Abstract
Over the past decade, numerous studies have shown that increased aortic stiffness is associated with major cardiovascular disease end points, including heart disease, stroke, and kidney disease. Cardiac abnormalities and enhanced atherogenesis in the setting of increased pulsatile load on heart and arteries have been well described. However, recent studies have shown a further association between excessive pressure pulsatility and a number of afflictions of aging that share a predominant microvascular etiology, including many forms of kidney disease and cognitive impairment. In these disorders, microvascular remodeling and impaired regulation of local blood flow, which are related to large artery stiffness and pressure pulsatility, are associated with evidence of diffuse microscopic tissue damage. This brief review will summarize age-related changes in aortic and peripheral vascular function and will discuss potential mechanisms leading from changes in properties of large arteries to excessive pressure pulsatility, abnormal microvascular structure and function, and end-organ dysfunction and damage.
Keywords: aorta, pulse pressure, pulse wave velocity, hemodynamics, stiffness, microcirculation
over the past decade, numerous studies have shown that abnormal aortic function is associated with major cardiovascular disease end points, including heart disease, stroke, and chronic kidney disease. Recent studies have shown an association between excessive pressure pulsatility and a number of afflictions of aging that share abnormal microvascular structure and function as important elements of their pathogenesis, including kidney disease and cognitive impairment. These disorders are characterized by impaired regulation of local blood flow and diffuse microscopic tissue damage. This brief review will describe age-related changes in the properties of large- and medium-sized arteries that promote transmission of potentially deleterious pulsatile energy into the microcirculation. Associated abnormalities in small artery and end-organ structure and function will be discussed, with a special emphasis on the brain and kidneys, which are high-flow, low-impedance organs that are, as a result, particularly susceptible to pulsatile damage. The goal of this mini-review is to summarize a growing body of evidence supportive of the hypothesis that excessive pressure pulsatility resulting from abnormal large artery stiffness promotes abnormalities in microvascular structure and function that contribute to end-organ damage and dysfunction with advancing age.
A BRIEF SYNOPSIS OF PULSATILE HEMODYNAMICS
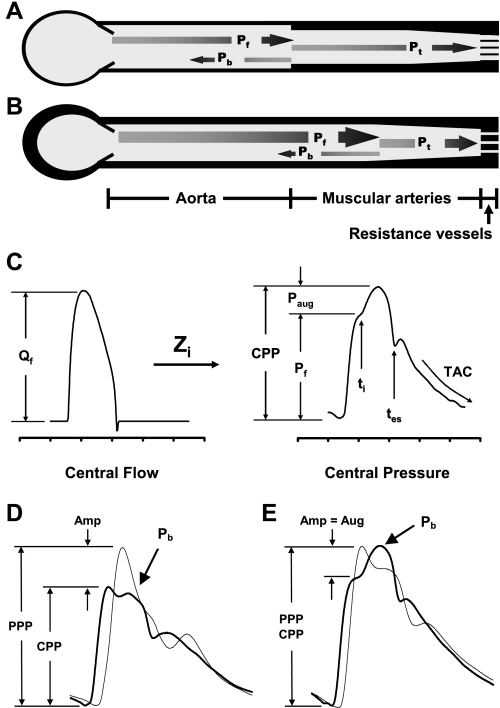
When the heart contracts, the resulting forward flow wave interacts with characteristic impedance of the aorta to produce a forward pressure wave that normally travels down the aorta at a relatively low pulse wave velocity (PWV) (Fig. 1, A and C). Characteristic impedance, the impedance to pulsatile flow in the absence of reflections, is simply the ratio of the amplitudes of the forward pressure and flow waves. As the forward wave propagates distally, it encounters regions of impedance mismatch due to variable wall properties and diameter, which amplifies the waveform (Amp; Fig. 1D) and produces a partial wave reflection. Reflected waves from throughout the arterial system summate to form an aggregate backward-traveling reflected wave that normally returns to the central aorta in late systole and early diastole (Pb; Fig. 1, A and D), producing a favorable secondary diastolic pressure rise that enhances coronary perfusion. Wave reflection is also helpful for the periphery because partial reflection means that less pulsatile energy is transmitted distally into the microcirculation (Fig. 1A). When reflecting sites are located closer to the heart or PWV is moderately elevated, the reflected wave can return to the central aorta in early systole and augment (Aug) central pulse pressure (Fig. 1E). Note that central pressure augmentation obscures the normal pattern of peripheral pressure amplification and produces an increment to central pulse pressure that would not be evident from the change in peripheral pulse pressure assessed during a routine blood pressure measurement in the arm. This stealthy increment in central pulse pressure produces an undetected increase in pulsatile hemodynamic stress in the heart and brain because these organs are exposed to central (aortic or carotid) pulse pressure rather than peripheral (brachial) pulse pressure.
Fig. 1.
A brief synopsis of pulsatile hemodynamics. A: a simple model of the arterial system with a single dominant reflecting site at the interface between aorta and muscular arteries. When the forward wave (Pf) encounters the reflecting site, a portion is reflected (Pb) and most is transmitted (Pt). B: when the aorta stiffens (denoted by a thicker wall), impedance mismatch is reduced, the reflecting site shifts distally, a smaller fraction of Pf is reflected, and therefore more is transmitted into the microcirculation, which may trigger hypertrophic remodeling. C: the input flow wave interacts with input impedance (Zi) to produce the resulting pressure wave. The forward-flow wave (Qf) interacts with characteristic impedance (Zc) to produce Pf. Wave reflection and overlap between Pf and Pb creates late systolic augmentation (Paug), which contributes to central pulse pressure (CPP). The timings of wave reflection (ti) and systolic ejection (tes) are noted, and considerable overlap between Pf and Pb is evident (ti < tes). Total arterial compliance (TAC) can be computed by analyzing the pressure decay in diastole. PPP, peripheral pulse pressure. D: representative carotid (dark) and brachial (light) waveforms in a young healthy person. Note substantial amplification (Amp) of the pulse pressure with distal propagation. E: similar waveforms in an older individual with prominent central augmentation (Aug), which obscures normal peripheral amplification (Amp) of the forward-wave peak. [A and B reproduced with permission from Vyas et al. (96).]
