Abstract
Both endogenous and exogenous estrogen decrease pulmonary artery (PA) vasoconstriction. Whether these effects are mediated via estrogen receptor (ER)-α or ER-β, and whether the contribution of ERs is stimulus-dependent, remains unknown. We hypothesized that administration of the selective ER-α agonist propylpyrazole triol (PPT) and/or the selective ER-β agonist diarylpropiolnitrile (DPN) rapidly decreases PA vasoconstriction induced by pharmacologic and hypoxic stimuli via a nitric oxide (NO)-dependent mechanism. PA rings (n = 3–10/group) from adult male Sprague-Dawley rats were suspended in physiologic organ baths. Force displacement was measured. Vasoconstrictor responses to phenylephrine (10−8M − 10−5M) and hypoxia (Po2 35–45 mmHg) were determined. Endothelium-dependent and -independent vasorelaxation were measured by generating dose-response curves to acetylcholine (10−8M − 10−4M) and sodium nitroprusside (10−9M − 10−5M). PPT or DPN (10−9M − 5 × 10−5M) were added to the organ bath in the presence and absence of the NO-synthase inhibitor Nω-nitro-l-arginine methyl ester (l-NAME) (10−4M). Selective ER-α activation (PPT, 5 × 10−5M) rapidly (<20 min) decreased phenylephrine-induced vasoconstriction. This effect, as well as PPT's effects on endothelium-dependent vasorelaxation, were neutralized by l-NAME. In contrast, selective ER-β activation (DPN, 5 × 10−5M) rapidly decreased phase II of hypoxic pulmonary vasoconstriction (HPV). l-NAME eliminated this phenomenon. Lower PPT or DPN concentrations were less effective. We conclude that both ER-α and ER-β decrease PA vasoconstriction. The immediate onset of effect suggests a nongenomic mechanism. The contribution of specific ERs appears to be stimulus specific, with ER-α primarily modulating phenylephrine-induced vasoconstriction, and ER-β inhibiting HPV. NO inhibition eliminates these effects, suggesting a central role for NO in mediating the pulmonary vascular effects of both ER-α and ER-β.
Keywords: propylpyrazole triol, diarylpropiolnitrile, phenylephrine, hypoxic pulmonary vasoconstriction, nongenomic effects
female sex is increasingly recognized as a protective factor in patients suffering from trauma and sepsis (12, 16). The critical role of sex hormones has recently been recognized. In particular, 17β-estradiol (E2) has been shown to improve outcomes in experimental trauma hemorrhage and shock (13, 14, 41), sepsis (5, 7, 12), myocardial ischemia-reperfusion (39, 40), and acute lung injury (30, 35, 42). The protective effects of E2 are mediated via estrogen receptor (ER)-α and ER-β. The effects of these ER subtypes are tissue and compartment specific. For example, ER-α decreases proinflammatory cytokine production by splenic macrophages and Kupffer cells after trauma-hemorrhage (33, 34). On the other hand, ER-β decreases inflammatory markers and mortality in shock-induced lung injury (5, 42).
In this context, the recently discovered nongenomic effects of estrogen have gained much attention (21). In contrast to the well-described genomic effects, which occur at a transcriptional level and which take hours to days to develop, nongenomic mechanisms use existing proteins and signaling pathways, therefore taking only seconds to minutes to mediate their effects.
Estrogens have also been shown to affect the pulmonary vasculature (18). In fact, both endogenous and exogenous estrogen decrease pulmonary artery (PA) vasoconstriction under normoxic as well as hypoxic conditions (8, 19). We have recently demonstrated that exogenous E2 acutely decreases PA vasoconstriction through a rapid and therefore most likely nongenomic mechanism (17).
However, it is unknown whether the acute pulmonary vascular effects of E2 are mediated by ER-α or ER-β. This is of importance, since a better mechanistic understanding of the pulmonary vascular effects of E2 may allow for future nonhormonal, targeted, therapeutic interventions in pulmonary hypertension and acute lung injury. In addition, better insights into pulmonary vascular ER signaling may improve our current understanding of why acute and chronic hypoxic pulmonary hypertension are less common and less pronounced in females, while idiopathic pulmonary arterial hypertension (iPAH) is more prevalent in the female population (18, 20, 27).
In the systemic vasculature, both ER-α and ER-β have been demonstrated to mediate the vasodilator effects of estrogen (26, 43). This effect is mediated by genomic as well as nongenomic mechanisms, some of which include increases in local nitric oxide (NO) release (28). While the exact roles of ER-α and ER-β in the systemic vasculature have not been entirely characterized, data regarding the pulmonary vasculature are sparse and conflicting (3, 4, 11). For example, Hisamoto et al. (11) suggested that E2 activates endothelial NO synthase (eNOS) through ER-α and not through ER-β, while Chambliss et al. (3) demonstrated that ER-β is indeed capable of activating eNOS. This discrepancy may be due to the use of different cell populations and differences in experimental settings. Furthermore, the contribution of ER-α and ER-β to the regulation of PA tone during hypoxia remains unknown. Only recently have selective ER agonists become available, allowing for detailed analysis of the role of ER-α and ER-β in the pulmonary vasculature. In particular, the effects of selective ER agonists on acute hypoxic pulmonary vasoconstriction (HPV) have not yet been investigated. It also remains unknown whether the contribution of ER-α and ER-β differs between hypoxic and nonhypoxic stimuli.
We therefore hypothesized that the administration of selective ER-α and/or ER-β agonists acutely decreases PA vasoconstriction induced by pharmacologic and hypoxic stimuli. In addition, we hypothesized that selective ER agonists exert their effects through a NO-dependent mechanism.
To test our hypotheses, we measured isometric force displacement in isolated PA rings from adult male Sprague-Dawley rats. Our objectives were 1) to determine whether the selective ER-α agonist propylpyrazole triol (PPT) rapidly decreases phenylephrine (PE)- and hypoxia-induced PA vasoconstriction, 2) to investigate whether the selective ER-β agonist diarylpropiolnitrile (DPN) rapidly decreases PE- and hypoxia-induced PA vasoconstriction, and 3) to measure whether the effects of PPT and DPN are attenuated in the presence of the NOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME).
MATERIALS AND METHODS
Animals.
All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication no. 85-23, revised 1985). All of the animal protocols were approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine. Adult age-matched male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 225–350 g were allowed ad libitum access to food and water up to the time of experimentation. All animals were cared for in a nonstressful environment for at least 1 wk prior to experimentation.
Isolated PA ring preparation.
Rats were anesthetized with intraperitoneal injections of pentobarbital (150 mg/kg). Median sternotomy was performed, and the heart and lungs were removed en bloc and placed in modified Krebs-Henseleit solution at 4°C. Under a dissecting microscope, extralobar PA branches were dissected out and cleared of surrounding tissue. The right and left main branches were cut into 2- to 3-mm wide rings and suspended on steel hooks connected to force transducers (ADInstruments, Colorado Springs, CO) for isometric force measurement. Care was taken during the entire process to avoid injury to the endothelium. PA rings were immersed in individual water-jacketed organ chambers containing modified Krebs-Henseleit solution bubbled with 95% O2-5% CO2 at 37°C. Force displacement was recorded using a PowerLab (ADInstruments) eight-channel data recorder on an Apple iMac PowerPC G4 Computer (Apple Computer, Cupertino, CA).
Experimental protocol and groups.
