Abstract
Activation of μ-opioid receptors makes animals hyperphagic and increases their preference for a high-fat diet. Previous studies have suggested that this receptor population plays a role in mediating the hyperphagia that is associated with food deprivation. In this paper, we tested the hypothesis that food deprivation will increase the expression of μ-opioid receptors in the ventral medial hypothalamus and arcuate nucleus (VMH/ARC). Food deprivation resulted in a significant increase in the mRNA expression of μ-opioid receptors in the VMH/ARC and the lateral hypothalamus (LH) after 48 h of fasting but not after 24 or 12 h of fasting in either the light or dark. We did not observe a change in the mRNA expression of κ- or δ-opioid receptors after food deprivation. When food-deprived animals were given a choice between a low-fat diet and a high-fat diet, they were hyperphagic and consumed significantly more of the high-fat diet. When the μ-opioid receptors were blocked with β-funaltrexamine (selective μ-opioid receptor antagonist), prior to giving food-deprived animals access to both a low-fat and high-fat diet, it significantly decreased the percentage of high-fat diet consumed. These data demonstrate that hypothalamic μ-opioid receptors may contribute to the hyperphagia and increased preference for a high-fat diet that is associated with food deprivation.
Keywords: food preference, hyperphagia
several changes occur in the central nervous system of animals that are food deprived. Food deprivation decreases the circulating levels of leptin (13), which enhances the synthesis of two orexigenic peptides, neuropeptide Y (NPY) and agouti gene-related peptide (AgRP; 5, 24, 41). With refeeding, leptin levels rise, suppressing the mRNA levels of NPY and AgRP. Increased levels of NPY and AgRP are believed to play an important role in the hyperphagia that is associated with food deprivation.
The opioid system may also play a role in modulating the hyperphagia of food deprivation. When food-deprived animals are given access to food, the hyperphagia that occurs is attenuated by administering opioid antagonists to μ-, κ-, or δ-opioid receptors (7, 10, 14). It has also been noted that food-deprived animals have a shift in their food preference toward a high-fat diet, a preference that could be stimulated by activation of the μ-opioid system (25, 26, 38).
In this paper we investigated the role of μ-opioid receptors in modulating the hyperphagia and fat preference that is associated with food-deprived animals. Activation of μ-opioid receptors results in hyperphagia and increased preference for a diet high in fat (7, 30, 31, 40). The location of μ-opioid receptors coincides with many areas of the central nervous system that regulate feeding, including the hypothalamus, nucleus accumbens, amygdala, ventral tegmental area, and the nucleus of the tractus solitarius (12, 15, 29). Because food-deprived animals are hyperphagic and prefer a high-fat diet when given a choice, the possibility exists that changes in the expression and activity of this receptor population in food-deprived animals contributes to the feeding behavior that is associated with these animals.
Thus, we hypothesized that changes in the expression of hypothalamic μ-opioid receptors positively contributes to the hyperphagia observed in food-deprived animals.
MATERIALS AND METHODS
Animals
Male Long-Evans rats (Harlan, Indianapolis IN) weighing 250 to 300 g were used in the experiments. Animals were individually housed with a 12:12-h light-dark cycle with ad libitum access to a nonpelleted low-fat diet, a nonpelleted high-fat diet, and water. Diets (3) were purchased from Research Diets (New Brunswick, NJ). All procedures were approved by the Institutional Animal Care and Use Committees located at Pennington Biomedical Research Center.
Experiment 1 — Food Preference of Food-Deprived Animals
Male Long-Evans rats were divided into an ad libitum group (n = 7) and groups that were food deprived for 48 h (n = 7), 24 h (n = 7), 12 h in the light (n = 7), or 12 h in the dark (n = 7) and given ad libitum access to a low-fat and a high-fat diet. On test days, diets were removed from the food-deprived animals for their respective time frame. After 48, 24, or 12 h in the light, or 12 h in the dark, both groups of animals (i.e., ad libitum fed and food deprived) were given preweighed low-fat and high-fat diet simultaneously. Food intake was measured 2 h after the food was given to the animals. Dietary preference was the diet that provided >50% of the daily intake.
Experiment 2 — mRNA Expression of μ-, κ-, and δ-Opioid Receptors in Ad Libitum-Fed and Food-Deprived Rats
Male Long-Evans rats were divided into an ad libitum-fed group (n = 7) and groups deprived of food for 48 h (n = 7), 24 h (n = 7), 12 h in the light (n = 7), 12 h in the dark (n = 7). All animals had ad libitum access to a nonpelleted low-fat and a high-fat diet. On test day, food was removed from the food-deprived animals. After the respective time of food deprivation, the brains of the ad libitum and food-deprived groups were removed and frozen on dry ice. Frozen sections of the hypothalamus were placed on a freezing vibratome. Anatomically appropriate micropunches of the ventral medial hypothalamus/arcuate nucleus (VMH/ARC), lateral hypothalamus (LH), and paraventricular nucleus (PVN) were collected and processed for isolation of RNA.
Real-time-PCR.