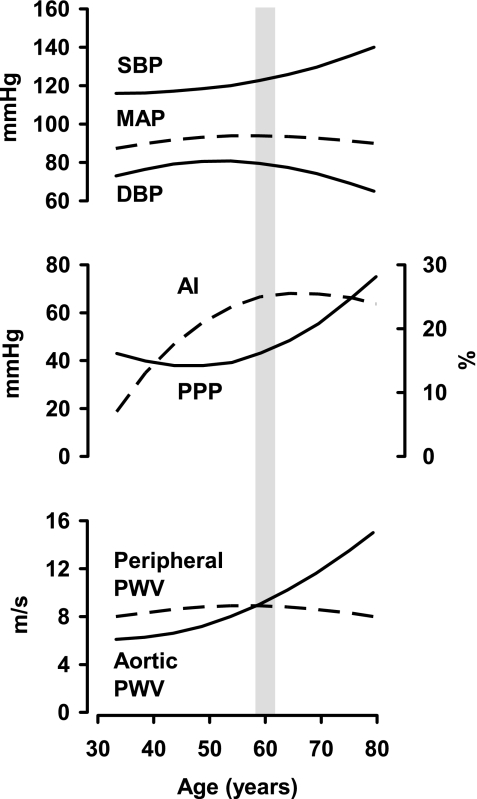
Aortic wall stiffness increases throughout the normal human lifespan, particularly in the presence of cardiovascular disease risk factors (60). However, because of differing nonlinear dependencies on aortic wall stiffness and geometry, PWV and measures of pressure pulsatility, which are closely related to characteristic impedance, change discordantly with advancing age. Before 50 yr of age, mean arterial pressure (MAP) increases modestly, producing comparable increments in systolic blood pressure (SBP) and diastolic blood pressure (Fig. 2). Carotid-femoral PWV increases modestly in parallel with the increase in MAP. Importantly, pulse pressure falls slightly during this phase, despite the increase in carotid-femoral PWV. In contrast, the proportion of central pulse pressure attributable to the secondary late systolic pressure peak increases dramatically (81). This late systolic pressure augmentation (Paug), which is evident on the carotid pressure waveform, is often expressed as the carotid augmentation index: AI = Paug/CPP (Fig. 1C), where CPP is central pulse pressure. Augmentation index depends on both timing and amplitude of the reflected pressure wave. Because relative wave reflection, location of reflecting sites and PWV can change separately with advancing age, augmentation index is a relatively imprecise measure of any particular property of the arterial system. However, augmentation index does provide a concise summary of the net effects of changes in the amplitude and timing of the aggregate global reflected wave. Thus early age-related hemodynamic change is dominated by increasing MAP and central wave reflection together with a modest reduction in peripheral pressure pulsatility.
Fig. 2.
A schematic representation of approximate patterns of change in key pulsatile hemodynamic measures with advancing age. Peripheral systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) all increase modestly before age 60 yr, whereas PPP falls slightly. During this early phase, augmentation index (AI) increases dramatically, whereas aortic and peripheral pulse wave velocity (PWV) change modestly. In contrast, after 60 yr of age, PPP increases dramatically and AI plateaus despite an ongoing increase in aortic PWV. At this transition point, aortic PWV meets then exceeds peripheral PWV, leading to a reduction in reflection at this junction (Fig. 1B), likely explaining the plateau in AI despite increasing aortic PWV.
A major hemodynamic transition occurs at around 60 yr of age (Fig. 2), when MAP reaches a maximum and begins to fall whereas peripheral pulse pressure reaches a minimum and increases dramatically thereafter. Because of rising pulse pressure with stable or falling MAP, SBP increases and diastolic pressure falls. This phase of rapidly increasing pulse pressure is accompanied by a parallel increase in forward wave amplitude and stable or falling augmentation index despite progressively increasing PWV, indicating that wave reflection does not contribute appreciably to increasing pulse pressure with age in older persons (62). A marked increase in aortic PWV and characteristic impedance, in association with a demonstrated lack of stiffening in large peripheral muscular arteries with advancing age (Fig. 2), diminishes the normal impedance mismatch between central aorta and periphery (compare Fig. 1A vs. 1B). As a result, reflection from proximal reflecting sites may be attenuated, leading to increased transmission of pulsatile energy into smaller arteries and the peripheral microcirculation (Fig. 1B). This “impedance matching” between aorta and muscular arteries provides an explanation for the observed reduction in relative wave reflection as pulse pressure increases beyond 60 yr of age (Fig. 1B) and may contribute to the association between aortic stiffening and diseases that share a microvascular etiology (62, 96).
CHANGES IN MUSCULAR ARTERY STRUCTURE AND FUNCTION WITH AGE
In contrast to the aorta, peripheral muscular arteries undergo relatively modest change with age (Fig. 2). Considerable work has been performed in the readily accessible brachial and radial arteries. Resting diameter increases modestly with advancing age and exposure to traditional cardiovascular disease risk factors, such as obesity, hypertension, and diabetes (7). Wall thickness increases, particularly in hypertensive individuals, but wall stiffness is normal or even reduced (29). This apparent paradox suggests that hypertrophy of relatively compliant smooth muscle cells in the arterial wall unloads stiffer components such as collagen, stabilizing arterial stiffness across a fairly broad range of distending pressures and maintaining muscular artery compliance at normal levels in the face of elevated distending pressure (20, 29, 45, 46, 66, 98). Similarly, functional measures of muscular artery stiffness, such as local distensibility or PWV, change relatively little or may even become less stiff (higher distensibility, lower PWV) with advancing age (8, 88), at a time when pulse pressure is increasing rapidly, suggesting that stiffening in the larger muscular arteries contributes little to age-related increases in pulse pressure. However, measures of local vascular distensibility are often confounded by pressure not having been measured at precisely the same point as diameter, resulting in amplification-related errors with a magnitude that depends on whether diameter or pressure was measured further downstream in the arterial system. Hypertrophy and remodeling in the peripheral muscular arteries may serve to offset the reduction in total arterial compliance that otherwise would occur at any given level of aortic stiffening (64). Furthermore, as noted above, marked stiffening of the aorta in the setting of unchanged or reduced stiffness of the muscular arteries reduces the normal gradient of increasing arterial stiffness moving centrifugally from aorta to periphery. Because this impedance gradient promotes wave reflection, reduced wave reflection and increased transmission of pulsatile energy into the microcirculation will occur as aortic stiffness meets then exceeds stiffness of the large muscular arteries (Fig. 2) (62).
In contrast to modest change in mechanical properties, measures of endothelial function in the brachial artery appear to deteriorate substantially with age and in the presence of cardiovascular disease risk factors, including increased pulse pressure (7, 63). Brachial artery endothelial function is frequently assessed by imaging the brachial artery before and after 5 min of forearm ischemia induced by cuff inflation above or below the elbow (7, 15). Cuff release is associated with a vigorous (6- to 7-fold) but variable increase in flow that stimulates release of nitric oxide from the brachial artery endothelium, leading to flow-mediated dilation (FMD) of the brachial artery. The percent change in diameter is taken as an index of brachial artery endothelial function. FMD assessed in this manner is blunted in the presence of many conventional cardiovascular disease risk factors, such as age, male sex, obesity, hypertension, and lipid disorders (7, 15). However, it is important to recall that an increase in local shear stress in the brachial artery due to the hyperemic forearm blood flow response is responsible for triggering release of nitric oxide, leading to the observed FMD. Variability in the flow response, if not considered in the model, will therefore confound interpretation of variability in the FMD response (30, 63).