Before starting experimental protocols, the PA rings were stretched to a predetermined optimal passive tension of 750 mg. The rings were allowed to equilibrate for 60 min, during which time the Krebs-Henseleit solution was changed every 15 min. Viability of PA rings was determined by measuring maximum contractile response to 8 × 10−2 M of KCl. The dosage of KCl was determined to produce maximal contractile response in previous experiments. After KCl washout, the integrity of each PA endothelium was evaluated by dilation with acetylcholine (ACh; 10−6 M) after PE (10−6 M) precontraction. Rings demonstrating < 200 mg contraction to PE were discarded. In endothelium-intact PA, rings demonstrating < 50% vasorelaxation to ACh were discarded as well. After washout of ACh, PA rings were allowed to equilibrate. After having established viability and endothelial integrity, and following equilibration, PA rings were exposed to pharmacologically or hypoxia-induced vasoconstriction, respectively. Experimental groups consisted of PA rings treated with various concentrations (10−9 M, 10−6 M, 5 × 10−5 M) of the selective ER-α agonist PPT or the selective ER-β agonist DPN. To compare the rapid effects of the selective ER-α and ER-β agonists with those of E2, PA rings were also treated with 5 × 10−4 M of E2. This concentration was shown to be effective in previous experiments (17). Control groups consisted of untreated and vehicle-treated animals.
Pharmacologic vasoconstriction.
To investigate the rapid effects of selective ER-agonists on PE-induced vasoconstriction, ER-agonists were added to the organ bath 10 min prior to PE (10−6 M). Dose-response curves for PPT and DPN (10−9 M, 10−6 M, 5 × 10−5 M) were generated. To investigate whether the pulmonary vascular effects of the selective ER-agonists are mediated by a NO-dependent mechanism, separate vasoreactivity experiments were performed in the presence and absence of the NOS inhibitor l-NAME (10−4 M). l-NAME was added to the organ bath 30 min prior to the ER-agonist. l-NAME concentration and timing of administration were based on previous experiments (37). PA vasoreactivity was investigated by generating dose-response curves to PE (10−8 M − 10−5 M). The ER agonist was added to the organ bath 10 min prior to PE. After PE-precontraction, endothelium-dependent and -independent vasorelaxation were measured by generating dose-response curves to ACh (10−8 M − 10−4 M) and sodium nitroprusside (SNP; 10−9 M − 10−5 M), respectively, as described previously (38). Vasoreactivity experiments were terminated after the highest concentration of ACh or SNP, respectively.
HPV.
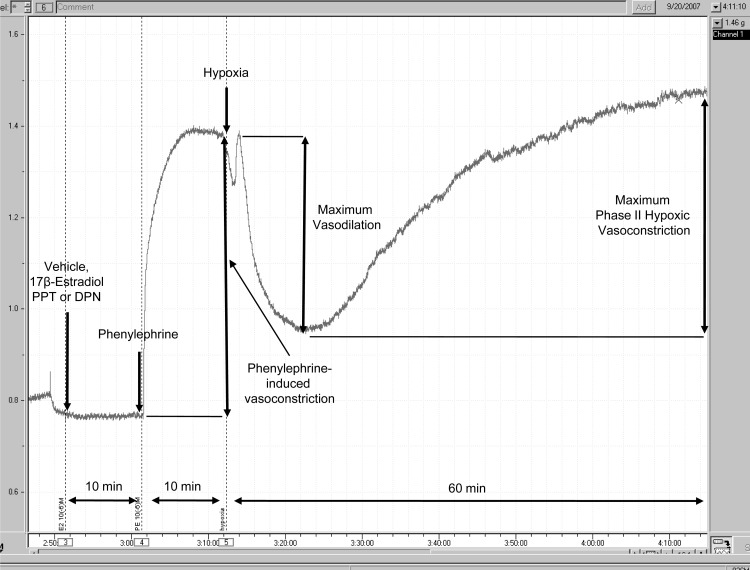
To measure the effect of hypoxia on PA rings, we gassed PE-precontracted PA rings with 95% N2-5% CO2 for 60 min. This produced a Po2 of 35–45 mmHg in the organ bath, which was measured with a blood gas analyzer (Synthesis 20; Instrumentation Laboratory, Lexington, MA). Dose-response curves for PPT and DPN (10−9 M, 10−6 M, 5 × 10−5 M) were generated. To investigate whether the vasoactive effects of ER-agonists are mediated by NO, additional experiments were performed in the presence and absence of l-NAME (10−4 M). l-NAME was added to the organ bath 30 min prior to the ER-agonist. As described in previous experiments, hypoxia caused a biphasic PA vasoconstriction: an early contraction (occurring 2–3 min after exposure to hypoxia) followed by a transient vasorelaxation and a late phase II (occurring 10–15 min after hypoxia exposure) contraction (Fig. 1). Due to its very brief and transient nature, phase I vasoconstriction was not measured. Maximum phase II vasoconstriction was measured as the difference between the highest and lowest force displacements during hypoxia and expressed as a percentage of maximum PE-precontraction. Maximum vasorelaxation was measured as the difference between PE-precontraction and the lowest force displacements during hypoxia and expressed as a percentage of maximum PE-precontraction. Hypoxia experiments were terminated after 60 min of hypoxia.
Fig. 1.
Representative pressure tracing of hypoxic pulmonary vasoconstriction (HPV) in an isolated pulmonary artery ring. Force in grams is depicted on the y-axis. Time in hours and minutes is represented on the x-axis. Pulmonary arteries precontracted using phenylephrine (PE) (10−6 M) were exposed to hypoxia (Po2 = 35–45 mmHg) for 60 min. Maximum vasorelaxation was measured as the difference between the tension measured when hypoxia was induced (PE-precontraction) and the lowest force preceding phase II HPV. Maximum phase II HPV was measured as the difference between the lowest force preceding contraction and the highest force during 60 min of hypoxia. To investigate for rapid (and, therefore, most likely nongenomic effects), vehicle, 17β-estradiol (E2), propylpyrazole triol (PPT), or diarylpropiolnitrile (DPN) were added to the organ bath 10 min prior to PE and 20 min prior to hypoxia. In the experiments investigating the effect of nitric oxide synthase (NOS)-inhibition on estrogen receptor (ER) signaling during HPV, Nω-nitro-l-arginine methyl ester (l-NAME) was added to the organ bath 30 min prior to the ER agonist.
Chemicals and reagents.
All chemical reagents were obtained from Sigma (St. Louis, MO) unless otherwise specified. PPT and DPN were obtained from Tocris Biosciences (Ellisville, MO). PPT and DPN were dissolved in ethanol (100%) according to the manufacturer's instructions. All other reagents were dissolved in deionized distilled water. Krebs-Henseleit solution is a physiologically balanced salt solution containing (in mmol/l): 127 NaCl, 4.7 KCl, 17 NaHCO3, 1.17 MgSO4, 1.18 KH2PO4, 2.5 CaCl2, and 5.5 d-glucose. The final pH of all solutions was 7.35–7.45.
Presentation of data and statistical analysis.
Force displacement after stimulation PE is expressed as percent change from baseline tension of 750 mg. Force displacement during hypoxia is expressed as percent change from the amount of PE-precontraction. All reported values are expressed as means ± SE. Experimental groups (n = 3–10/group) were compared by two-way ANOVA with post hoc Bonferroni test or Student's t-test (Prism 4; Graphpad Software, San Diego, CA). Differences at an alpha level of 0.05 (P < 0.05) were considered statistically significant.
