Abstract
Activation of neuronal ATP-sensitive potassium (KATP) channels is an important mechanism that protects neurons and conserves neural function during hypoxia. We investigated hypoxia (bath gassed with 95% N2-5% CO2 vs. 95% O2-5% CO2 in control)-induced changes in KATP current in second-order neurons of peripheral chemoreceptors in the nucleus of the solitary tract (NTS). Hypoxia-induced KATP currents were compared between normoxic (Norm) rats and rats exposed to 1 wk of either chronic sustained hypoxia (CSH) or chronic intermittent hypoxia (CIH). Whole cell recordings of NTS second-order neurons identified after 4-(4-(dihexadecylamino)styryl)-N-methylpyridinium iodide (DiA) labeling of the carotid bodies were obtained in a brain stem slice. In Norm cells (n = 9), hypoxia (3 min) induced an outward current of 12.7 ± 1.1 pA with a reversal potential of −73 ± 2 mV. This current was completely blocked by the KATP channel blocker tolbutamide (100 μM). Bath application of the KATP channel opener diazoxide (200 μM, 3 min) evoked an outward current of 21.8 ± 5.8 pA (n = 6). Hypoxia elicited a significantly smaller outward current in both CSH (5.9 ± 1.4 pA, n = 11; P < 0.01) and CIH (6.8 ± 1.7 pA, n = 6; P < 0.05) neurons. Diazoxide elicited a significantly smaller outward current in CSH (3.9 ± 1.0 pA, n = 5; P < 0.05) and CIH (2.9 ± 0.9 pA, n = 3; P < 0.05) neurons. Western blot analysis showed reduced levels of KATP potassium channel subunits Kir6.1 and Kir6.2 in the NTS from CSH and CIH rats. These results suggest that hypoxia activates KATP channels in NTS neurons receiving monosynaptic chemoreceptor afferent inputs. Chronic exposure to either sustained or intermittent hypoxia reduces KATP channel function in NTS neurons. This may represent a neuronal adaptation that preserves neuronal excitability in crucial relay neurons in peripheral chemoreflex pathways.
Keywords: chemoreflex, electrophysiology, respiration
arterial peripheral chemoreflex pathways mediate many of the physiological responses to hypoxia (e.g., sympathoexcitation, increased ventilation). Neurons in the nucleus of the solitary tract (NTS) receive the first central projections from arterial chemoreceptors located in the carotid bodies. NTS neurons receiving these excitatory afferent inputs then activate other central neural structures mediating the integrated response to hypoxia (27). Arterial hypoxemia can result in tissue hypoxia in brain structures. Tissue hypoxia disrupts synaptic transmission and causes neural dysfunction (5–7, 29, 33). Without proper neuroprotective mechanisms, tissue hypoxia could have detrimental effects on central neurons and impair the physiological response to hypoxia.
Various mechanisms exist to protect the neuron and conserve essential neural functions during tissue hypoxia. One such protective mechanism is the activation of ATP-sensitive potassium (KATP) channels (5, 7, 33). The KATP channel is a heterooctamer composed of two subunits: the pore-forming subunits (Kir6.1 and Kir6.2) and the regulatory sulfonylurea receptor subunits (SUR1 and SUR2) (1). Kir6.x subunits belong to the inward rectifier potassium channel family, while SUR subunits belong to the ATP-binding cassette protein superfamily. KATP channels were first identified in the heart and have been extensively studied in both cardiac myocytes and pancreatic β-cells (2, 9, 24). Metabolic stress such as hypoxia, ischemia, or hypoglycemia leads to depletion of intracellular ATP and activation of KATP channels. Thus membrane potential and excitability can be modulated by the metabolic state of the cell. In pancreatic β-cells, the increase in ATP concentration due to increased glucose metabolism closes the KATP channels (9). This depolarizes the β-cell membrane, leading to the opening of the voltage-dependent calcium channels, calcium influx, and exocytosis of insulin.
KATP channels are also found in brain. Neuronal KATP channels also consist of the Kir6.x potassium channel subunits and the sulfonylurea receptor subunits (1, 2, 29). Activation of this potassium channel during depletion of ATP hyperpolarizes the neuron membrane, reducing neuronal excitability and ATP consumption. Activation of neuronal KATP channels is thought to play a neuroprotective role during tissue hypoxia or ischemia. However, it has been suggested that excessive channel activation could silence neurons (19). It is crucial to keep a certain level of neuronal excitability in neurons involved in chemoreflex pathways so that changes in cardiorespiratory function can continue to be evoked to supply oxygen to vital organs. So far there is no information about the behavior of KATP channels activated by hypoxia in NTS neurons receiving peripheral chemoreceptor inputs.
Many pathological conditions are associated with chronic hypoxia. Patients with chronic heart and pulmonary disease or those who travel to high altitude can experience chronic sustained hypoxia (CSH), while sleep apnea patients experience chronic intermittent hypoxia (CIH). Both types of exposure to CSH and CIH cause significant changes in the neural control of cardiorespiratory function including persistent sympathoexcitation and an enhanced ventilatory response to acute hypoxia (4, 6, 14, 23). However, how different types of chronic hypoxia affect the function of central neurons in peripheral chemoreflex pathways is not well characterized. Prolonged tissue hypoxia can also exist in these situations, but the effect of chronic exposures to hypoxia on neuronal KATP channels in NTS neurons is unknown.
We investigated the function of neuronal KATP channels in NTS neurons receiving monosynaptic inputs from the carotid body chemoreceptors. Using whole cell patch-clamp recording in an in vitro brain stem slice preparation, we studied the activity of KATP channels in response to hypoxia in NTS neurons collected from normoxic control (Norm), CSH, and CIH rats. Our results demonstrated the presence of KATP channels in second-order NTS neurons, and we found that exposures to either CSH or CIH reduce the function and level of neuronal KATP channels in the NTS.
METHODS
All experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio.
Surgical preparation for labeling carotid body.
Male Sprague-Dawley rats (125–145 g, Charles River Laboratories, Wilmington, MA) were anesthetized with a combination of ketamine (75 mg/kg ip, Fort Dodge) and medetomidine (0.5 mg/kg ip, Pfizer). Under aseptic conditions, crystals of the anterograde fluorescent dye 4-(4-(dihexadecylamino)styryl)-N-methylpyridinium iodide (DiA) were gently applied unilaterally to the carotid body region. DiA dissolves in the nerve axons and diffuses centrally, permitting visualization of chemoreceptor synaptic terminals and neurons receiving these synaptic contacts as previously described (30, 36). The area was then embedded with silicone adhesive (Kwik-Sil, WPI, Sarasota, FL). Anesthesia was terminated with atipamezole (1 mg/kg ip, Pfizer) at the conclusion of the surgical procedures. Postoperative analgesics (nubaine im) were available as needed.
