Abstract
The inspiratory drive to hypoglossal (XII) motoneurons originates in the caudal medullary intermediate reticular (IRt) region. This drive is mainly glutamatergic, but little is known about the neurochemical features of IRt XII premotor neurons. Prompted by the evidence that XII motoneuronal activity is controlled by both muscarinic (M) and nicotinic cholinergic inputs and that the IRt region contains cells that express choline acetyltransferase (ChAT), a marker of cholinergic neurons, we investigated whether some IRt XII premotor neurons are cholinergic. In seven rats, we applied single-cell reverse transcription-polymerase chain reaction to acutely dissociated IRt neurons retrogradely labeled from the XII nucleus. We found that over half (21/37) of such neurons expressed mRNA for ChAT and one-third (13/37) also had M2 receptor mRNA. In contrast, among the IRt neurons not retrogradely labeled, only 4 of 29 expressed ChAT mRNA (P < 0.0008) and only 3 of 29 expressed M2 receptor mRNA (P < 0.04). The distributions of other cholinergic receptor mRNAs (M1, M3, M4, M5, and nicotinic α4-subunit) did not differ between IRt XII premotor neurons and unlabeled IRt neurons. In an additional three rats with retrograde tracers injected into the XII nucleus and ChAT immunohistochemistry, 5–11% of IRt XII premotor neurons located at, and caudal to, the area postrema were ChAT positive, and 27–48% of ChAT-positive caudal IRt neurons were retrogradely labeled from the XII nucleus. Thus the pre- and postsynaptic cholinergic effects previously described in XII motoneurons may originate, at least in part, in medullary IRt neurons.
Keywords: inspiratory drive, muscarinic receptors, nicotinic receptors, single-cell reverse transcription-polymerase chain reaction, upper airway
the inspiratory drive to hypoglossal (XII) motoneurons originates in cells located between the ventrolateral border of the XII nucleus and nucleus ambiguus in the caudal medullary intermediate reticular (IRt) region (7, 22, 40, 71). This drive is mainly glutamatergic (11, 58), consistent with the evidence that many IRt XII premotor neurons use glutamate as their main transmitter (61). Other than that, little is known about the neurochemical features of IRt XII premotor neurons.
XII motoneurons innervate muscles of the tongue, including the genioglossus. In healthy individuals, the tongue has multiple functions related mainly to food intake and phonation. However, in obstructive sleep apnea (OSA) patients, the genioglossus and other upper airway muscles exhibit prominent tonic and phasic inspiratory activity, the function of which is to maintain the airway patent despite anatomic conditions that make the pharyngeal airway of these patients vulnerable to collapse (38, 60). Upper airway motor tone in OSA patients is high in wakefulness, decreases during slow-wave sleep, and nearly disappears during rapid eye movement (REM) sleep (16, 21, 39, 48, 53). The neurochemical mechanisms that determine the state-dependent changes in upper airway motor tone are the subject of intense basic and clinical research motivated by prospects of uncovering a pharmacological treatment for OSA and other sleep-related respiratory disorders (19, 24, 34). To date, serotonin and norepinephrine have been identified as major stimulatory modulators that maintain activity in XII motoneurons during wakefulness but are withdrawn during sleep (8, 12, 25, 28, 62). Acetylcholine, a transmitter that is released, at least in part, from neurons that have high levels of activity in wakefulness and/or REM sleep (10, 36, 59), also exerts pre- and postsynaptic effects on XII motoneurons (4, 6, 9, 30, 43, 47, 55, 74). A portion of the cholinergic input to XII motoneurons originates in dorsal pontine cholinergic neurons that have state-dependent activity (52), but projections of these neurons to the XII nucleus are relatively limited compared with the numbers of pontomedullary serotonergic or noradrenergic neurons projecting to the XII nucleus (37, 51). In addition to those located in the dorsal pontine tegmentum, many cholinergic neurons are scattered throughout the medullary reticular formation, but the functions, activity patterns, and connectivity of these neurons are unknown. In particular, cells that express markers of cholinergic neurons [choline acetyltransferase (ChAT) or nitric oxide synthase (NOS)] are distributed within the IRt region in a pattern reminiscent of that of XII premotor neurons (1, 18, 64). Indeed, some ChAT- or NOS-immunoreactive neurons of the medullary reticular formation, including the IRt region, were previously shown to project to the trigeminal, facial, or XII motor nuclei (13, 14, 61). However, their localization relative to other premotor neurons and their other neurochemical markers have been little investigated. Using single-cell reverse transcription-polymerase chain reaction (RT-PCR) and then following up with ChAT immunohistochemistry, we found that half of the caudal IRt XII premotor neurons expressed mRNA for ChAT, that many also expressed mRNA for type 2 muscarinic (M2) receptors, and that >30% of ChAT-immunopositive caudal IRt neurons were retrogradely labeled from the XII nucleus. A preliminary report has been published (67).
MATERIALS AND METHODS
Experiments were conducted on seven 25- to 30-day-old male Sprague-Dawley rats [body wt 107 ± 9 (SE) g at time of tracer injection into the XII nucleus] and three adult male Sprague-Dawley rats (body wt 310–385 g) obtained from Charles River Laboratories (Wilmington, DE). Juvenile rats were used for single-cell mRNA profiling because cells from young rats survive dissociation procedures better than those from fully mature animals. Data indicate that most ingestive, respiratory, and sleep-wake behaviors reach maturity in rats older than 25 days (29). After tracer injections, the animals were housed individually under a 12:12-h light (0700–1900)-dark cycle and received standard rodent chow and water ad libitum. All surgical and animal handling procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania and followed the guidelines of the American Physiological Society for the care and use of animals in research.
Tracer injections, cell dissociation, and RT-PCR procedures.
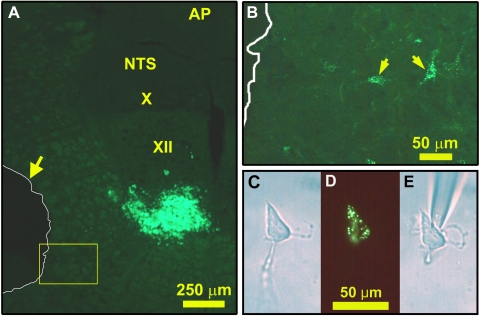
Fluorescein isothiocyanate (FITC)-labeled latex microspheres (LumaFluor, Naples, FL; 20–50 nl) were microinjected into the XII nucleus of pentobarbital-anesthetized Sprague-Dawley rats (Fig. 1A). After 5–8 days, the animals were killed by decapitation under deep isoflurane anesthesia (4–5%) and the medulla was extracted and placed in ice-cold buffer containing (in mM) 135 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, and 20 mannitol, pH 7.4 adjusted with NaOH, osmolarity 300 ± 5 (SE) mosM (72). One or two 400-μm-thick coronal slices were obtained from the level of the area postrema with a tissue slicer (VSLM1; Lafayette, IN). They were digested with papain (catalog no. LS003119, Worthington, Lakewood, NJ; 25–30 U/ml, 15–20 min), after which 700-μm micropunches were cut out from the IRt regions of both sides and cells from individual punches were mechanically dispersed and plated in separate, custom-made culture dishes, as described previously (66). The slices from which the punches were extracted were fixed in formalin, cut into 25-μm sections, and mounted to verify the antero-posterior level of the slice, the location of the punches, and FITC bead injection sites (Fig. 1, A and B).