Recent work from the Framingham Heart Study has shown that much of the age- and risk factor-related blunting of brachial artery FMD is attributable to variability in the flow response, which is a measure of microvascular function, rather than change in brachial diameter for a given flow stimulus (63). When variability in the shear response was ignored, many conventional cardiovascular disease risk factors, including higher pulse pressure, were related to FMD. However, these risk factor relations were markedly attenuated or no longer significant when variability in the hyperemic flow response was considered in the model, suggesting that abnormal microvascular function and blunted hyperemic reserve explained the blunted large artery dilation (63). For example, the slope of the relation between age and FMD was reduced by fourfold, and male sex and pulse pressure were no longer related to FMD. In summary, mechanical properties of the large muscular arteries change relatively little with advancing age. Furthermore, age- and risk factor-related abnormalities in peripheral muscular artery endothelial function have likely been overestimated in studies that evaluated FMD in response to reactive hyperemia because most studies failed to account for variability in the microvascular response.
PRESSURE PULSATILITY AND MICROVASCULAR FUNCTION
The microcirculation, arbitrarily defined as vessels smaller than ∼300 μm in diameter, is responsible for achieving a balance between several potentially conflicting goals. Microvascular control mechanisms must provide for carefully regulated blood flow adequate to meet local tissue metabolic demand while also limiting pressure exposure in the fragile capillaries (67, 77). In the presence of microvascular dysfunction, excessive capillary pressure may lead to hyperfiltration, protein leakage, edema formation, and damage to the capillaries and tissue. Global microvascular resistance is also a key determinant of systemic MAP, which is cardiac output multiplied by total peripheral resistance. Therefore, local influences on microvascular tone may be modified by global blood pressure control systems. Because of the many demands on microvascular function, tone in the resistance vessels is modulated by a variety of local and systemic control mechanisms, mediated through local and humoral vasoactive mediators and metabolites, local innervation, and myogenic tone. In addition, the microcirculation undergoes continual structural adaptation in response to biomechanical and biochemical forces, including steady and pulsatile circumferential wall stress, shear stress, and the local metabolic and neurohumoral milieu. These forces drive structure (diameter and wall thickness) and function (activation state) to an equilibrium that optimizes circumferential and shear stresses and perfusion within the context of overriding effects of the ambient neurohumoral and metabolic milieu (35, 71, 72).
Myogenic tone refers to an intrinsic level of vascular smooth muscle cell tone, in the absence of specific vasoactive mediators, that is enhanced when vascular smooth muscle cells are stretched. Myogenic contraction is mediated in part by tensile strain of integrins attached to the extracellular matrix and involves activation of calcium channels and enhanced sensitivity of contractile proteins to ambient calcium levels (31, 47). Myogenic tone plays an increasingly important role in modulating resistance to blood flow in progressively smaller vessels (17). In small arteries and arterioles, which have high relative wall thickness, a given change in tone and circumferential shortening has an enhanced effect on lumen diameter because of incompressibility of wall mass. Myogenic tone plays a key role in short-term regulation of local blood flow, particularly in autoregulated organs such as the brain and kidneys, because an increase in mean perfusion pressure is rapidly offset by a proportionate increased in local resistance to flow, which serves to stabilize perfusion across a fairly broad autoregulated pressure range.
In the long term, persistent elevation of microvascular tone is replaced by remodeling of wall components, which restores wall stress and tone to nominal levels while maintaining a new, elevated level of microvascular resistance (35). As with myogenic tone, there is an anatomic gradient of hypertrophy. Proximal small arteries, which exhibit relatively less myogenic tone, undergo a combination of hypertrophy and rearrangement to a smaller relaxed lumen diameter (hypertrophic remodeling). The hypertrophic response may be driven in part by the limited myogenic response, which results in persistent elevation of wall stress following an increase in distending pressure (1, 22). In contrast, smaller arterioles, which mount a more vigorous myogenic response because of geometric factors noted above, tend to remodel wall components around a smaller lumen with no change in myocyte mass (eutrophic remodeling). Transduction of physical forces through interactions between the extracellular matrix, integrins, and the cytoskeleton of vascular smooth muscle cells activates the RhoA pathway, which orchestrates migration and possibly also hypertrophy or hyperplasia of microvascular smooth muscle cells, resulting in a remodeled vascular wall (31). In addition, pulsatile strain in the microcirculation upregulates local production of angiotensin II and increases generation of reactive oxygen species (76, 87). The resulting shift in the balance between nitric oxide and reactive oxygen species in favor of the latter creates oxidative stress in the microcirculation, which may promote myocyte hypertrophy through activation of MAP kinases or NF-κB (48). Inward remodeling, whether hypertrophic or eutrophic, increases the ratio of the media cross-sectional area to lumen area. Microvascular remodeling also limits hyperemic flow reserve because even at full vasorelaxation, the remodeled microvascular lumen area is reduced (by definition). Thus microvascular remodeling may interfere with steady-state and dynamic control of local blood flow and may contribute to or complicate the pathogenesis of some forms of hypertension. Increased media-to-lumen ratio is common in microvessels from hypertensive individuals and conveys increased risk for adverse clinical events (75).
Like myogenic tone, microvascular remodeling classically has been interpreted as an adaptive mechanism that increases local resistance to limit mean flow to the tissues. However, recent work has challenged the concept that myogenic tone and microvascular remodeling serve primarily to regulate tissue perfusion relative to changing MAP. Studies in animal models and in humans have demonstrated that microvascular endothelial function (11), remodeling (5, 6, 13, 36), and myogenic tone (53, 57, 82) may be more sensitive to alterations in pulse pressure than MAP. The resulting enhanced myogenic tone and microvascular remodeling in response to pulsatile strain may serve to protect the microcirculation from pulsatile barotrauma in the face of elevated SBP and pulse pressure (53, 54). If this hypothesis is correct, hypertrophic remodeling and increased tone in the microcirculation in response to excessive pressure pulsatility may represent a mechanism whereby a primary abnormality in aortic stiffness and pulse pressure could promote secondary elevation of MAP, culminating in systolic hypertension, which is by far the most common form of hypertension in middle-aged and older adults.