RESULTS
ER-α agonist PPT rapidly decreases pharmacologically-induced vasoconstriction.
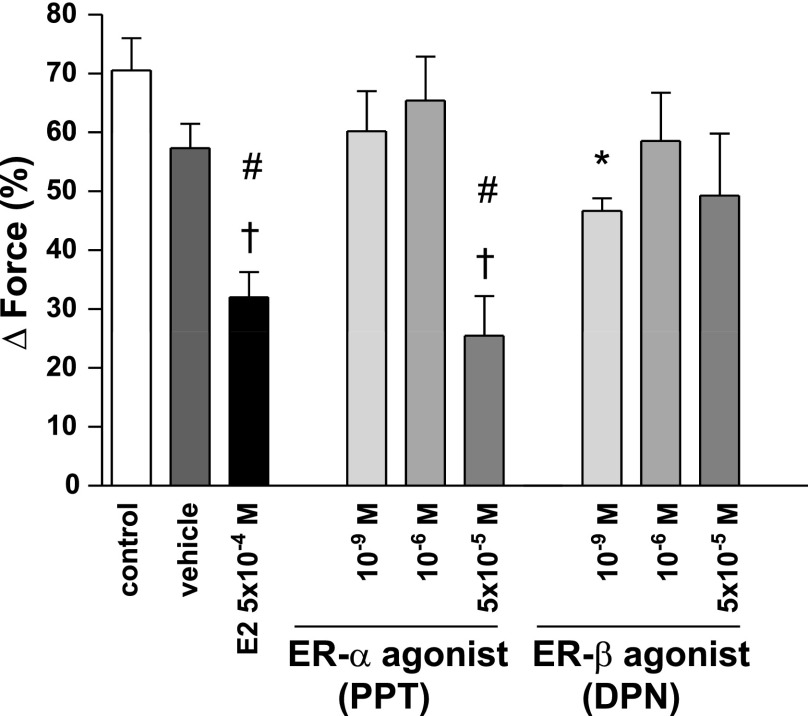
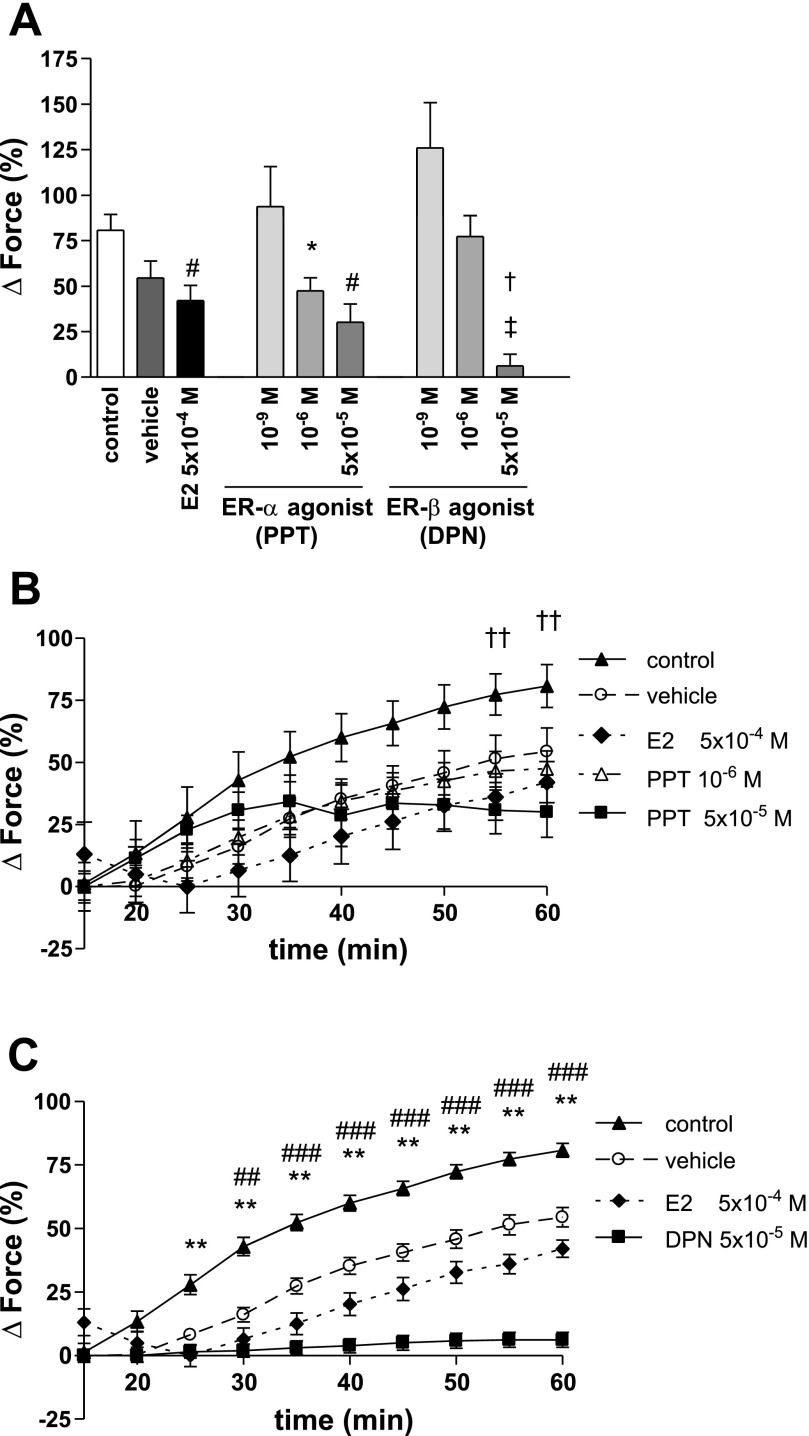
The selective ER-α agonist PPT at a 10−9 M or a 10−6 M concentration did not significantly affect PE-induced vasoconstriction compared with control or vehicle (PPT 10−9 M: 60.21 ± 6.83%; PPT 10−6 M: 65.43 ± 7.48%; control: 70.57 ± 5.43%; vehicle: 57.34 ± 4.16%) (Fig. 2). However, when given at a 5 × 10−5 M concentration, PPT significantly and rapidly (<20 min) decreased PE-mediated vasoconstriction similar to E2 (PPT 5 × 10−5 M: 25.52 ± 6.74%; E2: 32.03 ± 4.28%; P = not significant for PPT 5 × 10−5 M vs. E2). In contrast, the selective ER-β agonist DPN slightly decreased PE-mediated vasoconstriction at a 10−9 M concentration (46.67 ± 2.19%). However, this attenuation was less pronounced than that of PPT and E2 and did not differ from vehicle. DPN at higher concentrations did not affect PE-induced vasoconstriction (10−6 M: 58.55 ± 8.21%; 5 × 10−5 M: 49.32 ± 10.51%).
Fig. 2.
PE-induced vasoconstriction for PPT and DPN. Vehicle, control, and E2 are demonstrated for comparison. Force was measured immediately before the induction of hypoxia and is expressed as %change from baseline contraction. The ER-α agonist at a 5 × 10−5 M concentration rapidly decreased PE-induced vasoconstriction similar to E2. N = 4–10/group. †P < 0.001 vs. control; #P < 0.01 vs. vehicle; *P < 0.05 vs. control, E2 and PPT 5 × 10−5 M.
ER-α agonist PPT rapidly decreases pharmacologically-induced vasoconstriction by a NO-dependent mechanism.