Fig. 1.
Retrograde labeling with fluorescent latex beads of XII premotor neurons and their identification after cell dissociation. A: fluorescein isothiocyanate (FITC)-labeled bead injection site in the ventral part of the XII nucleus and location of tissue micropunch taken from the medullary intermediate reticular (IRt) region (outlined in white and shown by arrow). B: expanded image of the region framed in yellow in A located just medial to the tissue punch; arrows point to 2 retrogradely labeled cells that remained in the slice. C–E: a cell dissociated from an IRt tissue punch and containing FITC-labeled beads, as seen under phase contrast (C), fluorescent illumination (D), and again phase contrast when the cell was attached to the tip of the sampling pipette (E). AP, area postrema; NTS, nucleus of the solitary tract; X, dorsal motor nucleus of the vagus; XII, hypoglossal motor nucleus.
Dispersed cells containing FITC-labeled beads were identified with an inverted phase-contrast microscope equipped with a fluorescent light source and FITC filters (IMT2, Olympus) (Fig. 1, C–E). Individual cells, both labeled and unlabeled, were aspirated into glass pipettes and then transferred to PCR tubes. The samples were then subjected to reverse transcription (RT) with SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA) in a total buffer volume of 20 μl as described previously (73). The resulting cDNA aliquots (3–5 μl) were then used in separate two-round, nested or seminested PCRs with different sets of primers listed in Table 1. Seminested PCRs with 3-μl aliquots of the RT product and Titanium Taq DNA polymerase (Clontech, Mountain View, CA) were performed to amplify cDNAs for ChAT, α4-subunit of nicotinic receptor (Nα4), and neuron-specific enolase (NSE), as described previously (69). To test for the presence of mRNAs for all muscarinic (M) receptor subtypes in each cell, degenerate nested primers were used. First-round PCRs were performed with two sets of degenerate primers (20 nM) targeting the homologous regions of M1–3 or M4–5 receptors with separate 5-μl aliquots of the RT product. The first-round product was then used as a template to amplify cDNAs for distinct M receptor subtypes in separate second-round PCRs with specific primers (200 nM). The first round of amplification (35–37 cycles) was performed with a conventional thermal cycler (PCR Sprint; Thermo Hybaid, Ashford, UK) and the second with a real-time cycler (LightCycler; Roche Diagnostics, Indianapolis, IN). The primers were designed with Vector NTI software (Invitrogen). The criteria for primer specificity, reaction quality control, and interpretation of negative PCRs and the strategy to optimize PCR conditions were described previously (66). Cell samples that yielded negative results with all primer sets of interest were tested for NSE. If that reaction was also negative, the sample was disregarded (11 of 77 cells collected tested negative for all primers, including NSE).
Table 1.
PCR primers used in the study
| Gene, Accession Number | Primer | Sequence (5′–3′) |
|---|---|---|
| ChAT, XM_001061520 | Sense, external/internal | GAAGCAGAAATACAGCCCCG |
| Antisense, external | CACGGGTCCATAACAGCAGA | |
| Antisense, internal | GCAGCCTTGTGGTCAGTCAT | |
| M1–M3 receptors (degenerate) | Sense, external | GCCTGTGCWGACCTSATCAT |
| Antisense, external | CAGAASARRATGGCWGGRGC | |
| M1 receptor (specific), NM_080773 | Sense, internal | CCATGAACCTCTATACCACG |
| Antisense, internal | CGAGAAGTAACGGTCAAAGC | |
| M2 receptor (specific), NM_031016 | Sense, internal | CTACACTGTGATTGGCTACTGG |
| Antisense, internal | AGAGGATGAAGGAAAGGACC | |
| M3 receptor (specific), NM_012527 | Sense, internal | TGGGCACTGGGGAACTTAGC |
| Antisense, internal | GCCAGACCAATCATCACACC | |
| M4–M5 receptors (degenerate) | Sense, external | GCCATTGCTGCCTTCTACMT |
| Antisense, external | GGGTTGATGGTGCTGTTGAC | |
| M4 receptor (specific), NM_031547 | Sense, internal | TGCCACCCAGAACACCAAGG |
| Antisense, internal | AGCAGAGCCAGTAGCCGATG | |
| M5 receptor (specific), NM_017362 | Sense, internal | AGCCAAGAAGAGAGAGCCAG |
| Antisense, internal | AGCCAGTAACCCAAGTGCCA | |
| α4-Subunit of nicotinic receptor, NM_024354 | Sense, external/internal | ATCGTGCCTCGCCTCCTCTT |
| Antisense, external | TTTGGTGCCTCCCGCCTTGA | |
| Antisense, internal | ACTGCTGTGTCCGTGGGGTT | |
| Neuron-specific enolase, M11931 | Sense, external | CTCAAGGGGGTCATCAAGGA |
| Antisense, external/internal | GCTTGGATGGCTTCTGTGAC | |
| Sense, internal | CAAGGCTGGCTACACGGAAA |
ChAT, choline acetyltransferase.
The position and size of the melting curve peaks provided an initial assessment as to whether the expected cDNA was generated in the second round of PCR. Some PCR products were then cooled and displayed on GelRed-stained 2% agarose gels to further verify that they were of the expected size. To control for false-positive PCRs caused by amplification of genomic DNA, in one previous study (69) we tested non-reverse-transcribed single-neuron samples from 15 cells from 12 rats, and in each of another two studies (65, 68) we tested at least 8 single-cell samples. None of those samples was ever positive. In this study, we tested three non-reverse-transcribed single-cell samples from three rats with all primers, and none was positive. To control for contamination of the culture medium or reagents with target mRNAs or cDNAs, one sample of the fluid from above the plated cells was collected at the end of each experiment and submitted to the same RT-PCR procedures as single-cell samples. None of those reactions was positive.
ChAT immunohistochemistry on retrogradely labeled XII premotor neurons.