Fresh tissue was homogenized in QIAzol (Trizol) using a motorized tissue homogenizer (Tekmar, Cincinnati, OH). Homogenate was transferred to a phase-lock gel tube, and chloroform was added. Samples were centrifuged, and the upper aqueous phase was placed in a new collection tube containing 70% ethanol. RNA from the whole hypothalamus was isolated using RNeasy Kit (Qiagen, Valencia, CA). Reverse transcriptase (RT) was conducted using Moloney murine leukemia virus procedures (Promega, Madison WI). For RT, 2 μg of RNA from each sample was added to random primers (Promega) and incubated in a thermal cycler (model PTC-100; MJ Research, Watertown, MA) for 5 min at 70°C. Tubes were removed, placed on ice, and a mixture of 5 × Moloney murine leukemia virus, 10 mM dNTP, and RT buffer was added. Tubes were returned to the thermal cycler for 60 min at 37°C and then 15 min at 70°C. Real-time PCR was conducted with primers designed for rat NPY and rat AgRP.
For real-time PCR, SYBR Green 2 × Master Mix (Applied Systems, Foster City, CA), forward and reverse primers (10 μM), and RT product (10 ng) were added to 384-well plates. The cycling parameters consisted of initial 2-min incubation at 50°C, followed by 10 min at 95°C, and then 15 s at 95°C and a 1-min annealing step at 60°C (40 cycles). A dissociation step (15 s at 95°C) was added following 40 cycles to determine the specificity of primers. Quantity of NPY and μ-opioid receptors was based on a standard curve and normalized to cyclophillin RNA (ABI Prism 7900 Sequence Detection System; Applied Biosystems).
Experiment 3: Effect of β-Funaltrexamine (Selective μ-Opioid Receptor Antagonist) on High-Fat Intake of Animals Food Deprived for 48 h
Male Long-Evans rats were anesthetized with a ketamine cocktail (ketamine/acepromazine/xylazine, 80 mg/ml × 1.6 mg/ml × 5 mg/ml, respectively) and fitted with a 26-gauge stainless steel guide cannula (Plastic One, Austin, TX) aimed at the third ventricle. The coordinates (anterior-posterior, −2.3; medial-lateral, 0; and dorsal-ventral, −8.5 from bregma) were determined from the rat atlas of Paxinos and Watson (27). Animals were allowed to recover for 1 wk. On test days, food was removed from the animals, and they were divided into groups that received an intracerebroventricular injection of either saline (n = 6) or 10 nmol β-funaltrexamine (n = 6). Forty-eight hours after the injection, animals were given ad libitum access to both a low-fat and a high-fat diet. Food intake was measured 2 h after food was provided to the animals.
Materials
β-Funaltrexamine was purchased from Sigma-Aldrich (St. Louis, MO). Primers were designed using Primer Express (Applied Biosystems). The following primers were used for μ-opioid receptor (MOR-forward)-gTAgTgggccTcTTcggAAAc; (MOR-reverse)-gTTggTggCAgTcTTcATTTTg; κ-opioid receptor (KOR-forward)-TCAGGGAAGATGTGGATGTCATT; (KOR-reverse)-TGAAGAGGTCCCACCAGGAA; δ-opioid receptor (DOR-forward)-TGGGTCTTGGCTTCAGGTGT; (DOR-reverse)-CGTGCATACCACTGCTCCAT. The primers used for cyclophillin were: (cyclophillin-forward)-CCCACCGTGTTCTTCGACAT, (cyclophillin-reverse)-CTGTCTTTGGAACTTTGTCTGCAA.
Statistical Analysis
Data are presented as means ± SE. Statistical analyses were performed using Graphpad software (Graphpad, LaJolla, CA). Results were analyzed by using two-way ANOVA for repeated measures or by using t-tests where appropriate. Differences were considered statistically significant at P ≤ 0.05.
RESULTS
Experiment 1: Food Intake and Dietary Preference of Animals Deprived of Food for 48, 24, or 12 h in the Light or 12 h in the Dark
Animals that are food deprived for 48, 24, or 12 h in the light, or 12 h in the dark prefer to eat a high-fat diet. When food-deprived animals were given a choice between a low-fat and a high-fat diet, the animals ate significantly more of the high-fat diet (P < 0.001) (Figs. 1, 2, 3, and 4). There was not a significant difference in the type of diet the ad libitum-fed animals consumed.