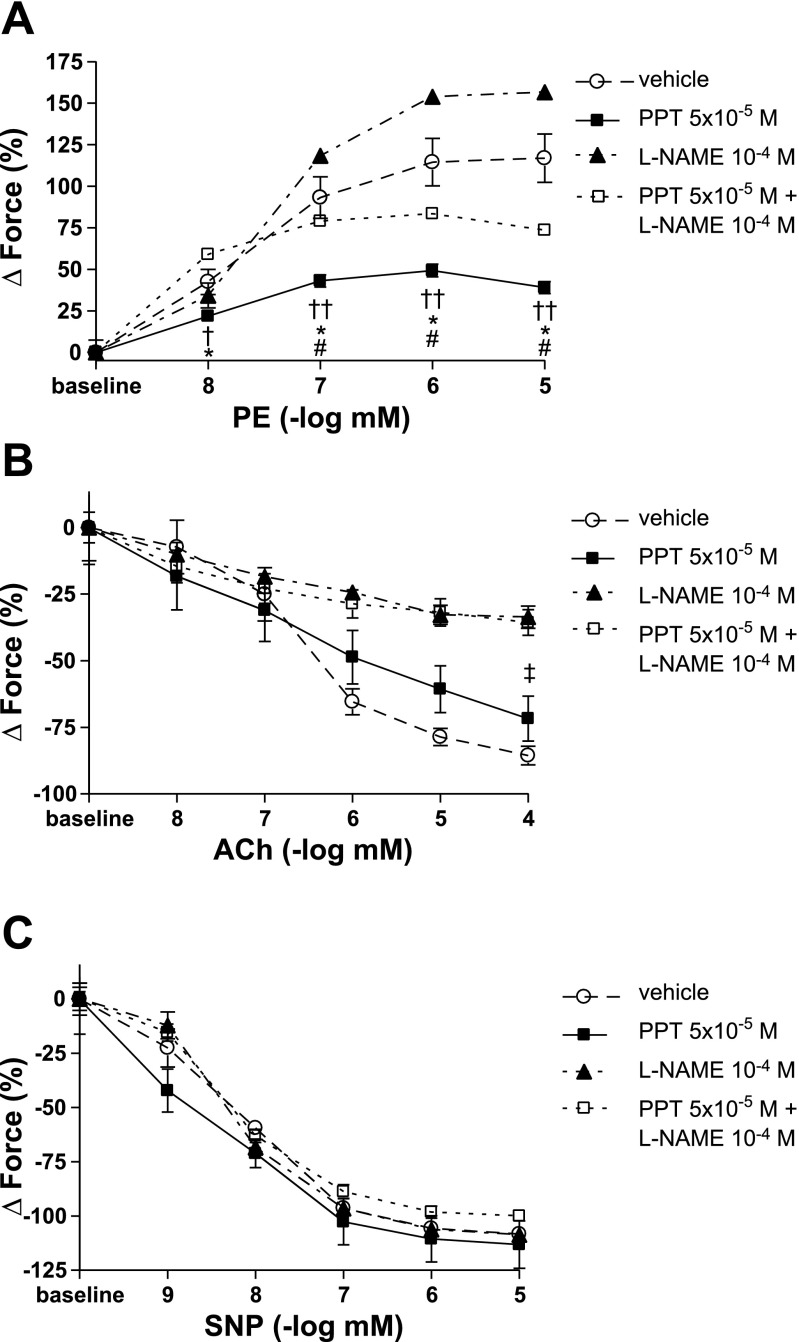
To confirm the above described results, and to further investigate whether the observed PPT-related decrease in PE-induced vasoconstriction is mediated by a NO-dependent mechanism, we performed vasoreactivity studies by generating dose-response curves to PE. After PE precontraction, endothelium-dependent and -independent vasorelaxation were measured by generating dose-response curves to ACh and SNP, respectively. The PPT concentration found to be effective in the above-described experiments (5 × 10−5 M) was used for the vasoreactivity studies. PPT significantly decreased vasoconstriction at all PE concentrations (Fig. 3A). However, in the presence of the nonselective NOS inhibitor l-NAME, this effect was significantly attenuated, indicating that PPT decreases PE-induced vasoconstriction by a NO-dependent mechanism. The vasodilator effects of PPT in the presence of ACh were attenuated by l-NAME as well, again indicating that PPT exerts its vasodilator effects through NO (Fig. 3B). As expected, NOS-inhibition did not affect endothelium-independent vasorelaxation (Fig. 3C).
Fig. 3.
Vasoreactivity studies for PPT with and without NOS-inhibition using l-NAME. PPT was added to the organ bath 10 min prior to PE. l-NAME was added 40 min prior to PE. A: force is expressed as %change from baseline. B and C: force is expressed as %change from PE-precontraction. PPT rapidly and significantly decreased PE-mediated vasoconstriction. This effect was attenuated in the presence of l-NAME (A). l-NAME also attenuated PPT vasodilator effects in the presence of ACh (B). In contrast, endothelium-independent vasorelaxation was not affected by NOS-inhibition (C). SNP, sodium nitroprusside. N = 3–7/group. †P < 0.05 vs. vehicle; ††P < 0.001 vs. vehicle; *P < 0.001 vs. PPT + l-NAME; #P < 0.001 vs. l-NAME; ‡P < 0.05 vs. PPT + l-NAME.
ER-α agonist PPT and ER-β agonist DPN do not affect vasorelaxation during hypoxia.
