Abstract
Considerable evidence suggests that the loss of strength and muscle mass appear to be inevitable consequences of aging. Moreover, aging is associated with an increase in body fat. This study examined whether increased physical activity could prevent or reverse the losses of strength and skeletal muscle mass as well as the gain in fat in older adults. Eleven men and 31 women completed a randomized trial consisting of either a physical activity (PA; n = 22) or successful aging health educational control (SA; n = 20) group. Isokinetic knee extensor strength and computed tomography-derived midthigh skeletal muscle and adipose tissue cross-sectional areas (CSA) were assessed at baseline and at 12 mo following randomization. Total body weight and muscle CSA decreased in both groups, but these losses were not different between groups. Strength adjusted for muscle mass decreased (−20.1 ± 9.3%, P < 0.05) in SA. The loss of strength was completely prevented in PA (+2.5 ± 8.3%). In addition, there was a significant increase (18.4 ± 6.0%) in muscle fat infiltration in SA, but this gain was nearly completely prevented in PA (2.3 ± 5.7%). In conclusion, regular physical activity prevents both the age-associated loss of muscle strength and increase in muscle fat infiltration in older adults with moderate functional limitations.
Keywords: aging, muscle quality, adipose tissue, intermuscular
muscle strength and muscle quality are important predictors of incident mobility limitations in older adults (25). Sarcopenia, the reduction in muscle mass that normally occurs with aging, has been interpreted as the primary reason for the age-related loss of strength (1, 5, 20). However, loss of muscle strength in aging cannot be solely attributed to decreased muscle mass (2, 14, 18, 24). Age-related changes in muscle mass and muscle “quality,” defined by strength per unit muscle size, that is, specific torque, may each contribute separately to the loss of muscle function as well as increased risk for mortality (17) in old age.
We have reported that higher intramuscular lipid content defined by attenuation characteristics on computed tomography (CT) is associated with lower isokinetic strength (8) and greater risk for future mobility limitations (25) independent of the cross-sectional area (CSA; size) of muscle. This suggests that higher muscle lipid content is associated with muscle weakness and physical function independent of muscle mass. Interestingly, muscle-wasting diseases such as Duchenne muscular dystrophy are also characterized by reductions in both muscle mass and greater fat infiltration within muscle coincident with impaired muscle function (16). It is unclear, however, how an increased fatty infiltration that may occur with aging may contribute to loss of muscle strength in old age.
Several studies have examined the effects of physical activity on muscle mass and strength in older adults (for a review see Ref. 3). Far fewer studies have examined the effects of physical activity on muscle quality. Moreover, few objective data exist, particularly in the context of randomized controlled trials, concerning the potential of physical activity to prevent the loss of muscle mass and strength with age. Moreover, no studies have examined whether physical activity may prevent the decline in muscle quality and increase in muscle fat accumulation that normally occurs with age. Sipila et al. (21) found that there was a trend for aerobic exercise training (mostly walking) to improve the specific torque (21) but with no effect on the amount of lean tissue (22). These interventions, however, were conducted over a relatively short (18 wk) period. Thus the effects of increased physical activity on age-associated loss of muscle strength, increase in skeletal muscle lipid accumulation, and diminished function require further investigation.
This study sought to determine whether modest increases in physical activity through structured exercise would improve muscle strength and decrease muscle fat infiltration in older adults with moderate functional limitations. This longer-term (1 yr) randomized controlled trial provides novel insight into the ability of physical activity to prevent the negative consequences of aging on muscle and strength.
METHODS
Design.
This study was performed as an ancillary project to the Lifestyle Interventions and Independence for Elders pilot (LIFE-P) study (15, 19). It was conducted to examine whether a modest physical activity program could prevent some of the deleterious effects of aging on body composition and muscle strength in older men and women with moderate functional capacity. This parent study was designed as a multicenter pilot to help plan a definitive phase 3 randomized controlled trial to examine the efficacy of a program of physical activity compared with attention-control (i.e., a health education “successful aging” workshop) on the incidence of major mobility-disability as defined as the inability to walk 400 m or death in at-risk older men and women.