Three adult rats were anesthetized with isoflurane (3%) followed by Nembutal (60 mg/kg ip). The head was placed in a stereotaxic holder, and the skin, muscles, and membranes overlying the fourth ventricle were cut along the midline and retracted laterally. A glass pipette (A-M Systems, Carlsborg, WA; tip diameter 20–25 μm) filled with Fluoro-Gold (FG; Fluorochrome, Denver, CO) or cholera toxin B subunit (CTb, List Biological Laboratories, Campbell, CA) was inserted into the XII nucleus at the level 0.15 mm rostral to the caudal end of the calamus scriptorius and 0.2 mm lateral to the midline. One animal received unilateral FG injection (1%, 5 nl), and the other two received CTb injections (1%, 5–10 nl). The tracers were injected over 10–20 s by applying pressure to the fluid in the pipette while monitoring the movement of meniscus through a calibrated microscope. The micropipette was left in place for 5–10 min and then slowly withdrawn. The muscles and skin overlying the operated area were sutured in layers. Five to seven days after tracer injections, the rats were deeply anesthetized with Nembutal (100 mg/kg ip) and transcardially perfused with phosphate-buffered saline (PBS) with heparin (1,000 U/ml) and 0.003% lidocaine, followed by 4% paraformaldehyde in PBS. The brains were removed, postfixed in the same solution for 48 h at 4°C, cryoprotected in 30% sucrose-PBS, and sectioned on a cryostat (CM1850; Leica, Bannockburn, IL) into three series of 35-μm coronal sections.
In the experiment with FG injection, one series was mounted on gelatin-coated glass slides, dried, dehydrated, and coverslipped, and another was subjected to immunohistochemistry to visualize ChAT. For the latter, sections were incubated for 24 h at 4°C in goat anti-ChAT antibodies (1:3,000, catalog no. AB144P, lot no. LV1375874; Millipore, Temecula, CA) in PBS containing 0.2% Triton X-100 and 5% horse serum, then for 2 h in biotinylated anti-goat antibodies at room temperature, and then for 1.5 h in 5-(4,6-dichlorotriazinyl) aminofluorescein (DTAF)-conjugated streptavidin (1:1,000; Jackson Laboratories, West Grove, PA). In the experiments with CTb injections, the series of sections designated for double labeling were first processed to visualize CTb and then subjected to ChAT immunohistochemistry. For CTb immunohistochemistry, sections were incubated for 24 h at 4°C in goat choleragenoid (β-subunit of cholera toxin-CTb) antiserum (1:20,000, catalog no. 703, lot no. 70732A2; List Biological Labs) in PBS containing 0.2% Triton X-100 and 5% donkey serum. They were subsequently incubated for 2 h in Cy3-labeled donkey anti-goat antibodies (1:200; Jackson Laboratories). They were then processed for ChAT immunohistochemistry as described above but with rabbit anti-ChAT antibodies (1:200, catalog no. AB143, lot no. LV1462024; Millipore) in donkey serum followed by incubation in biotinylated anti-rabbit antibodies (1:200; Jackson Laboratories) for 1 h and then in DTAF-conjugated streptavidin.
Double-labeled sections from the antero-posterior levels from −14.6 to −11.0 mm caudal to bregma that matched the standard levels shown in a rat brain atlas (44) (11 sections from 11 different levels per rat) were examined for the presence of the retrograde tracer and/or ChAT immunoreactivity in the IRt region and its rostral extension with a Leica DMLB microscope and appropriate filter sets (FG, Cy3, FITC). Cells were regarded as retrogradely labeled when they contained FG or CTb grains within a clearly identifiable cell body, the nucleus was visible, and the grains were not visible under filters other than the one appropriate for the tracer. ChAT-positive cells were identified by uniform distribution of immunofluorescence (DTAF) within the cell body and proximal dendrites.
Every section analyzed was redrawn under low magnification and then reviewed under high magnification for ChAT-positive and retrogradely labeled cells; the locations of those found in the IRt region were marked on the drawing of the section. The maps of cell distributions obtained from the three rats were then transferred onto the matching standard medullary cross sections from a rat brain atlas (44). Photographs were taken with a digital camera (PDMC-2; Polaroid, Cambridge, MA) and then enhanced with Photoshop software (version 5.0; Adobe, San Jose, CA). The processing was limited to adjustments of the color balance and contrast in order to best represent the image seen under direct microscopic observation.
Statistical analysis.
The variability of the means is characterized by the standard error (SE). Statistical comparisons of the proportions of cells expressing different mRNAs were conducted with a two-tailed Fisher exact test (Analyse-It Software, Leeds, UK). Labeled cell densities in different regions were compared with paired Student's t-tests. Differences were regarded as significant at P < 0.05.
RESULTS
ChAT and cholinergic receptor mRNAs are expressed in XII premotor neurons and other medullary IRt region neurons.
FITC-labeled latex beads were injected into the XII nucleus of seven rats, with most injections placed in the ventral part of the nucleus at the level of the area postrema (Fig. 1A). Unlike water-soluble tracers, latex beads do not diffuse away from the injection site, and they emit a strong fluorescent signal that can be unmistakably recognized in cells in situ and after their dissociation. Examination of tissue sections from all seven 400-μm-thick medullary slices from which IRt region micropunches were extracted revealed that five slices were obtained from the antero-posterior levels between −13.68 and −12.80 mm from bregma, one from ∼400 μm caudal, and one from ∼400 μm rostral to that range. Figure 1B shows cells, retrogradely labeled with latex beads, located just medial to a tissue punch, and Fig. 1, C–E, show a bead-containing IRt cell after its dissociation.
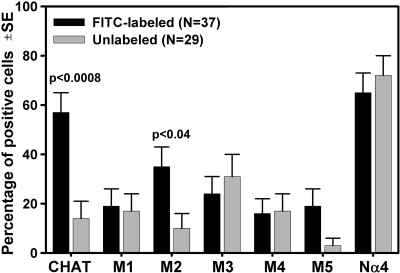
Thirty-seven IRt cells retrogradely labeled from the XII nucleus and twenty-nine unlabeled cells were found to be positive for at least one transcript after RT-PCRs with primers for ChAT, five muscarinic receptor subtypes, Nα4, and/or NSE. Over one-half of retrogradely labeled cells (21/37) expressed mRNA for ChAT, and one-third (13/37) also had type 2 muscarinic (M2) receptor mRNA. In contrast, among the IRt neurons that were not retrogradely labeled, only 4 of 29 expressed ChAT mRNA; this was significantly less than for the retrogradely labeled group (P < 0.0008). M2 receptor mRNA was also less commonly expressed in unlabeled neurons than in the retrogradely labeled group (in 3 of 29 cells, P < 0.04) (Fig. 2). Among the retrogradely labeled cells that expressed ChAT mRNA, seven were positive for M2 receptor mRNA (33%); this proportion was not different from that for M2 receptor mRNA-expressing XII premotor cells that were ChAT negative. The percentages of cells expressing other cholinergic receptor mRNAs (M1, M3, M4, M5, and Nα4) did not differ between IRt XII premotor and unlabeled IRt cells (Fig. 2). Although most retrogradely labeled IRt cells (33 of 37) were dissociated from a punch taken contralaterally to the tracer injection site (to maximize the distance between the site of tracer injection and the region from which cells were sampled), ChAT mRNA also was detected in three of the four retrogradely labeled cells that were obtained from a punch taken from the IRt region on the side of tracer injection, and two of these cells coexpressed M2 receptor mRNA.