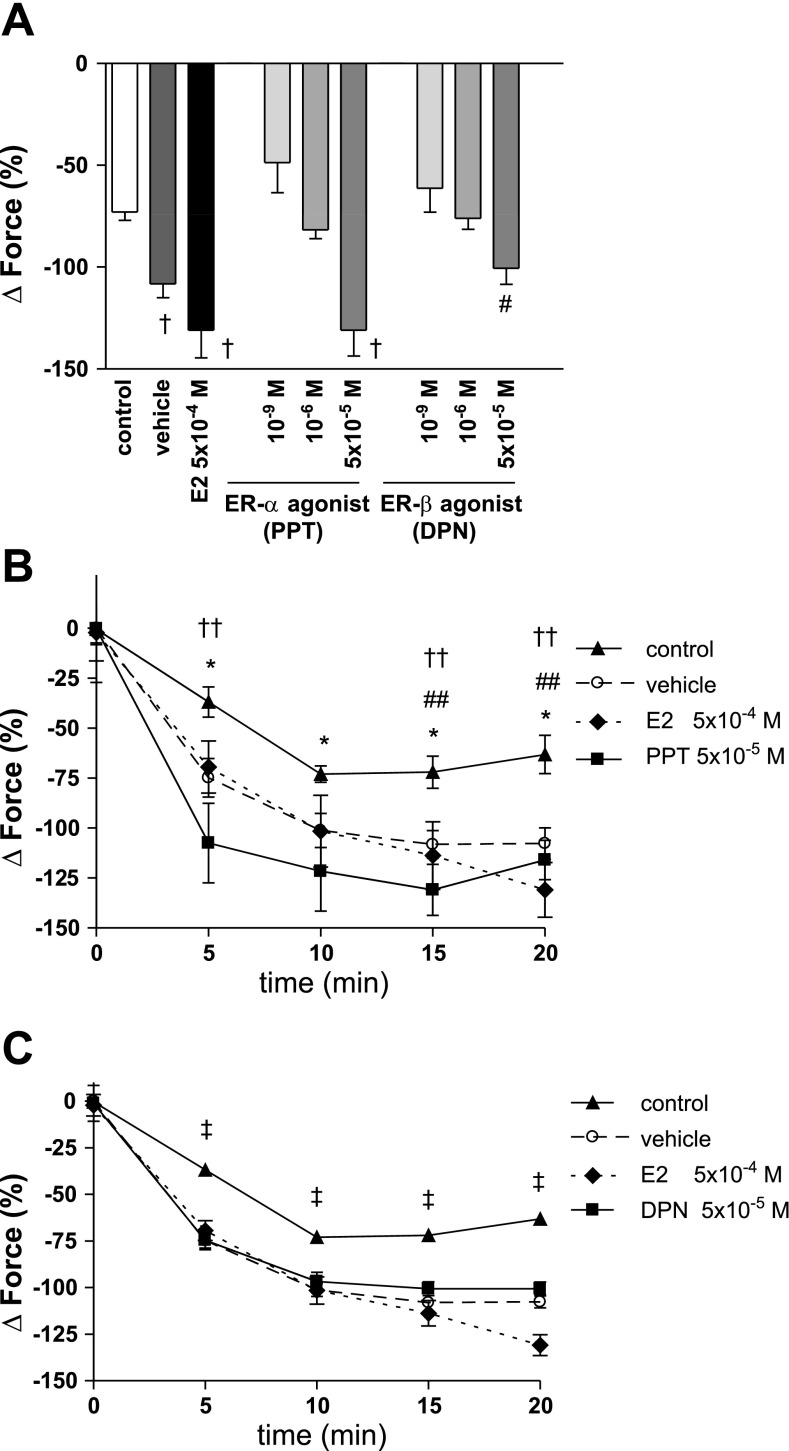
Both, PPT and DPN in the highest concentrations enhanced vasorelaxation during hypoxia similar to E2 (PPT: −130.90 ± 12.73%, DPN: −100.55 ± 7.84%, E2: −130.90 ± 13.70%, control: −72.94 ± 4.16%). However, this effect was not different from vehicle (−108.13 ± 6.91%). There were no significant differences in vasorelaxation between the highest concentrations of PPT and DPN, as well as E2 and vehicle when measuring maximum vasorelaxation (Fig. 4A) or individual time points during the vasodilatory phase of hypoxia (Fig. 4, B and C).
Fig. 4.
Maximal vasorelaxation (A) and timeline for PPT (B) and DPN (C) during the vasodilatory phase of hypoxia. Force is expressed as %change from PE-precontraction. PPT and DPN in the highest concentrations enhanced vasorelaxation during hypoxia similar to E2. However, this effect was not different from vehicle. N = 4–10/group. A: †P < 0.001 for PPT 5 × 10−5 M, E2 and vehicle vs. control; #P < 0.01 for DPN 5 × 10−5 M vs. control. B: *P < 0.05 for PPT 5 × 10−5 M vs. control; ##P < 0.05 for E2 vs. control; ††P < 0.05 for vehicle vs. control. C: ‡P < 0.001 for DPN 5 × 10−5 M, E2, and vehicle vs. control.
ER-β agonist DPN rapidly decreases phase II HPV.
PPT at 10−6 M and 5 × 10−5 M concentrations decreased maximal phase II of HPV (47.48 ± 7.12% and 30.04 ± 10.16%) compared with control (80.79 ± 8.61%). However, this effect was not different from vehicle (54.48 ± 9.39%). In contrast, DPN (5 × 10−5 M) significantly and rapidly decreased maximal phase II HPV (6.20 ± 6.47%) compared with vehicle and control (Fig. 5A). This effect was significantly more pronounced than the effects of PPT. In addition, the DPN-induced decrease in HPV was readily detectable 25 min after induction of hypoxia (Fig. 5, B and C).
Fig. 5.
Maximal phase II HPV (A) and timeline for PPT (B) and DPN (C) during HPV. Force is expressed as %change from PE-precontraction. While PPT (10−6 M and 5 × 10−5 M) decreased HPV compared with control, this effect was not different from vehicle. In contrast, DPN (5 × 10−5 M) significantly and rapidly (<25 min after induction of hypoxia) decreased HPV compared with vehicle and control. N = 4–10/group. A: *P < 0.05 vs. control; #P < 0.01 vs. control; ‡P < 0.0001 vs. control; †P < 0.01 vs. vehicle, E2 and PPT 10−6 M. B: ††P < 0.05 for PPT 5 × 10−5 M vs. control. (C) **P < 0.001 for DPN 5 × 10−5 M and E2 vs. control; ##P < 0.05, ###P < 0.001 for DPN 5 × 10−5 M vs. vehicle.
ER-β agonist DPN rapidly decreases phase II HPV by a NO-dependent mechanism.
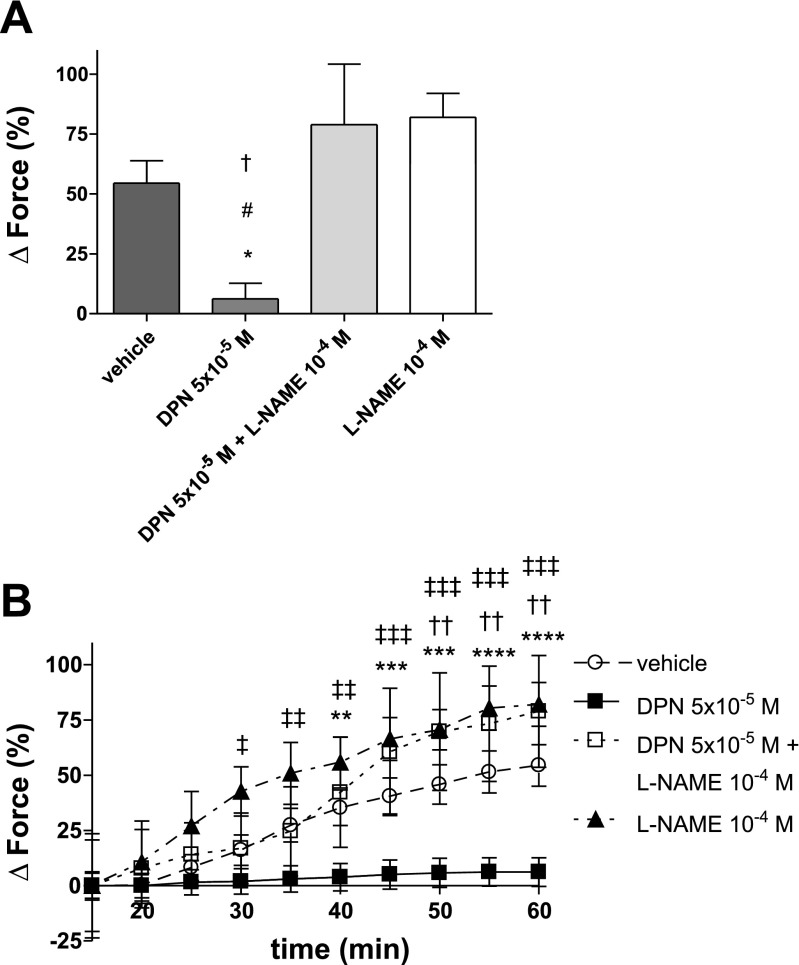
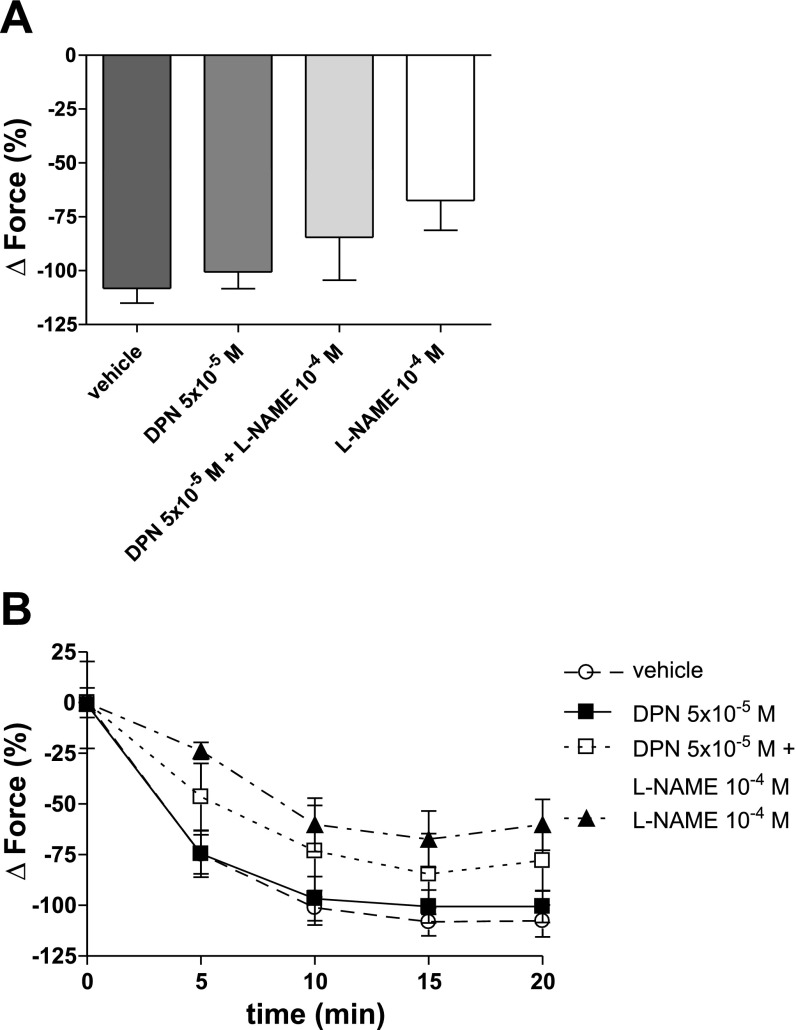
To investigate whether the DPN-related decrease of HPV is mediated by NO, HPV was examined in the presence of the NOS inhibitor l-NAME. Interestingly, the effects of DPN on phase II HPV were neutralized by l-NAME (78.87 ± 25.31%), indicating that the DPN-related decrease in HPV is mediated by NO (Fig. 6, A and B). In contrast, vasorelaxation during hypoxia was not affected by NO-inhibition (Fig. 7).
Fig. 6.
