Abstract
Orthostatic stress such as head-up tilt (HUT) elicits a wide range of heart rate (HR) and arterial pressure (AP) responses among healthy individuals. In this study, we evaluated cardiovascular dynamics in healthy subjects with different HR responses to HUT, but without autonomic dysfunction. We measured AP (brachial artery) and HR (ECG) during 5 min of 60° HUT in 76 healthy normotensive individuals. We then chose individuals on the basis of the extremes of HR responses to HUT (high = ΔHR ≥ 20 beats/min, and low = ΔHR ≤ 10 beats/min; n = 15 per group). Peak HR during HUT was 87 ± 10 beats/min in the high and 69 ± 14 beats/min in the low group (P < 0.05). High HR responders had lower systolic pressure at baseline (121 ± 9 vs. 129 ± 11 mmHg, P < 0.05) and during HUT (120 ± 10 vs. 131 ± 13 mmHg, P < 0.05), and higher plasma norepinephrine (NE) response to HUT (ΔNE: 156.9 ± 17.8 vs. 89.0 ± 17.2 pg/ml; P < 0.05). ΔNE during HUT was also significantly correlated with ΔHR when all 76 subjects were included in a regression analysis (r = 0.39; P < 0.001). Pulse pressure was lower during HUT in high HR responders compared with low HR responders (45 ± 1 vs. 55 ± 2 mmHg, P < 0.05). High HR responders also had larger fluctuations in systolic and pulse pressure during HUT (coefficient of variation = 10.7 ± 0.7 vs. 5.7 ± 0.3%; 7.9 ± 0.5 vs. 4.1 ± 0.4%, respectively, P < 0.05). Sex distribution was different between groups (high: 5 women, 10 men; low: 10 women, 5 men). Higher HR with lower AP during HUT is consistent with normal baroreflex mechanisms of integration. Although interindividual variability appears to be a fundamental part of cardiovascular regulation, the mechanisms of these differences and the sex discrepancy requires further investigation.
Keywords: baroreflex, arterial pressure, orthostasis, sympathetic nervous system, norepinephrine
normal cardiovascular dynamics during orthostasis include the displacement of blood from the central circulation into the lower extremities, reduction of venous return, and decreased stroke volume and cardiac output (10). Arterial pressure (AP) is usually not significantly altered during orthostasis in normal individuals due to rapid baroreflex-mediated increases in heart rate (HR) and sympathetic vasoconstrictor nerve activity (5, 22), resulting in increased total peripheral resistance via norepinephrine (NE) secretion (8, 10). These well-synchronized cardiovascular events prevent major abrupt changes in AP and the development of symptoms during orthostatic stress. In autonomic disorders such as the postural tachycardia syndrome (POTS), patients experience exaggerated increases in HR responses (≥30 beats/min) to head-up tilt (HUT). These patients also present with symptoms of heart palpitations, dizziness, and headaches in the upright posture even when AP appears well maintained although less stable than “normal” (11, 15, 28).
Potential mechanisms in POTS appear to include low blood volume, reduced stroke volume, impaired vasoconstriction, and reduced blood flow (11, 15, 28); however, the pathophysiology of this clinical syndrome is heterogeneous and not completely understood. For example, although POTS patients may exhibit anxious responses to their symptoms during upright posture, our laboratory previously showed that anxiety is not the cause of the excessive HR response to orthostasis in POTS patients (14). Furthermore, these patients showed normal baroreflex control of HR but greater fluctuations in systolic and pulse pressure (15). Other investigators have shown higher supine and upright plasma NE levels in POTS patients compared with healthy controls (11). Additionally, interventions that decrease effective circulating volume, such as bed rest deconditioning, can also create “POTS-like” conditions in which heart rate responses to orthostasis are augmented and blood pressure (BP) variability during tilt is also increased (27).
It is well known that orthostatic stress elicits a wide range of HR and AP responses among healthy individuals; this interindividual variability has been documented in previous work (1, 23, 24, 27). In the present study, we hypothesized that POTS is the extreme end of a continuum of cardiovascular responses to orthostasis and that healthy individuals with augmented HR response to HUT would exhibit cardiovascular dynamics during HUT that were more similar to POTS patients than healthy individuals with lower HR responses. We tested whether healthy individuals with a high HR response to HUT would have greater AP variability during HUT and higher plasma NE compared with individuals with low HR response.