Fig. 2.
Percentages of IRt cells expressing mRNA for choline acetyltransferase (ChAT) and different cholinergic receptors among the 37 cells retrogradely labeled from the XII nucleus (XII premotor) and 29 nonretrogradely labeled from the XII nucleus neurons dissociated from the medullary IRt region. Each cell was tested for the presence of all 7 mRNA species. Over half (57%) of XII premotor neurons expressed ChAT mRNA, whereas only 14% of nonretrogradely labeled IRt cells expressed this message (P < 0.0008). The percentage of XII premotor neurons expressing type 2 muscarinic receptor mRNA (M2; 35%) was also higher than for nonretrogradely labeled IRt cells (10%; P < 0.04). The percentages of cells expressing other muscarinic receptor mRNAs (M1, M3, M4, and M5) were relatively low and not significantly different between XII premotor neurons and nonretrogradely labeled IRt cells. Over 60% of IRt cells expressed mRNA for the α4 nicotinic receptor subunit (Nα4), a dominant nicotinic subunit in the brain stem (17, 36), but the percentages were not different between XII premotor neurons and nonretrogradely labeled IRt cells. Error bars represent SE of the estimated proportions, as determined by the size of each population and the assumption of a random bimodal distribution (Analyse-It Software, Leeds, UK).
ChAT immunoreactivity in IRt XII premotor neurons.
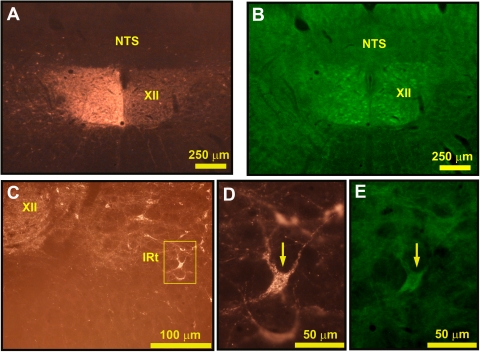
In agreement with previous studies (1, 18), many ChAT-positive cells were present in the IRt region spanning from the ventrolateral border of the XII nucleus to the nucleus ambiguus and in the reticular formation rostral to this region. In agreement with the study of Travers et al. (61), we found that many of these were retrogradely labeled from the XII nucleus. Figure 3A shows the injection site in one of the three rats used in this part of the study, and Fig. 3, D and E, show an IRt neuron that was both retrogradely labeled from the XII nucleus of the opposite side and ChAT positive.
Fig. 3.
ChAT-expressing cells of the medullary IRt region are retrogradely labeled from the XII nucleus. A: cholera toxin B subunit (CTb) injection site in the left XII nucleus. B: the same frame as in A seen under the filters for ChAT shows immunostaining of XII motoneurons on both sides. C: retrogradely labeled cells located in the IRt region ventrolateral to the XII nucleus on the side opposite to tracer injection. D: enlarged image of one of the cells framed in C, as seen under filters for CTb. E: the same frame as in D but shown with filters for FITC demonstrates ChAT immunoreactivity in the cell indicated by the arrows here and in D.
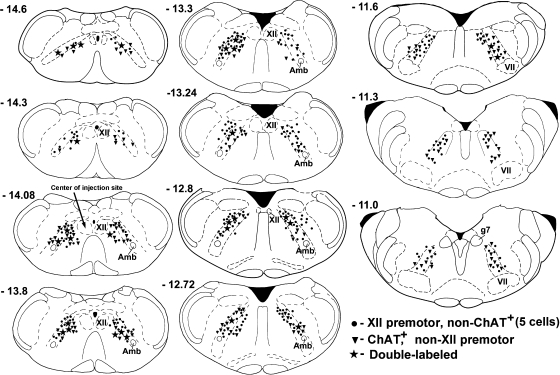
In the three rats, we found a total of 710, 1,434, and 1,728 neurons retrogradely labeled from the XII nucleus in the IRt regions ipsi- and contralateral to the injection site at the 11 antero-posterior levels analyzed (the lowest number being from the experiment with FG). XII premotor neurons were located bilaterally, with only a modest ipsilateral predominance that yielded an ipsi- to contralateral premotor neurons ratio of 1.03–1.35. Circles and stars in Fig. 4 show an approximate distribution of XII premotor cells in one experiment with CTb. The longitudinal distribution of XII premotor cells across all antero-posterior levels analyzed is then shown for each of the three animals in Fig. 5A. Most of the XII premotor cells located within the IRt and its rostral extension were found between levels −14.1 and −11.6 mm from bregma (44).
Fig. 4.
Distribution of neurons retrogradely labeled from the XII nucleus, all ChAT-positive (ChAT+) cells, and ChAT+ XII premotor neurons found at different rostro-caudal levels in 1 of the 2 rats with CTb used as retrograde tracer. •, non-ChAT cells retrogradely labeled from the XII nucleus (5 cells per symbol); ▾, ChAT+ neurons that were not retrogradely labeled from the XII nucleus (one cell per symbol); ★, all cells retrogradely labeled from the XII nucleus that were ChAT+ (one cell per symbol). Cell locations were superimposed onto standard medullary sections taken from a rat brain atlas (44). Most cholinergic XII premotor neurons were located within the caudal part of the IRt region. Amb, nucleus ambiguus; g7, genu of the facial nerve; VII, facial nucleus; XII, hypoglossal nucleus.
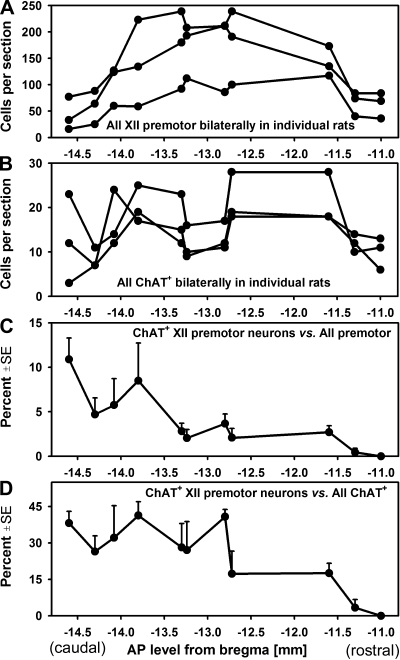
Fig. 5.
