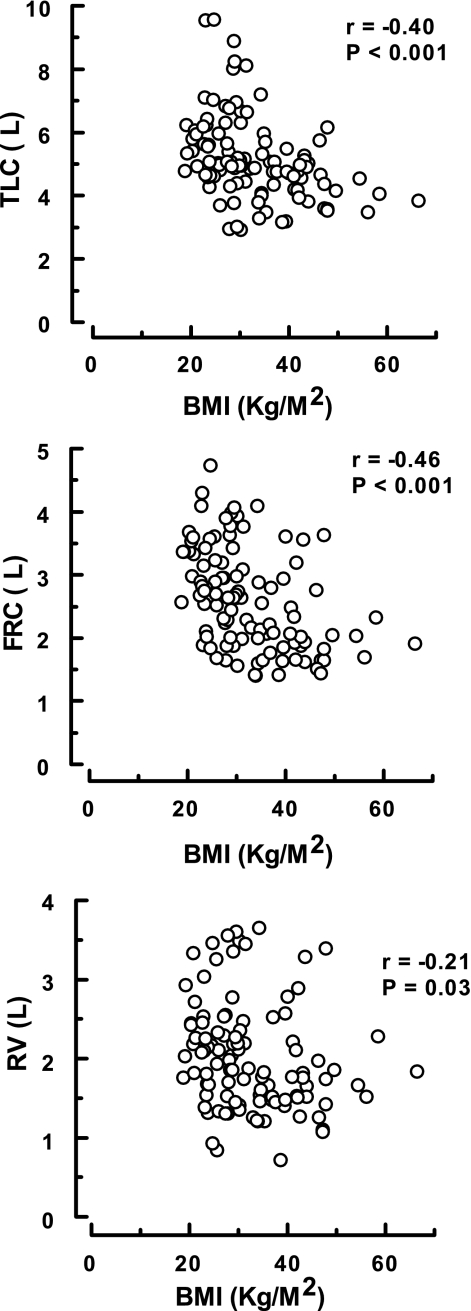
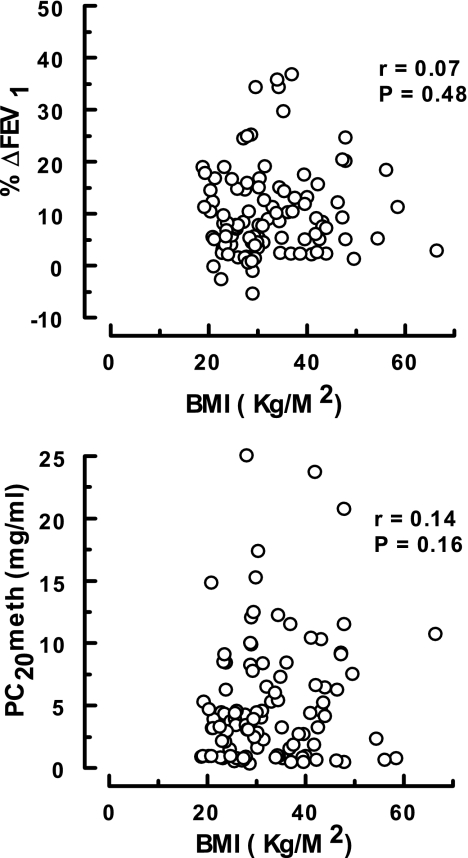
The associations between pulmonary mechanics and BMI for all of the asthmatics (n = 106) are presented in Figs. 4–6. As shown, significant relationships were only recorded between the degree of adiposity and those indexes dependent on the volume of gas in the thorax [i.e., FEV1 and FVC (Fig. 4) and TLC, FRC, and RV (Fig. 5)]. No correlations were found between BMI and airway geometry or bronchial responsiveness [i.e., sGaw and FEF25-75 (Fig. 4) or %ΔFEV1 and PC20meth (Fig. 6)].
Fig. 4.
Association between body mass index (BMI) and the indexes of airway function in asthmatics. These data were derived from the 106 subjects in the A and OA groups.
