Abstract
Chlorine (Cl2) is a highly reactive oxidant gas used extensively in a number of industrial processes. Exposure to high concentrations of Cl2 results in acute lung injury that may either resolve spontaneously or progress to acute respiratory failure. Presently, the pathophysiological sequelae associated with Cl2-induced acute lung injury in conscious animals, as well as the cellular and biochemical mechanisms involved, have not been elucidated. We exposed conscious Sprague-Dawley rats to Cl2 gas (184 or 400 ppm) for 30 min in environmental chambers and then returned them to room air. At 1 h after exposure, rats showed evidence of arterial hypoxemia, respiratory acidosis, increased levels of albumin, IgG, and IgM in bronchoalveolar lavage fluid (BALF), increased BALF surfactant surface tension, and significant histological injury to airway and alveolar epithelia. These changes were more pronounced in the 400-ppm-exposed rats. Concomitant decreases of ascorbate (AA) and reduced glutathione (GSH) were also detected in both BALF and lung tissues. In contrast, heart tissue AA and GSH content remained unchanged. These abnormalities persisted 24 h after exposure in rats exposed to 400 ppm Cl2. Rats injected systemically with a mixture of AA, deferoxamine, and N-acetyl-l-cysteine before exposure to 184 ppm Cl2 had normal levels of AA, lower levels of BALF albumin and normal arterial Po2 and Pco2 values. These findings suggest that Cl2 inhalation damages both airway and alveolar epithelial tissues and that resulting effects were ameliorated by prophylactic administration of low-molecular-weight antioxidants.
Keywords: ascorbate, N-acetyl-l-cysteine, deferoxamine, arterial blood gases, alveolar permeability, minimum surface tension, airway epithelium, histology
chlorine (Cl2) is the ninth largest produced chemical by volume in the United States, most of it being transported by rail to manufacturing plants (20). Current uses include pulp bleaching, waste sanitation, organic compound and pharmaceutical manufacturing, drinking water treatment, and maintenance of pathogen-free swimming pools. Accidental or deliberate release of Cl2 into the atmosphere has been associated with significant morbidity and mortality (20, 53). In addition, during the last few years, Cl2 cylinders have been bundled with traditional explosives, raising significant concerns regarding the possible reemergence of this agent as a chemical weapon against both combatants and civilians (5).
The toxicity of Cl2 is related to its oxidizing potential. Cl2 has higher solubility in water than other strong oxidant gases such as oxygen (O2), nitrogen dioxide (NO2), and ozone (O3) (52). Consequently, when inhaled Cl2 gas readily dissolves in the epithelial lining fluid (ELF) and either undergoes hydrolysis to generate hypochlorous (HOCl) and hydrochloric (HCl) acids or reacts directly with a number of biomolecules. HOCl (pKa = 7.5) and its conjugate base (OCl−) are also powerful oxidants and react rapidly with a variety of biomolecules in the ELF including 1) ascorbate (AA) and reduced glutathione (GSH), 2) the sulfur amino acids cysteine and methionine, 3) histidine, 4) the α-amino groups of various amino acids and protein disulfides, and 5) the side chains of tryptophan, lysine, and tyrosine (17, 21, 27).
The sites of actions of Cl2 depend on its inhaled concentration. At <50 ppm, Cl2 will react with cellular components in the upper airways (47), resulting in reversible bronchospasm and increased airway resistance (7, 11, 14, 15, 33, 43). At concentrations >50 ppm (likely to be encountered during industrial accidents and terrorist attacks), Cl2 molecules penetrate to more distal lung regions (47). Evidence of Cl2-induced alveolar epithelial injury was provided by the studies of Weill et al. (60), who reported that a significant fraction of industrial accident victims exposed to 400 ppm Cl2 developed pulmonary edema. A recent report also indicated that individuals exposed to Cl2 in Iraq developed severe acute lung injury and required mechanical ventilation and supplemental oxygen to alleviate arterial hypoxemia (5).