Antero-posterior distributions of XII premotor and ChAT+ neurons in the IRt region and its rostral extension in 3 rats. A: all cells retrogradely labeled from the XII nucleus. B: all ChAT+ neurons. C: average percentage of ChAT+ XII premotor neurons relative to all retrogradely labeled neurons in 3 rats. D: average percentage of ChAT+ cells retrogradely labeled from the XII nucleus relative to all ChAT+ neurons. The antero-posterior axis is based on a rat brain atlas (44). ChAT+ XII premotor neurons were predominantly located at the level of the XII nucleus, at and caudal to −12.8 mm from bregma.
In the same sets of brain sections, we found a total of 128, 170, and 206 ChAT-positive cells in the IRt of both sides (Fig. 4, triangles). The density of their longitudinal distribution over the antero-posterior levels analyzed was relatively even, with a small tendency for a decrease around −13 mm from bregma and a more pronounced decline at levels rostral to −11.8 mm (Fig. 5).
Whereas the numbers of both XII premotor and ChAT-positive cells were relatively constant at the antero-posterior levels from −14.6 to −11.6 mm from bregma, the distribution of double-labeled cells was not constant across this range. The percentage of ChAT-positive cells retrogradely labeled from the XII nucleus relative to all XII premotor neurons was on average 4.0% ± 0.7, but it was very low at the levels rostral to −13.3 mm from bregma and gradually increased at more caudal levels (Fig. 4, stars and Fig. 5C). The difference between the percentage of ChAT-positive cells found caudal and rostral to the level −13.3 mm from bregma was significant (6.5% ± 1.4 vs. 1.8% ± 0.6; P < 0.01, t-test). Similarly, when the percentage of double-labeled cells was determined relative to all ChAT-positive neurons, the percentage of those that were retrogradely labeled from the XII nucleus was higher at caudal than rostral levels (Fig. 5D). The mean percentage of double-labeled cells versus all ChAT-positive neurons at, and caudal to, −12.8 mm from bregma was 33.4% ± 2.5, but it was only 9.5% ± 4.6 at more rostral levels (P < 0.001). Thus axonal projections to the XII nucleus were most common for the caudally located ChAT-positive IRt neurons.
DISCUSSION
We found that a high percentage of cholinergic neurons located in the caudal medullary IRt region have axonal projections to the XII motor nucleus. Although relative to the total number of IRt XII premotor neurons those immunoreactive for ChAT represented a minority, ChAT mRNA was present in over half of IRt neurons retrogradely labeled from the XII nucleus. We also found that many XII premotor neurons expressed mRNA for M2 receptors, a cholinergic receptor subtype that is often expressed at presynaptic terminals and acts to inhibit the release of the transmitter(s) used by the parent neuron. Together, these findings show that cells of the IRt region may be an important source of previously described pre- and postsynaptic cholinergic effects in XII motoneurons.
Cholinergic and cholinoceptive nature of projections from the IRt region to the XII nucleus.
The medullary reticular formation has been shown previously to contain cholinergic neurons with axonal projections to the facial, trigeminal, and XII motor nuclei (13, 14, 61), but the incidence of such projections appeared to be relatively small. The cells retrogradely labeled from these nuclei were scattered widely, making it difficult to appreciate the potential importance of these projections.
Our estimate of the incidence of ChAT mRNA in caudal IRt XII premotor neurons suggests that ∼50% of them can be cholinergic. This is higher than the previous estimate that ChAT-immunopositive XII premotor neurons represent 4–6% of all lateral medullary neurons projecting to the XII nucleus (61). The difference may be due, in part, to the higher sensitivity of RT-PCR than immunohistochemistry. Indeed, in the immunohistochemical part of our study, we found that an average of 4% of IRt XII premotor cells were ChAT positive. However, we also found that this percentage was unevenly distributed rostro-caudally; it was over three times higher at the caudal levels than at the rostral levels. In addition, at least 30% of ChAT-immunopositive neurons located in the caudal half of the IRt region had projections to the XII nucleus. By comparison, only a few cholinergic cells of the thousands located in the dorsal pontine tegmentum had axonal projections to the XII nucleus (52). Thus the caudal IRt region may represent a major source of cholinergic afferents to the XII nucleus. The cholinergic XII premotor neurons, albeit less numerous than glutamatergic neurons, which were estimated to represent >60% of the IRt XII premotor population (61), may represent the second largest neurochemically distinct input to the XII nucleus from the caudal medullary IRt region.
Radiolabeled quinuclidinyl benzilate, a ligand for all muscarinic receptors, exhibits prominent binding in the IRt region, suggesting that many IRt neuron cell bodies express functional muscarinic receptors (70). A novel finding of our study is that caudal IRt cells with projections to the XII nucleus have a significantly higher probability of expressing M2 receptor mRNA than IRt cells that were not retrogradely labeled from the XII nucleus. In agreement with earlier in situ hybridization results (Ref. 35; see Ref. 26 for a review), we also found other muscarinic receptor mRNAs in IRt neurons, but only the M2 subtype was significantly enriched in the XII premotor population. Similarly, mRNA for the Nα4, a dominant subunit of cholinergic nicotinic receptors in the brain stem (20, 46), was expressed with comparable probability in the IRt neurons that were and were not retrogradely labeled from the XII nucleus. Thus, while our mRNA results show a potential for multiple types of muscarinic and nicotinic receptors to be expressed in IRt neurons, only the M2 subtype tends to be preferentially expressed in XII premotor neurons.
Muscarinic M2 receptors are often located on terminals of cholinergic and other neurons, where they presynaptically inhibit transmitter release (2, 32, 50, 63), but there is also evidence for their postsynaptic action as autoreceptors in cholinergic neurons (63) and as heteroreceptors in orofacial motoneurons (e.g., Ref. 15). While mRNA findings alone do not allow one to predict where and how M2 receptors may function in XII premotor neurons, we found M2 receptor mRNA in both ChAT mRNA-positive and ChAT mRNA-negative neurons. IRt neurons may have both pre- and postsynaptic M2 receptors, but the relative distribution of these distinct receptor sites among the glutamatergic, cholinergic, and other XII premotor neurons remains to be determined.
Potential functional significance of ChAT and M2 receptors in XII premotor neurons of IRt region.