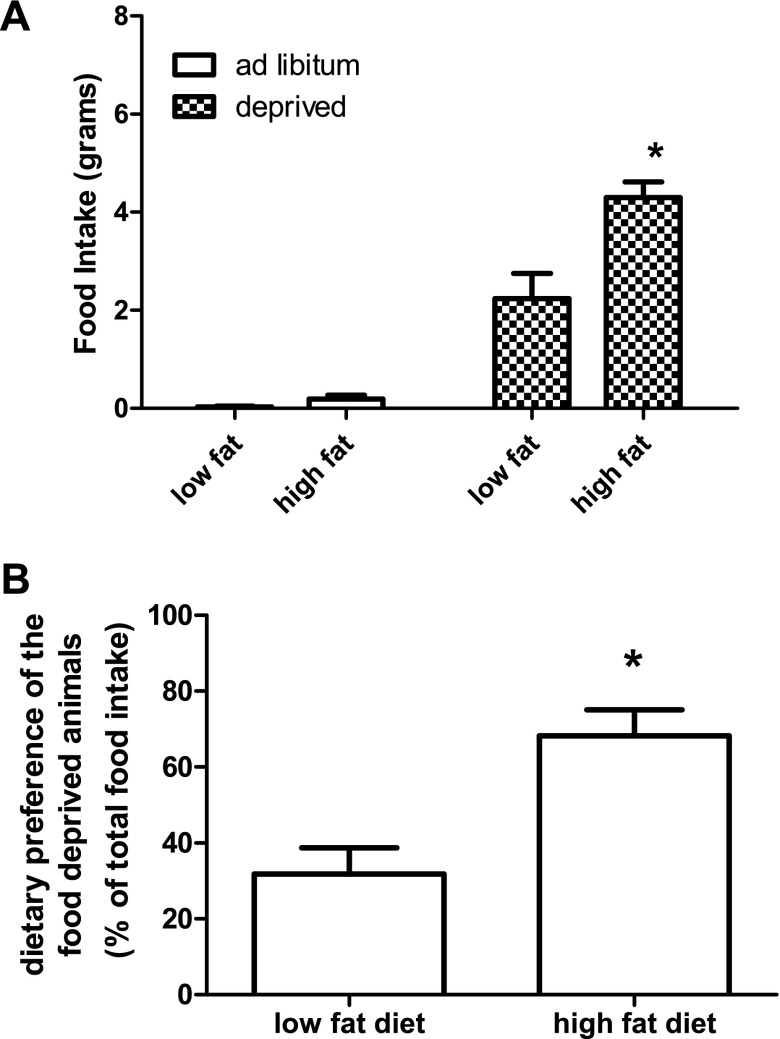
Fig. 1.
Food intake and dietary preference of animals food deprived for 48 h. Food intake is represented in grams. Ad libitum-fed animals did not eat significantly more of one diet compared with the other. Animals that were food deprived ate significantly more of the high-fat diet compared with the low-fat control. Values are expressed as means ± SE. *P ≤ 0.05.
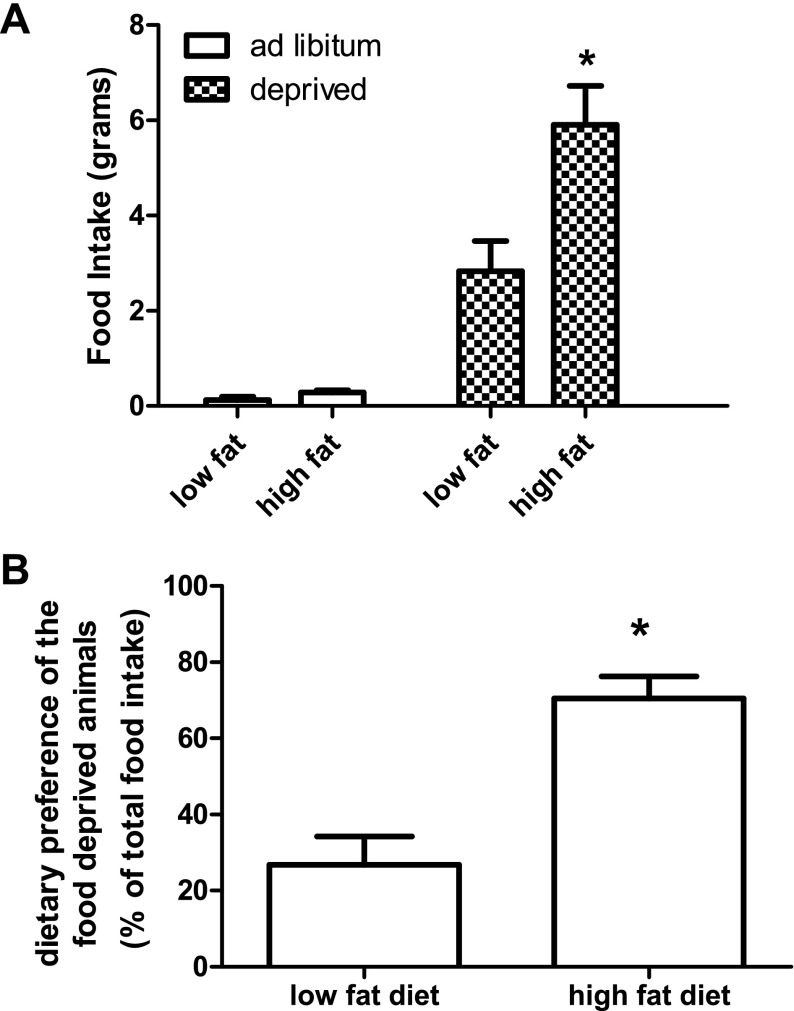
Fig. 2.
Food intake and dietary preference of animals food deprived for 24 h. Food intake is represented in grams. Ad-libitum fed animals did not eat significantly more of one diet compared with the other. Animals that were food deprived ate significantly more of the high-fat diet compared with the low-fat control. Values are expressed as means ± SE. *P ≤ 0.05.
