Abstract
The present study was designed to take advantage of telemetry data acquisition and develop an easy and reliable system to servocontrol renal perfusion pressure (RPP). Digitized pressure signals from lower abdominal aorta in rats, reflecting RPP, was obtained by a telemetry device and dynamically exported into an Excel worksheet. A computer program (LabVIEW) compared the RPP data with a preselected pressure range and drove a bidirectional syringe pump to control the inflation of a vascular occluder around the aorta above renal arteries. When RPP was higher than the preselected range, the syringe pump inflated the occluder and decreased RPP, and vice versa. If RPP was within range, there was no action. In this way, RPP was servocontrolled within the desired range. In experiments with norepinephrine- or ANG II-induced acute increases in systemic arterial pressure (120–145 mmHg), the system controlled RPP at a constant range of 100–105 mmHg within 30–50 s and differentiated the pressure-dependent and -independent effects on renal functions. In Dahl S rats with high-salt-induced hypertension, this system maintained RPP at 100–120 mmHg over 10 days, while systemic arterial pressures were 150 ± 5.9 mmHg in uncontrolled animals. This system also has the ability of simultaneity and multiplexing to control multiple animals. Our results suggest that this is an effective and reliable system to servocontrol RPP, which can be easily established with general computer knowledge. This system provides a powerful tool and may greatly facilitate the studies in pressure-dependent/-independent effects of a variety of cardiovascular factors.
Keywords: LabVIEW, pressure dependent, urinary sodium excretion, renal blood flow
renal perfusion pressure (RPP) is significantly involved in the regulation of renal functions, such as blood flow autoregulation, renin release, and sodium excretion. Meanwhile, a variety of physiological controllers regulate renal function through their direct effects on renal tubular functions and intrinsic hemodynamics within the kidneys as well as indirect effects by altering RPP, which activates the intrarenal mechanisms. Moreover, many pathogenic factors may induce renal injury also through their direct damage actions on the kidneys (RPP independent) and indirect damage actions by changing RPP that causes renal dysfunction (RPP dependent) (4, 5, 7, 13, 15). To study the impact of RPP on renal function and dissect the pressure-dependent and -independent effects of different cardiovascular or renal controllers, a device to control the RPP is required. In previous studies, several servocontrol systems have been developed for use in different animal models, such as dogs, rabbits, and rats (8, 14, 17, 19). However, these servocontrol systems were designed to use analog signals of blood pressure obtained from indwelling arterial catheters and are difficult to build and maintain. This is especially true for bioscience researchers who lack electronic engineering knowledge and facilities, which significantly limited the use of this technique. Today's advanced computer technology makes auto instrument control much easier and allows us to develop a simple and reliable servocontrol system for RPP.
The use of telemetric measurement of arterial blood pressure in animal models is becoming more and more popular, and the American Heart Association recommends this technique for its numerous advantages over other methods in different animal experiments (9). Therefore, it is imperative to establish a method that can utilize digitized signals of blood pressure obtained from telemetry devices to servocontrol organ perfusion pressure, such as renal, coronary, and cerebral vascular beds. Additionally, digitized RPP data obtained from a telemetry system also makes it simple to employ computer technology for the servocontrol of RPP. In the present study, we developed an RPP servocontrol system using a pressure telemetry device and LabVIEW computer software (National Instruments). This system effectively achieved a stable servocontrol of RPP under different acute and chronic experimental conditions and can be easily built with general computer knowledge.
METHODS
Animals.
Experiments were performed on male Sprague-Dawley rats (Harlan), SS13BN and Dahl S rats (Charles River) weighing 250 to 300 g. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University.
Telemetry measurement of RPP.
RPP was monitored as we described previously (10). In brief, a catheter connected to a telemetry transmitter was implanted into the infrarenal aorta via cannulating the femoral artery and the transmitter was placed subcutaneously; the RPP signal from the transmitter was received by a remote receiver and then recorded by a computer program [Data Sciences International (DSI)].
Implantation of inflatable Silastic aorta occluder.
To control RPP, an inflatable Silastic vascular occluder (customer made, 1.5-mm lumen diameter, 2.5-mm width; DocXS Biomedical Products) was implanted around the aorta above the renal arteries through a left-mid-sagittal incision, and the extension tubing was tunneled out at the back of the neck and passed through a flexible spring that was attached to the rat with a skin button. This spring was then held by the arm of a Raturn cage (stand-alone Raturn cage for rat; Bioanalytical Systems) in which the rat was housed. The aorta occluder extension tubing was infused with sterile saline to adjust the pressure in the occluder using a bidirectional syringe pump (model NE-1000; New Era Pump Systems), which was controlled by a computer program. The Raturn cage is a servocontrolled turning cage, which automatically counterrotates the circular cage in a direction opposite to the rat movement. This device allows the rat to freely move and prevents any twist of the tubing implanted around the aorta, which warrants a continuous infusion without interruption by rat movement. After surgery, animals were given a 1-wk recovery period before experiments started. Sham-controlled animals were subjected to the same surgery without inflation of the aorta occluders. Animals learned to use the Raturn system quickly, and the recovery period after surgery conditioned all rats to the Raturn cage for 1-wk before experiments.
Telemetric signal-driven servocontrol of RPP.
The servocontrol of RPP is accomplished by a feedback loop developed using LabVIEW, an instrument-controled computer software. The LabVIEW program dynamically analyzes RPP signals and drives infusion or withdrawal of the bidirectional syringe pump to control the inflation of the aorta occluder and, consequently, regulate RPP. The upper and lower points of preselected RPP range are set first. When the RPP is higher than the upper preset point, the syringe pump starts infusion to inflate the aorta occluder that decreases RPP. When the RPP is lower than the lower preset point, the syringe pump starts to withdraw to deflate the aorta occluder that increases RPP. If RPP is within the preselected range, the syringe pump stops infusion/withdrawal. In this way, a stable RPP is maintained at a desired range no matter what the systemic arterial pressure is. The system set up is described in detail in the following paragraphs.