A complete description of the LIFE-P study design has been reported previously (19). Subjects were enrolled in the study if they were between the ages of 70 and 89 yr and met the study inclusion/exclusion criteria, which included a score of ≤9 on the Short Physical Performance Battery (13); being able to walk 400 m within 15 min without sitting and without use of any assistive device; and sedentary lifestyle (<20 min/wk spent in structured physical activity during the past month). Study exclusions included severe heart failure; uncontrolled angina; severe pulmonary disease; chest pain or severe shortness of breath during the 400-m walk test; cancer requiring treatment in the past 3 yr; Parkinson's disease or other serious neurological disorders; other illness with life expectancy of <12 mo; or a Mini Mental State exam score of <21.
Sixty-five men and women only at the Pittsburgh site were asked to participate in this ancillary study as they were enrolled in the parent study. All subjects were randomized after their baseline visits. Similar inclusion/exclusion criteria were used. Fifty-nine subjects agreed to participate in this ancillary study. Of these, only 52 subjects could be scheduled for baseline strength testing and CT scanning and were randomized into either a physical activity (PA) or successful aging health educational control (SA) group. Randomization was stratified by sex. Follow-up testing was not completed in 10 subjects (5 in each group) because of unwillingness or inability to return for the follow-up clinic visit. Eleven men and 31 women (PA, n = 22; SA, n = 20) completed the trial, including 12-mo follow-up testing. Isokinetic knee extensor strength, body weight, and CT-derived midthigh skeletal muscle and adipose tissue CSAs were assessed at baseline and at 12 mo following randomization. All assessors were blinded to intervention group. All participants signed an informed consent, and the study was approved by the Institutional Review Board of the University of Pittsburgh.
Physical activity intervention.
The physical activity intervention is described elsewhere (15, 19) in greater detail. Briefly, it included aerobic, strength, flexibility, and balance training. Walking was employed as the primary mode of physical activity, given its widespread popularity and ease of administration across a broad segment of the older adult population. The intervention was structured into three phases: adoption (weeks 1–8); transition (weeks 9–24); and maintenance (week 25 to end of trial). The initial contacts were primarily center-based with a shift to home-based activity in the transition and maintenance phases.
For the adoption phase, three 40- to 60-min supervised center-based physical activity sessions per week were conducted. During the transition phase of the program, the number of center-based sessions was reduced to two times per week. These sessions were supplemented by home-based endurance/strengthening/flexibility exercises. In the maintenance phase participants were encouraged to perform home-based physical activity a minimum of 5 days/wk. An optional once-per-week center-based group physical activity session was offered to all PA participants. To assess levels of participation in moderate physical activity defined as activities ≥3.0 metabolic equivalents (METs), we also used the Community Healthy Activities Model Program for Seniors (CHAMPS) physical activity questionnaire in all subjects (23).
Health education control.
A successful aging health education intervention was used as the active control and was designed to provide attention and health education. Participants met in small groups weekly for the first 26 wk and then monthly. Sessions included health topics relevant to older adults such as nutrition, medications, foot care, and recommended preventive services at different ages. Basic educational information related to physical activity was provided. Telephone calls were made after each missed session to encourage regular participation, and participants received a monthly newsletter.
CT.
Axial CT scans (9800 Advantage, General Electric, Milwaukee, WI) at the midthigh level were obtained on each participant during both their baseline and 12-mo follow-up examination of the LIFE study protocol. Patients were imaged in the supine position with the arms above the head and toes directed toward the top of the gantry, with legs extended flat on the table. An anterior-posterior scout scan of the entire femur was used to localize the midthigh position. The femoral length was measured in cranial-caudal dimension, and the scan position was determined as the midpoint of the distance between the medial edge of the greater trochanter and the intercondyloid fossa. A single, 10-mm-thick, axial image was obtained at the femoral midpoint, making sure that the entire circumference of both thighs was included in the field of view. The scanning parameters for this image were 120 kVp and 200–250 mA. A quality review was performed on each subject's images to ensure that all images were present, that the proper scan techniques were used, and that the image was of appropriate quality for analysis.