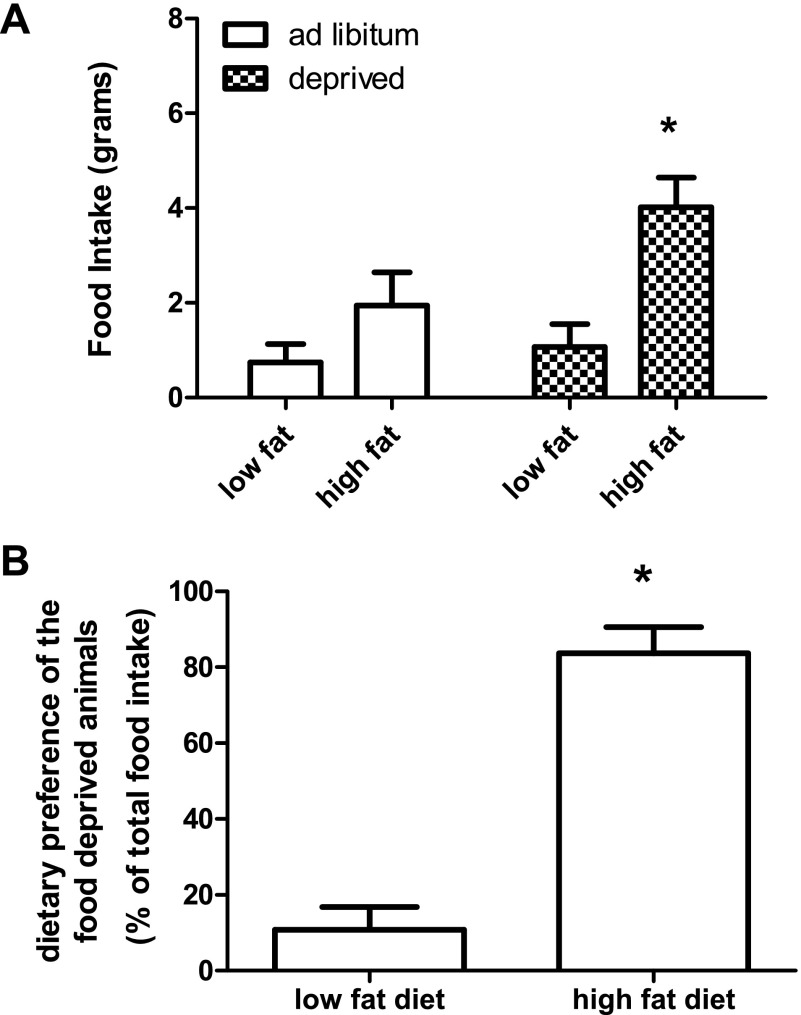
Fig. 3.
Food intake and dietary preference of animals food deprived for 12 h in the light. Food intake is represented in grams. Ad libitum-fed animals did not eat significantly more of one diet compared with the other. Animals that were food deprived ate significantly more of the high-fat diet compared with the low-fat control. Values are expressed as means ± SE. *P ≤ 0.05.
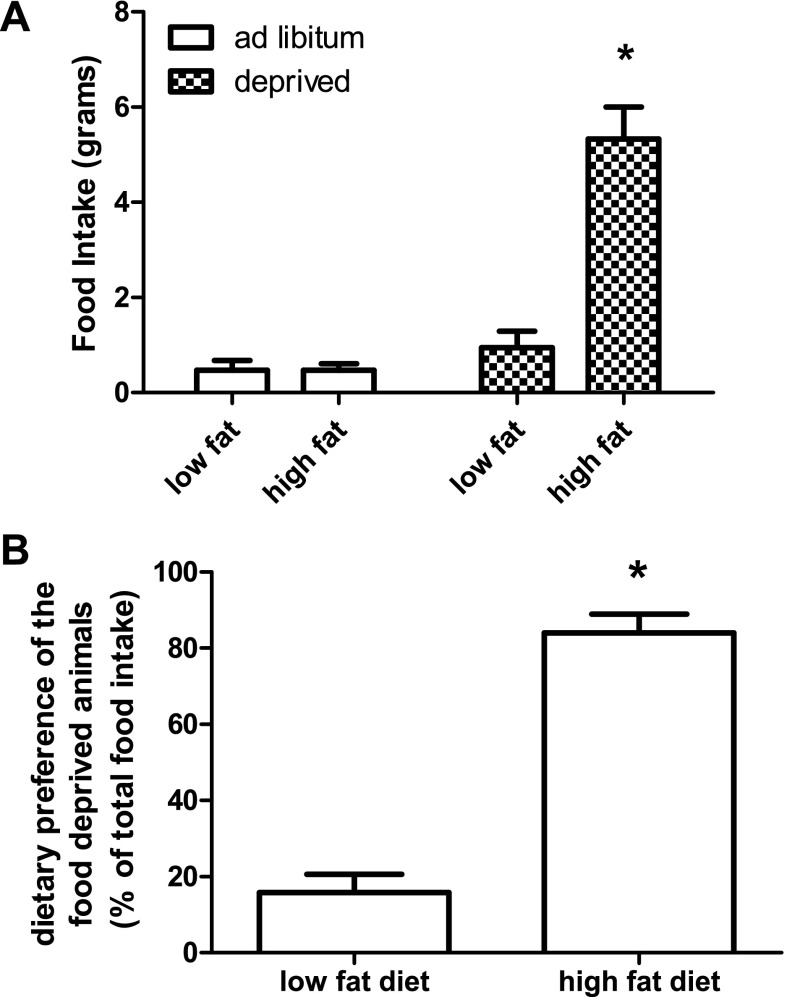
Fig. 4.