Chronic sustained hypoxia exposure.
Rats in the CSH group were housed in their home cages within a normobaric hypoxia chamber at oxygen levels of 10 ± 0.5% for 7 days as described previously (20, 21, 30). The air within the chamber was recycled and the oxygen level controlled by a computer-driven set of valves and pumps. A hypoxic environment was achieved by the addition of nitrogen gas to room air. Oxygen levels within the chamber were monitored with an electrochemical sensor, and this information was fed into the computer-driven feedback circuit so that deviations in the oxygen level from the preset value of 10% were rapidly corrected by addition of appropriate gas. Temperature and humidity were monitored, and the recycled air was passed through a desiccant and CO2 scrubber. Rats were taken out of the sustained hypoxia chamber immediately before decapitation. Normal control (Norm) rats were maintained in a similar environment while breathing room air.
Chronic intermittent hypoxia exposure.
CIH rats were housed in hypoxia chambers with ad libitum food and water access as previously described (11, 18). Over a 3-min period, chambers were flushed with 100% N2 until a fractional O2 concentration of 10 ± 1% was reached and maintained for ∼75 s. After that, the chambers were flushed with room air (21% O2) for 3 min. Exposures to CIH occurred during the light period (0800-1600) for 7 days. The animals were not exposed to CIH during the remainder of the light period (1600-2100) and during the dark period (2100-0700). Norm rats were kept in the same types of chambers as CIH-exposed rats, and gas switching occurred in the same time frame as in CIH-exposed rats. However, in Norm rats, gas switching was between sources of room air so that Norm rats always inspired room air (21% O2). For whole cell recording experiments, CIH rats were taken out of the intermittent hypoxia chamber in the morning following the seventh and last day of exposure and were therefore decapitated 14–18 h after the last exposure to intermittent hypoxia.
Brain slice preparation.
Rats were anesthetized with isoflurane, and the brain stem was rapidly removed and placed in ice-cold, high-sucrose artificial cerebrospinal fluid (aCSF) that contained (in mM) 3 KCl, 1 MgCl2, 1 CaCl2, 2 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, and 206 sucrose, pH 7.4 when continuously bubbled with 95% O2-5% CO2. The brain stem was mounted in a vibrating microtome (VT1000E, Leica Microsystems, Bannockburn, IL), and horizontal slices (250-μm thickness) were cut with a sapphire knife (Delaware Diamond Knives, Wilmington, DE). The slices were incubated for at least 1 h in normal aCSF that contained (in mM) 124 NaCl, 3 KCl, 2 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, and 2 CaCl2, pH 7.4 when continuously bubbled with 95% O2-5% CO2.
Electrophysiological recording.
A single slice was transferred to the recording chamber on an upright epifluorescent microscope (Olympus BX50WI, Tokyo) equipped with infrared differential interference contrast (IR-DIC) and an optical filter set for visualization of DiA. The slice was held in place with a nylon mesh, submerged in normal aCSF equilibrated with 95% O2-5% CO2 and perfused at a rate of ∼2 ml/min. All images were captured with a charge-coupled device (CCD) camera (IR-1000, CCD-100; Dage-MTI, Michigan City, IN) displayed on a TV monitor. Patch pipettes were pulled from borosilicate glass capillaries with an inner filament (0.90-mm ID, 1.2-mm OD, WPI) on a pipette puller (model P-2000, Sutter Instrument, Novato, CA) and were filled with a solution of the following composition (in mM): 145 K-gluconate, l MgCl2, 10 HEPES, 1.1 EGTA, 2 Mg2ATP, and 0.3 Na3GTP. The pH was adjusted to 7.3 with KOH. The pipette resistance ranged from 3 to 5 MΩ. A seal resistance of ≥1 GΩ and an access resistance <20 MΩ that changed <15% during recording were considered acceptable. Recordings of postsynaptic currents began 5 min later, after the whole cell access was established and the holding current reached a steady state. Recordings were made with an AxoPatch 200B patch-clamp amplifier and pCLAMP software version 8 (Molecular Devices, Union City, CA). Whole cell currents were filtered at 2 kHz, digitized at 10 kHz with the DigiData 1200 Interface (Molecular Devices), and stored in a PC computer for off-line analysis. All experiments were performed at room temperature.
Whole cell voltage-clamp recordings were performed on second-order NTS peripheral chemoreceptor neurons labeled with fluorescent DiA as previously described (Refs. 11, 30, 36; see also Fig. 1A). Cells were clamped at a membrane potential of −60 mV. Input resistance was monitored by frequent application of a 10-mV hyperpolarizing voltage step (100-ms duration) from a holding potential of −60 mV. A current-voltage (I-V) curve was established by changing clamping membrane potentials from −130 mV to −40 mV in 10-mV steps. The duration of each step was 500 ms, and each voltage step was applied every 2 s. The I-V curve was obtained during control conditions and at the end of hypoxia. In all patch-clamp experiments, the bath solution included the sodium channel blocker TTX (1 μM), the non-NMDA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 μM), and the GABAA receptor antagonist gabazine (25 μM).
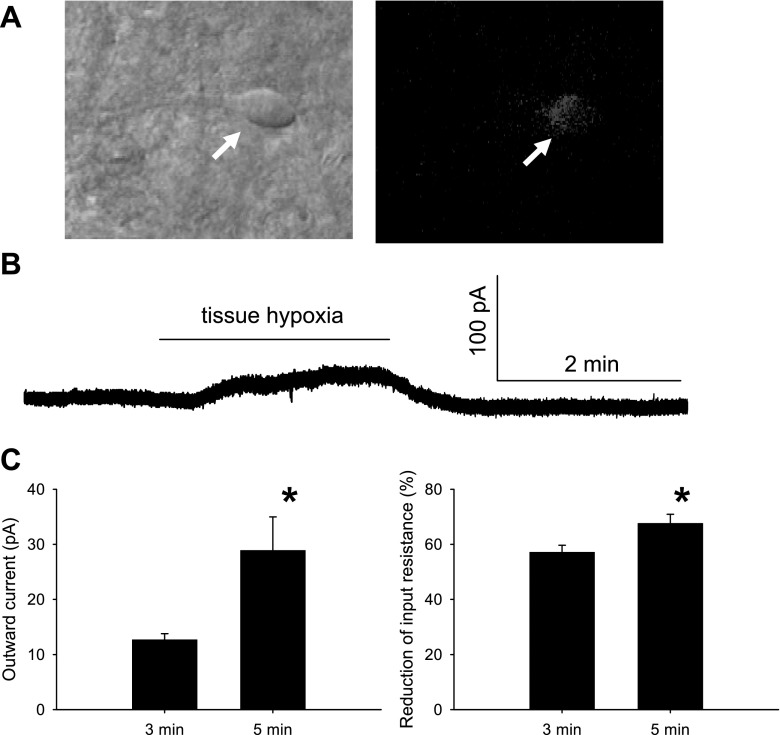
Fig. 1.