It has been suggested that XII premotor neurons of the IRt region control tongue movements in a wide range of behaviors (41, 61), but their best-documented role is to mediate inspiratory drive to XII motoneurons (22, 40, 45, 71). Importantly, a recent study has shown that the caudal XII premotor neurons of the IRt region are involved in this function, whereas those located more rostrally may mediate reflexes from upper airway receptors (7). Since our data suggest that cholinergic XII premotor neurons are particularly numerous in the caudal IRt region, it would be of interest to determine whether a portion of inspiratory drive to XII motoneurons is mediated by cholinergic neurons. If this is so, such effects could be mediated by nicotinic cholinergic receptors that are expressed in XII motoneurons (6, 9, 49, 56, 57, 74), as well as postsynaptic M2 muscarinic receptors (5, 15, 30, 56, 70). In anesthetized rats, microdialysis delivery of nicotinic receptor agonists to the XII nucleus region increased XII motoneuronal activity, whereas muscarinic receptor agonists elicited suppressant effects (31). However, these results could be confounded by the use of 1-mm-long microdialysis probes that extended beyond the XII nucleus and could deliver drugs directly to the IRt region. In a neonatal medullary rhythmic slice preparation, a component of cholinergic activation of XII motoneurons was mediated by muscarinic receptors located within the XII nucleus (54, 56).
Both muscarinic M2 and nicotinic receptors can presynaptically inhibit excitatory glutamatergic inputs to XII motoneurons (4, 47) and, at least in neonatal rats, also suppress inhibitory transmission (43). Since many glutamatergic and adjacently located cholinergic XII premotor neurons of the caudal IRt region project to the XII nucleus, it is possible that the two neuronal populations interact, with interactions occurring at the level of both their cell bodies and their terminals within the XII nucleus. One role of such an interaction could be to control the magnitude of inspiratory drive to XII motoneurons.
In healthy humans and normocapnic rodents, respiratory modulation of XII motoneurons is minimal or absent (21, 33), but it may become greatly enhanced as a result of a disease or experimental conditions. For example, both the tonic and phasic inspiratory components of upper airway motor tone are significantly higher during wakefulness in OSA patients than in healthy persons (16, 21, 38, 60). This represents an adaptation to compromised upper airway anatomy that allows OSA patients to generate adequate ventilation when awake. Vagotomy is a frequently used experimental manipulation that greatly increases inspiratory modulation of XII motoneurons (e.g., Ref. 3; reviewed in Ref. 23). The mechanisms and central pathways underlying these two forms of enhancement of inspiratory drive are unknown, but they are likely to be mediated by inspiratory-modulated neurons of the caudal IRt region (6, 22, 40, 45, 71). Both of these conditions may result entirely from an increased activation in pathways upstream from IRt XII premotor neurons or may involve altered interactions between glutamatergic and cholinergic XII premotor neurons of the IRt region. Testing whether the increase of the wakefulness drive to upper airway motoneurons in OSA patients involves either glutamatergic or cholinergic IRt XII premotor neurons may be technically challenging, but it should be feasible to determine whether the vagotomy-induced enhancement of respiratory modulation of XII motoneurons is mediated by cholinergic and/or glutamatergic IRt premotor neurons.
In relation to the control of upper airway motor tone across the sleep-wake cycle, it is of note that the number of medullary cholinergic neurons surviving neurotoxic lesions was positively correlated with the amount of REM sleep (17), suggesting that these neurons have increased activity during REM sleep. Other than that, it is not known whether medullary cholinergic neurons exhibit increased activity during either REM sleep or wakefulness, as is typical of pontine cholinergic neurons (reviewed in Ref. 26). What is known is that many inspiratory-modulated IRt neurons have increased activity during REM sleep (42) and pharmacologically induced REM sleep-like state (27, 71). If some of these neurons are cholinergic, this could elicit a wide range of effects in XII motoneurons, including presynaptic suppression of either glutamatergic excitation (4) or active inhibition (43), postsynaptic cholinergic activation (5, 6, 74), or a shift in the relative contribution of glutamatergic and cholinergic drives in XII motoneurons.
Perspectives
Many caudal medullary IRt region XII premotor neurons may use acetylcholine as their transmitter, and many may also express muscarinic M2 receptors. M2 receptors are often presynaptic and may function in XII premotor neurons as inhibitory modulators of transmitters released from their terminals. Notably, the XII premotor neurons that express cholinergic markers are mainly located in the caudal part of the IRt region known to be the main source of inspiratory drive to XII motoneurons. In OSA patients, inspiratory activation of the muscles innervated by XII motoneurons is enhanced during wakefulness; this protects the upper airway from collapse. Considering that some caudal IRt XII premotor neurons may be activated during REM sleep, or both wakefulness and REM sleep, acetylcholine-containing IRt neurons may modulate transmission of inspiratory drive to XII motoneurons in a state-dependent manner.
GRANTS
The study was supported by National Heart, Lung, and Blood Institute Grant HL-47600.