Fig. 5.
Association between body mass index (BMI) and lung volumes in asthmatics. These data were derived from the 106 subjects in the A and OA groups.
Fig. 6.
Association between BMI and airway responsiveness in asthmatics. These data were derived from 106 subjects in the A and OA groups.
Exercise performance.
Twenty-three N, 15 A, 13 O, and 20 OA subjects completed the 6-min walks. There were significant differences between groups in the lengths ambulated (P = 0.002) (Table 5). The N and A subjects went statistically similar distances (P = 0.62). The O subjects ambulated less than N subjects (P = 0.001), but there were no differences between them and A (P = 0.18) or OA subjects (P = 0.38). All participants were asymptomatic before exercise. Borg scores increased between 0.7 and 1.2 units over control with exertion (P < 0.001), but there were no significant differences among O, A, and OA subjects and no significant interactions in the combination of OA. No significant alterations in Sp were observed.
were observed.
Table 5.
Exercise performance
| Variable | N | A | O | OA | P Value |
|---|---|---|---|---|---|
| n | 22 | 15 | 13 | 20 | |
| Distance, ft | 1595±55 | 1451±65 | 1233±68 | 1409±63 | 0.002 |
| Borg score | 0.8±0.2 | 1.5±0.4 | 1.9±0.4 | 2.0±0.4 | <0.001 |
| SpO2, % | 97.1±0.3 | 97.4±0.4 | 96.5±0.7 | 96.8±0.4 | 0.55 |
Data are means ± SE for n subjects in each group. Distance is the length walked by subjects, and the Borg score indicates the intensity of dyspnea during exertion. SpO2, average percent arterial oxygen saturation measured by pulse oximetry during the last minute of exercise. P values are derived from between-group comparisons.
Symptoms and medication use.
To determine whether the presence of obesity influenced the clinical features of asthma, we contrasted the symptoms and medication use in the obese and nonobese asthmatics. As shown in Table 6, no significant between-group differences were found for any variable.
Table 6.
Symptoms and medication use in the A and OA groups
| A | OA | P Value | |
|---|---|---|---|
| Symptoms | |||
| Cough | 47 | 44 | 0.85 |
| Wheeze | 63 | 38 | 0.15 |
| Dyspnea | 47 | 50 | 0.85 |
| Chest tightness | 33 | 44 | 0.08 |
| Medications | |||
| ICS | 55 | 60 | 0.50 |
| LABA | 55 | 55 | 1.00 |
| SABA | 74 | 74 | 1.00 |
| Ipratropium | 23 | 15 | 0.30 |
| Leukotrienes | 13 | 1 | 0.40 |
| Theophylline | 4 | 0 | 0.15 |
| None | 9 | 19 | 0.20 |
Data represent the percentage of affected subjects in each group. ICS, inhaled corticosteroids; LABA, long-acting bronchodilators; SABA, short-acting bronchodilators.
DISCUSSION
The results of the current study provide an in-depth physiological assessment of how obesity and asthma combine to influence lung function. By examining the impact of both conditions on multiple traditional tests of pulmonary performance, we could sequentially deduce the unique interactions that existed in each. Once this was established, combining those parameters that reflected specific areas such as pulmonary mechanics, airway responsivity, and exercise performance permitted us to draw a composite picture of the manner in which the illnesses associated. We readily acknowledge that this approach may not provide a complete description of all the potential events transpiring in comorbidity and that complex interactions could be occurring in systems that we did not examine. Nevertheless, we believe it provides valuable insights into the areas studied.
Our data demonstrate that each illness in isolation produces a specific set of abnormalities in pulmonary mechanics that coalesce in an algebraic fashion when the diseases coexist. As a result, the derangements that are unique to a given condition remain unchanged in comorbidity, whereas shared ones either add or subtract depending on the directional changes in the components. Synergistic interactions do not occur. These observations strongly suggest that asthma and obesity influence the respiratory system through different pathways. Asthma impacts bronchial tone and evokes the sequela of airway obstruction (8, 27). Obesity reduces lung volumes and interferes with chest movement. It does not appear to alter bronchial smooth muscle activity (7, 21, 23, 32).