Hypoxia-induced neuronal response in the nucleus of solitary tract (NTS). A: 4-(4-(dihexadecylamino)styryl)-N-methylpyridinium iodide (DiA)-labeled NTS second-order neuron (arrow) from a normoxic rat. Left: cell viewed with infrared-differential contrast. Right: same neuron viewed with fluorescence to reveal DiA-labeled somatic appositions. B: raw data showing that hypoxia induced an outward current in voltage-clamp mode. C: 5 min of hypoxia (n = 6) induced a significantly greater outward current (left) and reduction in input resistance (right) compared with 3 min of hypoxia (n = 9). All experiments were performed in the presence of 1 μM TTX, 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), and 25 μM gabazine. *P < 0.05.
Slices were exposed to hypoxia by switching the bath solution from aCSF continuously bubbled with 95% O2-5% CO2 to aCSF continuously bubbled with 95% N2-5% CO2. The pH in both solutions was frequently monitored and kept stable during the course of the experiment. In a similarly gassed brain slice preparation, superfusion with an anoxic aCSF solution lowered the partial pressure of oxygen within the slice to near zero (31).
Western blot analysis.
Western blot analysis was performed with modification as previously described (15, 22). Each rat was anesthetized with isoflurane and decapitated. The brain was removed from the skull, and the brain stem was placed in a commercially available brain matrix (Stoelting). The matrix was used to cut the brain stem into 1-mm-thick coronal sections with razor blades. Under a dissecting microscope the caudal NTS was dissected from the sections, placed in microcentrifuge tubes, and flash frozen with liquid nitrogen. Next, the caudal NTS was sonicated in 50 μl of modified RIPA buffer supplemented with protease and phosphatase inhibitors (Sigma, St. Louis, MO), followed by 30-min incubation on ice. The total homogenate was then centrifuged at 14,000 rpm for 30 min at 4°C. The total protein for each sample was determined with the Bradford method. Ten micrograms of total protein from each sample was loaded onto 10% acrylamide SDS gel, electrophoresed in Tris-glycine buffer under denaturing conditions, and transferred to nitrocellulose membrane (Bio-Rad) in Tris-glycine buffer with 20% methanol. Membranes were blocked for 1 h at room temperature with 5% nonfat milk in Tris-buffered saline-Tween 20 (TBS-Tween; 50 mM Tris base, 200 mM NaCl, 0.1% Tween 20). Membranes were then incubated overnight at 4°C with primary antibodies against Kir6.2, Kir6.1, or β-actin (all from Sigma). Blots were then rinsed three times for 10 min each with TBS-Tween and incubated at room temperature for 1 h in a horseradish peroxidase-conjugated secondary antibody against respective primary antibody host species (1:5,000, Sigma). The respective immunoreactive bands were detected by enhanced chemiluminescence (ECL reagents, Amersham) and exposed to radiographic film (Hyperfilm ECL, Amersham). Densitometry of the bands of interest was performed by acquiring digital images of radiographic film (Hyperfilm, Amersham).
Data analysis.
Data are presented as means ± SE. Differences in responses to tissue hypoxia or different chronic hypoxia protocols (sustained and intermittent) were tested by one-way ANOVA or unpaired t-test. For Western blot analysis, the immunoreactivity bands were quantified by densitometry with the Scion program and the respective values were normalized against β-actin densitometry in four independent experiments. Data are expressed as arbitrary densitometric units. Statistics tests were performed with SigmaStat (v2.03, SPSS software, Chicago, IL), and graphs were made in SigmaPlot (v8.0, SPSS software). Values of P < 0.05 were considered significant.
Drugs.
DiA was obtained from Molecular Probes (Eugene, OR). Gabazine (SR-95531 hydrobromide) and TTX were obtained from Tocris (Ballwin, MO). CNQX, tolbutamide, diazoxide, tetraethylammonium chloride (TEA-Cl), and other chemicals were obtained from Sigma.
RESULTS
Hypoxia-induced outward currents in Norm rats.
Similar to our previous studies (11, 30, 36), cells receiving monosynaptic chemoreceptor afferent inputs were usually spindle- or oval shaped. Fluorescent putative boutons were seen along the borders of the soma and proximal processes of these NTS neurons (Fig. 1A). These neurons had an average resting membrane potential of −55.0 ± 0.8 mV and input resistance of 1.2 ± 0.1 GΩ (n = 24).
We initially examined responses to 3-min and 5-min exposures to hypoxia. Both 3- and 5-min exposures to hypoxia elicited a clearly discernable outward current and reduced input resistance in NTS neurons receiving arterial chemoreceptor afferent inputs (Fig. 1B). After switching back to normal aCSF, current returned to control levels within 5 min. We did not observe any delayed changes in current indicative of a posthypoxia depolarization or hyperpolarization in any cells. The 5-min exposure to hypoxia induced significantly greater effects than the 3-min exposure (Fig. 1C). In six neurons, 5 min of hypoxia induced an outward current of 28.9 ± 6.1 pA and reduced input resistance by 68 ± 3%. In nine neurons, 3 min of hypoxia induced an outward current of 12.7 ± 1.1 pA and reduced input resistance by 57 ± 2%. In subsequent studies, only the 3-min hypoxia exposure was used.