METHODS
Subjects.
We studied 76 healthy, normotensive, recreationally active young adults (47 women, 29 men; age range = 18–40 yr) as part of an ongoing study evaluating cardiovascular responses to sympathoexcitation in healthy humans. Subjects were recruited by local advertisement from Rochester, MN, and surrounding areas. Exclusion criteria included any chronic disease or condition and any use of medication (including over-the-counter medication) except oral contraceptives. We also excluded highly trained athletes from our subject group. Of these, five (3 women, 2 men) exhibited symptoms of presyncope during the HUT protocol and were excluded from further analysis. To minimize any variability in autonomic control mechanisms due to reproductive hormones, female participants were studied during the phase of the cycle when estrogen and progesterone are at the lowest levels: either during the early follicular phase of the menstrual cycle (between days 2 and 6) or during the low-hormone phase of the oral contraceptive cycle (3, 18, 19). All participants gave their written informed consent to participate in the study. The study protocol was approved by the Institutional Review Board of the Mayo Foundation.
We then divided individuals into groups based on the extremes of HR responses. Those with a HR change ≥20 beats/min were classified as high HR responders, and those with a HR change ≤10 beats/min were classified as low HR responders. This resulted in 15 participants in each of the high and low HR responder groups, and 46 in the middle group. There were 10 men and 5 women among the high HR responders, 5 men and 10 women among the low HR responders, and 30 women and 16 men in the middle group.
Instrumentation.
Subjects rested supine on a tilt table with a foot board while a 5-cm, 20-gauge catheter was inserted into the brachial artery of the nondominant arm using aseptic technique after local anesthesia (2% lidocaine). The catheter was connected to a pressure transducer for determination of AP. A three-lead electrocardiogram was used to measure HR continuously. A venous catheter was inserted in the dominant forearm for drug administration as part of the baroreceptor sensitivity (BRS) assessment.
BRS.
After a 5-min baseline period, a bolus of sodium nitroprusside (100 μg) was given intravenously followed after 1 min by a bolus of phenylephrine (150 μg) to transiently decrease and increase AP, respectively. Data were recorded for an additional 2 min after the phenylephrine bolus. Recordings of AP and ECG were sampled at 250 Hz using data-acquisition software (Windaq, Dataq Instruments, Akron, OH) and stored on a computer for offline analysis with signal-processing software (Windaq, Dataq Instruments). Cardiac BRS was assessed using the relationship between R-R interval (RRI) and systolic pressure during the vasoactive drug boluses as described previously (4, 18).
HUT.
We measured HR and AP (arterial catheter) during 5 min of 60° HUT after a period of 20 min of quiet supine rest. The average of 5 min baseline and HUT for HR and AP measurements is reported. These 5-min averages were used for calculation of the HR response to tilt; this difference was used to classify individuals as high or low HR responders, as described above. Arterial blood samples (5 ml) were taken during supine rest and during the last 30 s of HUT and transferred immediately to ice-chilled vacutainers containing anticoagulant and antioxidant. Participants then rested in the supine position for 10 min. Plasma was separated by refrigerated centrifugation and stored frozen until measured in reversed-phase high-performance liquid chromatography with electrochemical detection after extraction with activated alumina. Intra-assay coefficient of variation (CV) for NE was 4.5% at 224 pg/ml and 3.3% at 429 pg/ml, and interassay CV for NE was 7.9% at 49.3 pg/ml and 3.8% at 800 pg/ml.
Twenty-four hour BP and HR measurements.
Twenty-four hour ambulatory BP and HR recordings (Spacelabs) were obtained. Monitors were set to record BP and HR every 15 min from 6 AM to 10 PM, and every 20 min from 10 PM to 6 AM. The daytime period was defined as the interval from 8 AM to 10 PM, and nighttime from midnight to 6 AM (21, 25). The night drop in BP was calculated as the difference between daytime and nighttime BP adjusted for the daytime BP level and expressed in percentages. The SDs and CVs of the 24-h recordings were used as an index of HR, systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse pressure (PP) variability.
Statistical analysis.