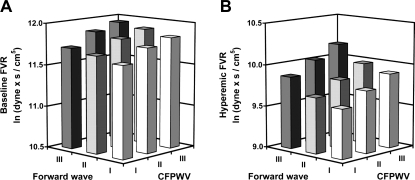
To test the hypothesis that large artery stiffness has a direct effect on microvascular structure and function, relations between aortic stiffness and forearm microvascular function were evaluated in the Framingham Offspring cohort. Resting and hyperemic forearm blood flow and local vascular resistance were assessed before and after a 5-min period of forearm ischemia produced by occlusion of a cuff placed just below the elbow (65). Forearm vascular resistance was increased moderately at rest and markedly during hyperemia in proportion to stiffness of the aorta, whether measured in terms of carotid-femoral PWV or forward pressure wave amplitude (Fig. 3). For example, in the subgroup with forward wave amplitude and carotid-femoral PWV in the highest compared with the lowest tertiles, hyperemic forearm vascular resistance was increased by nearly twofold (Fig. 3, note the logarithmic scale). Similarly, in two additional population-based studies, diameter of retinal arterioles, which are directly measurable microvessels, was shown to be reduced in individuals with a stiffer aorta or carotid artery (12, 50). These studies demonstrate that in large, community-based samples, microvascular structure and function are altered in the presence of increased aortic stiffness.
Fig. 3.
Forearm vascular resistance (FVR) at rest (A) and during hyperemia (B) induced by 5 min of forearm ischemia. Data are least square means of natural log-transformed FVR, plotted according to tertiles of carotid-femoral PWV (CFPWV) and forward pressure wave amplitude, and adjusted for age, sex, body mass index, heart rate, total-to-high-density lipoprotein cholesterol ratio, triglycerides, fasting glucose, prevalent cardiovascular disease, diabetes, use of lipid-lowering or antihypertensive therapy, smoking, hormone replacement in women, timing of walk test. and daily aspirin use. [Reproduced with permission from Mitchell et al (65).]
In the Framingham analysis, forearm vascular resistance was related to forward-wave amplitude and aortic PWV even when both stiffness measures were considered together in a model, suggesting that each variable contributed separately to the observed alterations in microvascular structure and function (Fig. 3). These two measures of aortic function have comparable relations to aortic wall stiffness and thickness but have markedly different inverse dependencies on aortic diameter, with pulse pressure being more sensitive to diameter (61). As a result, the variables may change discordantly in the presence of altered aortic diameter, which recently has been shown to play an important role in the pathogenesis of elevated pulse pressure in older people (16, 21, 58, 59, 61). Furthermore, the variables convey differing information about the energy content of the incident pressure waveform. Forward-wave amplitude serves as an indicator of potential energy of the waveform, while PWV represents a marker of waveform momentum (kinetic energy). Elevation of either component can enhance transmission of pulsatile energy into the microcirculation, leading to activation of mechanosensitive genes, increased oxidative stress, and altered structure and function. The Framingham findings provide support for the hypothesis that excessive pressure pulsatility has important effects on resting and dynamic microvascular structure and tone, even in relatively healthy, unselected individuals. However, the alternative hypothesis that microvascular abnormalities may initiate or exacerbate aortic stiffening should also be considered (84). Whereas microvascular remodeling may be protective with respect to microvascular barotrauma in the periphery, these structural changes potentially result in impaired matching between metabolic demand and local perfusion at rest and especially in response to an acute change in demand.
POTENTIAL HEMODYNAMIC MECHANISMS LINKING AORTIC STIFFNESS AND MICROVASCULAR DYSFUNCTION TO END-ORGAN DAMAGE
Regional flow, local pulse pressure, and tissue-specific differences in microvascular structure modulate the potentially harmful effects of aortic stiffening on structure and function in various organs. In tissues with low flow (high impedance), such as resting skeletal muscle, feeding arteries and arterioles provide ∼75% of the series resistance of the bed and dissipate most of the mean and pulsatile energy content of advancing pressure and flow waveforms proximal to the capillaries. These organs may be relatively less sensitive to excessive pressure pulsatility. In high-flow (low-impedance) organs, like the brain and kidneys, pressure pulsatility penetrates further into the microcirculation. Capillaries in these organs are potentially exposed to damaging levels of pressure pulsatility throughout the day if aortic stiffness and pulse pressure are elevated. In addition, the heart and brain are relatively more severely affected by aortic stiffening because loss of apparent amplification as the aorta stiffens equates to a greater increase in (local) central pulse pressure compared with peripheral pulse pressure (Fig. 1D). Finally, glomerular capillaries are particularly vulnerable to pulsatile damage because they are positioned between afferent (proximal) and efferent (distal) arterioles. Because efferent arteriolar resistance is normally greater than afferent resistance, the pressure drop across the afferent arteriole is low and mean and pulsatile pressure in the glomerulus is high, approaching values in the large arteries.
Blood flow to critical organs with high resting demand, such as the kidneys and brain, is normally autoregulated, meaning that flow remains relatively constant across a wide range of mean perfusion pressures. Myogenic tone and microvascular remodeling are thought to play key roles in short- and long-term control, respectively, of regional blood flow in the face of changing MAP. Alternatively, if myogenic tone and microvascular remodeling are more sensitive to pulse pressure, the microvascular response may serve to limit penetration of excessive pulsatility into the capillaries, but at a cost. It is important to consider that increasing vascular resistance in response to elevated pulse pressure at unchanged MAP will necessarily reduce mean blood flow, which is proportional to MAP divided by local resistance. Thus increased pulse pressure with unchanged or falling MAP, as commonly occurs beyond 60 yr of age (Fig. 2) (25), could potentially interfere with tissue blood flow (65). The resulting mismatch between local resistance and MAP may contribute to age-related declines in blood flow in the kidney and brain. Furthermore, transduction of excessive pressure pulsatility contributes to oxidative stress in the microcirculation (48). Oxidation of bystander lipids and proteins may cause collateral damage in the tissues. As noted above, resistance vessel remodeling in response to increased pressure pulsatility (5, 6, 13, 36) limits vasodilatory reserve (65). Blood pressure instability, which is common in older people, sensitizes high-flow organs to the harmful effects of limited vasoreactivity. Labile blood pressure is often a manifestation of arterial stiffening, which increases volume-dependency of blood pressure and reduces baroreceptor sensitivity (41). The resulting concurrence of labile blood pressure and impaired microvascular reactivity in an individual with a stiff aorta transiently reduces MAP below the lower limit of the (right-shifted) autoregulatory range, exposing critical organs to repeated episodes of microvascular ischemia, which, when superimposed on impaired resting flow, may produce cumulative ischemic damage, leading to fixed deficits in these critical high-flow organs.