Skeletal muscle and adipose tissue CSAs of the thigh were calculated from the axial CT images using commercially available software (Slice-O-Matic, Tomovision, Montreal, Canada). Muscle and adipose tissue areas were calculated by multiplying the area of a given pixel as extracted from the image header. The mean attenuation coefficient values of muscle within the regions outlined on the images were determined by averaging the CT number (pixel intensity) in Hounsfield units (HU). The methodological variability of this measure is quite small (12). Skeletal muscle and adipose tissue areas were calculated by the range of attenuation values for skeletal muscle (0 to 100 HU) and adipose (−190 to −30 HU) tissue. Intermuscular adipose tissue (IMAT) was distinguished from the subcutaneous adipose tissue by manually drawing a line along the deep fascial plane surrounding the thigh muscles. Once the adipose tissue was segmented from the images, the individual muscles were identified. Muscle borders that were not already defined by adipose tissue were outlined manually, ensuring that no pixels for bone were included in the muscle area. Quadriceps muscles were separated from hamstring muscles with manual tracing.
Isokinetic strength testing.
Isokinetic strength of the knee extensors was determined at 60°/s with a Kin-Com dynamometer (125 AP, Chattanooga, TN). Before strength testing, participants had a period of warm-up by performing a long-distance corridor walk as another part of the LIFE study protocol. The right leg was tested unless injured or weaker by self-report, or restricted in motion. After giving instructions on the procedure, the person was positioned so that the lateral femoral epicondyle of the knee joint was aligned with the rotational axis of the dynamometer. The participant's limb was weighed for gravity correction, and start-stop angles were set at 90° and 30°. Two practice trials were performed at 50% effort to familiarize the participant with the procedure and to provide a warm-up period. At least three maximal efforts were performed by each volunteer. Beginning with the first maximal effort, the torque production over the entire range of motion was plotted, and the plot of each subsequent effort was overlaid on the previous efforts until three similar curves were obtained. Participants were not asked to perform more than six trials. Maximal torque production was recorded as the mean peak torque production from three similar trials.
Data analysis.
Data are presented as means ± SE, unless otherwise indicated. Statistical analyses were performed using JMP version 5.0 for the Macintosh (SAS Institute, Cary, NC). Skeletal muscle CSA, strength, and adipose tissue changes were analyzed using a two-way (group × time) ANOVA. Adjustments for multiple comparisons were made using Bonferroni correction. The probability of detecting significant changes resulting from the intervention was set at an alpha level of P = 0.05. Bivariate correlational analyses were performed using simple linear regression.
RESULTS
Attendance rates for physical activity sessions were 66% during the adoption phase and 69% during the transition phase (Table 1). In addition, home-based participation in physical activity was sustained throughout the trial (16). Fifty-six percent of PA participants turned in at least one home activity log during the maintenance phase. Participation in the successful aging sessions in SA was at least 70% throughout the trial. Moreover, the estimated weekly energy expenditure during walking determined by CHAMPS was higher in PA than in SA at both six and 12 mo of intervention (Table 2). None of the participants in this ancillary study was suspended from the program due to medical reasons. All results are collapsed with respect to sex because we did not have sufficient power to determine whether any of the between-group differences was influenced by sex.
Table 1.
Supervised physical activity performed by PA group during intervention
| Phase 1 | Phase 2 | Phase 3 | |
|---|---|---|---|
| Sessions per week | 1.98±0.18 | 1.38±0.10 | 0.56±0.07 |
| %Sessions attended | 66.1±6.2 | 68.9±5.2 | 55.8±7.0 |
| Minutes per session | 35.1±1.9 |
Values are means ± SE. PA, physical activity. Phase 1: adoption phase, 3 prescribed supervised sessions per week; phase 2: transition phase, 2 prescribed supervised sessions per week; phase 3: maintenance phase, 1 session per week; Minutes per session, average minutes for phases 1–3.
Table 2.