Means, SDs, and CVs were computed for all variables of interest during resting supine and during HUT. Independent t-tests were used to determine group differences in age, height, weight, body mass index (BMI), BRS, and 24-h BP assessments. One-way ANOVA was used to compare change in the HR, systolic arterial pressure (SAP), diastolic arterial pressure (DAP), PP, MAP, and NE from baseline to HUT between high, middle, and low HR responders. The average of the given variable over the 5 min of HUT was used for these analyses. To further assess potential difference over time, or between groups during HUT, a two-way repeated-measures ANOVA was used with group (high vs. low) as a between-subject effect and time as within-subject effect. We also performed regression analysis between the change in HR with HUT and BP and norepinephrine changes. An alpha < 0.05 was accepted as statistically significant. All analyses were done using SAS, version 9.1 (SAS Institute, Cary, NC) or Stata, version 9.1 (Stata, College Station, TX). Unless indicated otherwise, data are presented as means ± SE.
RESULTS
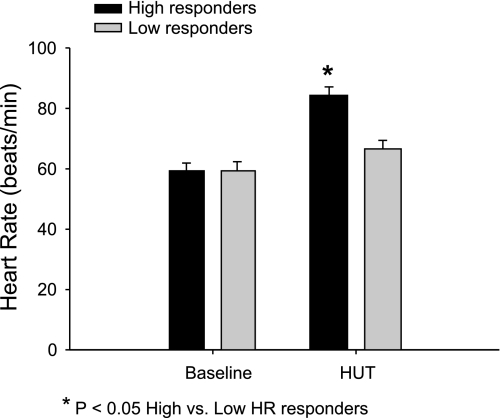
Figure 1 shows the mean HR at baseline and during HUT in the group with a high HR response (ΔHR ≥ 20 beats/min) and low HR response to tilt (ΔHR ≤ 10 beats/min). General characteristics of low, middle, and high HR responders are shown in Table 1. Table 2 shows the sex distribution of the data for the subjects in the low and high HR responder groups. High HR responders were mostly men, while low HR responders were mostly women. Age, height, weight, BMI, and BRS were not different between groups. Mean 24-h HR, SBP, DBP, PP, and night percent drop in SBP were also not different between high and low HR responders; however, the CV for the 24-h HR and 24-h PP were significantly higher in the high HR responders (see Table 3).
Fig. 1.
Heart rate (HR) at baseline and head-up tilt (HUT) in high vs. low HR responders. Although HR at baseline was not different between the 2 groups, HR during HUT was significantly higher in the high responder group.
Table 1.
Descriptive characteristics of high, low, and middle HR responders to HUT
| Variable | High | Low | Middle |
|---|---|---|---|
| Sex | 10 men, 5 women | 10 women, 5 men | 30 women, 16 men |
| Age, yr | 25.7±1.6 | 28.9±1.7 | 28.1±1.0 |
| Height, cm | 168.5±7.1 | 169.7±2.1 | 166.0±3.1 |
| Weight, kg | 74.9±3.2 | 69.5±2.6 | 70.5±1.6 |
| BMI, kg/m2 | 24.2±0.5 | 24.0±0.5 | 24.2±0.3 |
| BRS (RRI), ms/mmHg | 22.3±1.8 | 25.5±2.7 | 21.0±1.2 |
| Baseline HR, beats/min (CV, %) | 59±2 (10±1) | 59±2 (9±1) | 63±1 (9±1) |
| HUT HR, beats/min (CV, %) | 84±3 (9±1) | 67±3* (11±1) | 78±1 (10±1) |
| Baseline SAP, mmHg (CV, %) | 121±3* (3±0) | 129±3 (3±0) | 124±2 (3±0) |
| HUT SAP, mmHg (CV, %) | 120±3 (6±1) | 131±3* (4±1*) | 124±2 (6±0) |
| Baseline DAP, mmHg (CV, %) | 68±1 (5±0) | 72±1 (5±0) | 70±1 (5±0) |
| HUT DAP, mmHg (CV, %) | 75±1 (7±1) | 77±3 (6±1) | 77±1 (8±1) |
| Baseline MAP, mmHg (CV, %) | 86±2* (4±0) | 92±2 (4±0) | 90±1 (4±0) |
| HUT MAP, mmHg (CV, %) | 89±2 (6±1) | 94±2 (5±1) | 92±1 (7±0) |
| Baseline PP, mmHg (CV, %) | 53±2 (7±1) | 58±2 (6±1) | 54±1 (6±0) |
| HUT PP, mmHg (CV, %) | 45±2 (11±1) | 55±2* (8±1*) | 47±2 (10±1) |
| Delta NE, pg/ml (CV, %) | 156.9±16.2 | 89.0±15.6* | 138.1±9.2 |
Values are means ± SE. CV, coefficient of variation; BMI, body mass index; BRS, baroreflex sensitivity; RRI, R-R interval; HR, heart rate; HUT, head-up tilt; SAP, systolic arterial pressure; DAP, diastolic arterial pressure; MAP, mean arterial pressure; PP, pulse pressure; NE, norepinephrine. High = HUT ΔHR > 20 beats/min. Low = HUT ΔHR < 10 beats/min.