The asthmatic patients demonstrate the usual physiological alterations seen during remission. They have hypersensitivity to adrenergic and cholinergic agonists, reduced flows in the mid vital capacity range, and hyperinflated RV (8, 27). The attenuated sGaw and FEV1 values coupled with normal values for FVC, TLC, and FRC are also typical (8, 27). Their PC20meth values are well within diagnostic norms, and the bronchodilator effect size for albuterol is appropriate for the magnitude of the prechallenge FEV1 (8, 27). In composite, these findings nicely match the published phenotype for chronic asthma and support the representative nature of the subjects in this group.
Adiposity mainly compresses the chest, limiting the expansion of the lungs, and only secondarily influences the airways (21, 26, 31, 35, 38). Functionally, the thorax acts as though it were immersed in water, and the obese participants have reduced values for TLC, FRC, and FVC and a normal RV. Their small thoraxes also produce low forced expiratory volumes and flows. Like asthma, these physiological derangements mirror previous findings (7, 21, 22, 30, 33, 42) and demonstrate the similarities between our subjects and those in the literature.
In our experiments, sGaw and the indexes of bronchial responsiveness in obesity are not different from normal. Such phenomena have been noted previously (15, 18, 30, 33, 42). Airway resistance, as an isolated measurement, can indeed be high in this situation (30, 42), but only because of the small lungs and not because of intrinsic bronchial pathology. When corrected for volume, as in our case, and those of others (30, 42), airway sizes are appropriately normal. A critical evaluation of the data concerning the association of obesity and airway hyperreactivity follows below.
Comorbidity joins the above patterns in a noninteractive fashion. As a result, the combined state has the heightened airway reactivity and diminished sGaw that adiposity lacks and the reduced TLC and FRC that asthma lacks. The low lung volumes of obesity combine with the delayed emptying of asthma to cause the forced expiratory volumes in the union to be smaller than with either alone. Finally, the air trapping of asthma appears to be opposed by the diminished chest expansion of obesity, causing the FRC and RV in the combination to lie between the individual extremes. The only exception to simple combination effects is the FEF25-75 value. In this case, the flows in the comorbid state are better preserved than expected and principally reflect the presence of asthma even when corrected for expired volume.
The regression data offer further insights into the interplay between obesity and asthma. BMI correlated weakly with lung volumes and related indexes but not with sGaw or airway reactivity. If obesity and asthma influenced lung function through a similar mechanism, stronger associations would have been expected and would have involved all variables.
The exercise studies were designed to explore potential limitations that could interfere with daily activities and not to examine detailed cardiopulmonary function (2). The expectation was that the combined illness would cause more respiratory distress than either alone. This did not appear to be the case. It needs to be noted that the number of participants in this phase is relatively small, and type II statistical errors are possible. It is also possible that those who volunteered had better exercise tolerance.
Benther et al. (6) have postulated that corpulence is associated with a pattern of rapid shallow breathing at lung volumes near closing volume, which produces limited lung distension. This, in turn, purportedly leads to a perturbed equilibrium of myosin binding in airway smooth muscle, causing an increase in airway reactivity. Although this is an appealing hypothesis, it lacks supporting data. Prevention of deep inhalations during a methacholine challenge amplifies airway responsiveness, but this is only seen in normal subjects (36). It is not observed in asthmatics (36) and has never been reported in obesity. Although respiratory system compliance and lung volumes are lowered in obesity (26, 35), affected individuals can still take deep breaths. As shown in Table 3, TLC, although reduced from control, remained within predicted norms (22).
Furthermore, if the Benther et al. hypothesis (6) were correct, obese subjects would be expected to show an augmented sense of dyspnea at rest that worsens with exertion. None of this was observed experimentally. Neither was there an interaction with asthma. Like the data of Dixon et al. (15), our observations in Tables 5 and 6 do not demonstrate differences in the sensation of breathlessness between obese and nonobese asthmatics. Moreover, if the airways are truly smaller in obesity because of an abnormal latch state of myosin, rather than a passive loss of volume, and if the former augmented the asthma diathesis, then the responses to albuterol and methacholine should have exceeded normal. They also should have exceeded, or at least equaled, the effect sizes in the comorbid group. Neither of these events occurred.