A brief introduction of LabVIEW.
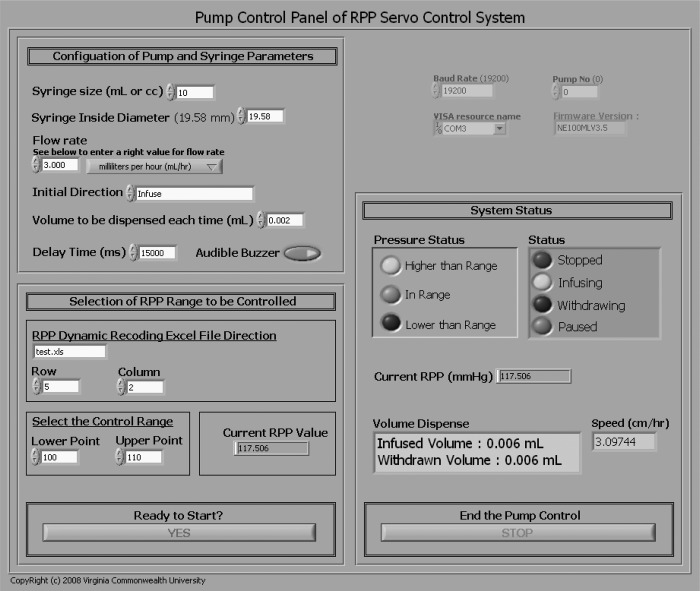
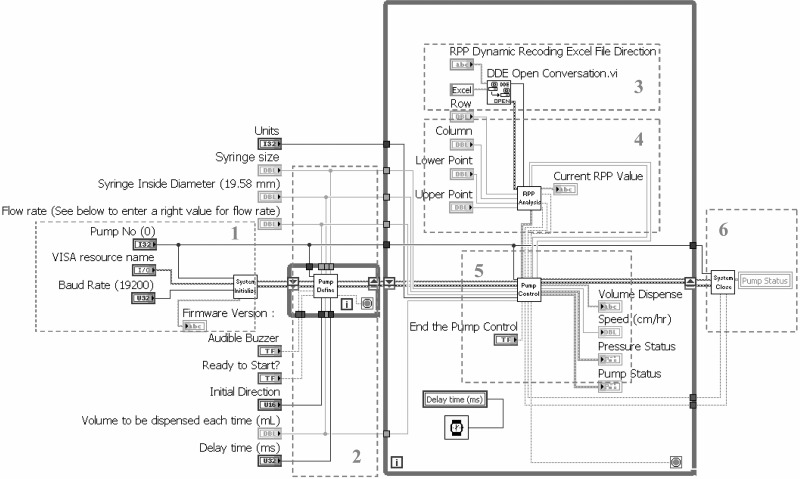
LabVIEW is a very user-friendly software for developing computer programs for data acquisition and instrument control. It does not require comprehensive computer knowledge and is easy to learn. LabVIEW uses graphical programming language. Its graphical dataflow language and block diagram approach naturally represent the flow of data and intuitively map user interface controls to data, so novice programmers can build automated systems quickly and easily. Strong technique support warrants the grasp of the software by a new user. Figure 1 is the front panel of the system, which is the interface for an operator to run the system. (Color versions of the figures are posted with the online version of this article.) Figure 2 is a diagram of our program for servocontrol. The programmer defines the flow of data and the execution of the application through a concept known as dataflow programming. Block diagrams consist of functions that are represented by icons. What the programmer needs to do is to wire the connections of these icons and structures that control execution logic.
Fig. 1.
Front panel of system description in LabVIEW. It contains four areas: initialization (top), parameters configuration (left middle), system status (right middle), and system on and off switches (bottom).
Fig. 2.
Block diagram of system programmed in LabVIEW. It can be described by 6 blocks: 1) system initialization; 2) system configuration; 3) Digital Data Exchange connection; 4) RPP data analysis; 5) pump control and system status monitoring; and 6) system close. A color version of this figure is posted with the online version of this article.
Dynamic input of RPP data into Excel.
During RPP data acquisition, the DSI system can export the pressure data dynamically into an Excel worksheet at the same time. However, the data in the Excel worksheet is updated by adding RPP data into the new cells at the end of a column in the worksheet, and, therefore, the RPP value in a certain cell in the worksheet is not updated. Because LabVIEW can only request data from a fixed Excel cell, it is unable to read dynamic RPP data in this condition. This problem can be solved by setting the dynamic time period short (e.g., <1 min) when exporting RPP data into Excel, which updates the RPP data within a certain Excel worksheet cell real time. In other words, in DSI dynamic data export function, there is an option that allows the change of dynamic time period (time span) from seconds to hours or days, and a 1-min time period contains four 15-s data in four Excel cells in our case. The data update/addition of new RPP data is to shift the four 15-s data up within these four Excel cells, which makes the old number at the top moved out and a new number added at the bottom of these four Excel cells. The positions/locations of these four Excel cells in a worksheet are fixed and not changed. What changes is the data within these four Excel cells. In initial setup, Excel file name and location (row, column) of the Excel cell that contains updated RPP data are input into the control panel of the system (Fig. 1), and then LabVIEW is able to request dynamic RPP data by reading only a certain cell in an Excel worksheet. Dynamic data export does not alter the normal data-saving and data-analysis functions of DSI.
Data exchange between Excel worksheet and LabVIEW.
LabVIEW cannot directly read data from an Excel worksheet to obtain RPP data. However, both Excel and LabVIEW support Digital Data Exchange (DDE) protocol, which is a protocol to establish communications among multiple applications. In LabVIEW, a Virtual Instrument can be set up as a DDE client to request and import the RPP data from Excel into LabVIEW. A free DDE Client virtual instrument for LabVIEW can be downloaded from the National Instruments website (http://zone.ni.com/devzone/cda/epd/p/id/2231).