Acknowledgments
The authors thank Kate Benincasa for assistance with immunohistochemistry.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Armstrong DM, Saper CB, Levey AI, Wainer BH, Terry RD. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol 216: 53–68, 1983. [DOI] [PubMed] [Google Scholar]
- 2.Baghdoyan HA, Lydic R, Fleegal MA. M2 muscarinic autoreceptors modulate acetylcholine release in the medial pontine reticular formation. J Pharmacol Exp Ther 286: 1446–1452, 1998. [PubMed] [Google Scholar]
- 3.Bailey EF, Jones CL, Reeder JC, Fuller DD, Fregosi RF. Effect of pulmonary stretch receptor feedback and CO2 on upper airway and respiratory pump muscle activity in the rat. J Physiol 532: 2–34, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellingham MC, Berger AJ. Presynaptic depression of excitatory synaptic inputs to rat hypoglossal motoneurons by muscarinic M2 receptors. J Neurophysiol 76: 3758–3770, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Bellingham MC, Ireland MF. Contribution of cholinergic systems to state-dependent modulation of respiratory control. Respir Physiol Neurobiol 131: 135–144, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlin NL, Bocchiaro CM, Greene RW, Feldman JL. Nicotinic excitation of rat hypoglossal motoneurons. Neuroscience 115: 861–870, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol 579: 515–526, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med 174: 1264–1273, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Dehkordi O, Millis RM, Dennis GC, Coleman BR, Johnson SM, Changizi L, Ovid TC. Alpha-7 and alpha-4 nicotinic receptor subunit immunoreactivity in genioglossus muscle motoneurons. Respir Physiol Neurobiol 145: 153–161, 2005. [DOI] [PubMed] [Google Scholar]
- 10.El Mansari M, Sakai K, Jouvet M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exp Brain Res 76: 519–529, 1989. [DOI] [PubMed] [Google Scholar]
- 11.Feldman JL, Neverova NV, Saywell SA. Modulation of hypoglossal motoneuron excitability by intracellular signal transduction cascades. Respir Physiol Neurobiol 147: 131–143, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med 172: 1322–1330, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fort P, Luppi PH, Sakai K, Salvert D, Jouvet M. Nuclei of origin of monoaminergic, peptidergic, and cholinergic afferents to the cat trigeminal motor nucleus: a double-labeling study with cholera-toxin as a retrograde tracer. J Comp Neurol 301: 262–275, 1990. [DOI] [PubMed] [Google Scholar]
- 14.Fort P, Sakai K, Luppi PH, Salvert D, Jouvet M. Monoaminergic, peptidergic, and cholinergic afferents to the cat facial nucleus as evidenced by a double immunostaining method with unconjugated cholera toxin as a retrograde tracer. J Comp Neurol 283: 285–302, 1989. [DOI] [PubMed] [Google Scholar]
- 15.Hellström J, Oliveira AL, Meister B, Cullheim S. Large cholinergic nerve terminals on subsets of motoneurons and their relation to muscarinic receptor type 2. J Comp Neurol 460: 476–486, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Hendricks JC, Petrof BJ, Panckeri K, Pack AI. Upper airway dilating muscle hyperactivity during non-rapid eye movement sleep in English bulldogs. Am Rev Respir Dis 148: 185–194, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Holmes CJ, Jones BE. Importance of cholinergic, GABAergic, serotonergic and other neurons in the medial medullary reticular formation for sleep-wake states studied by cytotoxic lesions in the cat. Neuroscience 62: 1179–1200, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Holmes CJ, Mainville LS, Jones BE. Distribution of cholinergic, GABAergic and serotonergic neurons in the medial medullary reticular formation and their projections studied by cytotoxic lesions in the cat. Neuroscience 62: 1155–1178, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Horner RL Respiratory motor activity: influence of neuromodulators and implications for sleep disordered breathing. Can J Physiol Pharmacol 85: 155–165, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Itier V, Bertrand D. Neuronal nicotinic receptors: from protein structure to function. FEBS Lett 504: 118–125, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Katz ES, White DP. Genioglossus activity during sleep in normal control subjects and children with obstructive sleep apnea. Am J Respir Crit Care Med 170: 553–560, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Koizumi H, Wilson CG, Wong S, Yamanishi T, Koshiya N, Smith JC. Functional imaging, spatial reconstruction, and biophysical analysis of a respiratory motor circuit isolated in vitro. J Neurosci 28: 2353–2365, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubin L, Davies RO. Central pathways of pulmonary and airway vagal afferents. In: Regulation of Breathing, edited by Dempsey JA, Pack AI. New York: Dekker, 1995, p. 219–284.
- 24.Kubin L, Davies RO. Mechanisms of airway hypotonia. In: Sleep Apnea. Pathogenesis, Diagnosis, and Treatment, edited by Pack AI. New York: Dekker, 2002, p. 99–154.
- 25.Kubin L, Davies RO, Pack AI. Control of upper airway motoneurons during REM sleep. News Physiol Sci 13: 91–97, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Kubin L, Fenik V. Pontine cholinergic mechanisms and their impact on respiratory regulation. Respir Physiol Neurobiol 143: 235–249, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Kubin L, Kimura H, Tojima H, Pack AI, Davies RO. Behavior of VRG neurons during the atonia of REM sleep induced by pontine carbachol in decerebrate cats. Brain Res 592: 91–100, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett 139: 243–248, 1992. [DOI] [PubMed] [Google Scholar]
- 29.Kubin L, Volgin DV. Developmental profiles of neurotransmitter receptors in respiratory motor nuclei. Respir Physiol Neurobiol (April 26, 2008); doi: 10.1016/j.resp.2008.04.012. [DOI] [PMC free article] [PubMed]
- 30.Lape R, Nistri A. Current and voltage clamp studies of the spike medium afterhyperpolarization of hypoglossal motoneurons in a rat brain stem slice preparation. J Neurophysiol 83: 2987–2995, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Sood S, Liu H, Horner RL. Opposing muscarinic and nicotinic modulation of hypoglossal motor output to genioglossus muscle in rats in vivo. J Physiol 565: 965–980, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loffelholz K Muscarinic receptors and cell signalling. Prog Brain Res 109: 191–194, 1996. [PubMed] [Google Scholar]
- 33.Lu JW, Mann GL, Ross RJ, Morrison AR, Kubin L. Differential effect of sleep-wake states on lingual and dorsal neck muscle activity in rats. Respir Physiol Neurobiol 147: 191–203, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Magalang UJ, Mador MJ. Behavioral and pharmacologic therapy of obstructive sleep apnea. Clin Chest Med 24: 343–353, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Mallios VJ, Lydic R, Baghdoyan HA. Muscarinic receptor subtypes are differentially distributed across brain stem respiratory nuclei. Am J Physiol Lung Cell Mol Physiol 268: L941–L949, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Maloney KJ, Mainville L, Jones BE. Differential c-Fos expression in cholinergic, monoaminergic and GABAergic cell groups of the pontomesencephalic tegmentum after paradoxical sleep deprivation and recovery. J Neurosci 19: 3057–3072, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manaker S, Tischler LJ. Origin of serotonergic afferents to the hypoglossal nucleus in the rat. J Comp Neurol 334: 466–476, 1993. [DOI] [PubMed] [Google Scholar]
- 38.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Invest 89: 1571–1579, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okabe S, Hida W, Kikuchi Y, Taguchi O, Takishima T, Shirato K. Upper airway muscle activity during REM and non-REM sleep of patients with obstructive apnea. Chest 106: 767–773, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Ono T, Ishiwata Y, Inaba N, Kuroda T, Nakamura Y. Hypoglossal premotor neurons with rhythmical inspiratory-related activity in the cat: localization and projection to the phrenic nucleus. Exp Brain Res 98: 1–12, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Ono T, Ishiwata Y, Inaba N, Kuroda T, Nakamura Y. Modulation of the inspiratory-related activity of hypoglossal premotor neurons during ingestion and rejection in the decerebrate cat. J Neurophysiol 80: 48–58, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Orem JM, Lovering AT, Vidruk EH. Excitation of medullary respiratory neurons in REM sleep. Sleep 28: 801–807, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Pagnotta SE, Lape R, Quitadamo C, Nistri A. Pre- and postsynaptic modulation of glycinergic and GABAergic transmission by muscarinic receptors on rat hypoglossal motoneurons in vitro. Neuroscience 130: 783–795, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (compact 3rd Ed.). San Diego, CA: Academic, 1997.