Food intake and dietary preference of animals food deprived for 12 h in the dark. Food intake is represented in grams. ad libitum fed animals did not eat significantly more of one diet compared with the other. Animals that were food deprived ate significantly more of the high-fat diet compared with the low-fat control. Values are expressed as means ± SE. *P ≤ 0.05.
Experiment 2: mRNA Expression of μ-, κ-, and δ-Opioid Receptors in Ad Libitum-Fed and Food-Deprived Animals
Table 1 shows the mRNA expression of μ-, κ-, and δ-opioid receptors, respectively, of animals that were food deprived for 48 h. mRNA expression of μ-opioid receptors was significantly increased in the VMH/ARC (P = 0.0290) and LH (P = 0.0194). The mRNA expression of μ-opioid receptors in the PVN was not statistically different. Forty-eight-hour food deprivation did increase the mRNA expression of κ-opioid receptors in the LH (P = 0.0186).
Table 1.
mRNA expression of μ-, κ-, and δ-opioid receptors within the ventral medial hypothalamus/arcuate nucleus (VMH/ARC), lateral hypothalamus (LH), and paraventricular nucleus (PVN) of animals that had ad libitum access to diet or were food deprived for 48 h
| Brain Site | Mean | P Value | |
|---|---|---|---|
| μ-Opioid Receptors | |||
| Ad libitum | VMH/ARC | 0.81±0.09 | |
| Deprived | VMH/ARC | 1.13±0.09 | 0.03 |
| Ad libitum | LH | 1.19±0.03 | |
| Deprived | LH | 1.37±0.06 | 0.01 |
| Ad libitum | PVN | 2.64±0.28 | |
| Deprived | PVN | 2.27±0.14 | 0.12 |
| κ-Opioid Receptors | |||
| Ad libitum | VMH/ARC | 0.99±0.09 | |
| Deprived | VMH/ARC | 1.08±0.21 | 0.75 |
| Ad libitum | LH | 0.60±0.05 | |
| Deprived | LH | 0.78±0.05 | 0.02 |
| Ad libitum | PVN | 0.23±0.03 | |
| Deprived | PVN | 0.42±0.09 | 0.09 |
| δ-Opioid Receptors | |||
| Ad libitum | VMH/ARC | 1.36±0.26 | |
| Deprived | VMH/ARC | 1.36±0.22 | 0.99 |
| Ad libitum | LH | 1.499±0.14 | |
| Deprived | LH | 1.39±0.09 | 0.56 |
| Ad libitum | PVN | 0.53±0.05 | |
| Deprived | PVN | 0.67±0.15 | 0.44 |
Values are expressed as means ± SE. Gene expression of μ-opioid receptors in food-deprived animals was significantly greater than the ad libitum-fed group within the VMH/ARC and LH. mRNA expression of κ-opioid receptor was significantly greater in the LH. No change was observed in mRNA expression of δ-opioid receptors. P ≤ 0.05 vs. ad libitum.
Fasting rats for 24 h, 12 h in the light, or 12 h in the dark did not change the mRNA expression of μ-, κ-, and δ-opioid receptors (Tables 2, 3, 4).
Table 2.
mRNA expression of μ-, κ-, and δ-opioid receptors in the VMH/ARC, LH, and PVN of animals that had ad libitum access to their diets or were food deprived for 24 h
| Brain Site | Mean | P Value | |
|---|---|---|---|
| μ-Opioid Receptors | |||
| Ad libitum | VMH/ARC | 0.77±0.12 | |
| Deprived | VMH/ARC | 1.05±0.17 | 0.26 |
| Ad libitum | LH | 1.33±0.13 | |
| Deprived | LH | 1.66±0.08 | 0.14 |
| Ad libitum | PVN | 1.75±0.33 | |
| Deprived | PVN | 1.74±0.21 | 0.98 |
| κ-Opioid Receptors | |||
| Ad libitum | VMH/ARC | 1.07±0.08 | |
| Deprived | VMH/ARC | 1.02±0.11 | |
| Ad libitum | LH | 0.64±0.06 | |
| Deprived | LH | 0.81±0.05 | 0.16 |
| Ad libitum | PVN | 0.44±0.11 | |
| Deprived | PVN | 0.32±0.09 | |
| δ-Opioid Receptors | |||
| Ad libitum | VMH/ARC | 1.25±0.18 | |
| Deprived | VMH/ARC | 1.74±0.11 | 0.05 |
| Ad libitum | LH | 1.05±0.04 | |
| Deprived | LH | 1.23±0.11 | 0.14 |
| Ad libitum | PVN | 0.49±0.13 | |
| Deprived | PVN | 0.62±0.12 | 0.57 |
Values are means ± SE. Gene expression of μ-, κ-, and δ-opioid receptors was not significantly different between the ad libitum and the food-deprived group.