Estimated weekly energy expenditure assessed from self-reported walking activity at baseline and follow-up in PA and SA groups
| PA | SA | P Value | |
|---|---|---|---|
| Baseline, kcal/wk | 634±727 | 588±610 | 0.9577 |
| 6 mo, kcal/wk | 1,504±2,057 | 558±974 | 0.0007 |
| 12 mo, kcal/wk | 1,529±2,389 | 557±724 | 0.0091 |
| Changes | |||
| Baseline to 6-mo, kcal/wk | 918±2,059 | −26±828 | 0.0046 |
| Baseline to 12 mo, kcal/wk | 923±2,263 | −20±1,003 | 0.0080 |
Values are means ± SE. Data were obtained by Community Healthy Activities Model Program for Seniors (CHAMPS; Ref. 23) and analyzed using 1-way ANOVA. SA, successful aging health-educational control group.
Body composition.
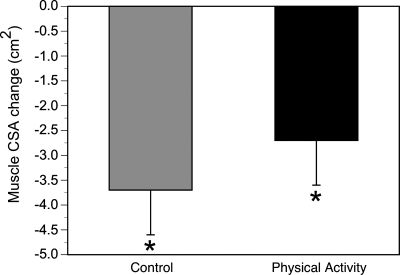
Baseline age, sex and racial composition, and body composition were similar in PA and SA (Table 3). Both PA and SA similarly lost a significant amount of body weight during the 12-mo trial (1.3 ± 0.7 kg in each group). This amounted to a loss of ∼1.4% of their body weight. Both the SA and PA group lost a significant amount of thigh muscle determined by midthigh CSA on CT (Fig. 1). This loss in thigh muscle CSA was not statistically different between SA (4 ± 1%) and PA (3 ± 1%). Similarly, the loss of quadriceps muscle mass was not statistically different between SA (3 ± 1%) and PA (1 ± 1%).
Table 3.
Baseline subject characteristics
| SA (Education) | PA (Exercise) | P Value | |
|---|---|---|---|
| n | 20 | 22 | |
| Sex, M/F | 6/14 | 5/17 | |
| Racial composition, AA/C | 4/16 | 6/16 | |
| Age, yr | 77.4±1.0 | 76.7±1.0 | 0.65 |
| Height, cm | 164.7±2.4 | 161.7±1.9 | 0.32 |
| Weight, kg | 83.2±3.9 | 80.8±4.2 | 0.69 |
| BMI, kg/m2 | 30.4±1.3 | 30.7±1.4 | 0.91 |
| Thigh muscle CSA, cm2 | 97.2±6.9 | 94.6±5.7 | 0.78 |
| Thigh muscle density, HU | 38.8±1.2 | 39.5±1.0 | 0.67 |
| Thigh total AT, cm2 | 124.2±12.8 | 133.8±14.1 | 0.61 |
| Thigh IMAT, cm2 | 4.9±1.0 | 5.1±1.0 | 0.87 |
| SPBB | 7.2±0.3 | 8.0±0.2 | 0.20 |
| Peak torque, N·m | 76.3±8.7 | 71.2±6.4 | 0.63 |
Values are means ± SE. M, male; F, female; AA, African-American; C, Caucasian; BMI, body mass index; CSA, cross-sectional area; HU, Hounsfield units; AT, adipose tissue; IMAT, intermuscular adipose tissue; SPBB, Short Physical Performance Battery.
Fig. 1.
Changes in midthigh skeletal muscle cross-sectional area (CSA) in the physical activity and control groups. Both groups lost muscle (within-group change: *P < 0.05), although this loss was not statistically different between the 2 groups.