Our observations regarding the lack of concordance between adiposity and airway hyperreactivity are part of a growing body of evidence (1, 15, 18, 32, 33, 36, 37, 39). In early positive studies, Litonjua et al. (24) and Chinn et al. (12) reported that methacholine responsiveness correlates with BMI, but a reevaluation of their data raises important concerns. In the first investigation, individuals with both high and low BMI were found to be at increased risk for the development of bronchial hypersensitivity, thereby raising issues about the selectivity and sensitivity of the findings (24). Interestingly, a subsequent study in asthmatic children by the same investigative group (39) failed to find any relationship between BMI and methacholine susceptibility. In the second investigation, hyperresponsivity could only be detected in males and not females. Such observations have no apparent clinical corollary. In fact, several studies suggest that obesity is a risk factor for asthma in women and not men (5, 10, 18). It is pertinent to note that the magnitude of the difference in reactivity in the Chinn et al. study between the obese and normal subjects is only 0.3 of a doubling dose of agonist (12). Changes greater than or equal to two doubling doses are usually considered meaningful (3, 27).
These issues may not just represent methodological shortcomings. Schachter et al. (33) were unable to find any relationship between BMI and airway hyperreactivity in a survey of over 1,900 adults, whereas Hancox et al. (18) could not detect any association between obesity and either cholinergic or adrenergic sensitivity in their cohort of 1,000 subjects. At the opposite end of the spectrum, weight reductions ≥20 kg, although improving lung function and reducing symptoms, do not have any influence on airway responsiveness (1). Finally, since there is general agreement that cholinergic and sympathomimetic sensitivity occur concomitantly (27), one would expect bronchodilator responsiveness to be part of the obesity syndrome. This does not seem to be the case. Our obese subjects, like those of Hancox et al. (18), did not respond any differently than normal individuals to albuterol. Furthermore, our obese asthmatics, again like those of Dixon et al. (15), reacted identically to the nonobese, suggesting that adiposity did not add anything to asthma with regard to this phenomenon.
We do not believe that our results are adversely influenced by protocol design or the choice of measurements. Since our investigation is not a longitudinal study or an epidemiological survey, it is limited in sample selection. Because it was not possible to observe temporally how obesity and asthma merged to form the combined condition, we characterized each population separately and computed the relative contributions. This analysis is only valid if the effect in each subgroup is established and representative of patients with the illnesses in question and if clinical features (symptoms and medication use) are similar between the obese and nonobese asthmatics. We believe that these conditions have been fully met (Tables 1–4 and 6) (7, 8, 21, 27, 30, 34).
A salient feature of the current work is that, unlike many population-based trials that involve individuals with self-reported diagnoses that may or may not account for their symptoms (17, 41), our subjects were well defined and documented to have the conditions of interest. Eighteen obese (34.6%) and 14 obese asthmatics (26.4%) also had obstructive sleep apnea (OSA) diagnosed by polysomnography. The existence of this illness did not influence our results. There was no significant difference in the distribution among groups (P = 0.83). Moreover, subgroup analysis demonstrated that the subjects with OSA had statistically higher BMI values, but there were no other differences among groups. All of our subjects were under the care of pulmonary specialists who used standard diagnostic criteria, and we did not make any of the diagnoses of the study illnesses in our participants. All of the functional observations reported were prospectively obtained. The clinical diagnoses in the chart at the time of enrollment were the sole criteria used to place individuals into the various groups, and the population assignments were done before testing. Once placed into a specific population, membership therein remained inviolate irrespective of the subsequent physiological results. No attempt was made to include or exclude asthmatic or obese patients of a predetermined level of severity or with a particular set of abnormalities.