P < 0.05 vs. the other two groups (High vs. Low vs. Middle).
Table 2.
Descriptive characteristics of male and female participants in the Low and High HR responder groups
| Variable | Women (n = 15) | Men (n = 15) |
|---|---|---|
| Age, yr | 28.7±1.6* | 26.0±1.8 |
| Height, cm | 159.5±4.6* | 178.7±4.6 |
| Weight, kg | 64.1±2.1* | 80.4±2.1 |
| BMI, kg/m2 | 23.2±0.5* | 25.1±0.5 |
| BRS (RRI), ms/mmHg | 22.9±2.3 | 24.8±2.3 |
| Baseline HR, beats/min | 59±3 | 59±3 |
| HUT HR, beats/min | 72±4 | 79±4 |
| Baseline SAP, mmHg | 124±3 | 127±3 |
| HUT SAP, mmHg | 124±3 | 127±3 |
| Baseline DAP, mmHg | 69±1 | 71±1 |
| HUT DAP, mmHg | 75±2 | 78±2 |
| Baseline MAP, mmHg | 89±2 | 89±2 |
| HUT MAP, mmHg | 92±2 | 92±2 |
| Baseline PP, mmHg | 55±2 | 56±2 |
| HUT PP, mmHg | 49±2 | 50±2 |
| Baseline NE, pg/ml | 131.5±15.9 | 109.5±15.4 |
| HUT NE, pg/ml | 225.6±21.7 | 257.0±20.9 |
Values are means ± SE.
P < 0.05, women vs. men.
Table 3.
Twenty-four hour blood pressure characteristics of High and Low HR responders to HUT
| Variable | High | Low |
|---|---|---|
| 24 h HR, beats/min (CV, %) | 73.6±2.4 (21.1±2.6*) | 67.9±2.9 (17.1±1.1) |
| 24 h SBP, mmHg (CV, %) | 117.6±2.1 (10.6±0.9) | 115.7±1.9 (9.6±0.5) |
| 24 h PP, mmHg (CV, %) | 50.1±2.2 (16.4±2.4*) | 49.9±1.6 (14.8±2.1) |
| Night %Drop in SBP | 11.6±1.7 | 12.8±1.5 |
| Night %Drop in DBP | 19.1±2.3 | 19.8±2.1 |
| Night %Drop in MAP | 15.4±1.9 | 16.3±1.7 |
Values are means ± SE.
P < 0.05, High vs. Low. SBP, systolic blood pressure; DBP, diastolic blood pressure.
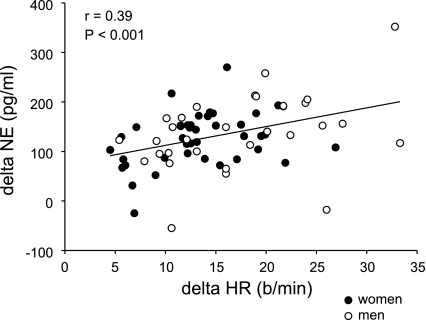
The increase in NE from baseline to HUT was significantly higher in the high HR responders than in the low HR responders (see Table 1). Furthermore, the change in NE during tilt was significantly correlated with the change in HR during tilt when all 76 subjects were included in the analysis (r = 0.39; P < 0.001; see Fig. 2). However, average absolute plasma NE values at baseline and during HUT were not different between the two groups. At baseline, for high HR responders NE was 109.7 ± 16.6 pg/ml, and for low HR responders NE was 129.8 ± 16.0 pg/ml. During HUT, the NE for the high HR responders was 266.6 ± 21.0 pg/ml, and for the low HR responders NE was 218.8 ± 20.3 pg/ml.