INCREASED AORTIC STIFFNESS IS ASSOCIATED WITH MICROVASCULAR BRAIN AND KIDNEY DAMAGE, COGNITIVE IMPAIRMENT, AND KIDNEY DISEASE
Several clinical studies involving diverse participants have demonstrated that increased aortic stiffness and microvascular dysfunction are risk factors for abnormal structure and function in high-flow organs like the brain and kidneys. There is an extensive literature relating arterial stiffness to abnormalities in kidney function, as recently reviewed by Safar et al. (78). Higher pulse pressure is associated with reduced kidney function, as assessed by measured glomerular filtration rate (91), and is associated with accelerated decline in kidney function over time (23). Albuminuria, a marker of microvascular kidney damage, is associated with various cardiovascular disease risk factors, including increased aortic stiffness (34, 83, 100). Increased pulse pressure was associated with albuminuria in patients with known cardiovascular disease (69) and in a nondiabetic community-based sample (14). Diabetes, a powerful risk factor for cardiovascular disease, is a major risk factor for albuminuria and reduced glomerular filtration rate. Diabetes is initially associated with generalized hyperperfusion and microvascular hypertension and is also associated with increased stiffness of the aorta. As noted above, the combination of aortic stiffening and hyperperfusion is particularly pernicious because a greater percentage of the already increased pressure pulsatility penetrates into the microcirculation, creating a synergistic adverse effect that may sensitize diabetics to pulsatile microvascular damage (65). Relations between kidney disease and arterial stiffness appear to be bidirectional. Numerous mechanisms leading from abnormal kidney function to aortic stiffening have been identified (78). This bidirectionality complicates interpretation of cross-sectional associations and also sets up a potential vicious cycle wherein a primary abnormality in either aorta or kidney function could lead to accelerated deterioration in the structure and function of both organs. Large, prospective studies will be required to determine the contribution of aortic aging and subsequent microvascular damage to the well-known age-related decline in kidney function.
Vascular cognitive impairment and Alzheimer's disease have been related to cardiovascular disease risk factors, including increased SBP and pulse pressure, elevated fasting glucose and LDL cholesterol, reduced HDL cholesterol, and reduced physical activity in midlife (10, 42, 44, 70, 73, 74, 89, 97). Various structural lesions in the brain have been related jointly to vascular abnormalities and cognitive deficits. Silent cortical and subcortical infarcts are associated with impaired cognitive function and progressive cognitive decline and are also related to cardiovascular disease risk factors and blood pressure (2, 33, 37–39, 51, 92, 93). Cerebral microbleeds, which are localized hemosiderin deposits that are thought to be related to small vessel pathology and transient self-limited hemorrhage, are increased in proportion to pulse pressure (94). Brain atrophy is also related to cardiovascular disease risk factors, microvascular abnormalities and cognitive decline (24, 32, 79, 80, 99). Furthermore, chronic kidney disease, microvascular brain pathology, and cognitive impairment can cluster, suggesting shared elements of risk or pathogenesis (40, 43, 95). The association of brain pathology and cognitive impairment with SBP and pulse pressure suggests a relation with aortic stiffness, which recent studies have begun to explore using more direct measures of aortic stiffness (27, 80, 85, 86, 97).
Several studies provide evidence that microvascular dysfunction contributes to the relation between pulse pressure and cognitive impairment. White matter lesions in the brain, regions of hyperintensity on transverse relaxation time-weighted MRI scans that become increasingly prevalent with age, are associated with impaired cognitive function, dementia, Alzheimer's disease, and symptoms of depression (10, 26, 52). White matter lesions are thought to reflect cumulative adverse effects of microvascular dysfunction, impaired autoregulation and intermittent relative ischemia (68). Pathological studies reveal arteriosclerotic changes in small vessels in regions with white matter lesions, with deposition of hyaline material in the thickened media of the arteriole, reduced internal diameter, and a markedly increased media-to-lumen ratio (68, 90).
Presence and severity of white matter lesions are related to elevated SBP and pulse pressure as well as various other cardiovascular disease risk factors (10, 18, 28, 32, 49, 52, 68). Elevated midlife SBP is associated with brain atrophy, increased white matter lesions, and cognitive decline proportional to the changes in brain structure (9). White matter lesion severity is also related to blood pressure lability in elderly individuals (4, 19, 28, 56). Thus large-artery stiffness may promote microvascular dysfunction while simultaneously rendering an individual susceptible to intermittent hypotension and relative ischemia, particularly in the periventricular white matter watershed region where lesions are prominent. Functional studies have demonstrated impaired cerebrovascular blood flow reserve in individuals with white matter lesions and other structural abnormalities, providing additional support for the hypothesis that microvascular dysfunction is involved in the genesis of these lesions (3, 55). However, it is possible that a primary defect in brain structure or function alters blood flow characteristics because of impaired demand or chronic low flow, leading to the cross-sectional associations that have been described. Properly powered prospective studies are needed to determine whether abnormal microvascular function in an asymptomatic person with normal brain structure predisposes to subsequent premature cognitive decline and structural damage. Furthermore, detailed evaluations of continuous relations between direct quantitative measures of aortic stiffness and brain and kidney structure and function in a community-based setting are needed to better assess the burden of disease attributable to increased aortic stiffness.
CONCLUSIONS
With advancing age, the aorta undergoes marked but highly variable stiffening, which increases aortic PWV and pressure pulsatility. In contrast, stiffness of large muscular arteries changes relatively little. Aortic stiffening results in reduction of the protective stiffness gradient normally present between heart and periphery and enhances transfer of excessive, potentially harmful pulsatile energy into the periphery. Changes in central hemodynamics are associated with reactive changes in microvascular structure and function that increase peripheral resistance and limit vasodilatory reserve. These secondary changes in the microcirculation may compromise resting flow and flow reserve in vital, high-flow organs such as the heart, brain, and kidneys, leading to the observed associations between aortic stiffness and end-organ damage. The combination of major effects of stiffening on peripheral microvascular function and end-organ structure and function, a marked increase in prevalence of abnormal aortic stiffness with age and rapid aging of the population suggests that an epidemic of aortic stiffness-related disease is on the horizon unless measures to prevent or reverse aortic stiffening are identified and successfully implemented (60).
GRANTS
G. F. Mitchell's work has been supported by National Heart, Lung, and Blood Institute Grants N01-HC-25195, HL-60040, HL-70100, HL-71039, HL-73551, HL-075795, HL-77234, HL-77447, and HL-80124 and by the Donald W. Reynolds Foundation.
DISCLOSURES
G. F. Mitchell is owner of Cardiovascular Engineering, Inc, a company that designs and manufactures devices that measure vascular stiffness. The company uses these devices in clinical trials that evaluate the effects of diseases and interventions on vascular stiffness. Additional research support has been provided by Bristol-Myers Squibb Pharmaceutical Research Institute, AstraZeneca, and Brigham and Women's Hospital.