Syringe pump control by LabVIEW.
Syringe pumps used in the present study were from New Era Pump Systems. This vender has developed a LabVIEW driver program to control their syringe pumps, which can be downloaded for free from the National Instruments website (http://sine.ni.com/apps/utf8/niid_web_display.download_page?p_id_guid=10DD8233D9A108ECE0440003BA230ECF). The syringe pump control commands to servocontrol RPP in the present study were based on this program.
Program of the feedback loop for servocontrol of RPP in LabVIEW.
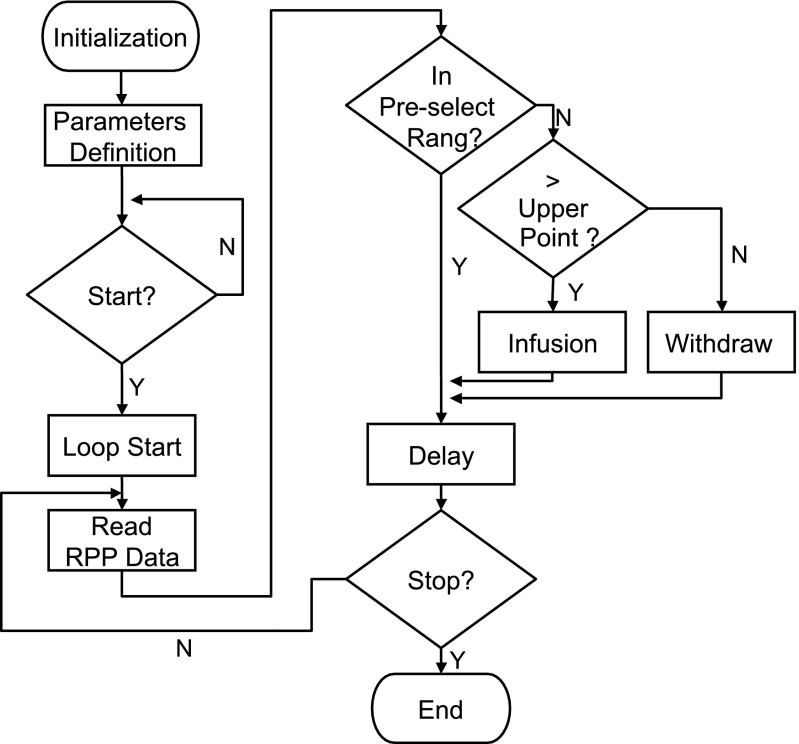
Design flow of the feedback loop for servocontrol of RPP is elucidated in Fig. 3, which corresponds to the blocks in Fig. 2, as described below. In brief, the first step is to initialize the communication between LabVIEW and the syringe pump, which involves basic parameters including pump model, pump address, and connecting port (block 1 in Fig. 2). The second is to define the parameters, which acquires syringe size, speed, and dispensing volume (block 2 in Fig. 2), as well as Excel cell locations where RPP data and upper and lower points of preselected RPP range are input (block 4). After completion of all the above parameters, a feedback loop is followed (frame consisting of blocks 3, 4, and 5) after the start switch is clicked. The gray frame is a While Loop structure in LabVIEW, which repeats the execution of the blocks within the structure in a specific order. The logic of the feedback loop is as follow: DDE client reads the dynamic RPP data (block 3) and imports them into a comparator (block 4), in which the RPP is estimated to see whether it is within the preselected range. If yes, the system maintains the current status. Otherwise, another comparator is activated to determine whether the RPP is higher than the upper point of the preselected range. If yes, an infusion signal is generated to inflate the aorta occluder, which decreases RPP. If not, a “withdraw” signal is generated to deflate the aorta occluder, which increases RPP. After any of the above three actions, i.e., maintaining current statement, infusing, or withdrawing the syringe pump (block 5), the program is wired back to DDE reading RPP (block 3) to create a feedback loop. Therefore, the above steps are repeated for the analyses of RPP against the preselected range and the adjustment of the syringe pump, therefore accomplishing servocontrol of RPP.
Fig. 3.
Design flow of the feedback loop for servocontrol of renal perfusion pressure (RPP).
RPP data acquisition in the DSI system is set for a period of 5 s, and the interval between every data acquisition is 15 s, because the interval for repeating data acquisition in DSI could not be set < 10 s. For this reason, a 10-s synchronous delay is incorporated in the loop. After all, the loop analyzes RPP and adjusts syringe pump every 15 s and is able to continuously run until an “interrupt” command is present, which will turn off the loop and end the system. Simultaneous control of multiple animals is achieved by defining different addresses/numbers of syringe pumps and linking the pumps to specific Excel cells of different RPP data from corresponding animals. The system can control up to 250 animals, depending on the setup of the DSI system. A DSI system can accommodate up to 33 data exchange matrices and each matrix accepts 16 inputs of RPP data. However, a software limitation of 250 parameters allows servocontrol of a maximum of 250 rats.
Servocontrol of RPP in acute experiments.
Animals were prepared for acute renal functional study as we described previously (10, 12). In brief, male Sprague-Dawley (SD) rats were anesthetized with ketamine (30 mg/kg im) (Phoenix) and thiobutabarbital (inaction, 50 mg/kg ip) (Sigma), and then placed on a thermostatically controlled warming table to maintain body temperature at 37°C. Servocontrol of RPP was performed as described above. A catheter was placed in the left femoral vein for intravenous infusion. For renal blood flow (RBF) measurement, a transonic flow probe (2 mm) was placed around the left renal artery and RBF was measured with a flowmeter (Transonic Systems) as described previously (11). A plastic catheter was inserted into the left ureter for urine collection. After surgery, the animals received a continuous infusion of 0.9% NaCl solution containing 2% albumin at a rate of 1 ml·h−1·100 g body wt−1 throughout the experiment to replace fluid losses and maintain a stable hematocrit of ≈ 41.3 ± 1.5%. The urine volume (U·V) was determined gravimetrically, and urinary sodium (Na+) concentrations were measured using a flame photometer. RBF, U·V, and urinary Na+ excretion (UNa·V) were factored per gram kidney weight.