Fig. 2.
Linear regression analysis of the relationship between the change in HR and the change in plasma norepinephrine (NE) during HUT, showing a significant positive correlation between these 2 variables.
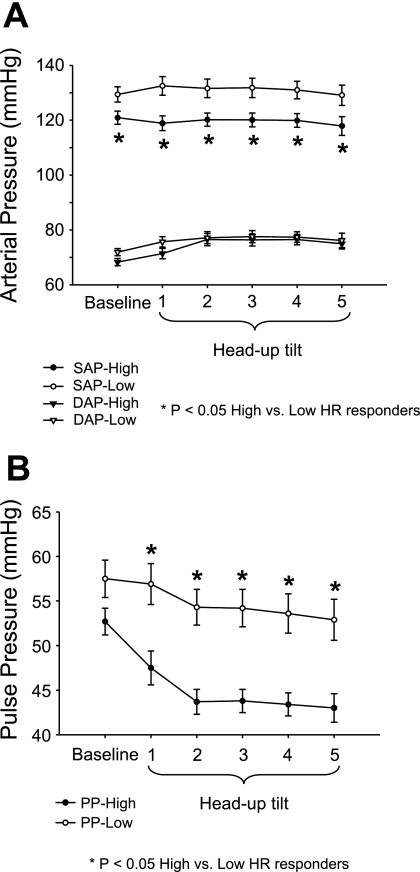
DAP did not differ significantly between groups at baseline or during HUT (repeated-measures ANOVA P = 0.34; Fig. 3A). SAP was significantly lower at baseline for the high HR responders compared with low HR responders and remained lower during HUT (repeated-measures ANOVA P < 0.01; Fig. 3A). MAP was significantly lower at baseline in the high HR responders compared with the low HR responders, but no differences were observed during HUT (Table 1). Baseline PP did not differ significantly between groups but was significantly lower during HUT among high HR responders compared with low HR responders (repeated-measures ANOVA P < 0.01; Fig. 3B). Additionally, PP during tilt was significantly correlated with ΔHR when all 76 subjects were included in the analysis (r = 0.44, P < 0.0001). Moreover, there were significantly greater fluctuations in SAP and PP during HUT among the high HR responders compared with the low HR responders (see Table 1). However, these data did not show significant correlations when all 76 subjects were included in regression analysis.
Fig. 3.
Systolic arterial pressure (SAP) and diastolic arterial pressure (DAP) (A), and pulse pressure (PP) responses (B) during each minute of HUT in high vs. low HR responders.
DISCUSSION
Our major new findings in the present study are that healthy adults with a high HR response to HUT had larger fluctuations in SAP and PP during HUT compared with low HR responders and exhibited larger increases in circulating NE during HUT. Our goal in these studies was to evaluate the idea that the interindividual variability in cardiovascular responses to orthostasis exists in a continuum, of which symptomatic syndromes such as POTS are at the high end. Our present findings that healthy, asymptomatic adults with high HR responses to tilt exhibited several cardiovascular changes similar to those seen in POTS are supportive of this idea.
The major cardiovascular reflex controlling BP during orthostasis is the arterial baroreflex. Supine cardiac BRS has been found to be similar between POTS and control subjects in previous studies (15); our present findings indicated no difference between high and low HR responders in terms of supine BRS in our healthy subjects as well. Another indication of appropriate baroreflex function in the present study was our observation that individuals with higher HR responses to tilt also had lower systolic pressures during tilt. That is, an appropriate baroreflex response would result in a higher HR when BP is lower.
Individuals with lower systolic pressure, and higher HR, during tilt also had a greater change in plasma NE during tilt. We found a significant positive correlation between the change in HR and the change in plasma NE during HUT across all 76 of our subjects (see Fig. 2). Sympathetic neurogenic vasoconstriction is quantitatively very important in terms of successful responses to orthostasis (5). Although we did not directly measure sympathetic nerve activity in the present study, this relationship suggests that those subjects “requiring” more of a HR response during HUT also had greater sympathetically mediated vasoconstriction.