Currently, management of both animals and people exposed to Cl2 consists of administration of supplemental oxygen to alleviate hypoxemia, β2 agonists and corticosteroids to reverse bronchoconstriction and inflammation, and, in more severe cases, mechanical ventilation (20, 61). The potential role of lung antioxidant defenses in limiting injury to the airway and alveolar epithelia during exposure of conscious animals to Cl2 concentrations likely to be encountered in industrial accidents has not been documented. Low-molecular-weight antioxidants (such as AA and GSH), present in relatively high concentrations in the ELF and lung tissues (34), are likely to interact directly with Cl2 and HOCl/OCl− molecules as well as secondary reactive species formed by Cl2/HOCl reactions with sulfhydryls and amines. For example, AA ameliorated the oxidation of low-density lipoprotein (LDL) by HOCl/OCl− and chloramines (9), while GSH reduced oxidant-induced cellular injury both in vitro and in vivo (1, 25, 49) and reverted chloramines to the original amine moieties (50).
Here we show that exposure of conscious rats to either 184 or 400 ppm Cl2 for 30 min caused significant depletion of AA and GSH in the bronchoalveolar lavage fluid (BALF) and lung tissue, increased alveolar permeability to solute, decreased the ability of pulmonary surfactant to reach a low minimum surface tension on dynamic compression, and resulted in severe hypoxemia in a Cl2 dose-dependent fashion. Furthermore, systemic injections of a cocktail containing AA, deferoxamine (Def), an iron chelator that also decreases nitrotyrosine levels (51), and N-acetyl-l-cysteine [NAC; a cell-permeant precursor of glutathione and scavenger of reactive intermediates (1)], mitigated Cl2-induced injury to the alveolar epithelium and improved gas exchange.
MATERIALS AND METHODS
Animals.
All experiments were performed on specific pathogen-free Sprague-Dawley male rats (200–250 g; Harlan Sprague Dawley, Indianapolis IN). All procedures were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Exposure to Cl2.
Rats were placed inside a cylindrical glass chamber (Specialty Glass, Houston, TX; part no. X02AI99C15A57H5) and exposed to either 184 or 400 ppm Cl2 for 30 min (Fig. 1A). Two mass flow controllers (MFCs) with Kalrez seals (Scott Specialty Gases, Los Angeles, CA; part no. 05236A1V5K) and a microprocessor control unit (Scott Specialty Gases; part no. 05236E4) were used to control the compressed air and Cl2 (1,000 ppm Cl2 in air; Airgas, Birmingham, AL) flow rates to achieve the chamber Cl2 target concentrations (184 or 400 ppm). A bubble flowmeter was used to validate MFC performance on a weekly basis. Air and Cl2 were initially mixed at a three-way junction, and they were further mixed by passing through a diffuser located inside the top lid of the exposure chamber. Gases exited the chamber via two large-bore diameter ports in its bottom half. Chamber Cl2 concentrations were monitored continuously with an electrochemical reduction detector (Interscan, Chatsworth, CA; model RM34-100m). The detector was connected to an IBM computer for real-time display of Cl2 concentration and data storage. Both the exposure chamber and the detector were placed inside a chemical fume hood located in a negative-pressure room. At the end of each exposure, the Cl2 gas was turned off, the chamber was vented with compressed air for 2–3 min, the two halves were separated, and the rats were removed and returned to their cages, where they breathed room air for 1–24 h. Food and water were provided ad libitum.