Effects of NOS inhibition on maximal phase II HPV (A) and timeline of hypoxic vasoconstriction (B). Force is expressed as %change from PE-precontraction. The decrease of phase II HPV by DPN was neutralized in the presence of the NOS inhibitor l-NAME, indicating that DPN decreases HPV by a NO-dependent mechanism. l-NAME was added to the organ bath 50 min prior to the onset of hypoxia. N = 3–6/group. A: *P < 0.05 vs. vehicle, #P < 0.05 vs. DPN + l-NAME, †P < 0.001 vs. l-NAME. B: ‡P < 0.05, ‡‡P < 0.01, ‡‡‡P < 0.001 for DPN vs. l-NAME; **P < 0.05, ***P < 0.01, ****P < 0.001 for DPN vs. vehicle; ††P < 0.05 for DPN vs. DPN + l-NAME.
Fig. 7.
Effects of NOS inhibition on maximal vasorelaxation (A) and timeline of vasorelaxation during hypoxia (B). Vasorelaxation during hypoxia is not affected by NOS inhibition. l-NAME was added to the organ bath 50 min prior to the onset of hypoxia. Force is expressed as %change from PE-precontraction. N = 3–6/group.
DISCUSSION
Our data indicate that activation of ER-α or ER-β rapidly decreases PA vasoconstriction. The selective ER-α agonist PPT rapidly attenuated PE-induced vasoconstriction, while the selective ER-β agonist DPN rapidly decreased phase II of acute HPV. These effects occurred within minutes of administration, suggesting a nongenomic mechanism. The observation that this decrease in PA vasoconstriction was attenuated by NOS-inhibition indicates that the pulmonary vascular effects of ER-α and ER-β are mediated by a NO-dependent mechanism. These data are in concordance with previous cell culture data demonstrating that both ERs can rapidly activate eNOS (3, 4, 11). The novelty of our results is the finding that the contribution of specific ERs appears to be stimulus-specific, with ER-α primarily modulating PE-induced vasoconstriction, and ER-β mediating the inhibitory effect on acute HPV. To our knowledge, the effects of selective ERs on acute HPV have not been investigated yet.
Our findings of ER-α and ER-β mediating their effect by a NO-dependent mechanism provide a physiologic mechanism for the previously reported rapid vasodilator effects of E2 (8, 17). These rapid vasodilator effects of E2 were reproduced in the present study. As described and discussed in our previous paper (17), as well as by English et al. (8), E2 exerted its effects in isolated PA rings only at relatively high concentrations which may be difficult to achieve in vivo. In contrast, PPT and DPN exerted their vasoactive effects at 10-fold lower concentrations than E2, making these compounds a more feasible therapeutic option. A possible explanation for these findings is that only a small amount of E2 may interact with ER-α and ER-β to evoke acute, nongenomic effects, while a substantial amount of E2 may bind to cytosolic ERs and diffuse into the nucleus to exert slower, genomic effects. Alternatively, it is possible that PPT and DPN have higher affinities for ERs than E2 itself. In this context, it is of interest to note that PPT has a 410-fold higher binding affinity for ER-α than ER-β, while DPN has a 70-fold higher affinity for ER-β over ER-α (23, 31). The rapid onset of the ER-α- and ER-β-mediated decrease in PA vasoconstriction (<20 min for PE-induced vasoconstriction and <45 min for HPV) is suggestive of a nongenomic increase in NO release. The concentrations required to mediate these effects were similar to those reported by other investigators demonstrating vasorelaxation in isolated systemic arteries (6, 36).