After a 1.5-h equilibration period and two 20-min control collections of urine and blood samples, norepinephrine (NE) was infused intravenously for 50 min (a 10-min clearance period and two 20-min sample collections), followed by two 20-min postcontrol collections after the infusion solution was switched back to saline and a 10-min clearance period. Experiments with RPP servocontrolled and noncontrolled were performed. Additional rats were infused with different concentrations of ANG II to test the sensitivity, accuracy, and stability of the servocontrol system in the regulation of RPP. At the end of the experiment, increased systemic arterial pressure in servocontrolled animals were confirmed by totally releasing the aorta occluder, which allows RPP to represent systemic arterial pressure.
Servocontrol of RPP in chronic experiment.
Rats were implanted with vascular occluders and telemetry transmitters as described above. After recovery from surgery, blood pressures for two control days were recorded, and then animals were challenged with a high-salt diet (8% NaCl; Dyete) for 10 days. The RPP were servocontrolled to a preselected range of 100–120 mmHg. Three groups were included: Dahl S servocontrol, Dahl S sham control, and SS13BN sham control. At the end of the experiment, increased systemic arterial pressure in servocontrolled Dahl S rats were confirmed by total deflation of aorta occluder, and the kidneys from all groups were collected and fixed in formalin for morphological examination of glomerular injury. Glomeruli were evaluated on the basis of the degree of glomerulosclerosis and mesangial matrix expansion (scored from 0 to 4), as previously described (18). In brief, a 1-lesion represented an involvement of 25% of the glomerulus while a 4-lesion indicated that 100% of the glomerulus was involved. The index of glomerular damage was obtained by averaging the scores of 20 glomeruli in each specimen, which was performed independently by two investigators without prior knowledge of the group to which the rat belonged.
To effectively control RPP and produce constant smooth RPP tracings rather than wide fluctuations in RPP, the syringe pump needs to be controlled by two parameters, “Flow Rate/Pump Speed” and “Volume to be Dispensed Each Time” (see Figs. 1 and 2). In our preliminary experiments, we found that a high pump speed could quickly inflate the occluder and bring down the RPP, but easily make the RPP out of the lower point of preset range when the pump continuously infused for 15 s between RPP data acquisitions. The results were that the pump kept infusing and then withdrawing immediately in circles of 15 s, which caused wide oscillations in RPP. This problem is solved by setting a volume to dispense each time the pump action is triggered. For example, when the pump is triggered to infuse at a speed of 50 μl/min for 15 s, it dispenses about 12 μl and causes overshooting that brings RPP too low. However, when we set a dispensing volume of 6 μl, the infusion stops after 6 μl is delivered; and, therefore, during the 15-s interval between RPP data acquisitions the pump only infuses for about 8 s, which avoids the overshoot but reserves the quick response of the system.