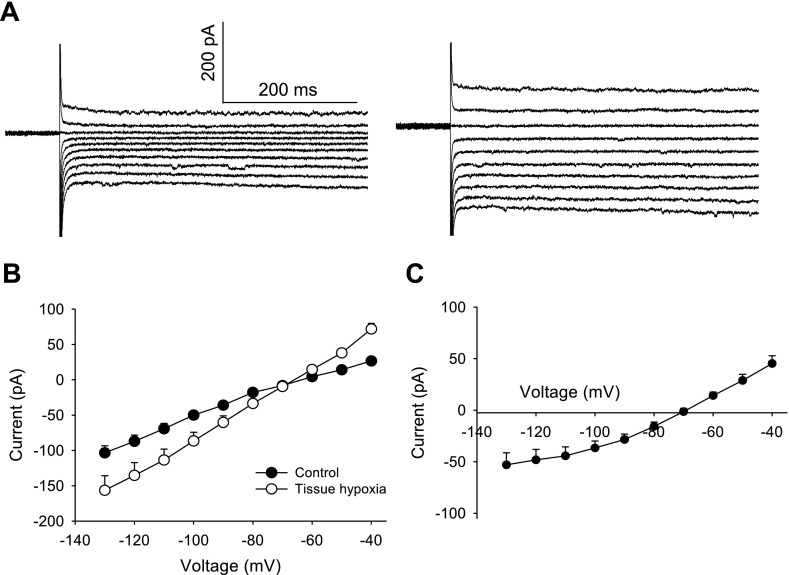
The reversal potential of the hypoxia-induced outward current was −73.1 ± 2.1 mV (n = 8) as determined from I-V curves obtained before and during the response to hypoxia (Fig. 2). In our preparation, the calculated junction potential was 15.6 mV; thus the actual calculated reversal potential was about −88.7 mV, close to the calculated potassium reversal potential of −97.7 mV. This result suggests that hypoxia-induced outward current is primarily mediated by potassium. After replacement of K-gluconate with 120 mM CsCl and 10 mM TEA-Cl in the pipette solution to block potassium channels, hypoxia evoked a small inward current of 4.6 ± 2.1 pA (n = 5). Bath application of the potassium channel blocker TEA-Cl (10 mM) did not significantly alter hypoxia-induced outward currents (14.1 ± 3.1 pA, n = 5; P > 0.05). This result suggests that the hypoxia-induced outward current is primarily mediated by a TEA-insensitive potassium current.
Fig. 2.
Hypoxia-induced alteration of current-voltage (I-V) relationship in second-order NTS neurons. A: current responses to a series of voltage steps (from −130 to −40 mV in 10-mV steps) applied before (left) and at the peak of (right) the hypoxia-induced outward current. Note the larger currents during hypoxia. B: hypoxia for 3 min increased the slope of the I-V curve (n = 8). C: net hypoxia-induced current determined by subtraction of I-V curves displayed in B. All experiments were performed in the presence of 1 μM TTX, 10 μM CNQX, and 25 μM gabazine.
Hypoxia-induced current was mediated by KATP channels.
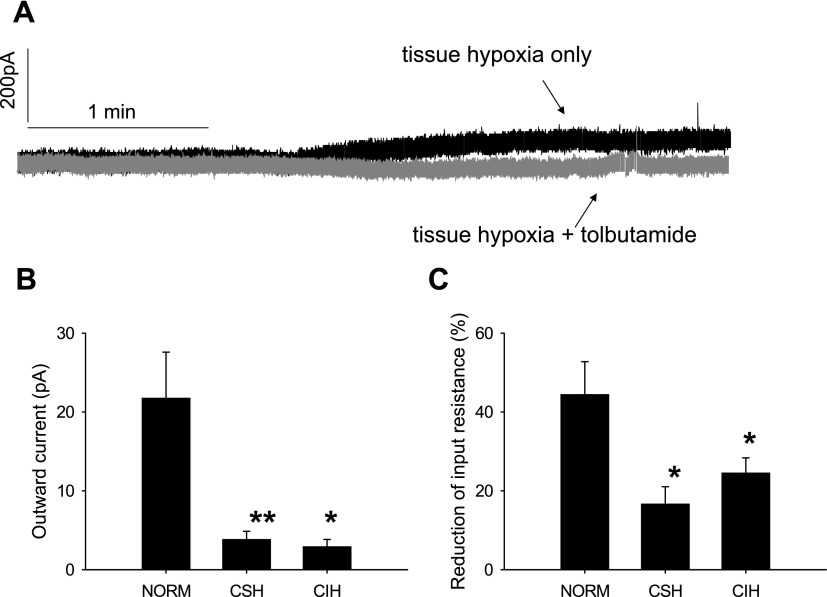
Application of the KATP channel blocker tolbutamide alone (100 μM) induced a small inward current of 5.6 ± 2.3 pA (n = 3). Tolbutamide abolished the hypoxia-induced outward current and revealed an inward current during hypoxia (6.1 ± 3.1 pA, n = 3) (Fig. 3A) with little change in input resistance (7 ± 4%). The I-V curves obtained at the end of tolbutamide application revealed a parallel rightward shift with no change in the reversal potential (data not shown). Bath application of the KATP channel opener diazoxide (200 μM) in the absence of any tissue hypoxia for 3 min evoked an outward current (21.8 ± 5.8 pA; Fig. 3B) and decreased input resistance (42 ± 11%, Fig. 3C) in six second-order NTS neurons. The reversal potential of the diazoxide-evoked outward current in these neurons was 75.6 ± 3.6 mV, which was not significantly different from that of the hypoxia-induced outward current.
Fig. 3.
Activity of neuronal ATP-sensitive potassium (KATP) channel in the NTS attenuated by chronic hypoxia exposure. A: raw data showing that hypoxia-induced outward current is abolished by the KATP channel blocker tolbutamide (100 μM). B: diazoxide (200 μM)-induced outward currents were significantly smaller in chronic sustained hypoxia (CSH; n = 3) and chronic intermittent hypoxia (CIH; n = 3) neurons compared with Norm neurons (n = 6). C: diazoxide-induced decrease in input resistance was significantly attenuated in the same CSH and CIH neurons as in B. There was no significant difference between CSH and CIH rats. All experiments were performed in the presence of 1 μM TTX, 10 μM CNQX, and 25 μM gabazine. *P < 0.05, **P < 0.01, compared with Norm neurons.
KATP channel activity in CSH neurons.