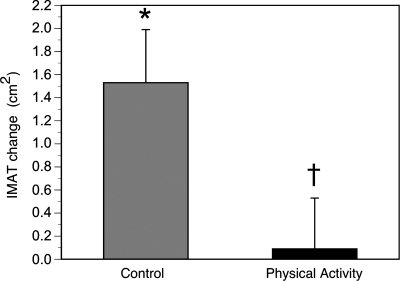
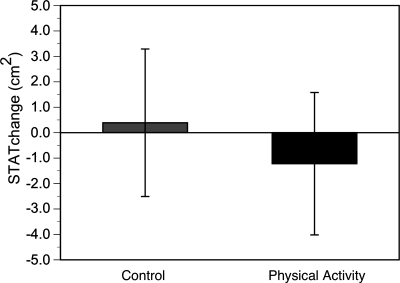
The effects of physical activity were examined on specific adipose tissue depots of the thigh. There was an 18% gain of IMAT in the SA controls in this 1-yr trial. This gain was nearly completely prevented by physical activity (Fig. 2). This gain of fat, however, was clearly depot specific; the subcutaneous thigh adipose tissue did not significantly change in either group (Fig. 3). The mean muscle attenuation value in the thigh decreased (P < 0.05) in the controls (38.9 ± 1.0 to 37.5 ± 1.1 HU) but not in PA (39.5 ± 1.0 to 38.9 ± 1.1 HU), reflecting an increase in the muscle tissue's lipid content in controls (9). These changes in muscle attenuation, however, were not different between groups (P = 0.13).
Fig. 2.
Changes in midthigh intermuscular adipose tissue (IMAT) in the physical activity and control groups. IMAT increased in the control group (within-group change: *P < 0.05, adjusting for multiple comparisons) but not in the physical activity group (†P < 0.05 for between-group change).
Fig. 3.
No statistically significant change in subcutaneous thigh adipose tissue (STAT) in either control or physical activity groups.
Muscle strength.
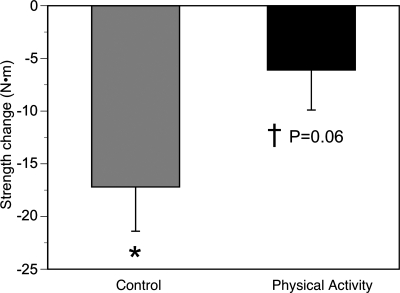
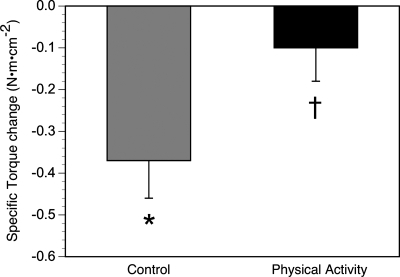
The control group lost a significant (21.6 ± 10%) amount of knee extensor (quadriceps) strength (Fig. 4). While the PA group only lost 1.5% of their muscle strength (not significant), the between-group change did not quite reach significance (P = 0.06). A similar pattern was observed for changes in specific torque (knee extensor strength per unit area of quadriceps muscle) as one measure of muscle quality. SA, but not PA, lost a substantial amount of muscle quality (Fig. 5), and now, the loss of muscle quality was significantly (P < 0.05) greater in SA compared with PA. None of the measured changes in body composition, including changes in muscle CSA, intermuscular fat, or muscle density, was significantly associated with changes in muscle strength.
Fig. 4.
Changes in knee extensor strength in the physical activity and control groups. The control group lost muscle (within-group change: *P < 0.05, adjusting for multiple comparisons). This effect was not observed in the physical activity group, although the between-group change did not reach significance (†P = 0.06).
Fig. 5.
Changes in specific torque (knee extensor peak torque/quadriceps area) in the physical activity and control groups. Specific torque was reduced in the control group (within-group change: *P < 0.05, adjusting for multiple comparisons). This effect was not observed in the physical activity group, with a between-group difference in the change in specific torque (†P < 0.05).
DISCUSSION
A primary finding from this study was that modest amounts of physical activity prevented the further loss of muscle strength in older adults who were presumably on the steep slope of functional decline. The degree of strength loss (∼22%) during 1 yr of aging in the control group was extraordinary compared with other longitudinal studies observing an approximate 2–3% loss per year (7). The large variability in the loss of strength could certainly explain part of this unusually large decrease in strength in these control subjects. On the other hand, the subjects' functional capacity assessed by the Short Physical Performance Battery in the present study was considerably less than that in this other longer longitudinal study (7). Subjects in the present study were also slightly older. Thus it is likely that greater strength losses are partly due to their lower functional capacity at baseline.