Because of the different plasticity of each individual vascular occluder, pump speed and dispensing volume need to be pretested at the very beginning of experiment by setting a preset RPP range with the lower point that is 20 mmHg below the basal MAP level, and then the RPP range is set at 100–120 mmHg after pretest. This process only takes several minutes and should not cause impact on animals. We chose a 20-mmHg difference between the maximum and minimum values for preset RPP range, because a range smaller than 20 mmHg triggers pump withdrawal more often, and easily results in wide fluctuations in RPP. Also, MAPs of most normal rats fall into 100–120 mmHg, and a range of 20 mmHg is acceptable as sufficient control of RPP in previous reports using the servocontrol system, which set a pressure range of ±10 mmHg (14, 16).
Statistics.
Data are presented as means ± SE. The significance of differences in mean values within and between multiple groups was evaluated using an ANOVA followed by a Duncan's multiple range test. Student's t-test was used to evaluate statistical significance of differences between two groups. P < 0.05 was considered statistically significant.
RESULTS
Response of servocontrol system to different levels of RPP after ANG II infusion.
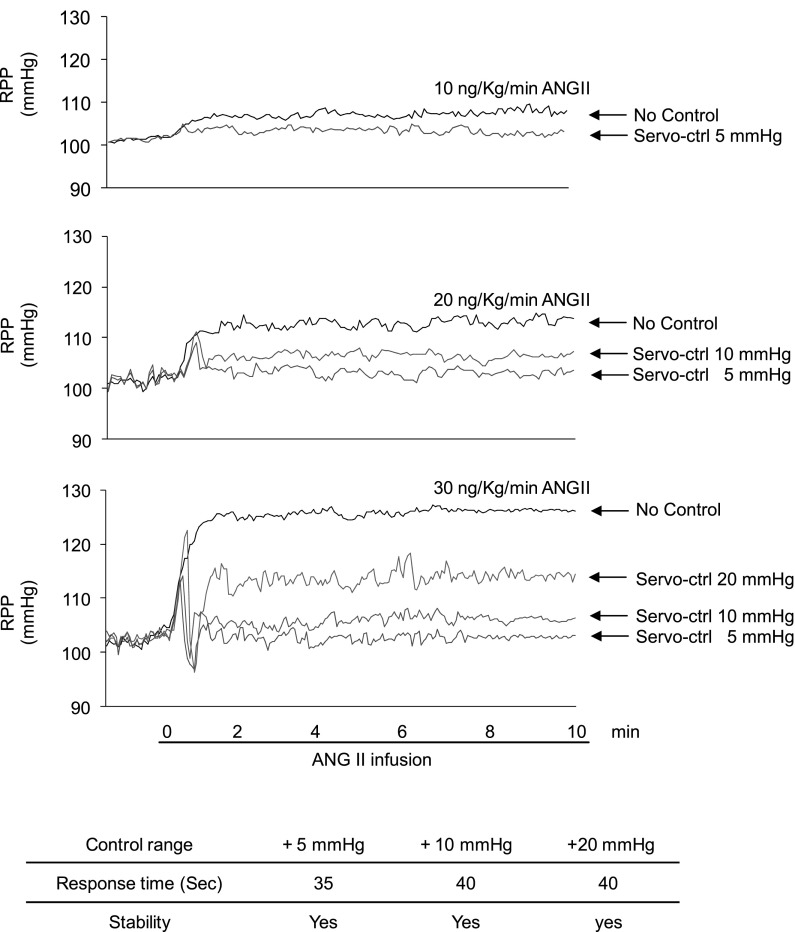
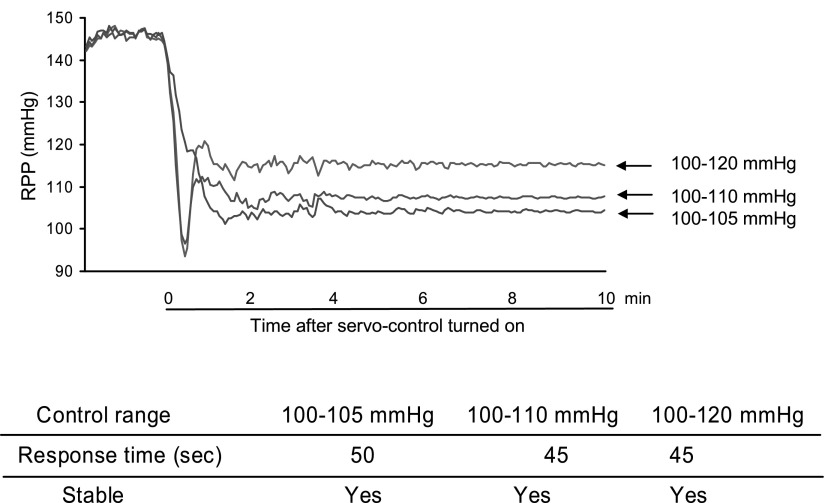
Infusion of ANG II at doses of 10, 20, and 30 ng·kg−1·min−1 induced increases of RPP by 8 ± 2, 16 ± 2.4, and 24 ± 3.7 mmHg from baseline RPP, respectively. In RPP servocontrolled animals, all three doses of ANG II-induced transit RPP increases that were brought into preset ranges quickly and were maintained stable. Three target control ranges were tested. They were 5, 10, and 20 mmHg above the baselines. For example, when baseline RPP was 100 mmHg, the three preset ranges would be 100–105, 100–110, and 100–120 mmHg. The time it took to bring increased RPP into preset ranges were 35, 40, and 40 s, respectively, for these three tested preset ranges. Figure 4 illustrates examples of original recording of RPP trace in servocontrolled and noncontrolled animals.
Fig. 4.
Responses of servocontrol system to the increases in RPP after intravenous infusion of ANG II. The system was turned on before ANG II infusion (10, 20, and 30 ng·kg−1·min−1) in servocontrolled animals. Representative original recording from 6 sets of experiments.
To test the system in a different way, the servocontrol was turned on after RPP had been elevated by ANG II. It took 45–50 s for the system to bring the increased RPP back to preset ranges. In this group of experiments, three target RPP ranges were preset, i.e., 100–105, 100–110, and 100–120 mmHg. The servocontrol system quickly controlled the RPP to the desired ranges and maintained it stable (Fig. 5).
Fig. 5.
Responses of servocontrol system to the increases in RPP after intravenous infusion of ANG II. The system was turned on after ANG II infusion (40 ng·kg−1·min−1) in servocontrolled animals. Representative original recording from 6 sets of experiments.
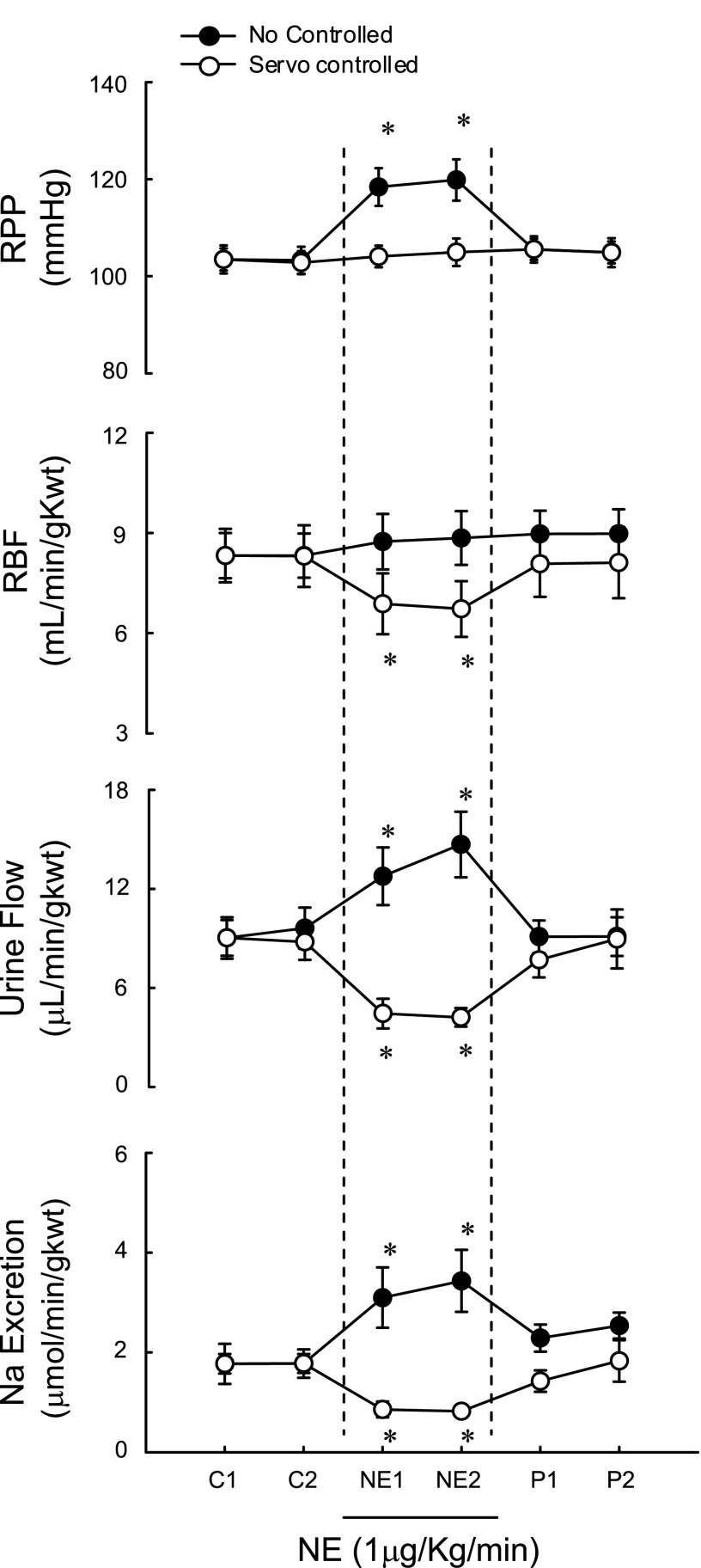
Effect of RPP servocontrol on intravenous infusion of NE-induced changes in renal functions.