The assessments of lung function employed were all standard, and we have long experience in their use. The variety of indexes examined was intended to explore a broad range of potential impairments so that subtle interactions could be detected where present. We realize that extrapolating the PC20meth values in the normal and obese participants did not produce physiological data, and this was not our intent. This procedure was undertaken solely to have continuous numeric values to make statistical comparisons between populations, rather than having to deal with cutoff limits. Such an approach had no impact on the results.
In summary, the findings in the current work indicate that asthma and obesity seem to influence the respiratory system through different pathways. Coexistence produces an algebraic summation of the individual abnormalities and not synergistic interactions. These observations indicate that the published epidemiological associations need to be supplemented by definitive mechanistic explanations so that advances can be made in the pathogenesis and therapy of comorbidity.
GRANTS
This work was supported in part by National Institutes of Health Grants HL-33791, HL-04140, and M01 RR-00080 and by an Institutional Incentive Grant from the MetroHealth Medical Center (to K. Nicolacakis).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aaron SD, Fergusson D, Dent R, Chen Y, Vandemheen KL, Dales RE. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest 125: 2046–2052, 2004. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 166: 111–117, 2002. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society. Guidelines for methacholine and exercise challenge testing—1999. Am J Respir Crit Care Med 161: 309–329, 2000. [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis 44: 1201–1218, 1991. [DOI] [PubMed] [Google Scholar]
- 5.Beckett WS, Jacobs DR Jr, Yu X, Iribarren C, Williams OD. Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med 164: 2045–2050, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Benther Weiss ST DA, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med 174: 112–119, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biring MS, Lewis MI, Liu JT, Mohsenifar Z. Pulmonary physiologic changes of morbid obesity. Am J Med Sci 318: 293–297, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Boulet LP, Turcotte H, Brochu A. Persistence of airway obstruction and hyperresponsiveness in subjects with asthma remission. Chest 105: 1024–1031, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Briscoe WA, DuBois AB. The relationship between airway resistance, airway conductance, and lung volume in subjects of different age and body size. J Clin Invest 37: 1279, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camargo CA Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med 159: 2582–2588, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Update: prevalence of overweight among children, adolescents, and adults. United States, 1988–1994. MMWR Morb Mortal Wkly Rep 46: 198–202, 1997. [PubMed] [Google Scholar]
- 12.Chinn S, Jarvis D, Burney P. European Community Respiratory Health Survey. Relation of bronchial responsiveness to body mass index in the ECRHS. Thorax 57: 1028–1033, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Division of Data Services. Asthma Prevalence: Health Care Use and Mortality 2000–2001. Hyattsville, MD: National Center for Health Statistics, 2002.
- 14.Division of Nutrition and Physical Activity, National Center for Chronic Disease Prevention and Health Promotion. Overweight and Obesity: Defining Overweight and Obesity (Online). Centers for Disease Control and Prevention, Department of Health and Human Services. http://www.cdc.gov/nccdphp/dnpa/obesity/defining.htm. [28 Feb 2005].
- 15.Dixon AE, Shade DM, Cohen RI, Skloot GS, Holbrook JT, Smith LJ, Lima JJ, Allayee H, Irvin CG, Wise RA. Effect of obesity on clinical presentation and response to treatment in asthma. J Asthma 43: 553–558, 2006. [DOI] [PubMed] [Google Scholar]
- 16.DuBois AB, Botelho SY, Comroe JH Jr. A new method for measuring airway resistance in man using a body plethysmograph: values in normal subjects and in patients with respiratory disease. J Clin Invest 35: 327, 1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford ES The epidemiology of obesity and asthma. J Allergy Clin Immunol 115: 897–909, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Hancox RJ, Milne BJ, Poulton D, Taylor DR, Greene JM, McLachlan CR, Cowan JO, Flannery EM, Herbison GP, Sears MR. Sex differences in the relation between body mass index and asthma and atopy in a birth cohort. Am J Respir Crit Care Med 171: 440–445, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 159: 179–187, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Hollenberg MD, Severson DL. Drug receptors and receptors/mechanisms. In: Principles of Pharmacology. Basic Concepts and Clinical Applications, edited by Munson PL, Mueller RA, Breese GR. New York: Chapman & Hall, 1995, p. 7–37.