REFERENCES
- 1.Allen SP, Wade SS, Prewitt RL. Myogenic tone attenuates pressure-induced gene expression in isolated small arteries. Hypertension 30: 203–208, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Aono Y, Ohkubo T, Kikuya M, Hara A, Kondo T, Obara T, Metoki H, Inoue R, Asayama K, Shintani Y, Hashimoto J, Totsune K, Hoshi H, Satoh H, Izumi S, Imai Y. Plasma fibrinogen, ambulatory blood pressure, and silent cerebrovascular lesions: the Ohasama study. Arterioscler Thromb Vasc Biol 27: 963–968, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bakker SL, de Leeuw FE, De Groot JC, Hofman A, Koudstaal PJ, Breteler MM. Cerebral vasomotor reactivity and cerebral white matter lesions in the elderly. Neurology 52: 578–583, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Ballard C, O'Brien J, Barber B, Scheltens P, Shaw F, McKeith I, Kenny RA. Neurocardiovascular instability, hypotensive episodes, and MRI lesions in neurodegenerative dementia. Ann NY Acad Sci 903: 442–445, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Baumbach GL Effects of increased pulse pressure on cerebral arterioles. Hypertension 27: 159–167, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Baumbach GL, Siems JE, Heistad DD. Effects of local reduction in pressure on distensibility and composition of cerebral arterioles. Circ Res 68: 338–351, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF Jr,. Lehman BT, Fan S, Osypiuk E, Vita JA. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation 109: 613–619, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Cameron JD, Bulpitt CJ, Pinto ES, Rajkumar C. The aging of elastic and muscular arteries: a comparison of diabetic and nondiabetic subjects. Diabetes Care 26: 2133–2138, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Carmelli D, Swan GE, Reed T, Miller B, Wolf PA, Jarvik GP, Schellenberg GD. Midlife cardiovascular risk factors, ApoE, and cognitive decline in elderly male twins. Neurology 50: 1580–1585, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Carmelli D, Swan GE, Reed T, Wolf PA, Miller BL, DeCarli C. Midlife cardiovascular risk factors and brain morphology in identical older male twins. Neurology 52: 1119–1124, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Ceravolo R, Maio R, Pujia A, Sciacqua A, Ventura G, Costa MC, Sesti G, Perticone F. Pulse pressure and endothelial dysfunction in never-treated hypertensive patients. J Am Coll Cardiol 41: 1753–1758, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Cheung N, Sharrett AR, Klein R, Criqui MH, Islam FM, Macura KJ, Cotch MF, Klein BE, Wong TY. Aortic distensibility and retinal arteriolar narrowing: the multi-ethnic study of atherosclerosis. Hypertension 50: 617–622, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Christensen KL Reducing pulse pressure in hypertension may normalize small artery structure. Hypertension 18: 722–727, 1991. [DOI] [PubMed] [Google Scholar]
- 14.Cirillo M, Stellato D, Laurenzi M, Panarelli W, Zanchetti A, De Santo NG. Pulse pressure and isolated systolic hypertension: association with microalbuminuria. The GUBBIO Study Collaborative Research Group. Kidney Int 58: 1211–1218, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Dart AM, Kingwell BA, Gatzka CD, Willson K, Liang YL, Berry KL, Wing LM, Reid CM, Ryan P, Beilin LJ, Jennings GL, Johnston CI, McNeil JJ, Macdonald GJ, Morgan TO, West MJ, Cameron JD. Smaller aortic dimensions do not fully account for the greater pulse pressure in elderly female hypertensives. Hypertension 51: 1129–1134, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Davis MJ Myogenic response gradient in an arteriolar network. Am J Physiol Heart Circ Physiol 264: H2168–H2179, 1993. [DOI] [PubMed] [Google Scholar]
- 18.de Leeuw FE, De Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, Breteler MM. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain 125: 765–772, 2002. [DOI] [PubMed] [Google Scholar]
- 19.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, Jack L, Carmelli D. Predictors of brain morphology for the men of the NHLBI twin study. Stroke 30: 529–536, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Drzewiecki G, Pilla JJ. Noninvasive measurement of the human brachial artery pressure-area relation in collapse and hypertension. Ann Biomed Eng 26: 965–974, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Farasat SM, Morrell CH, Scuteri A, Ting CT, Yin FC, Spurgeon HA, Chen CH, Lakatta EG, Najjar SS. Pulse pressure is inversely related to aortic root diameter implications for the pathogenesis of systolic hypertension. Hypertension 51: 196–202, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Feihl F, Liaudet L, Levy BI, Waeber B. Hypertension and microvascular remodelling. Cardiovasc Res 78: 274–285, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Fesler P, Safar ME, du Cailar G, Ribstein J, Mimran A. Pulse pressure is an independent determinant of renal function decline during treatment of essential hypertension. J Hypertens 25: 1915–1920, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O'Brien JT, Ford GA. Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure: brain atrophy, WMH change and blood pressure. J Neurol 254: 713–721, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Franklin SS, Gustin W, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 96: 308–315, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Garde E, Mortensen EL, Krabbe K, Rostrup E, Larsson HB. Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet 356: 628–634, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Hanon O, Haulon S, Lenoir H, Seux ML, Rigaud AS, Safar M, Girerd X, Forette F. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke 36: 2193–2197, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: the Honolulu-Asia Aging study. Stroke 33: 26–30, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Hayoz D, Rutschmann B, Perret F, Niederberger M, Tardy Y, Mooser V, Nussberger J, Waeber B, Brunner HR. Conduit artery compliance and distensibility are not necessarily reduced in hypertension. Hypertension 20: 1–6, 1992. [DOI] [PubMed] [Google Scholar]
- 30.Hayoz D, Weber R, Rutschmann B, Darioli R, Burnier M, Waeber B, Brunner HR. Postischemic blood flow response in hypercholesterolemic patients. Hypertension 26: 497–502, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Heerkens EH, Izzard AS, Heagerty AM. Integrins, vascular remodeling, hypertension. Hypertension 49: 1–4, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Heijer T, Skoog I, Oudkerk M, de Leeuw FE, De Groot JC, Hofman A, Breteler MM. Association between blood pressure levels over time and brain atrophy in the elderly. Neurobiol Aging 24: 307–313, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Howard G, Wagenknecht LE, Cai J, Cooper L, Kraut MA, Toole JF. Cigarette smoking and other risk factors for silent cerebral infarction in the general population. Stroke 29: 913–917, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Ishimura E, Taniwaki H, Tsuchida T, Obatake N, Emoto M, Shoji T, Shioi A, Inaba M, Nishizawa Y. Urinary albumin excretion associated with arterial wall stiffness rather than thickness in type 2 diabetic patients. J Nephrol 20: 204–211, 2007. [PubMed] [Google Scholar]