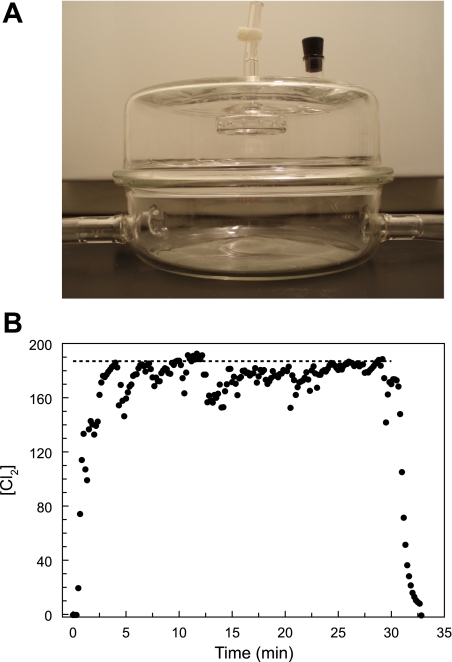
Fig. 1.
A: glass chamber used for exposing rats to either Cl2 or air (see materials and methods for details). B: record of Cl2 concentration ([Cl2]) in the exposure chamber with 2 rats present. Rats were placed in the chamber and breathed room air for ∼10 min. At time 0, one of the mass flow controllers was connected to a Cl2 cylinder (1,000 ppm in air) while the other one remained connected to room air. Relative flow rates were adjusted to achieve a nominal concentration of 184 ppm (shown by dotted line; total flow rate 5 l/min). [Cl2] was monitored continuously every 2 s, and the output was stored in a portable computer (as shown by individual symbols). At 30 min the Cl2 cylinder was switched off and the compressed air flow rate was increased to 5 l/min. Measured [Cl2] was lower than the nominal value because of the absorption of Cl2 by the rats.
Measurements of arterial blood gases and pH.
Rats were anesthetized with intraperitoneal injections of diazepam (0.16 mg/g body wt; Hospira, Lake Forest, IL) and Vetaket (ketamine HCl, 3.3 mg/g body wt; Phoenix Scientific, St. Joseph, MO). The right carotid artery was dissected and cannulated with an Exel Safelet 24-gauge catheter (Exel Intl., Culver City, CA), and 0.3 ml of arterial blood was drawn anaerobically into a heparanized syringe and placed in ice. Po2, Pco2, and pH were determined within 5 min of sampling with a blood gas analyzer (Instrumentation Laboratory, Lexington, MD; model 1640). In some cases, an additional arterial sample was drawn after the rats breathed 100% O2 for 15 min through a face mask.
Biochemical and biophysical measurements.
Rats were euthanized with an intraperitoneal injection of pentobarbital (0.3 mg/g body wt) followed by a bilateral thoracotomy. The lungs were then perfused through the right ventricle with 20 ml of saline. Subsequently, the trachea was exposed and 8 ml of normal saline was instilled and withdrawn slowly three times. Eighty and sixty percent of the instilled fluid were recovered from air- and Cl2-exposed rats, respectively. The BALF samples were spun at 150 g for 10 min at 4°C to pellet cells and cellular debris. The cell-free supernatant was split into 1-ml aliquots and placed on ice. One hundred forty-three microliters of 40% meta-phosphoric acid (MPA) in water was added to one of the aliquots to precipitate proteins and stabilize antioxidant redox couples. The heart and lung tissues were harvested and weighed. MPA (ml; 10% in water) equal to the sample weight in grams was added to each tube containing tissue samples, and the mixtures were homogenized with a tissue grinder and stored along with BALF samples at −80°C.
Measurements of AA, reduced and oxidized glutathione, and urate.