Higher HRs during HUT may be related to low stroke volume as has been shown to occur during physical deconditioning in bed rest studies (15). Low stroke volume and physical deconditioning are also possible explanations for the fluctuations in SAP and PP (16). For example, it has been suggested that a steeper Frank-Starling relationship could be operating after bed rest deconditioning in people with smaller and less distensible hearts (13), meaning that small changes in left ventricular end-diastolic volume could provoke greater changes in stroke volume that are translated into greater variability in SAP and PP. Furthermore, in a randomized clinical trial, it was shown that 12 wk of moderate-intensity endurance exercise training intervention in young men with symptoms of orthostatic intolerance resulted in symptom improvement, increased stroke volume and total peripheral resistance, and HR and NE reductions during upright posture (29). It may be that our high HR responders, as well as actual patients with POTS, have some of the changes associated with less distensible hearts and/or physical deconditioning.
Data collected over a 24-h period provide comprehensive information regarding variability in systemic hemodynamics that may affect cardiovascular responses during activities of daily living. We were therefore interested to note that 24-h variability in HR and PP in our subjects was consistent with the data we collected during 5 min of HUT. The group with the high HR response to orthostasis demonstrated a greater CV in HR over a 24-h period. Consistent with the increased PP variability during orthostasis, this group also had a greater PP CV over 24 h. Although variability in these indexes has not been obtained by 24-h ambulatory BP monitoring in POTS patients, the findings in the present study are generally consistent with the idea that patients with clinical or preclinical orthostatic tachycardia exhibit a wider range of HR and PP throughout the circadian cycle.
Although we observed several similarities between our high HR responders and patients with POTS, it is important to emphasize that there were several important differences as well. For example, our participants did not have tachycardia as defined for the POTS syndrome (average HR at baseline and HUT was < 100 beats/min in our subjects), and they were asymptomatic during orthostasis. Our goal was to study healthy individuals with no tachycardia (and no symptoms) during upright posture and compare their cardiovascular dynamics with those of people with POTS; in this way we were able to emphasize the wide spectrum of human responses to orthostatic stress from asymptomatic to symptomatic.
We were surprised to note in the present study that most of the subjects with high HR responses to tilt were male. This contrasts with the typical 5:1 female-to-male ratio among POTS patients (11). Reduced stroke volume and/or cardiac filling are often primary causes of HR increases during orthostasis. Women have, on average, smaller hearts and lower blood volume compared with men (9); therefore, we expected more women than men among the high HR responders to HUT. Also, higher SAP and PP variability is more likely to occur in people with smaller hearts (16). Although we did not assess heart size in this study, we compared body size between the high and low HR responders, and no differences were observed in height and weight. Furthermore, within each group, the average height and weight of women were lower than in the men; therefore, it is unlikely that body size or perhaps heart size would be plausible explanations for the unexpected sex distribution in the high vs. low HR responders to tilt. With regard to sex comparison, a limitation of our study is that we did not identify specific subgroups of women who were taking oral contraceptives and compare them to those who were not. All women were studied in the low-hormone phase, such that hormonal influences should have been minimized, but specific comparisons of women taking oral contraceptives compared with normally menstruating women were not performed.
Thus the mechanistic basis for the sex distributions we observed remains unclear. Another possible explanation for the sex distribution among POTS patients could be related to women being more likely to report symptoms of orthostasis than men. It has been previously observed that, regardless of age or physical conditioning, women generally report more physical symptoms than men, a phenomenon not associated with psychological disorders (2, 12, 20). Although the sex differences we report may point to previously unidentified contributors to HR responses to tilt, further studies with larger sample sizes will be needed to further address this issue.