- 35.Jacobsen JC, Mulvany MJ, Holstein-Rathlou NH. A mechanism for arteriolar remodeling based on maintenance of smooth muscle cell activation. Am J Physiol Regul Integr Comp Physiol 294: R1379–R1389, 2008. [DOI] [PubMed] [Google Scholar]
- 36.James MA, Watt PA, Potter JF, Thurston H, Swales JD. Pulse pressure and resistance artery structure in the elderly. Hypertension 26: 301–306, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Kario K, Ishikawa J, Eguchi K, Morinari M, Hoshide S, Ishikawa S, Shimada K. Sleep pulse pressure and awake mean pressure as independent predictors for stroke in older hypertensive patients. Am J Hypertens 17: 439–445, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Kario K, Matsuo T, Kobayashi H, Hoshide S, Shimada K. Hyperinsulinemia and hemostatic abnormalities are associated with silent lacunar cerebral infarcts in elderly hypertensive subjects. J Am Coll Cardiol 37: 871–877, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation 107: 1401–1406, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Khatri M, Wright CB, Nickolas TL, Yoshita M, Paik MC, Kranwinkel G, Sacco RL, DeCarli C. Chronic kidney disease is associated with white matter hyperintensity volume: the Northern Manhattan Study (NOMAS). Stroke 38: 3121–3126, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kingwell BA, Cameron JD, Gillies KJ, Jennings GL, Dart AM. Arterial compliance may influence baroreflex function in athletes and hypertensives. Am J Physiol Heart Circ Physiol 268: H411–H418, 1995. [DOI] [PubMed] [Google Scholar]
- 42.Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Iivonen S, Mannermaa A, Tuomilehto J, Nissinen A, Soininen H. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med 137: 149–155, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Kurella M, Chertow GM, Fried LF, Cummings SR, Harris T, Simonsick E, Satterfield S, Ayonayon H, Yaffe K. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol 16: 2127–2133, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA 274: 1846–1851, 1995. [PubMed] [Google Scholar]
- 45.Laurent S Arterial wall hypertrophy and stiffness in essential hypertensive patients. Hypertension 26: 355–362, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Laurent S, Girerd X, Mourad JJ, Lacolley P, Beck L, Boutouyrie P, Mignot JP, Safar M. Elastic modulus of the radial artery wall material is not increased in patients with essential hypertension. Arterioscler Thromb 14: 1223–1231, 1994. [DOI] [PubMed] [Google Scholar]
- 47.Lee DL, Webb RC, Jin L. Hypertension and RhoA/Rho-kinase signaling in the vasculature: highlights from the recent literature. Hypertension 44: 796–799, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Lehoux S Redox signalling in vascular responses to shear and stretch. Cardiovasc Res 71: 269–279, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Liao D, Cooper L, Cai J, Toole J, Bryan N, Burke G, Shahar E, Nieto J, Mosley T, Heiss G. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC Study. Neuroepidemiology 16: 149–162, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Liao D, Wong TY, Klein R, Jones D, Hubbard L, Sharrett AR. Relationship between carotid artery stiffness and retinal arteriolar narrowing in healthy middle-aged persons. Stroke 35: 837–842, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Longstreth WT, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol 55: 1217–1225, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Longstreth WT, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O'Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people The Cardiovascular Health Study. Stroke 27: 1274–1282, 1996. [DOI] [PubMed] [Google Scholar]
- 53.Loutzenhiser R, Bidani A, Chilton L. Renal myogenic response: kinetic attributes and physiological role. Circ Res 90: 1316–1324, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol 290: R1153–R1167, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marstrand JR, Garde E, Rostrup E, Ring P, Rosenbaum S, Mortensen EL, Larsson HB. Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke 33: 972–976, 2002. [DOI] [PubMed] [Google Scholar]
- 56.McQuinn BA, O'Leary DH. White matter lucencies on computed tomography, subacute arteriosclerotic encephalopathy (Binswanger's disease), and blood pressure. Stroke 18: 900–905, 1987. [DOI] [PubMed] [Google Scholar]
- 57.Mellander S, Arvidsson S. Possible 'dynamic’ component in the myogenic vascular response related to pulse pressure distension. Acta Physiol Scand 90: 283–285, 1974. [DOI] [PubMed] [Google Scholar]
- 58.Mitchell GF, Conlin PR, Dunlap ME, Lacourciere Y, Arnold JM, Ogilvie RI, Neutel J, Izzo JL Jr, Pfeffer MA. Aortic diameter, wall stiffness, and wave reflection in systolic hypertension. Hypertension 51: 105–111, 2008. [DOI] [PubMed] [Google Scholar]
- 59.Mitchell GF, Gudnason V, Launer LJ, Aspelund T, Harris TB. Hemodynamics of increased pulse pressure in older women in the community-based Age, Gene/Environment Susceptibility-Reykjavik Study. Hypertension 51: 1123–1128, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Cross-sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation 115: 2628–2636, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Mitchell GF, Lacourciere Y, Ouellet JP, Izzo JL Jr, Neutel J, Kerwin LJ, Block AJ, Pfeffer MA. Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic hypertension: the role of proximal aortic diameter and the aortic pressure-flow relationship. Circulation 108: 1592–1598, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 43: 1239–1245, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension 44: 134–139, 2004. [DOI] [PubMed] [Google Scholar]
- 64.Mitchell GF, Tardif JC, Arnold JM, Marchiori G, O'Brien TX, Dunlap ME, Pfeffer MA. Pulsatile hemodynamics in congestive heart failure. Hypertension 38: 1433–1439, 2001. [DOI] [PubMed] [Google Scholar]
- 65.Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D, Benjamin EJ. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation 112: 3722–3728, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Mourad JJ, Girerd X, Boutouyrie P, Safar M, Laurent S. Opposite effects of remodeling and hypertrophy on arterial compliance in hypertension. Hypertension 31: 529–533, 1998. [DOI] [PubMed] [Google Scholar]
- 67.O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 46: 200–204, 2005. [DOI] [PubMed] [Google Scholar]