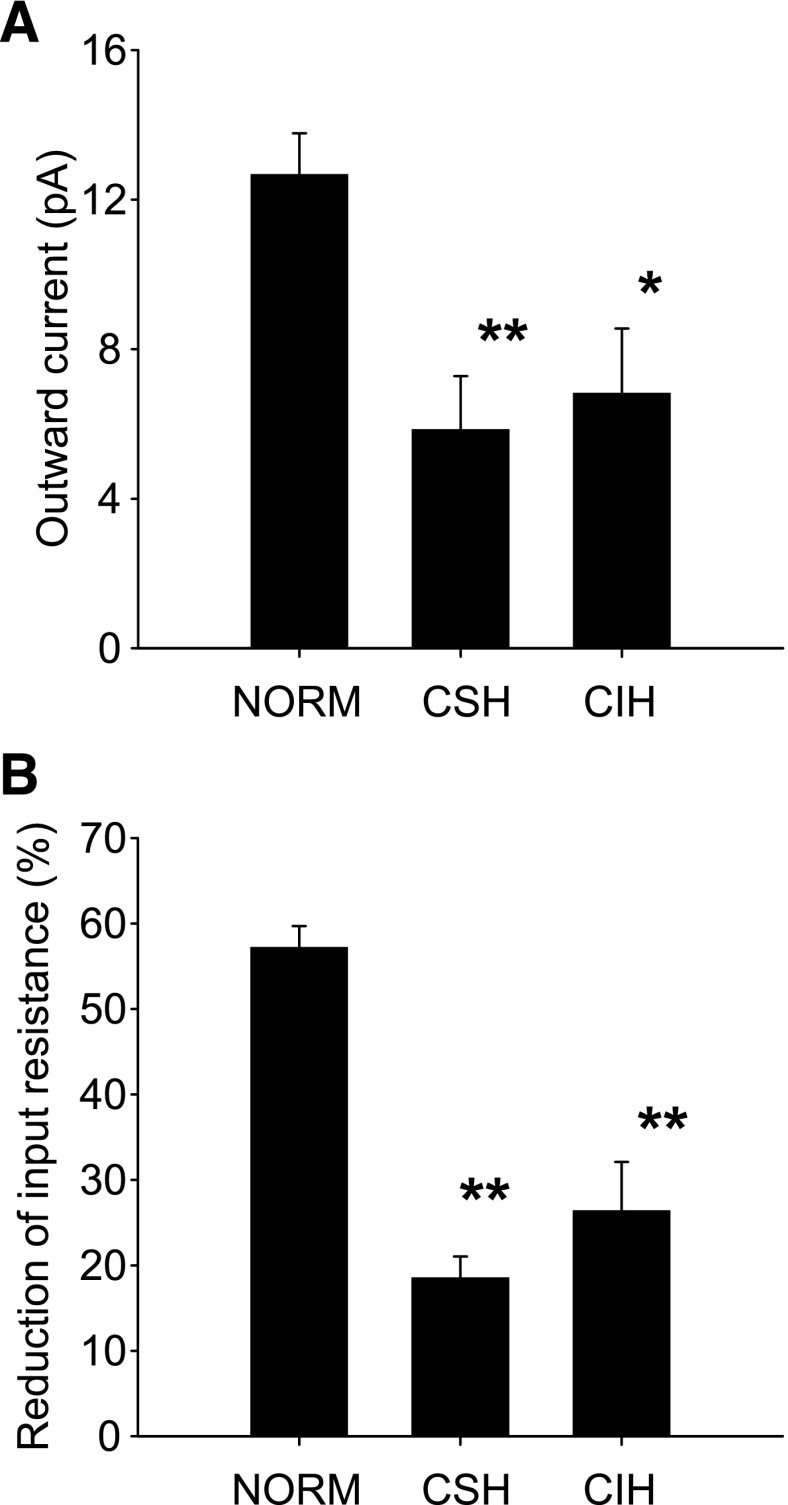
Second-order NTS neurons in CSH rats (n = 16) had a resting membrane potential (−55.0 ± 0.9 mV; P > 0.05) and input resistance (1.2 ± 0.1 GΩ; P > 0.05) no different from those of Norm neurons. However, in 10 CSH neurons 3 min of hypoxia induced an outward current (5.9 ± 1.4 pA; Fig. 4A) and reduced input resistance (18 ± 4%; Fig. 4B) that were significantly smaller than in Norm neurons (both P < 0.05). Similarly, in five CSH neurons the KATP channel opener diazoxide (200 μM), applied in the absence of any tissue hypoxia, evoked a significantly smaller outward current (3.9 ± 1.0 pA; Fig. 3B) and reduced input resistance (22 ± 6%; Fig. 3C) compared with Norm neurons (both P < 0.05).
Fig. 4.
Hypoxia-induced outward currents attenuated by chronic exposure to hypoxia. A: hypoxia-induced outward currents were significantly smaller in CSH (n = 10) and CIH (n = 6) neurons than in Norm (n = 9) neurons. B: hypoxia-induced decrease in input resistance was significantly attenuated in the same CIH and CSH neurons as in A. There was no significant difference between CSH and CIH rats. All experiments were performed in the presence of 1 μM TTX, 10 μM CNQX, and 25 μM gabazine. *P < 0.05, **P < 0.01, compared with Norm rats.
KATP channel activity in CIH neurons.
There was no significant difference in resting membrane potential (−56.2 ± 2.2 mV; P > 0.05) and input resistance (1.2 ± 0.1 GΩ; P > 0.05) in nine CIH neurons compared with Norm and CSH neurons. Similar to CSH neurons, in six CIH neurons 3 min of hypoxia induced a significantly smaller outward current (6.8 ± 1.7 pA; Fig. 4A) and reduced input resistance (26 ± 6%; Fig. 4B) compared with Norm rats. In three CIH neurons the KATP channel opener diazoxide (200 μM), applied in the absence of any tissue hypoxia, elicited significantly smaller outward currents (2.9 ± 0.9 pA; Fig. 3B) and decreases in input resistance (25 ± 4%; Fig. 3C) compared with Norm rats (both P < 0.05). There was no statistical difference in either hypoxia-induced responses or diazoxide-induced responses between CIH rats and CSH rats (Figs. 3 and 4).
Levels of KATP channel subunits.