Table 3.
mRNA expression of in the VMH/ARC, LH, and PVN of animals that had ad libitum access to their diets or were food deprived for 12 h in the light
| Brain Site | Mean | P Value | |
|---|---|---|---|
| μ-Opioid Receptors | |||
| Ad libitum | VMH/ARC | 1.08±0.11 | |
| Deprived | VMH/ARC | 0.79±0.14 | 0.12 |
| Ad libitum | LH | 1.31±0.08 | |
| Deprived | LH | 1.16±0.06 | 0.19 |
| Ad libitum | PVN | 2.15±0.46 | |
| Deprived | PVN | 2.05±0.22 | 0.82 |
| κ-Opioid Receptors | |||
| Ad libitum | VMH/ARC | 0.97±0.08 | |
| Deprived | VMH/ARC | 0.84±0.11 | 0.34 |
| Ad libitum | LH | 0.77±0.05 | |
| Deprived | LH | 0.73±0.05 | 0.61 |
| Ad libitum | PVN | 0.39±0.07 | |
| Deprived | PVN | 0.51±0.09 | 0.38 |
| δ-Opioid Receptors | |||
| Ad libitum | VMH/ARC | 0.82±0.07 | |
| Deprived | VMH/ARC | 1.38±0.31 | 0.09 |
| Ad libitum | LH | 0.93±0.08 | |
| Deprived | LH | 0.90±0.07 | 0.81 |
| Ad libitum | PVN | 0.32±0.06 | |
| Deprived | PVN | 0.37±0.06 | 0.55 |
Values are means ± SE. Gene expression of μ-, κ-, and δ-opioid receptors was not significantly different between the ad libitum and the food-deprived group.
Table 4.
mRNA expression in the VMH/ARC, LH, and PVN of animals that had ad libitum access to their diets or were food deprived for 12 h in the dark
| Brain Site | Mean | P Value | |
|---|---|---|---|
| μ-Opioid Receptors | |||
| Ad libitum | VMH/ARC | 0.75±0.06 | |
| Deprived | VMH/ARC | 0.73±0.09 | 0.88 |
| Ad libitum | LH | 1.24±0.09 | |
| Deprived | LH | 1.11±0.09 | 0.32 |
| Ad libitum | PVN | 1.63±0.08 | |
| Deprived | PVN | 1.40±0.12 | 0.14 |
| κ-Opioid Receptors | |||
| Ad libitum | VMH/ARC | 0.76±0.05 | |
| Deprived | VMH/ARC | 0.72±0.05 | 0.55 |
| Ad libitum | LH | 0.72±0.04 | |
| Deprived | LH | 0.69±0.05 | 0.61 |
| Ad libitum | PVN | 0.33±0.05 | |
| Deprived | PVN | 0.37±0.05 | 0.59 |
| δ-Opioid Receptors | |||
| Ad libitum | VMH/ARC | 0.61±0.04 | |
| Deprived | VMH/ARC | 0.67±0.04 | 0.28 |
| Ad libitum | LH | 0.53±0.03 | |
| Deprived | LH | 0.52±0.04 | 0.83 |
| Ad libitum | PVN | 0.21±0.03 | |
| Deprived | PVN | 0.16±0.02 | 0.14 |
Values are means ± SE. Gene expression of μ-, κ-, and δ-opioid receptors was not significantly different between the ad libitum and the food-deprived group.
Experiment 3: Effect of β-Funaltrexamine (Selective μ-Opioid Receptor Antagonist) on High-Fat Intake of Animals Food Deprived for 48 h
Administering β-funaltrexamine into the third ventricle of food-deprived animals resulted in a significant decrease in the percentage of high-fat diet consumed. The percentage of high-fat diet consumed decrease from 82% to 41% (P = 0.043).
DISCUSSION
In this paper, we present the novel observation that food deprivation results in a significant increase in the gene expression of hypothalamic μ-opioid receptors. Using real-time-PCR, we investigated the effect of food deprivation at 48, 24, 12 h in the light, or 12 h in the dark on the gene expression of μ-opioid receptors in three hypothalamic sites. Our data demonstrate that food deprivation for 48 h, but not shorter periods, can increase the density of μ-opioid receptors in the VMH/ARC and LH. We did not observe a change in the mRNA expression of μ-opioid receptors in the PVN.
The change in the mRNA expression of μ-opioid receptors in the VMH/ARC and the LH is supported by work that utilized c-Fos immunohistochemistry to localize cells that are mediated by an opioid antagonist under conditions of food deprivation (8). That data suggest that cells within the arcuate nucleus and lateral hypothalamus are under opioid-mediated inhibitory control during food restriction.
A couple of factors can contribute to the increased μ-opioid receptors we observed at 48 h. First of all, upregulation of μ-opioid receptors can occur between 1 h and several days (10). Demonstrations of the upregulation of μ-opioid receptors have occurred as a result of utilizing agonist and antagonist to this receptor population. Our findings are intriguing because we observed an increase in μ-opioid receptors simply by depriving animals of food for 48 h. The possibility exists that the increase of μ-opioid receptors is potentiating the animal's hyperphagia and desire to acquire a high-fat diet.
Another factor that could contribute to our observation is the change in β-endorphin levels that occur in food-deprived animals. β-Endorphin is located in neuronal populations that are located in the arcuate nucleus and the caudal nucleus tractus solitarus. This opioid peptide, which is derived after the breakdown of the polypeptide proopiomelanocortin, has high affinity for the μ-opioid receptor.