Another important difference between our participants and POTS patients was the level of plasma NE at baseline and during HUT. NE in POTS patients has been reported in the range of 154–309 pg/ml at baseline, and between 339 and 654 pg/ml during HUT (28). In our participants, plasma NE values ranged between 53 and 198 pg/ml at baseline, and between 110 and 534 pg/ml during HUT. However, eight of our participants at baseline (3 high HR responders and 5 low HR responders) and six during HUT (4 high HR responders and 2 low HR responders) had NE values within the range reported in POTS patients. Although higher plasma NE levels are expected from the activation of the sympathetic system during orthostasis, the exaggerated NE response in POTS patients could be not just the result of a higher NE spillover but also a possible reduced NE clearance (17). The impact of having high levels of NE circulating for a longer period of time in the pathophysiology of POTS is not clear, especially when the symptoms and the HR increment during HUT do not appear to be related to standing NE levels of 600 pg/ml or higher (28). The interindividual variability in plasma NE and the number of factors that can affect this value (release, reuptake, metabolism, etc.) make the contribution of this variable difficult to evaluate.
In the context of measuring cardiovascular responses to tilt, it is of interest to note that invasive instrumentation (such as the arterial and intravenous catheters used in this study) has been shown to be associated with decreases in orthostatic tolerance in cross-sectional analyses (6, 7, 26). Thus it is possible that the invasive nature of our studies may have increased the overall rate of presyncope in our study. In this context, five subjects exhibited symptoms of presyncope in our study and were excluded from further analysis. We used direct arterial catheterization as the most accurate method of measuring beat-to-beat arterial pressure, and we do not believe this affects our present conclusions since all subjects were similarly instrumented.
In summary, we report in the present study that individuals with higher HR responses during HUT exhibit several cardiovascular responses that are similar to those seen in POTS patients. In high HR responders, the BP variability and the plasma NE responses were all greater than those exhibited by individuals with lower HR responses. Our goal was to investigate the spectrum of cardiovascular responses within a healthy, asymptomatic population. In this context, therefore, it is important to emphasize that our high HR responders had several important differences from POTS patients as well: they were asymptomatic, and HR and plasma NE values were below those reported in POTS. Nonetheless, the similarities between our high HR responders and POTS patients add an additional step in our understanding of the pathophysiology of the syndrome. Future work investigating the role of interindividual variability in autonomic regulation of BP is needed to continue to provide insight into these important questions.
GRANTS
This study was supported by National Institutes of Health (NIH) Grants K23-RR-17520, NS-32352, and NIH-CTSA-1-KL2-RR-024151-01.
Acknowledgments
We thank Dr. Timothy Curry for medical assistance. We also thank Karen Krucker, Shelly Roberts, Pamela Engrav, Tasha Pike, Brian Welch, Amine Issa, Miguel Bernal-Restrepo, Madhuri Somaraju, and Maile Ceridon for technical assistance. Also, we give special thanks to all the participants.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Asmussen E, Christensen EH, Nielsen M. The regulatory circulation in different postures. Surgery 8: 604, 1940. [Google Scholar]
- 2.Barsky AJ, Peekna HM, Borus JF. Somatic symptom reporting in women and men. J Gen Intern Med 16: 266–275, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charkoudian N Influences of female reproductive hormones on sympathetic control of the circulation in humans. Clin Auton Res 11: 295–301, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Charkoudian N, Eisenach JH, Joyner MJ, Roberts SK, Wick DE. Interactions of plasma osmolality with arterial and central venous pressures in control of sympathetic activity and heart rate in humans. Am J Physiol Heart Circ Physiol 289: H2456–H2460, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Cooper VL, Hainsworth R. Effects of head-up tilting on baroreceptor control in subjects with different tolerances to orthostatic stress. Clin Sci (Lond) 103: 221–226, 2002. [DOI] [PubMed] [Google Scholar]