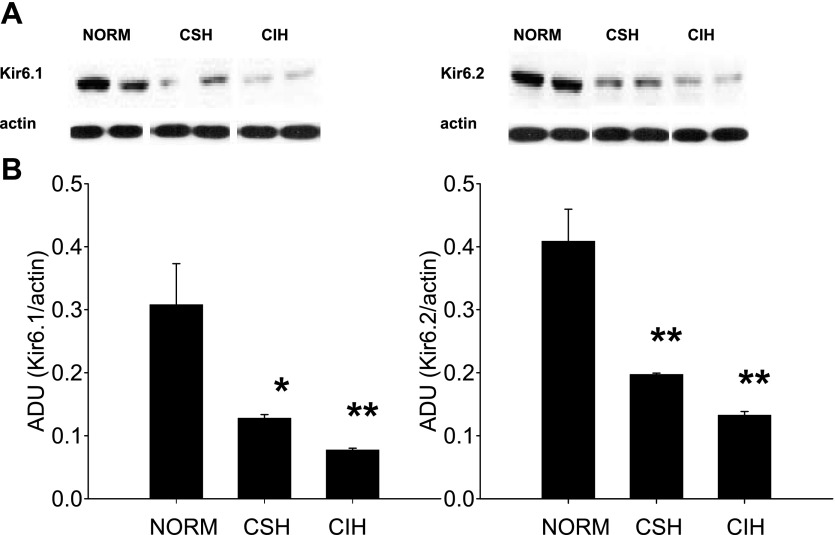
Compared with Norm rats (n = 4), the levels of the KATP channel subunits Kir6.1 and Kir6.2 in caudal NTS were significantly reduced in both CSH [n = 3; 58 ± 2% (P < 0.05) and 75 ± 2% (P < 0.01)] and CIH (n = 4; 52 ± 1% and 68 ± 2%, P < 0.01) rats. There was no difference in the level of these subunits between CSH rats and CIH rats (Fig. 5). The intensity of the β-actin bands was similar across all conditions, indicating that CIH and CSH did not alter β-actin levels and that sample loading was equal across all lanes.
Fig. 5.
Level of KATP channel potassium channel subunits in the NTS are reduced by chronic exposures to hypoxia. A: raw data of Western blot showing the Kir6.1 and Kir6.2 subunit levels in caudal NTS collected from Norm, CSH, and CIH rats. B: group data showing downregulation of potassium channel subunits in caudal NTS from CSH (n = 3) and CIH (n = 4) rats compared with Norm rats (n = 4). There was no significant difference between CSH and CIH rats. *P < 0.05, **P < 0.01, compared with Norm rats. ADU, arbitrary densitometric unit.
Responses of non-DiA-labeled NTS neurons.
To determine whether responses to acute hypoxia were specific to NTS neurons receiving arterial chemoreceptor inputs, we examined the responses of 13 non-DiA-labeled NTS neurons from Norm rats to 3-min hypoxia. We selected spindle-shaped or oblong neurons of size similar to nearby DiA-labeled neurons. Unlike DiA-labeled second-order neurons, the responses in non-DiA-labeled neurons were heterogeneous. In 5 of 13 neurons, hypoxia induced an inward current that averaged 3.9 ± 1.0 pA. In the remaining 8 of 13 non-DiA-labeled neurons, hypoxia induced an outward current that averaged 6.5 ± 2.3 pA, ranging from 0.6 to 19.5 pA. While there was overlap between the amplitudes of hypoxia-induced outward current in DiA-labeled vs. non-DiA-labeled NTS neurons, the mean value for the non-DiA-labeled population was significantly less than that observed in DiA-labeled NTS neurons (P = 0.023). In five non-DiA-labeled NTS neurons from CSH rats 3 min of hypoxia induced outward currents of 1.5 and 7.6 pA in two neurons, while in the remaining three neurons inward currents of −3.5 ± 0.9 pA were observed. These results suggest that compared with second-order neurons receiving peripheral chemoreceptor inputs, other NTS neurons have heterogeneous responses to tissue hypoxia that do not appear to be altered by chronic exposure to sustained hypoxia.
DISCUSSION
We have shown that hypoxia induces an outward current in NTS neurons receiving arterial chemoreceptor afferent inputs. This outward current is primarily mediated by potassium and exhibits sensitivity to known pharmacological modulators of neuronal KATP channels. The hypoxia-induced current was attenuated in NTS neurons after exposure to CIH or CSH and was associated with a reduction in NTS levels of neuronal KATP channel subunits. If the outward current evoked by activation of KATP channels during hypoxia normally damps NTS neuronal responses to excitatory chemoreceptor afferent inputs, reduced KATP channel function could lead to increased NTS neuronal discharge during hypoxia. Such a mechanism might contribute to the enhanced chemoreflex responses observed after CSH (6) and CIH (4, 23). An attenuation of the function of KATP channels in the NTS during CSH and CIH establishes a new, central neuronal adaptive mechanism(s) to chronic exposures to hypoxia.
Activation of neuronal KATP channels hyperpolarizes neurons and reduces excitability, protecting the neurons during periods of reduced ATP (5). Previous studies have detected KATP channels in the NTS (10, 13); however, these studies did not examine second-order peripheral chemoreceptor neurons. Furthermore, we found that in non-DiA-labeled NTS neurons the currents induced by tissue hypoxia were heterogeneous in both sign and amplitude. We provide electrophysiological evidence of functional KATP channels in second-order neurons of peripheral chemoreceptors. Our results suggest the physiological significance of neuronal KATP channels in peripheral chemoreflexes. Hypoxia increases excitatory, glutamatergic chemoreceptor afferent inputs to second-order neurons in the NTS (27, 35). Activation of KATP channels in these neurons during hypoxia could damp responses to excitatory synaptic inputs from peripheral chemoreceptors. This could protect neurons from overexcitation due to excessive presynaptic glutamate release and optimize the reflex performance during hypoxia.
Responses to the KATP channel agonist diazoxide were examined in the absence of tissue hypoxia. Therefore, the fact that the diazoxide-induced outward current was less in the CSH and CIH rats is consistent with our finding of reduced hypoxia-induced KATP currents and KATP receptor subunit levels in these models of hypoxia. In our opinion, this reflects a general deficit in KATP channel function following chronic exposures to sustained and intermittent hypoxia, whether KATP channel function is gauged by neuronal responses to tissue hypoxia or diazoxide.
An additional novel finding is that after exposure to either CIH or CSH there is downregulation of neuronal KATP channels in second-order neurons of peripheral chemoreceptors in the NTS. Studies in heart and brain have shown that short-term hypoxia/ischemia has a preconditioning effect and enhances KATP channel activity to provide additional protection from subsequent episodes of hypoxia/ischemia (8, 26, 33). However, there is little information about the effect of long-term hypoxia/ischemia on the function of KATP channels (3, 12, 32). Both electrophysiological and Western blot analyses indicate that chronic exposures to either sustained or intermittent hypoxia are associated with reduced KATP channel function in the NTS. This could be a crucial adaptive mechanism of the neurons in central peripheral chemoreflex pathways.