The duration of food deprivation plays an important role in the level of β-endorphin that is in the circulation. Majeed et al., (28) found that 24 h of food deprivation increased β-endorphin in the hypothalamus. However, findings by Bi et al., (5), Knuth and Friesen (22), and Kim et al., (21) demonstrated that food deprivation for 48 h or longer decreased the gene expression of β-endorphin and its precursor proopiomelanocortin. We hypothesize that we did not observe an increase in μ-opioid receptors during the 24 or 12 h in the light, or 12 h in the dark food deprivation, because the ligand for μ-opioid receptors was increased. Thus, the μ-opioid receptors were being activated by the increased levels of β-endorphin. However, after extended food deprivation (i.e., 48 h), β-endorphin levels decreased, and as a consequence, the population of μ-opioid receptors increased.
We also investigated the effect of food deprivation on δ- and κ-opioid receptors. δ-Opioid receptors are located predominately in intracellular compartments of neurons within the central nervous system that are related to pain. Food deprivation did not have any effect on the mRNA expression of δ-opioid receptors.
κ-Opioid receptors have been implicated in the control of food intake (15). However, our data demonstrated that food deprivation did not have an effect on the mRNA expression of κ-opioid receptors except in the lateral hypothalamus after 48 h of food deprivation. A lack of a change of κ-opioid receptors is supportive of work by Hadjimarkou et al., (18). Their work demonstrated that selective antagonists to κ-opioid receptors did not reduce deprivation-induced intake.
The feeding behavior of animals food deprived within each of our experimental designs was consistent. Food deprivation made the animals hyperphagic and significantly increased their preference for a high-fat diet. To determine whether μ-opioid receptors played a role in the animals feeding behavior, we administered β-funaltrexamine (μ-opioid receptor antagonist) to animals that were food deprived for 48 h. When we reintroduced the low-fat and high-fat diets, the preference for high-fat diet was attenuated in the animals that were pretreated with β-funaltrexamine. This data suggest that μ-opioid receptors play a role in the hyperphagia and diet preference that is associated with food deprivation.
Perspectives and Significance
Food deprivation initiates a series of events within the central nervous system to reestablish a homeostatic environment. These events include, but are not limited to, an increase in orexigenic peptide (i.e., NPY) and a decrease in anorexic peptide (i.e., proopiomelanocortin) and hormone (i.e., leptin). Data from this manuscript provide another event that occurs in food-deprived animals, which could help to reestablish homeostasis. Increases in the mRNa expression of μ-opioid receptors after prolonged fastings is significant because activation of this receptor population makes animals hyperphagic and increases their preference for a high-fat diet.
GRANT
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R34-DK-32089.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arjune D, Bowen WD, Bodnar RJ. Ingestive behavior following central [D-Ala2,Leu5,Cys6]-enkephalin (DALCE), a short-acting agonist and long-acting antagonist at the delta opioid receptor. Pharmacol Biochem Behav 39: 429–436, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Banks AS, Davis SM, Bates SH, Myers MG Jr. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 275: 14563–14572, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Barnes MJ, Holmes G, Primeaux SD, York DA, Bray GA. Increased expression of μ-opioid receptors in animals susceptible to diet-induced obesity. Peptides 27: 3292–3298, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Baslom DG, Breomomger JF, Schwartz MW. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes 48: 828–833, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol 285: R1030–R1036, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Bihua B, Zhizhong ZP. Trafficking of central opioid receptors and descending pain inhibition. Mol Pain 3: 37–44, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DR, Holtzman SG. Suppression of deprivation-induced food and water intake in rats and mice by naloxone. Pharmacol Biochem Behav 11: 567–573, 1979. [DOI] [PubMed] [Google Scholar]
- 8.Calcagnetti DJ, Reid LD. Morphine and acceptability of putative reinforcers. Pharmacol Biochem Behav 18: 567–567, 1983. [DOI] [PubMed] [Google Scholar]
- 9.Carr KD, Park TH, Yi Z, Stone EA. Neuroanatomical patterns of Fos-like immunoreactivity induced by naltrexone in food-restricted and ad libitum fed rats. Brain Res 779: 26–32, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Collin E, Cesselin F. Neurobiological mechanisms of opioid tolerance and dependence. Clin Neuropharmacol 14: 465–488, 1991. [DOI] [PubMed] [Google Scholar]
- 11.Cooper SJ Naloxone: effects on food and water consumption in the non-deprived and deprived rat. Psychopharmacology 71: 1–6, 1980. [DOI] [PubMed] [Google Scholar]
- 12.Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol 367: 375–402, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Frederich RC, Lollmann B, Hamann A, Napolitano-Rosen A, Kahn BB, Lowell BB, Lowell BB, Flier JS. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest 96: 1658–1663, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frenk H, Rogers GH. The suppressant effects of naloxone on food and water intake in the rat. Behav Neural Biol 26: 23–41, 1979. [DOI] [PubMed] [Google Scholar]