Nongenomic signaling by estrogens appear to be particularly important in “nontraditional” sex hormone target tissues (12). Interestingly, while ERs are present in almost all cells, they have tissue- and compartment-specific roles in mediating the protective effects of E2 on immune, metabolic, and organ functions following trauma hemorrhage, shock, and sepsis. For example, the liver is rich in ER-α, while ER-β seems to more be predominant in the lung (12). In addition, even within the same organ system, ER-α and ER-β may have complementary, redundant, and sometimes even antagonistic effects. For example, controversial data exist as to whether the protective effects of E2 during myocardial ischemia-reperfusion injury are mediated via ER-α, ER-β, or both (10, 39). Similarly, the specific roles of ER-α and ER-β in the systemic vasculature remain to be exactly determined (26, 43). Therefore, it is conceivable that the relative contribution of each receptor may depend on the clinical circumstance and the stimulus activating the receptor. The concept of stimulus-dependent ER subtype activation may explain some of the apparently discordant results of previous studies evaluating the relative importance of ER-α and ER-β in various organ systems. The relative contributions of ER-α and ER-β in the pulmonary vasculature are even less well defined, and previous data have been equivocal. Cell culture experiments by Chen et al. (4) and Hisamoto et al. (11) suggested that ER-α activates eNOS via nongenomic mechanisms, while Chambliss et al. (3) demonstrated that ER-β is capable of exerting this effect as well. However, none of these studies evaluated whether the contribution of ERs is stimulus-dependent. In addition, these experiments were performed with different cell populations and under normoxic conditions. Our data expand the current knowledge by evaluating the contribution of ERs during clinically relevant pharmacologic and hypoxic vasoconstrictor stimuli in a model of isolated PA rings. While the distal pulmonary arteries contribute to the majority of pulmonary vascular resistance, our results have clinical relevance since accumulating evidence suggests that altered physiological characteristics of the proximal segments of the pulmonary vascular bed can also negatively affect right ventricular function (32).
Interestingly, we observed an increase in vasorelaxation as well as a decrease in vasoconstriction during hypoxia with vehicle alone. The vehicle used in our experiments was ethanol, as this is the recommended carrier for both PPT and DPN, according to the manufacturer, due to solubility. While studies investigating the effect of ethanol on systemic vasomotor tone are conflicting (2, 29), we are not aware of any data demonstrating the effects of ethanol on PA vasomotor tone. However, when evaluating pharmacologically induced vasoconstriction, we did not observe any significant effects of vehicle (Fig. 2), and the vasorelaxatory effects of the highest concentration of DPN during hypoxia were clearly more pronounced than those of the vehicle alone (Fig. 5), indicating that both PPT and DPN have independent vasorelaxatory properties.
Our findings have potential implications for the treatment of pulmonary hypertension or acute lung injury and its more severe form, the acute respiratory distress syndrome. In these conditions, PA vasoconstriction can lead to right ventricular strain and decompensation (24). Since most of the currently available treatments for pulmonary hypertension and acute lung injury are limited by expense, side effects and lack of survival benefit (20, 24), new treatment alternatives are clearly needed. Improved understanding of the rapid and nongenomic signaling pathways used by sex and steroid hormone receptors represents a promising opportunity for the development of nonhormonal and targeted treatment alternatives for these conditions. The most impressive work by Dr. Chaudry's group as well as other investigators in the areas of trauma-hemorrhagic shock (13, 14, 41), sepsis (5, 7, 12), myocardial ischemia-reperfusion injury (10, 25, 39, 40), and acute lung injury (30, 35, 42) highlights the significant therapeutic potential of sex hormones and selective ER agonists in restoring the dysregulated immune response that characterizes these conditions (1, 22, 44). Along these lines, our laboratory has recently been able to demonstrate that estradiol-treated mesenchymal stem cells improve myocardial recovery after global ischemia in an isolated heart model (9). Whether ER-α and ER-β are capable of decreasing the inflammatory response and remodeling within the vessel wall, which are associated with hypoxic pulmonary hypertension as well as iPAH (15, 32), will need to be explored in future experiments.
Further insights into sex hormone receptor pathways may also improve the current knowledge of why acute and chronic hypoxic pulmonary hypertension are less common in females (18, 20, 27), while iPAH, a disabling condition characterized by PA vasoconstriction and remodeling as well as in situ thrombosis and eventually right heart failure, occurs twice as frequently in the female population (20). Furthermore, oral contraceptives have been considered to be a potential risk factor for iPAH (20). It is conceivable that a defect in ER pathways or intracellular signaling may contribute to these disparate findings. In this context, it is interesting to note that ER-α activation attenuated hypoxia-induced vasoconstriction, while ER-β activation decreased PA vasoconstriction induced by a nonhypoxic stimulus. This raises the question of whether the previously described sex differences in hypoxic pulmonary hypertension and iPAH may, at least in part, be due to differences in ER-α and/or ER-β signaling.
Perspectives and Significance
Selective ER agonists could provide a promising new strategy to further explore the role of sex differences and the roles of ER-α and ER-β in animal models of pulmonary hypertension. The findings of the present study advance the current knowledge of the pulmonary vascular effects of estrogen by demonstrating that both ER-α and ER-β decrease vasoconstriction in a model of isolated PA rings in a rapid and, therefore, most likely nongenomic manner. The contribution of specific ERs appears to be stimulus specific, with ER-α primarily modulating PE-induced vasoconstriction and ER-β inhibiting acute HPV. The observation that NOS inhibition eliminates these effects suggests a central role for NO in mediating the rapid pulmonary vascular effects of both ER-α and ER-β. Since l-NAME is a nonspecific NOS inhibitor, the present study does not allow for any conclusions as to which specific NOS isoform is mediating the vasorelaxatory effects of selective ER agonists. However, data from the systemic vasculature and from experiments with isolated PA endothelial cells suggest that eNOS most likely represents the responsible isoform. In future experiments, we will try to identify which NOS isoform is responsible for the ER-α- and ER-β-induced generation of NO observed in our model. Current research efforts in our laboratory aim to identify the mediators of ER-induced pulmonary vasorelaxation that act both upstream and downstream of NO, as this represents an area that holds promise to identify potential future therapeutic targets. Finally, the use of ER-α- and ER-β-specific antagonists in the presence and absence of PPT and DPN may allow for further characterization of the roles of ER-α and ER-β in the pulmonary vasculature.