However, this neuroprotective mechanism could be deleterious to chemoreflex function. While the activation of KATP channels can reduce neuronal excitability, excessive activation of this channel can ultimately silence neurons (19). In addition, excessive activation of KATP channels could increase extracellular potassium levels and intracellular potassium loss and eventually lead to neuronal apoptosis (34). These factors could prove disadvantageous to the chemoreflex since it is necessary to maintain some degree of neuronal excitability in peripheral chemoreflex pathways to maintain chemoreflex function and attempt to supply oxygen to crucial organs. Reduced KATP channel function could represent an adaptive mechanism to ensure the proper function of peripheral chemoreflexes during long-term exposures to hypoxia. Thus, when facing chronic exposure to hypoxia, reduced KATP channel function could provide a survival advantage to the organism that helps maintain the ability to respond to arterial chemoreceptor afferent inputs. However, this proposed survival advantage to the organism may ultimately prove disadvantageous to the neuron. Both sustained and intermittent hypoxia have been reported to induce apoptosis in PC-12 cultures (17).
The cellular mechanisms whereby chronic exposures to sustained and intermittent hypoxia induce downregulation of neuronal KATP channels are currently unknown. Our Western blot analysis results suggest that the reduced KATP current is due to changes in the expression of KATP channel proteins, although changes in channel structure cannot be excluded. Alternatively, hypoxia-induced downregulation of neuronal KATP channels could be due to a direct postsynaptic effect of hypoxia, because chronic hypoxia can alter ATP synthesis (12). During hypoxia, anaerobic ATP production can be upregulated to compensate for reduced mitochondrial oxidative phosphorylation. Therefore, increases in intracellular ATP could conceivably reduce the KATP currents recorded in neurons from our chronic hypoxic rats. However, the function of KATP channels is more dependent on local ATP levels in the vicinity of the channels than on the concentration of ATP in the bulk cytosolic solution (31). Furthermore, our patch recording pipette contained the same concentration of ATP in all experiments; therefore, the intracellular level of ATP was likely similar in the cells we studied. It should be noted that our pipette solution contained 2 mM Mg2ATP. The inclusion of ATP in the pipette solution may inhibit the activation of KATP channels. Normal intracellular ATP is thought to be in the millimolar range, so that KATP channels are strongly inhibited under normal cellular conditions (25). The ability of diazoxide to bind to the KATP channels also depends on the intracellular level of ATP. Diazoxide opens KATP channels in the presence of 0.3 mM ATP, but is ineffective with 5 mM ATP (28). These data suggest that the KATP currents we have observed in NTS neurons are very likely underestimated. Under physiological conditions, we expect that these channels would have faster and larger responses to hypoxia.
Another potential mechanism that mediates chronic hypoxia-induced downregulation of neuronal KATP channels could be hypoxia-induced neurochemical changes. Extracellular adenosine levels increase in the NTS during hypoxia (16). Adenosine has been shown to induce PKC-mediated downregulation of neuronal KATP channels mediated by receptor internalization (19). The precise mechanism(s) underlying our observation requires further investigation.
We did not observe significant differences between CIH and CSH neurons in both electrophysiological responses and KATP channel subunit levels. Because the activity of KATP channels is minimal after chronic exposures to either sustained or intermittent hypoxia, it is likely that our protocols of CIH and CSH exposure have already induced maximal reduction of KATP channels in the NTS, as strongly suggested by the Western blotting results. However, it is unclear whether the downregulation of KATP channels in these two different chronic hypoxia exposure protocols develops in a similar pattern. We also do not know whether or how the downregulation recovers after chronic exposures to hypoxia. This information is crucial to our understanding of neuronal adaptations to chronic exposures to sustained and intermittent hypoxia.
Our electrophysiological data suggest that the reduction in hypoxia-induced outward current occurs specifically in cells receiving peripheral arterial chemoreceptor afferent inputs. It may seem surprising that an electrophysiologically observed change in a subpopulation of NTS neurons would be reflected in an immunoblot obtained from an NTS region containing many neurons in addition to the cells from which recordings have been obtained. One might expect that changes in the subpopulation would be obscured by different or no changes in the other (non-DiA labeled) neurons in the sample. However, we cannot exclude the possibility that some of the non-DiA-labeled neurons undergo the same changes in hypoxia-induced outward current as the DiA-labeled cells and contribute to the reduced level of KATP channel proteins as measured in Western blots. The heterogeneity of the amplitudes of the hypoxia-induced outward currents observed in the population of non-DiA-labeled NTS neurons precluded us from making a meaningful comparison to rats chronically exposed to sustained or intermittent hypoxia. This provides strong support that our use of DiA labeling is identifying a homogeneous subpopulation of NTS neurons and emphasizes the value of reducing heterogeneity in the sample population by identification of afferent input.
Perspectives and Significance
We found that long-term exposure to either intermittent or sustained hypoxia downregulates KATP channel function in the NTS. Acute hypoxia activates arterial peripheral chemoreceptors, which increases excitatory glutamatergic inputs to NTS neurons. At the same time, hypoxia activates KATP channels, which reduce the excitability of NTS second-order neurons. Activation of KATP channels could protect neurons from potential damage caused by excitation of chemoreceptor afferent inputs and excitotoxicity due to increased afferent fiber release of glutamate. During chronic exposures to intermittent or sustained hypoxia, persistent physiological responses to hypoxia are crucial to guarantee oxygen supply. The downregulation of NTS neuronal KATP channels could contribute to maintain the excitability of the second-order neurons and peripheral chemoreflex pathways. We suggest that this is an example of hypoxia-induced neural plasticity in peripheral chemoreflex pathways following chronic exposures to intermittent or sustained hypoxia.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-41894 and HL-88052 (to S. W. Mifflin) and HL-62579 (to J. T. Cunningham).