- 15.George SR, Zastawny RL, Briones-Urbina R, Cheng R, Nguyen T, Heiber M, Kouvelas A, Chan AS, O'Dowd BF. Distinct distributions of mu, delta and kappa opioid receptor mRNA in rat brain. Biochem Biophys Res Commun 205: 1438–1444, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Glass MJ, Billington CJ, Levine AS. Opioid and food intake: distributed functional neuronal pathways? Neuropeptides 33: 360–368, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Gong Y, Ishida-Takahashi R, Villanueva EC, Finger DC, Munzberg H, Myers MG Jr. The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 vial alternate mechanism. J Biol Chem 282: 31019–31027, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Hadjimarkou MM, Khaimova E, Pan YX, Rossi GC, Pasternak GW, Bodnar RJ. Feeding induced by food deprivation is differentially reduced by opioid receptor antisense oligodeoxynucleotide probes in rats. Brain Res 987: 223–232, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Colocalization of agouti-related protein and neuropeptide Y in arcuate nucleus neurons activated by fasting. Nat Neurosci 1: 271–272, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Hakansson ML, Meister B. Transcription factor STAT3 in leptin target neurons of the rat hypothalamus. Neuroendocrinology 68: 420–427, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Kim EM, Welch CC, Grace MK, Billington CJ, Levine AS. Chronic food restriction and acute food deprivation decrease mRNA levels of opioid peptides in arcuate nucleus. Am J Physiol Regul Integr Comp Physiol 270: R1019–R1024, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Knuth UA, Friesen HG. Changes of β-endorphin and somatostatin concentrations in different hypothalamic areas of female rats after chronic starvation. Life Sci 33: 827–833, 1983. [DOI] [PubMed] [Google Scholar]
- 23.Koch JE, Bodnar RJ. Selective alterations in macronutrient intake of food-deprived or glucoprivic rats by centrally-administered opioid receptor subtype antagonists in rats. Brain Res 657: 191–201, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Korner J, Savontaus E, Chua SC Jr, Leibel RL, Wardlaw SL. Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J Neuroendocrinol 13: 959–966, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Larue-Achagiotis C, Thouzeau C. Refeeding after prolonged fasting in rats: nycthemeral variations in dietary self-selection. Physiol Behav 59: 1033–1037, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Lax P, Larue-Achagiotis C, Martel P, Madrid JA, Verger P. Repeated short-fasting modifies the macronutrient self-selection pattern in rats. Physiol Behav 65: 69–76, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Levine AS, Grace M, Billington CJ. B-funaltrexamine (B-FNA) decreases deprivation and opioid-induced feeding. Brain Res 562: 281–284, 1991. [DOI] [PubMed] [Google Scholar]
- 28.Majeed NH, Lason W, Przewlocka B, Przewlocki R. Brain and peripheral opioid peptides after changes ingestive behavior. Neuroendocrinology 42: 267–272, 1986. [DOI] [PubMed] [Google Scholar]
- 29.Mansour A, Khachaturian H, Lewis ME, Akil HK, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci 7: 2445–2465, 1987. [PMC free article] [PubMed] [Google Scholar]
- 30.Marks-Kaufman R, Kanarek RB. Diet selection following a chronic morphine and naloxone regimen. Pharmacol Biochem Behav 35: 665–669, 1990. [DOI] [PubMed] [Google Scholar]
- 31.Marks-Kaufman R, Kanarek RB. Morphine selectively influences macronutrient intake in the rat. Pharmacol Biochem Behav 12: 427–430, 1980. [DOI] [PubMed] [Google Scholar]
- 32.Mazarakou G, Georgoussi Z. STAT5A interacts with and is phosphorylated upon activation of the mu-opioid receptor. J Neurochem 93: 918–931. [DOI] [PubMed]
- 33.Morton GJ, Schwartz MW. The NPY/AgRP neuron and energy homeostasis. Int J Obes Relat Metab Disord 25, Suppl 5: S56–S62, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Mutze J, Roth J, Gerstberger R, Huschle T. Nuclear translocation of the transcription factor STAT5 in the rat brain after systemic leptin administration. Neurosci Lett 417: 286–291, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The Rat Brain in Steretoxic Coordinates (2nd ed.). New York: Academic, 1986.
- 36.Schwartz MW, Erickson JC, Baskin DG, Palmiter RD. Effect of fasting and leptin deficiency on hypothalamic neuropeptide Y gene transcription in vivo revealed by expression of a lacZ reporter gene. Endocrinology 139: 2629–2635, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Simone DA, Bodnar RJ, Goldman EJ, Pasternak GW. Involvement of opioid receptor subtypes in rat feeding behavior. Life Sci 36: 829–833, 1985. [DOI] [PubMed] [Google Scholar]
- 38.Smith BK, Berthoud HR, York DA, Bray GA. Differential effects of baseline macronutrient preferences on macronutrient selection after galanin, NPY, and an overnight fast. Peptides 18: 207–211, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Tartaglia LA The leptin receptor. J Biol Chem 272: 6093–6096, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Welch CC, Grace MK, Billington CJ, Levine AS. Preference and diet type affect macronutrient selection after morphine, NPY, norepinephrine, and deprivation. Am J Physiol Regul Integr Comp Physiol 266: R426–R433, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Wilson BD, Bagnol D, Kaelin CB, Ollmann MM, Gantz I, Watson SJ, Barsh GS. Physiological and anatomical circuitry between Agouti-related protein and leptin signaling. Endocrinology 140: 2387–2397, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Will MJ, Vanderheyden WM, Kelley AE. Striatal opioid peptide gene expression differentially tracks short-term satiety but does not vary with negative energy balance in a manner opposite to hypothalamic NPY. Am J Physiol Regul Integr Comp Physiol 292: R217–R226, 2007. [DOI] [PubMed] [Google Scholar]