There is a loss of muscle mass and accompanying decrease in strength with age (1, 5). Recent longitudinal studies of older men and women, however, indicate that the loss of muscle and loss of strength are not tightly linked (7). Evidence from our group also indicates that aging is associated with an increase in muscle fat infiltration that is in turn associated with muscle weakness (8) and poor function (26). Although an extensive body of evidence indicates that vigorous exercise has many positive effects on skeletal muscle, considerably less is known about the potential of moderate exercise to prevent sarcopenia and progressive muscle weakness in older adults. This study examined whether moderate physical activity can reverse or prevent these apparently negative consequences of aging in a group of older adults with moderate functional limitations.
The prevention of this strength loss is of particular significance considering that this physical activity program did not significantly prevent the loss of muscle mass, implicating the ability of physical activity to affect muscle quality. These results are supported by many previous studies demonstrating greater effects of exercise on muscle strength than on mass in older adults (6, 22). This intervention study also supports our recent findings of a dissociation between the loss of muscle mass and strength in older men and women (7). Taken together, these results indicate that physical activity may prevent the loss of strength in older adults who are at a high risk for disability and that these effects are not explained by the prevention of sarcopenia or the loss of muscle mass.
Another primary novel finding in this study was that physical activity prevented the age-associated increase in muscle fat infiltration. This ability of physical activity to prevent the gain of fat was more remarkable considering that these changes were clearly not generalized to all body fat depots, since changes in subcutaneous gluteal-femoral fat were not significantly different between groups, nor was this depot affected by physical activity. This large (∼18%) increase in muscle fat infiltration during 1 yr of aging in the control group has been associated with insulin resistance (11), the metabolic syndrome (10), as well as muscle weakness and poor muscle function (8, 25, 26) in older adults. Thus these novel effects of physical activity to prevent this age-associated increase in muscle fat infiltration may represent a novel pathway to prevent the progressive decline in function.
There were significant limitations to our study. The relatively small sample size did not permit us to examine whether these important effects of physical activity were similar in men and women or across various racial groups. It is also possible that a longer intervention or follow-up period would have allowed us to observe significant effects of physical activity to prevent the loss of muscle mass. As a randomized controlled trial, we did not analyze the data according to an arbitrary level of compliance. Nearly 70% of the offered sessions were attended in the adoption and transition phases of the intervention, with a mean walking time of 35 min per session. This is similar to that observed for the main study (4). It is possible that the observed effects of exercise on muscle fat infiltration and muscle strength would have been even stronger with higher compliance. The study was simply not adequately powered to examine dose-response associations between adherence or the amount of exercise performed and the key outcomes. Nevertheless, we demonstrate that increased physical activity results in positive effects on the key outcomes of strength and muscle fat infiltration. The study was also limited by an inability to discern causality; many factors not measured in the study, including exercise-induced changes in metabolic or contractile machinery, could influence muscle fat infiltration or muscle strength. The lack of adequate power also limited our ability to determine whether these changes in muscle and fat might be related to strength or functional outcomes that were determined in the parent study, including functional performance and incident mobility limitations. Further studies are clearly needed to address these issues and to determine whether skeletal muscle and muscle fat infiltration are key mediators of the ability of physical activity to prevent the further loss of function and disability in older men and women.
In summary, this is the first randomized controlled trial demonstrating clear effects of moderate physical activity to prevent progressive muscle weakness in older adults. In addition, the significant age-associated increase in muscle fat infiltration was prevented with increased physical activity. This effect of physical activity on fat within muscle was depot specific; similar effects were not observed in subcutaneous adipose tissue. These data are the first to indicate specific effects of physical activity to prevent both further losses of muscle strength and increases in muscle fat infiltration in older age. Further studies are needed to determine whether these effects on strength and muscle fat infiltration are mechanistically linked and further, whether either of these mediate the ability of physical activity to prevent disability in older men and women.