Acknowledgments
The authors acknowledge the expert technical assistance of Myrna Herrera-Rosales and Melissa Vitela.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev 20: 101–135, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft FM Adenosine 5′-triphosphate-sensitive potassium channels. Annu Rev Neurosci 11: 97–118, 1988. [DOI] [PubMed] [Google Scholar]
- 3.Asemu G, Papousek F, Ostádal B, Kolár F. Adaptation to high altitude hypoxia protects the rat heart against ischemia-induced arrhythmias. Involvement of mitochondrial KATP channel. J Mol Cell Cardiol 31: 1821–1831, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Baker TL, Fuller DD, Zabka AG, Mitchell GS. Respiratory plasticity: differential actions of continuous and episodic hypoxia and hypercapnia. Respir Physiol 129: 25–35, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Ballanyi K Protective role of neuronal KATP channels in brain hypoxia. J Exp Biol 207: 3201–3212, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Bisgard GE, Neubauer JA. Peripheral and central effects of hypoxia. In: Regulation of Breathing (2nd ed.), edited by Dempsey JA, Pack A. New York: Dekker, 1995, p. 617–668.
- 7.Busija DW, Lacza Z, Rajapakse N, Shimizu K, Kis B, Bari F, Domoki F, Horiguchi T. Targeting mitochondrial ATP-sensitive potassium channels—a novel approach to neuroprotection. Brain Res Brain Res Rev 46: 282–294, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MV, Baines CP, Downey JM. Ischemic preconditioning: from adenosine receptor to KATP channel. Annu Rev Physiol 62: 79–109, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic β-cells. Nature 311: 271–273, 1984. [DOI] [PubMed] [Google Scholar]
- 10.Dallaporta M, Perrin J, Orsini JC. Involvement of adenosine triphosphate-sensitive K+ channels in glucose-sensing in the rat solitary nucleus. Neuroscience 278: 77–80, 2000. [DOI] [PubMed] [Google Scholar]
- 11.de Paula PM, Tolstykh G, Mifflin S. Chronic intermittent hypoxia alters NMDA and AMPA-evoked currents in NTS neurons receiving carotid body chemoreceptor inputs. Am J Physiol Regul Integr Comp Physiol 292: R2259–R2265, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Eells JT, Henry MM, Gross GJ, Baker JE. Increased mitochondrial KATP channel activity during chronic myocardial hypoxia: is cardioprotection mediated by improved bioenergetics? Circ Res 87: 915–921, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira M, Browning KN, Sahibzada N, Verbalis JG, Gillis RA, Travagli RA. Glucose effects on gastric motility and tone evoked from the rat dorsal vagal complex. J Physiol 536: 141–152, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher EC Physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol 90: 1600–1605, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Foley CM, Stanton JJ, Price EM, Cunningham JT, Hasser EM, Heesch CM. GABAA alpha1 and alpha2 receptor subunit expression in rostral ventrolateral medulla in nonpregnant and pregnant rats. Brain Res 975: 196–206, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Gourine AV, Llaudet E, Thomas T, Dale N, Spyer KM. Adenosine release in nucleus tractus solitarii does not appear to mediate hypoxia-induced respiratory depression in rats. J Physiol 544: 161–170, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gozal EL, Sachleben LR, Rane MJ, Vega C, Gozal D. Mild sustained and intermittent hypoxia induce apoptosis in PC-12 cells via different mechanisms. Am J Physiol Cell Physiol 288: C535–C542, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Hinojosa-Laborde C, Mifflin SW. Sex differences in blood pressure response to intermittent hypoxia in rats. Hypertension 46: 1016–1021, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Hu K, Huang CS, Jan YN, Jan LY. ATP-sensitive potassium channel traffic regulation by adenosine and protein kinase C. Neuron 38: 417–432, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Ilyinsky O, Mifflin S. Chronic hypoxia abolishes expiratory prolongation following carotid sinus nerve stimulation in the anesthetized rat. Respir Physiol Neurobiol 146: 269–277, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Ilyinsky O, Tolstykh G, Mifflin S. Chronic hypoxia abolishes posthypoxia frequency decline in the anesthetized rat. Am J Physiol Regul Integr Comp Physiol 285: R1322–R1330, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Mueller PJ, Foley CM, Heesch CM, Cunningham JT, Zheng H, Patel KP, Hasser EM. Increased nitric oxide synthase activity and expression in the hypothalamus of hindlimb unloaded rats. Brain Res 1115: 65–74, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Neubauer JA Physiological and pathophysiological responses to intermittent hypoxia. J Appl Physiol 90: 1593–1599, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Noma A ATP-regulated K+ channels in cardiac muscle. Nature 305: 147–148, 1983. [DOI] [PubMed] [Google Scholar]
- 25.Ohno-Shosaku T, Yamamoto C. Identification of an ATP-sensitive K+ channel in rat cultured cortical neurons. Pflügers Arch 422: 260–266, 1992. [DOI] [PubMed] [Google Scholar]
- 26.O'Rourke B Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res 94: 420–432, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sapru HN Carotid chemoreflex. Neural pathways and transmitters. Adv Exp Med Biol 410: 357–364, 1996. [PubMed] [Google Scholar]
- 28.Schwanstecher M, Löser S, Rietze L, Panten U. Phosphate and thiophosphate group donating adenine and guanine nucleotides inhibit glibenclamide binding to membranes from pancreatic islets. Naunyn Schmiedebergs Arch Pharmacol 343: 83–89, 1991. [DOI] [PubMed] [Google Scholar]
- 29.Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol 81: 133–176, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Tolstykh G, Belugin S, Mifflin S. Responses to GABAA receptor activation are altered in NTS neurons isolated from chronic hypoxic rats. Brain Res 1006, 107–113, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Trapp S, Ballanyi K. KATP channel mediation of anoxia-induced outward current in rat dorsal vagal neurons in vitro. J Physiol 487: 37–50, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia Y, Eisenman D, Haddad GG. Sulfonylurea receptor expression in rat brain: effect of chronic hypoxia during development. Pediatr Res 34: 634–641, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Yamada K, Inagaki N. Neuroprotection by KATP channels. J Mol Cell Cardiol 38: 945–949, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Yu SP, Yeh CH, Sensi SL, Gwag BJ, Canzoniero LMT, Farhangrazi ZS, Ying HS, Tian M, Dugan LL, Choi DW. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science 278: 114–117, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Mifflin SW. Excitatory amino acid receptors within NTS mediate arterial chemoreceptor reflexes in rats. Am J Physiol Heart Circ Physiol 265: H770–H773, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, Mifflin SW. Modulation of synaptic transmission to second-order peripheral chemoreceptor neurons in caudal nucleus tractus solitarius by alpha1-adrenoreceptors. J Pharmacol Exp Ther 320: 670–677, 2007. [DOI] [PubMed] [Google Scholar]