- 68.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke 28: 652–659, 1997. [DOI] [PubMed] [Google Scholar]
- 69.Pedrinelli R, Dell'Omo G, Penno G, Bandinelli S, Bertini A, Di B, V, Mariani M. Microalbuminuria and pulse pressure in hypertensive and atherosclerotic men. Hypertension 35: 48–54, 2000. [DOI] [PubMed] [Google Scholar]
- 70.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes 51: 1256–1262, 2002. [DOI] [PubMed] [Google Scholar]
- 71.Pries AR, Reglin B, Secomb TW. Structural adaptation of microvascular networks: functional roles of adaptive responses. Am J Physiol Heart Circ Physiol 281: H1015–H1025, 2001. [DOI] [PubMed] [Google Scholar]
- 72.Pries AR, Reglin B, Secomb TW. Remodeling of blood vessels: responses of diameter and wall thickness to hemodynamic and metabolic stimuli. Hypertension 46: 725–731, 2005. [DOI] [PubMed] [Google Scholar]
- 73.Qiu C, von Strauss E, Fastbom J, Winblad B, Fratiglioni L. Low blood pressure and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Arch Neurol 60: 223–228, 2003. [DOI] [PubMed] [Google Scholar]
- 74.Qiu C, Winblad B, Fastbom J, Fratiglioni L. Combined effects of APOE genotype, blood pressure, and antihypertensive drug use on incident AD. Neurology 61: 655–660, 2003. [DOI] [PubMed] [Google Scholar]
- 75.Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation 108: 2230–2235, 2003. [DOI] [PubMed] [Google Scholar]
- 76.Ryan SM, Waack BJ, Weno BL, Heistad DD. Increases in pulse pressure impair acetylcholine-induced vascular relaxation. Am J Physiol Heart Circ Physiol 268: H359–H363, 1995. [DOI] [PubMed] [Google Scholar]
- 77.Safar ME, Lacolley P. Disturbance of macro- and microcirculation: relations with pulse pressure and cardiac organ damage. Am J Physiol Heart Circ Physiol 293: H1–H7, 2007. [DOI] [PubMed] [Google Scholar]
- 78.Safar ME, London GM, Plante GE. Arterial stiffness and kidney function. Hypertension 43: 163–168, 2004. [DOI] [PubMed] [Google Scholar]
- 79.Schmidt R, Launer LJ, Nilsson LG, Pajak A, Sans S, Berger K, Breteler MM, de Ridder M, Dufouil C, Fuhrer R, Giampaoli S, Hofman A. Magnetic resonance imaging of the brain in diabetes: the Cardiovascular Determinants of Dementia (CASCADE) Study. Diabetes 53: 687–692, 2004. [DOI] [PubMed] [Google Scholar]
- 80.Scuteri A, Brancati AM, Gianni W, Assisi A, Volpe M. Arterial stiffness is an independent risk factor for cognitive impairment in the elderly: a pilot study. J Hypertens 23: 1211–1216, 2005. [DOI] [PubMed] [Google Scholar]
- 81.Segers P, Rietzschel ER, De Buyzere ML, Vermeersch SJ, De Bacquer D, Van Bortel LM, De Backer G, Gillebert TC, Verdonck PR. Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension 49: 1248–1255, 2007. [DOI] [PubMed] [Google Scholar]
- 82.Shepherd AP Effect of arterial pulse pressure and hypoxia on myogenic responses in the gut. Am J Physiol Heart Circ Physiol 235: H157–H161, 1978. [DOI] [PubMed] [Google Scholar]
- 83.Smith A, Karalliedde J, De Angelis L, Goldsmith D, Viberti G. Aortic pulse wave velocity and albuminuria in patients with type 2 diabetes. J Am Soc Nephrol 16: 1069–1075, 2005. [DOI] [PubMed] [Google Scholar]
- 84.Stefanadis C, Vlachopoulos C, Karayannacos P, Boudoulas H, Stratos C, Filippides T, Agapitos M, Toutouzas P. Effect of vasa vasorum flow on structure and function of the aorta in experimental animals. Circulation 91: 2669–2678, 1995. [DOI] [PubMed] [Google Scholar]
- 85.Tiemeier H, Bakker SL, Hofman A, Koudstaal PJ, Breteler MM. Cerebral haemodynamics and depression in the elderly. J Neurol Neurosurg Psychiatry 73: 34–39, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tiemeier H, Breteler MM, van Popele NM, Hofman A, Witteman JC. Late-life depression is associated with arterial stiffness: a population-based study. J Am Geriatr Soc 51: 1105–1110, 2003. [DOI] [PubMed] [Google Scholar]
- 87.Ungvari Z, Csiszar A, Kaminski PM, Wolin MS, Koller A. Chronic high pressure-induced arterial oxidative stress: involvement of protein kinase C-dependent NAD(P)H oxidase and local renin-angiotensin system. Am J Pathol 165: 219–226, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van der Heijden-Spek JJ, Staessen JA, Fagard RH, Hoeks AP, Boudier HA, and Van Bortel LM. Effect of age on brachial artery wall properties differs from the aorta and is gender dependent: a population study. Hypertension 35: 637–642, 2000. [DOI] [PubMed] [Google Scholar]
- 89.van Swieten JC, Geyskes GG, Derix MM, Peeck BM, Ramos LM, van Latum JC, van Gijn J. Hypertension in the elderly is associated with white matter lesions and cognitive decline. Ann Neurol 30: 825–830, 1991. [DOI] [PubMed] [Google Scholar]
- 90.van Swieten JC, van den Hout JH, van Ketel BA, Hijdra A, Wokke JH, van Gijn J. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly. A morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain 114 ( Pt 2): 761–774, 1991. [DOI] [PubMed] [Google Scholar]
- 91.Verhave JC, Fesler P, du CG, Ribstein J, Safar ME, Mimran A. Elevated pulse pressure is associated with low renal function in elderly patients with isolated systolic hypertension. Hypertension 45: 586–591, 2005. [DOI] [PubMed] [Google Scholar]
- 92.Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke 33: 21–25, 2002. [DOI] [PubMed] [Google Scholar]
- 93.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 348: 1215–1222, 2003. [DOI] [PubMed] [Google Scholar]
- 94.Vernooij MW, van der LA, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, Krestin GP, Breteler MM. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology 70: 1208–1214, 2008. [DOI] [PubMed] [Google Scholar]
- 95.Vupputuri S, Shoham DA, Hogan SL, Kshirsagar AV. Microalbuminuria, peripheral artery disease, and cognitive function. Kidney Int 73: 341–346, 2008. [DOI] [PubMed] [Google Scholar]
- 96.Vyas M, Izzo JL Jr, Lacourciere Y, Arnold JM, Dunlap ME, Amato JL, Pfeffer MA, Mitchell GF. Augmentation index and central aortic stiffness in middle-aged to elderly individuals. Am J Hypertens 20: 642–647, 2007. [DOI] [PubMed] [Google Scholar]
- 97.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension 51: 99–104, 2008. [DOI] [PubMed] [Google Scholar]
- 98.Weber R, Stergiopulos N, Brunner HR, Hayoz D. Contributions of vascular tone and structure to elastic properties of a medium-sized artery. Hypertension 27: 816–822, 1996. [DOI] [PubMed] [Google Scholar]
- 99.Wong TY, Mosley TH Jr, Klein R, Klein BE, Sharrett AR, Couper DJ, Hubbard LD. Retinal microvascular changes and MRI signs of cerebral atrophy in healthy, middle-aged people. Neurology 61: 806–811, 2003. [DOI] [PubMed] [Google Scholar]
- 100.Yokoyama H, Aoki T, Imahori M, Kuramitsu M. Subclinical atherosclerosis is increased in type 2 diabetic patients with microalbuminuria evaluated by intima-media thickness and pulse wave velocity. Kidney Int 66: 448–454, 2004. [DOI] [PubMed] [Google Scholar]