Acknowledgments
This study was supported by a Cooperative Agreement with the National Institute on Aging (UO1-AG-022376).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Brooks SV, Faulkner JA. Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exerc 26: 432–439, 1994. [PubMed] [Google Scholar]
- 2.Evans WJ Effects of exercise on body composition and functional capacity of the elderly. J Gerontol A Biol Sci Med Sci 50: 147–150, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Evans WJ What is sarcopenia?. J Gerontol A Biol Sci Med Sci 50: 5–8, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Fielding RA, Katula J, Miller ME, Abbott-Pillola K, Jordan A, Glynn NW, Goodpaster B, Walkup MP, King AC, Rejeski WJ. Activity adherence and physical function in older adults with functional limitations. Med Sci Sports Exerc 39: 1997–2004, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol 71: 644–650, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol 64: 1038–1044, 1988. [DOI] [PubMed] [Google Scholar]
- 7.Goodpaster B, Park S, Harris T, Kritchevsky S, Nevitt M, Schwartz A, Simonsick E, Tylavsky F, Visser M, Newman A. The loss of skeletal muscle strength, mass and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61: 1059–1064, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Stamm E, Harris TB, Newman AB. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol 90: 2157–2165, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol 89: 104–110, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, Nevitt M, Holvoet P, Newman AB. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med 165: 777–783, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, Schwartz AV, Kritchevsky S, Newman AB. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 26: 372–379, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Thaete FL, Kelley DE. Skeletal muscle composition evaluated with computed tomography. Ann NY Acad Sci 904: 18–24, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Guralnik JM, Simonsick EM, Ferrucci L, Glynn R, Berkman L, Blazer D, Scherr P, Wallace R. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49: M85–M94, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Jubrias SA, Odderson IR, Esselman PC, Conley KE. Decline in isokinetic force with age: muscle cross-sectional area and specific force. Pflügers Arch 434: 246–253, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Katula JA, Kritchevsky SB, Guralnik JM, Glynn NW, Pruitt L, Wallace K, Walkup MP, Hsu FC, Studenski SA, Gill TM, Groessl EJ, Wallace JM, Pahor M. Lifestyle Interventions and Independence for Elders pilot study: recruitment and baseline characteristics. J Am Geriatr Soc 55: 674–683, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Liu M, Chino N, Ishihara T. Muscle damage progression in Duchenne muscular dystrophy evaluated by a new quantitative computed tomography method. Arch Phys Med Rehabil 74: 507–514, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 61: 72–77, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Overend TJ, Cunningham DA, Kramer JF, Lefcoe MS, Paterson DH. Knee extensor and knee flexor strength: cross-sectional area ratios in young and elderly men. J Gerontol 47: M204–M210, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Pahor M, Blair SN, Espeland M, Fielding R, Gill TM, Guralnik JM, Hadley EC, King AC, Kritchevsky SB, Maraldi C, Miller ME, Newman AB, Rejeski WJ, Romashkan S, Studenski S. Effects of a physical activity intervention on measures of physical performance: results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci 61: 1157–1165, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Reed RL, Yochum K, Pearlmutter L, Meredith KE, Mooradian AD. The interrelationship between physical exercise, muscle strength and body adiposity in a healthy elderly population. J Am Geriatr Soc 39: 1189–1193, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Sipila S, Multanen J, Kallinen M, Era P, Suominen H. Effects of strength and endurance training on isometric muscle strength and walking speed in elderly women. Acta Physiol Scand 156: 457–464, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Sipila S, Suominen H. Effects of strength and endurance training on thigh and leg muscle mass and composition in elderly women. J Appl Physiol 78: 334–340, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc 33: 1126–1141, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Vandervoort AA, McComas AJ. Contractile changes in opposing muscles of the human ankle joint with aging. J Appl Physiol 61: 361–367, 1986. [DOI] [PubMed] [Google Scholar]
- 25.Visser M, Goodpaster B, Kritchevsky S, Newman A, Nevitt M, Rubin S, Simonsick E, Harris T. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol Biol Sci 60: 324–333, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc 50: 897–904, 2002. [DOI] [PubMed] [Google Scholar]