- 21.Jenkins SC, Moxham J. The effects of mild obesity on lung function. Respir Med 85: 309–311, 1991. [DOI] [PubMed] [Google Scholar]
- 22.Jones RL, Nzekwu MMU. The effects of body mass index on lung volumes. Chest 130: 827–833, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Ladosky W, Botelho MAM, Albuquerque JP Jr. Chest mechanics in morbidly obese non-hypoventilating patients. Respir Med 95: 281–286, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Litonjua AA, Sparrow D, Celedon JC, DeMolles D, Weiss ST. Association of body mass index with the development of methacholine airway hyperresponsiveness in men: the normative aging study. Thorax 57: 581–585, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardization of spirometry. Series “ATS/ERS task force: standardization of lung function testing” number 2. Eur Respir J 26: 319–338, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Naimark A, Cherniak RM. Compliance of the respiratory system and its components in health and obesity. J Appl Physiol 15: 337–382, 1960. [DOI] [PubMed] [Google Scholar]
- 27.National Asthma Education and Prevention Program Expert Panel. National Asthma Education and Prevention Program Expert Panel Report II: Guidelines for the Diagnosis and Management of Asthma (NIH Publication 97-4051). Bethesda, MD: National Institutes of Health, 1997.
- 28.National Center for Health Statistics. Asthma (Online). www.cdc.gov/nchs/fastats/asthma.htm.
- 29.Ross EM, Gilman Al. Pharmacodynamics: mechanisms of drug action and the relationship between drug concentration and effects. In: Goodman and Gillman's The Pharmacologic Basis of Therapeutics (7th ed.), edited by Gilman A, Goodman LS, Rall TW, Murad F. New York: McMillan, 1985, p. 35–48.
- 30.Rubinstein I, Zamel N, DuBarry L, Hoffstein V. Airflow limitation in morbidly obese, nonsmoking men. Ann Intern Med 112: 828–832, 1990. [DOI] [PubMed] [Google Scholar]
- 31.Santesson J, Nordenström J. Pulmonary function in extreme obesity. Influence of weight loss following intestinal shunt operation. Acta Chir Scand 482, Suppl: 36–40, 1978. [PubMed] [Google Scholar]
- 32.Sahebjami H, Gartside PS. Pulmonary function in obese subjects with a normal FEV1/FVC ratio. Chest 119: 1425–1429, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Schachter LM, Salome CM, Peat JK, Woolcock AJ. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax 56: 4–8, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seidell JC, de Groot LCPGM, van Sonsbeek JL, Deurenberg P, Hautvast JGAJ. Association of moderate and severe overweight with self-reported illness and medical care in Dutch adults. Am J Public Health 76: 264–269, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharp JT, Henry JP, Sweany SK, Meadoes WR, Pietras RJ. The total work of breathing in normal and obese men. J Clin Invest 43: 728–739, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest 96: 2393–2403, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sykes TW, Hyanes RL, McFadden ER Jr. On line determinations of lung volumes by plethysmography and digital computer. Am Rev Respir Dis 115: 581–585, 1977. [DOI] [PubMed] [Google Scholar]
- 38.Weiner P, Waizman J, Weiner M, Rabner M, Magadle R, Zamir D. Influence of excessive weight loss after gastroplasty for morbid obesity on respiratory muscle performance. Thorax 53: 39–42, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss ST, Fuhlbrigge AL. Childhood Asthma Management Program Research Group. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP). Thorax 58: 1036–1041, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation on Obesity. Geneva: WHO, 1997.
- 41.Wilson MM, Irwin RS. The association of asthma and obesity. Arch Intern Med 159: 2513–2514, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Zerah F, Harf A, Perlemuter L, Lorino H, Lorino AM, Atlan G. Effects of obesity on respiratory resistance. Chest 103: 1470–1476, 1993. [DOI] [PubMed] [Google Scholar]