Intravenous infusion of NE at a dose of 1 μg·kg−1·min−1 significantly elevated RPP and increased both urine volume and sodium excretion, but had no effect on RBF. However, when RPP was servocontrolled, NE significantly decreased RBF, urine volume, and sodium excretion, indicating that at the dose used in the present study, pressure-independent effects of NE on RBF and renal sodium excretion are inhibitory, while its pressure-dependent effects are stimulatory. The RPP servocontrol system successfully controlled RPP and distinguished pressure-dependent/-independent effects of NE on the renal functions. (Fig. 6).
Fig. 6.
Effect of RPP servocontrol on intravenous infusion of NE-induced changes in renal functions. Labels on x-axes: C, intravenous infusion of saline; NE, norepinephrine (1 μg·kg−1·min−1), P, postcontrol. *P < 0.05 vs. control, n = 5.
Effect of RPP servocontrol on glomerular injury in high-salt-induced hypertensive Dahl S rats.
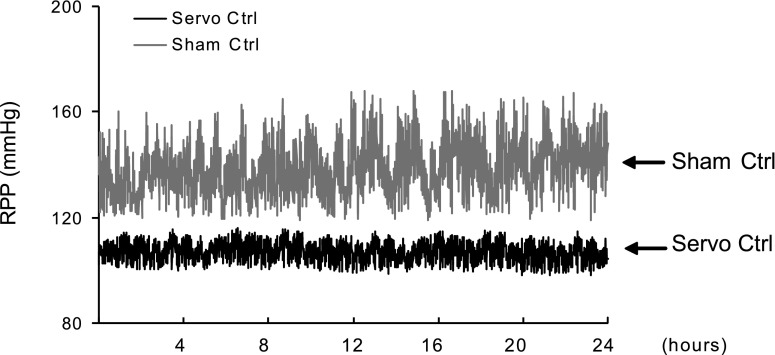
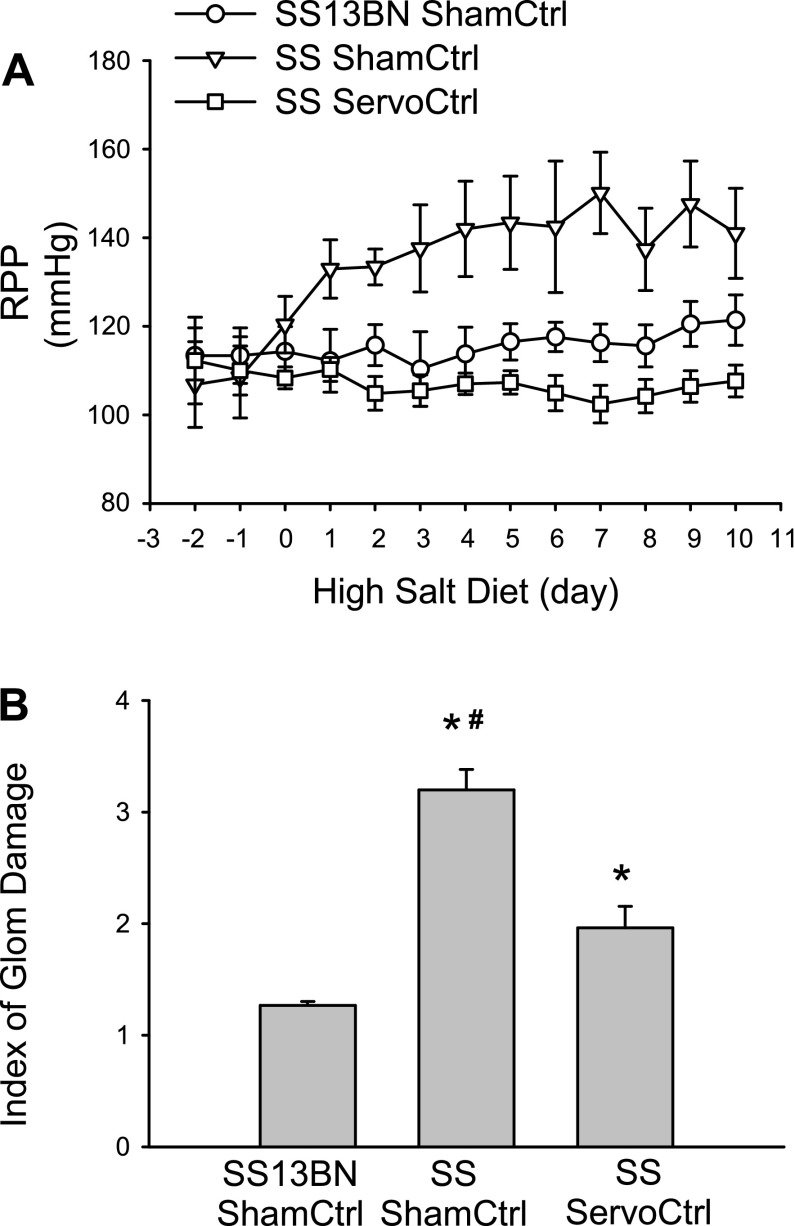
Ten-day high-salt challenge induced a considerable increase of RPP and remarkable glomerular injury in Dahl S rats compared with SS13BN control rats. The RPP servocontrol system effectively maintained a stable RPP within normal range throughout the whole experimental period in Dahl S rats fed with a high-salt diet that was accompanied by a significant attenuation of glomerular injury in these animals. Figure 7 shows 24-h RPP recordings as 1-min averages, which demonstrates that servocontrol maintained a stable RPP within normal range in Dahl S rat after high-salt diet. Although the RPP was dramatically changed between 118–163 mmHg in uncontrolled rats, the RPP in controlled rats was kept within preselected range. Figure 8 summarizes the data of RPP and glomerular damage in different groups of rats.