GRANTS
This work was supported in part by National Institutes of Health Grants R01-GM-070628, R01-HL-085595, K99/R00-HL-0876077-01, and F32-HL-085982, an American Heart Association Grant-in-aid, and American Heart Association Postdoctoral Fellowship 0725663Z.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ao L, Song Y, Fullerton DA, Dinarello CA, Meng X. The interaction between myocardial depressant factors in endotoxemic cardiac dysfunction: role of TNF-α in TLR4-mediated ICAM-1 expression. Cytokine 38: 124–129, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boban M, Modun D, Music I, Vukovic J, Brizic I, Salamunic I, Obad A, Palada I, Dujic Z. Red wine induced modulation of vascular function: separating the role of polyphenols, ethanol, and urates. J Cardiovasc Pharmacol 47: 695–701, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERβ has nongenomic action in caveolae. Mol Endocrinol 16: 938–946, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor-α mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest 103: 401–406, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cristofaro PA, Opal SM, Palardy JE, Parejo NA, Jhung J, Keith JC Jr, Harris HA. WAY-202196, a selective estrogen receptor-β agonist, protects against death in experimental septic shock. Crit Care Med 34: 2188–2193, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Cruz MN, Douglas G, Gustafsson JA, Poston L, Kublickiene K. Dilatory responses to estrogenic compounds in small femoral arteries of male and female estrogen receptor-β knockout mice. Am J Physiol Heart Circ Physiol 290: H823–H829, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Deitch EA, Feketeova E, Lu Q, Zaets S, Berezina TL, Machiedo GW, Hauser CJ, Livingston DH, Xu DZ. Resistance of the female, as opposed to the male, intestine to I/R-mediated injury is associated with increased resistance to gut-induced distant organ injury. Shock 29: 78–83, 2008. [DOI] [PubMed] [Google Scholar]
- 8.English KM, Jones RD, Jones TH, Morice AH, Channer KS. Gender differences in the vasomotor effects of different steroid hormones in rat pulmonary and coronary arteries. Horm Metab Res 33: 645–652, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Erwin GS, Crisostomo PR, Wang Y, Wang M, Markel TA, Guzman M, Sando IC, Sharma R, Meldrum DR. Estradiol-treated mesenchymal stem cells improve myocardial recovery after ischemia. J Surg Res. In press. [DOI] [PMC free article] [PubMed]
- 10.Gabel SA, Walker VR, London RE, Steenbergen C, Korach KS, Murphy E. Estrogen receptor-β mediates gender differences in ischemia/reperfusion injury. J Mol Cell Cardiol 38: 289–297, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, Adachi K, Tasaka K, Miyoshi E, Fujiwara N, Taniguchi N, Murata Y. Estrogen induces the Akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem 276: 3459–3467, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh YC, Frink M, Choudhry MA, Bland KI, Chaudry IH. Metabolic modulators following trauma sepsis: sex hormones. Crit Care Med 35: S621–S629, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh YC, Yu HP, Frink M, Suzuki T, Choudhry MA, Schwacha MG, Chaudry IH. G protein-coupled receptor 30-dependent protein kinase A pathway is critical in nongenomic effects of estrogen in attenuating liver injury after trauma-hemorrhage. Am J Pathol 170: 1210–1218, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu JT, Kan WH, Hsieh CH, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Mechanism of estrogen-mediated attenuation of hepatic injury following trauma-hemorrhage: Akt-dependent HO-1 up-regulation. J Leukoc Biol 82: 1019–1026, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43: 13S–24S, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Kher A, Wang M, Tsai BM, Pitcher JM, Greenbaum ES, Nagy RD, Patel KM, Wairiuko GM, Markel TA, Meldrum DR. Sex differences in the myocardial inflammatory response to acute injury. Shock 23: 1–10, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Lahm T, Crisostomo PR, Markel TA, Wang M, Wang Y, Weil B, Meldrum DR. Exogenous estrogen rapidly attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction. Shock. In press. [DOI] [PubMed]
- 18.Lahm T, Crisostomo PR, Markel TA, Wang M, Weil BR, Novotny NM, Meldrum DR. The effects of estrogen on pulmonary artery vasoreactivity and hypoxic pulmonary vasoconstriction: potential new clinical implications for an old hormone. Crit Care Med 36: 2174–2183, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Lahm T, Patel KM, Crisostomo PR, Markel TA, Wang M, Herring C, Meldrum DR. Endogenous estrogen attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction: the effects of sex and menstrual cycle. Am J Physiol Endocrinol Metab 293: E865–E871, 2007. [DOI] [PubMed] [Google Scholar]
- 20.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation 114: 1417–1431, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Meldrum DR G-protein-coupled receptor 30 mediates estrogen's nongenomic effects after hemorrhagic shock and trauma. Am J Pathol 170: 1148–1151, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meldrum DR Tumor necrosis factor in the heart. Am J Physiol Regul Integr Comp Physiol 274: R577–R595, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-β potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem 44: 4230–4251, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Morrell ED, Tsai BM, Crisostomo PR, Wang M, Markel TA, Lillemoe KD, Meldrum DR. Therapeutic concepts for hypoxic pulmonary vasoconstriction involving ion regulation and the smooth muscle contractile apparatus. J Mol Cell Cardiol 40: 751–760, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Nikolic I, Liu D, Bell JA, Collins J, Steenbergen C, Murphy E. Treatment with an estrogen receptor-β-selective agonist is cardioprotective. J Mol Cell Cardiol 42: 769–780, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME. Estrogen receptor-α mediates the protective effects of estrogen against vascular injury. Circ Res 90: 1087–1092, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Rabinovitch M, Gamble WJ, Miettinen OS, Reid L. Age and sex influence on pulmonary hypertension of chronic hypoxia and on recovery. Am J Physiol Heart Circ Physiol 240: H62–H72, 1981. [DOI] [PubMed] [Google Scholar]
- 28.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407: 538–541, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spaak J, Merlocco AC, Soleas GJ, Tomlinson G, Morris BL, Picton P, Notarius CF, Chan CT, Floras JS. Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter. Am J Physiol Heart Circ Physiol 294: H605–H612, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Speyer CL, Rancilio NJ, McClintock SD, Crawford JD, Gao H, Sarma JV, Ward PA. Regulatory effects of estrogen on acute lung inflammation in mice. Am J Physiol Cell Physiol 288: C881–C890, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-α-selective agonists. J Med Chem 43: 4934–4947, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki T, Shimizu T, Yu HP, Hsieh YC, Choudhry MA, Bland KI, Chaudry IH. 17β-estradiol administration following trauma-hemorrhage prevents the increase in Kupffer cell cytokine production and MAPK activation predominately via estrogen receptor-α. Surgery 140: 141–148, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki T, Shimizu T, Yu HP, Hsieh YC, Choudhry MA, Chaudry IH. Salutary effects of 17β-estradiol on T-cell signaling and cytokine production after trauma-hemorrhage are mediated primarily via estrogen receptor-α. Am J Physiol Cell Physiol 292: C2103–C2111, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Toth B, Schwacha MG, Kuebler JF, Bland KI, Wang P, Chaudry IH. Gender dimorphism in neutrophil priming and activation following trauma-hemorrhagic shock. Int J Mol Med 11: 357–364, 2003. [PubMed] [Google Scholar]
- 36.Traupe T, Stettler CD, Li H, Haas E, Bhattacharya I, Minotti R, Barton M. Distinct roles of estrogen receptors-α and -β mediating acute vasodilation of epicardial coronary arteries. Hypertension 49: 1364–1370, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Tsai BM, Wang M, Clauss M, Sun P, Meldrum DR. Endothelial monocyte-activating polypeptide II causes NOS-dependent pulmonary artery vasodilation: a novel effect for a proinflammatory cytokine. Am J Physiol Regul Integr Comp Physiol 287: R767–R771, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Tsai BM, Wang M, Pitcher JM, Kher A, Meldrum DR. Disparate IL-1β and iNOS gene expression in the aorta and pulmonary artery after endotoxemia. Surg Infect (Larchmt) 7: 21–27, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Wang M, Crisostomo P, Wairiuko GM, Meldrum DR. Estrogen receptor-α mediates acute myocardial protection in females. Am J Physiol Heart Circ Physiol 290: H2204–H2209, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Wang M, Tsai BM, Reiger KM, Brown JW, Meldrum DR. 17-β-Estradiol decreases p38 MAPK-mediated myocardial inflammation and dysfunction following acute ischemia. J Mol Cell Cardiol 40: 205–212, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Yu HP, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Mechanism of the nongenomic effects of estrogen on intestinal myeloperoxidase activity following trauma-hemorrhage: up-regulation of the PI-3K/Akt pathway. J Leukoc Biol 82: 774–780, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Yu HP, Hsieh YC, Suzuki T, Shimizu T, Choudhry MA, Schwacha MG, Chaudry IH. Salutary effects of estrogen receptor-β agonist on lung injury after trauma-hemorrhage. Am J Physiol Lung Cell Mol Physiol 290: L1004–L1009, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 295: 505–508, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Zou N, Ao L, Cleveland JC Jr, Yang X, Su X, Cai GY, Banerjee A, Fullerton DA, Meng X. Critical role of extracellular heat shock cognate protein 70 in the myocardial inflammatory response and cardiac dysfunction after global ischemia-reperfusion. Am J Physiol Heart Circ Physiol 294: H2805–H2813, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]