- 6.de Jong-de Vos van Steenwijk CC, Wieling W, Harms MP, Wesseling KH. Variability of near-fainting responses in healthy 6–16-year-old subjects. Clin Sci (Lond) 93: 205–211, 1997. [DOI] [PubMed] [Google Scholar]
- 7.de Jong-de Vos van Steenwijk CC, Wieling W, Johannes JM, Harms MP, Kuis W, Wesseling KH. Incidence and hemodynamic characteristics of near-fainting in healthy 6- to 16-year old subjects. J Am Coll Cardiol 25: 1615–1621, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Fu Q, Shook RP, Okazaki K, Hastings JL, Shibata S, Conner CL, Palmer MD, Levine BD. Vasomotor sympathetic neural control is maintained during sustained upright posture in humans. J Physiol 577: 679–687, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol 289: R109–R116, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Gabbett T, Gass G, Gass E, Morris N, Bennett G, Thalib L. Norepinephrine and epinephrine responses during orthostatic intolerance in healthy elderly men. Jpn J Physiol 50: 59–66, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology 69: 790–798, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Kroenke K, Spitzer RL. Gender differences in the reporting of physical and somatoform symptoms. Psychosom Med 60: 150–155, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation 96: 517–525, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Masuki S, Eisenach JH, Johnson CP, Dietz NM, Benrud-Larson LM, Schrage WG, Curry TB, Sandroni P, Low PA, Joyner MJ. Excessive heart rate response to orthostatic stress in postural tachycardia syndrome is not caused by anxiety. J Appl Physiol 102: 896–903, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Masuki S, Eisenach JH, Schrage WG, Dietz NM, Johnson CP, Wilkins BW, Dierkhising RA, Sandroni P, Low PA, Joyner MJ. Arterial baroreflex control of heart rate during exercise in postural tachycardia syndrome. J Appl Physiol 103: 1136–1142, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Masuki S, Eisenach JH, Schrage WG, Johnson CP, Dietz NM, Wilkins BW, Sandroni P, Low PA, Joyner MJ. Reduced stroke volume during exercise in postural tachycardia syndrome. J Appl Physiol 103: 1128–1135, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Meredith IT, Eisenhofer G, Lambert GW, Jennings GL, Thompson J, Esler MD. Plasma norepinephrine responses to head-up tilt are misleading in autonomic failure. Hypertension 19: 628–633, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Minson CT, Halliwill JR, Young TM, Joyner MJ. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation 102: 1473–1476, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Movahed MR, Martinez A, Morrell H, Greaves S, Greaves J, Sattur S. Differences according to gender in reporting physical symptoms during echocardiographic screening in healthy teenage athletes. Cardiol Young 18: 303–306, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Narkiewicz K, Winnicki M, Schroeder K, Phillips BG, Kato M, Cwalina E, Somers VK. Relationship between muscle sympathetic nerve activity and diurnal blood pressure profile. Hypertension 39: 168–172, 2002. [DOI] [PubMed] [Google Scholar]
- 22.O'Leary DD, Kimmerly DS, Cechetto AD, Shoemaker JK. Differential effect of head-up tilt on cardiovagal and sympathetic baroreflex sensitivity in humans. Exp Physiol 88: 769–774, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Smith JJ Circulatory Response to the Upright Posture. Boca Raton, FL: CRC Press, 1990, p. 33–36.
- 24.Smith JJ, Bush JE, Wiedmeier VT, Tristani FE. Application of impedance cardiography to study of postural stress. J Appl Physiol 29: 133–137, 1970. [DOI] [PubMed] [Google Scholar]
- 25.Staessen J, Bulpitt CJ, O'Brien E, Cox J, Fagard R, Stanton A, Thijs L, Van Hulle S, Vyncke G, Amery A. The diurnal blood pressure profile. A population study. Am J Hypertens 5: 386–392, 1992. [DOI] [PubMed] [Google Scholar]
- 26.Stevens PM Cardiovascular dynamics during orthostasis and the influence of intravascular instrumentation. Am J Cardiol 17: 211–218, 1966. [DOI] [PubMed] [Google Scholar]
- 27.ten Harkel AD, Beck L, Karemaker JM. Influence of posture and prolonged head-down tilt on cardiovascular reflexes. Acta Physiol Scand Suppl 604: 77–87, 1992. [PubMed] [Google Scholar]
- 28.Thieben MJ, Sandroni P, Sletten DM, Benrud-Larson LM, Fealey RD, Vernino S, Lennon VA, Shen WK, Low PA. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc 82: 308–313, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Winker R, Barth A, Bidmon D, Ponocny I, Weber M, Mayr O, Robertson D, Diedrich A, Maier R, Pilger A, Haber P, Rudiger HW. Endurance exercise training in orthostatic intolerance: a randomized, controlled trial. Hypertension 45: 391–398, 2005. [DOI] [PubMed] [Google Scholar]