Fig. 7.
Example of 24-h RPP recording in Dahl S rats after high-salt diet for 6 days.
Fig. 8.
Effect of RPP servocontrol on high-salt-induced glomerular damage in Dahl S rats. A: renal perfusion pressure (RPP). B: index of glomerular damages after high-salt challenge. SS, Dahl salt-sensitive rat. *P < 0.05 vs. SS13BN, #P < 0.05 vs. SS servocontrol, n = 6.
DISCUSSION
The present study demonstrated that this telemetry signal-driven RPP servocontrol system adjusted the increased RPP into desired ranges within < 50 s and maintained a stable RPP in both acute and chronic experiments. This system successfully dissected the pressure-dependent and -independent effects of renal modulators in both acute and chronic experiments. The system worked without error for up to 2 wk and was capable of controlling multiple animals.
An easy system with capacity of controlling multiple animals.
This is a simple system that includes three components: DSI device, LabVIEW software, and syringe pump. Unlike the previous RPP servocontrol system utilizing analog signals, the present servocontrol system does not need extra electronic devices for the analyses of RPP signals and the control of the syringe pump, some of which needs analog-to-digital signal conversion. The system directly uses the preexisting digitized RPP data from the DSI telemetry system and controls the syringe pump using a computer program, which does not need complicated electronic engineering knowledge and devices, as in a traditional analog system. A simple connection between the syringe pump and computer makes the system ready to run. The control panel of the system is straightforward and very easy to operate. There is no need for system maintenance. User-friendly LabVIEW plus preexisting DDE and syringe pump control programs allowed us to build the system easily. Another advantage of the system is controlling multiple animals, which just requires simple additions and links of extra syringe pumps and RPP data into the program.
An effective, stable, and reliable system.
During the test of the system, it took < 50 s to bring the elevated RPP back to desired ranges, demonstrating the quick action of the system. In response to different levels of RPP elevation after various doses of ANG II, the response time was similar, indicating that the system functions effectively under a wide range of systemic arterial pressure. In addition, it worked effectively and similarly when different target RPP ranges were selected. In both acute and chronic experiments, the system ran well and maintained a stable RPP. This is a very reliable system, which continuously ran for up to 2 wk without error in the chronic experiments.
A verified system that differentiates pressure-dependent and -independent effects of renal modulators.
NE is a vessel constrictor and exhibits stimulatory effect on sodium reabsorption (1–3, 6), and therefore should decrease RBF and sodium excretion. However, due to the elevation of RPP induced by NE, the overall effect of NE on renal function was to increase sodium excretion with no change in RBF, indicating a counteraction or influence of increased-RPP on NE's direct effect on renal functions. Our servocontrol system successfully dissected NE's direct effect and pressure-dependent effect on renal function, which further validated the effectiveness of our system.
It should be noted that the use of this servocontrol system in anesthetized rats was to test the response of the system and to verify its effectiveness in dissection of pressure-dependent and -independent effect of vascular controller, which does not necessarily suggest that this is the most appropriate system in these acute experiments because there are easier and cost-effective methods to control RPP in anesthetized animals.
In addition to the evaluation of our servocontrol system in these acute experiments, we also successfully confirmed the usefulness of the system in chronic experiment in high-salt-induced hypertensive Dahl S rats. Servocontrol of RPP significantly protected the glomeruli from high-salt-induced injury in Dahl S rats, indicating that portion of glomerular injury is pressure-dependent, which is consistent with previous studies showing servocontrol of RPP protected the kidneys from high-salt-induced injury in Dahl S rats (16) and ANG II-induced injury in Sprague-Dawley rats (14).
Limitation of the system.
There is an interval of 15 s for repeating RPP adjustments because of the limitation in DSI data acquisition. This low frequency appears a concern because there might be big changes in RPP during this 15-s period. However, our data suggested that there was no significant alteration in RPP, during the interval once the system started running, from the following points. First, pulse pressure significantly decreased from 50 mmHg to 10 mmHg (data not shown) after the pressure was built up in the aorta occluder when the system was on, indicating that dramatic RPP changes are unlikely. Second, in our 10-day chronic experiments, none of the RPP data obtained as 1-min averages was out of the preset range in servocontrolled animals, while RPP had more than 60 mmHg (106–168 mmHg) variations in uncontrolled animals, indicating that changes of RPP during the 15 s, if any, were insignificant. To adjust and maintain a constant mean arterial pressure within 1-min time periods was also considered as effective servocontrol of RPP by other investigators (14, 16). Third, data from both the acute and chronic experiments showed that servocontrol of RPP effectively distinguished the RPP-dependent and -independent effects, which was consistent with previous reports, indicating that RPP is successfully controlled. Therefore, even if there were any undetectable changes of RPP during the 15-s interval, the impact would be negligible. Although the current system is not as fast as the previous analog systems in terms of frequency and response time, this weakness does not, apparently, undermine the effectiveness of our system in the practical application. In addition, this weakness may be corrected in the future when there are more advanced telemetry devices or DSI updates/improves its data acquisition.