- 45.Peever JH, Shen L, Duffin J. Respiratory pre-motor control of hypoglossal motoneurons in the rat. Neuroscience 110: 711–722, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Picciotto MR, Caldarone BJ, Brunzell DH, Zachariou V, Stevens TR, King SL. Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications. Pharmacol Ther 92: 89–108, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Quitadamo C, Fabbretti E, Lamanauskas N, Nistri A. Activation and desensitization of neuronal nicotinic receptors modulate glutamatergic transmission on neonatal rat hypoglossal motoneurons. Eur J Neurosci 22: 2723–2734, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Remmers JE, DeGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 44: 931–938, 1978. [DOI] [PubMed] [Google Scholar]
- 49.Robinson DM, Peebles KC, Kwok H, Adams BM, Clarke LL, Woollard GA, Funk GD. Prenatal nicotine exposure increases apnoea and reduces nicotinic potentiation of hypoglossal inspiratory output in mice. J Physiol 538: 3–73, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roth MT, Fleegal MA, Lydic R, Baghdoyan HA. Pontine acetylcholine release is regulated by muscarinic autoreceptors. Neuroreport 7: 3069–3072, 1996. [DOI] [PubMed] [Google Scholar]
- 51.Rukhadze I, Kubin L. Differential pontomedullary catecholaminergic projections to hypoglossal motor nucleus and viscerosensory nucleus of the solitary tract. J Chem Neuroanat 33: 23–33, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Rukhadze I, Kubin L. Mesopontine cholinergic projections to the hypoglossal motor nucleus. Neurosci Lett 413: 121–125, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol 51: 160–170, 1976. [DOI] [PubMed] [Google Scholar]
- 54.Shao XM, Feldman JL. Acetylcholine modulates respiratory pattern: effects mediated by M3-like receptors in preBötzinger complex inspiratory neurons. J Neurophysiol 83: 1243–1252, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shao XM, Feldman JL. Mechanisms underlying regulation of respiratory pattern by nicotine in preBötzinger complex. J Neurophysiol 85: 2461–2467, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shao XM, Feldman JL. Cholinergic neurotransmission in the preBötzinger complex modulates excitability of inspiratory neurons and regulates respiratory rhythm. Neuroscience 130: 1069–1081, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shao XM, Tan W, Xiu J, Puskar N, Fonck C, Lester HA, Feldman JL. α4* nicotinic receptors in preBötzinger complex mediate cholinergic/nicotinic modulation of respiratory rhythm. J Neurosci 28: 519–528, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steenland HW, Liu H, Sood S, Liu X, Horner RL. Respiratory activation of the genioglossus muscle involves both non-NMDA and NMDA glutamate receptors at the hypoglossal motor nucleus in vivo. Neuroscience 138: 1407–1424, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Steriade M, Datta S, Paré D, Oakson G, Curró Dossi R. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci 10: 2541–2559, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suratt PM, McTier RF, Wilhoit SC. Upper airway muscle activation is augmented in patients with obstructive sleep apnea compared with that in normal subjects. Am Rev Respir Dis 137: 889–894, 1988. [DOI] [PubMed] [Google Scholar]
- 61.Travers JB, Yoo JE, Chandran R, Herman K, Travers SP. Neurotransmitter phenotypes of intermediate zone reticular formation projections to the motor trigeminal and hypoglossal nuclei in the rat. J Comp Neurol 488: 28–47, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Veasey SC, Panckeri KA, Hoffman EA, Pack AI, Hendricks JC. The effects of serotonin antagonists in an animal model of sleep-disordered breathing. Am J Respir Crit Care Med 153: 776–786, 1996. [DOI] [PubMed] [Google Scholar]
- 63.Vilaro MT, Palacios JM, Mengod G. Multiplicity of muscarinic autoreceptor subtypes? Comparison of the distribution of cholinergic cells and cells containing mRNA for five subtypes of muscarinic receptors in the rat brain. Mol Brain Res 21: 30–46, 1994. [DOI] [PubMed] [Google Scholar]
- 64.Vincent SR, Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience 46: 755–784, 1992. [DOI] [PubMed] [Google Scholar]
- 65.Volgin DV, Fay R, Kubin L. Postnatal development of serotonin 1B, 2A and 2C receptors in brainstem motoneurons. Eur J Neurosci 17: 1179–1188, 2003. [DOI] [PubMed] [Google Scholar]
- 66.Volgin DV, Mackiewicz M, Kubin L. α1B receptors are the main postsynaptic mediators of adrenergic excitation in brainstem motoneurons, a single-cell RT-PCR study. J Chem Neuroanat 22: 157–166, 2001. [DOI] [PubMed] [Google Scholar]
- 67.Volgin DV, Rukhadze I, Kubin L. Hypoglossal premotor neurons of the intermediate medullary reticular region express mRNA markers of cholinergic cells. Sleep 31, Suppl: A14–A15, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Volgin D, Saghir M, Kubin L. Developmental changes in the orexin 2 receptor mRNA in hypoglossal motoneurons. Neuroreport 13: 433–436, 2002. [DOI] [PubMed] [Google Scholar]
- 69.Volgin DV, Swan J, Kubin L. Single-cell RT-PCR gene expression profiling of acutely dissociated and immunocytochemically identified central neurons. J Neurosci Methods 136: 229–236, 2004. [DOI] [PubMed] [Google Scholar]
- 70.Wamsley JK, Lewis MS, Young WS III, Kuhar MJ. Autoradiographic localization of muscarinic cholinergic receptors in rat brainstem. J Neurosci 1: 176–191, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Woch G, Ogawa H, Davies RO, Kubin L. Behavior of hypoglossal inspiratory premotor neurons during the carbachol-induced, REM sleep-like suppression of upper airway motoneurons. Exp Brain Res 130: 508–520, 2000. [DOI] [PubMed] [Google Scholar]
- 72.Yamanaka A, Murak Y, Tsujino N, Goto K, Sakurai T. Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem Biophys Res Commun 303: 120–129, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Yan Z, Surmeier DJ. D5 dopamine receptors enhance Zn2+-sensitive GABAA currents in striatal cholinergic interneurons through a PKA/PP1 cascade. Neuron 19: 1115–1126, 1997. [DOI] [PubMed] [Google Scholar]
- 74.Zaninetti M, Tribollet E, Bertrand D, Raggenbass M. Presence of functional neuronal nicotinic acetylcholine receptors in brainstem motoneurons of the rat. Eur J Neurosci 11: 2737–2748, 1999. [DOI] [PubMed] [Google Scholar]