Price is an issue to take into consideration for building this servocontrol system. The telemetry blood pressure system used in our study was ∼ $40,000 and the syringe pump ∼ $900, while the Raturn cage was ∼ $4,000. Based on our experience in the experiment of continuous intrarenal interstitial infusion in conscious animals using swivel system, it probably will work fine to replace the Raturn cage with a swivel in this system, which will significantly reduce the cost. In addition, the use of the telemetry system is becoming more and more popular, and therefore, the price of this servocontrol system would be acceptable to many investigators who have already owned a telemetry system, such as the situation when we built our system, the additional expense for us was just for LabVIEW software, which has an academic price of ∼ $600, and the vascular occluder, ∼ $100.
Perspectives and Significance
The present study successfully established an easy, effective, and reliable system for servocontrol of RPP using the telemetry pressure device and computer software. This system provides an simple and powerful tool for researchers, especially for those who don't have access to a difficult analog system, and may greatly facilitate the studies in pressure-dependent and -independent effects of a variety of cardiovascular factors, not only in the kidneys, but also in other organs, such as brain, heart, limbs, etc., using different animals. It may also be a useful tool for some other studies, for example, in chronic organ ischemia, especially controlled different levels of ischemia and/or repeating ischemia/reperfusion. The principle of our system, i.e., using a telemetric signal to servocontrol a syringe pump, may also be applied in many different approaches, for example, servocontrol of drug administration, blood supply, or physical stress, etc., by a variety of physiological parameters, such as heart rate, blood flow, Po2/CO2, biopotential signals, and so on, or even combination and communication of various parameters to which the telemetry transmitters are available. There will certainly be many additional potential applications of the system to advance the researches in integrative physiology by other users who learn this system.
This servocontrol system is new and not perfect. There may be some issues that can be improved for this system. However, it not only provides an alternative RPP servocontrol system to the traditional analog one, but also brings out a new concept using telemetry and computer technology in servocontrol, which may stimulate the development of many new techniques in bioscience research.
GRANTS
This study was supported by National Institutes of Health Grants DK-54927, HL-70726 and HL-89563.
Supplementary Material
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Baines AD, Drangova R, Ho P. α1-Adrenergic stimulation of renal Na reabsorption requires glucose metabolism. Am J Physiol Renal Fluid Electrolyte Physiol 253: F810–F815, 1987. [DOI] [PubMed] [Google Scholar]
- 2.Besarab A, Silva P, Landsberg L, Epstein FH. Effect of catecholamines on tubular function in the isolated perfused rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 233: F39–F45, 1977. [DOI] [PubMed] [Google Scholar]
- 3.Correia AG, Madden AC, Bergstrom G, Evans RG. Effects of renal medullary and intravenous norepinephrine on renal antihypertensive function. Hypertension 35: 965–970, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Cowley AW Role of the renal medulla in volume and arterial pressure regulation. Am J Physiol Regul Integr Comp Physiol 273: R1–R15, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Cupples WA, Braam B. Assessment of renal autoregulation. Am J Physiol Renal Physiol 292: F1105–F1123, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Gullner HG Regulation of sodium and water excretion by catecholamines. Life Sci 32: 921–925, 1983. [DOI] [PubMed] [Google Scholar]
- 7.Hall JE The kidney, hypertension, obesity. Hypertension 41: 625–633, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Hester RL, Granger JP, Williams J, Hall JE. Acute and chronic servo-control of renal perfusion pressure. Am J Physiol Renal Fluid Electrolyte Physiol 244: F455–F460, 1983. [DOI] [PubMed] [Google Scholar]
- 9.Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. Recommendations for blood pressure measurement in humans and experimental animals: Part 2: blood pressure measurement in experimental animals: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 45: 299–310, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Li N, Chen L, Yi F, Xia M, Li PL. Salt-sensitive hypertension induced by decoy of transcription factor hypoxia-inducible factor-1α in the renal medulla. Circ Res 102:1101–1108, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li N, Yi F, Sundy CM, Chen L, Hilliker ML, Donley DK, Muldoon DB, Li PL. Expression and actions of HIF prolyl-4-hydroxylase in the rat kidneys. Am J Physiol Renal Physiol 292: F207–F216, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Li N, Zhang G, Yi FX, Zou AP, Li PL. Activation of NAD(P)H oxidase by outward movements of H+ ions in renal medullary thick ascending limb of Henle. Am J Physiol Renal Physiol 289: F1048–F1056, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Mattson DL Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol 284: R13–R27, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Mori T, Cowley AW Jr. Role of pressure in angiotensin ii-induced renal injury: chronic servo-control of renal perfusion pressure in rats. Hypertension 43: 752–759, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Mori T, Cowley AW Jr, Ito S. Molecular mechanisms and therapeutic strategies of chronic renal injury: physiological role of angiotensin II-induced oxidative stress in renal medulla. J Pharm Sci 100: 2–8, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Mori T, Polichnowski A, Glocka P, Kaldunski M, Ohsaki Y, Liang M, Cowley AW Jr. High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol. In press. [DOI] [PMC free article] [PubMed]
- 17.Nafz B, Persson PB, Ehmke H, Kirchheim HR. A servo-control system for open- and closed-loop blood pressure regulation. Am J Physiol Renal Fluid Electrolyte Physiol 262: F320–F325, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26: 137–143, 1984. [DOI] [PubMed] [Google Scholar]
- 19.Woods LL, Mizelle HL, Hall JE. Autoregulation of renal blood flow and glomerular filtration rate in the pregnant rabbit. Am J Physiol Regul Integr Comp Physiol 252: R69–R72, 1987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.