Heart and lung tissue homogenates were diluted with 5% MPA to a ratio of 1:20 (vol/wt). They were then spun along with BALF and plasma samples at 16,000 g for 3 min, and the supernatants were separated from the pellets and filtered through a 0.22-μm filter into HPLC vials on ice. Levels of AA, urate, GSH, and oxidized glutathione (GSSG) were quantified with an a LC-2010CHT HPLC (Shimadzu Scientific Instruments, Columbia, MD), equipped with an auto sampler with Peltier temperature control set to 4°C and an ESA CoulArray Detector (model 5600A; ESA Laboratories, Chelmsford, MI), by the method of Benavides et al. (6), optimized to allow the detection of these four analytes during the same chromatographic runs. Briefly, samples were separated on the column (Phenomenex Luna, reverse phase, C18, 250 × 4.60 mm; Phenomenex, Torrance, CA) provided with a Phenomenex guard column (ODS, 4-mm length × 3.0-mm ID) with an isocratic mixture of 50 mM phosphate buffer pH 3.3 containing 100 mM sodium octylsulfonate and CH3CN (at a ratio of 98.25:1.75) (Sigma-Aldrich, St. Louis, MO) at a flow rate of 0.6 ml/min. All samples and standards were run in duplicate, and the results were averaged. The collected data were analyzed with ESA CoulArray software (ESA).
Protein levels in BALF.
Protein concentrations in cell-free BALF samples were measured with the Micro BCA* Protein Assay Reagent Kit (Pierce, Rockford, IL) and the microtiter plate protocol as previously described (26). In each case, a standard curve was prepared by assaying known concentrations of BSA in 0.9% NaCl.
SDS and Western blotting studies.
In subsequent experiments, equal volumes of BALF proteins (40 μl) were separated by denatured SDS-PAGE (10%) and either visualized with Sypro Ruby staining or transferred to polyvinylidene difluoride (PVDF) membranes and blotted for albumin, IgG, and IgM. Goat anti-rat IgM (cat. no. GTX77095, GeneTex, San Antonio, TX) and goat anti-mouse albumin (cat. no. GTX77024, GeneTex) were used in conjunction with donkey anti-goat IgG-horseradish peroxidase (HRP) to detect rat IgM and albumin, respectively. Goat anti-mouse-HRP (Pierce Biotechnology, Rockford, IL) was used to detect the rat light and heavy IgG chains. Protein bands were revealed by enhanced chemiluminescence (Pierce Biotechnology, Rockford, IL) and exposed to X-ray films.
Phospholipid levels and surfactant surface tension in BALF.
Cell-free BALF samples were centrifuged at 12,500 g for 30 min to sediment lung phospholipids, which were quantitated as described previously (28). The composition of various phospholipid classes was determined based on phosphate measurements after extraction and one-dimensional thin-layer chromatography (TLC) on 250-μm-thick silica gel G (Analtech, Newark, DE) with a solvent system of chloroform-methanol-2-propanol-triethylamine-water (30:9:25:25:7 by volume) (18).
The ability of pulmonary surfactant to lower the surface tension of an air-liquid interface during dynamic compression was measured in vitro in a pulsating bubble surfactometer (General Transco, Largo, FL) as previously described (28). Briefly, cell-free BALF samples were evaporated under nitrogen and then resuspended to a concentration of 1.0 mg phospholipid/ml with hand vortexing in 0.15 M NaCl containing 2 mM CaCl2. Samples were introduced into a 40-μl plastic sample chamber mounted on the surfactometer pulsator unit, and an air bubble (communicating with ambient air) was formed and repetitively oscillated between radii of 0.55 and 0.4 mm at a rate of 20 cycles of compression-expansion per minute. The pressure drop across the air-water interface of the bubble was measured by a precision pressure transducer, and surface tension at minimum bubble radius (minimum surface tension) was calculated as a function of pulsation time with the Laplace equation for a spherical interface.
Lung histology.
After exposure of the trachea and opening of the thoracic cavity of euthanized rats, the lungs were inflated with 8 ml of buffered ethanolic formalin (75 parts ethyl alcohol:10 parts saturated formalin:15 parts distilled water) with a syringe. The trachea was ligated, and the lungs were removed en bloc and immersed in fixative for 24–48 h. After fixation the lung lobes were separated and trimmed for histological processing. The left lung was bisected lengthwise on a plane through the main stem bronchus and the margin of the lobe. The diaphragmatic (caudal) lobe of the right lung was cut transversely, at right angles to the main stem bronchus, in 2- to 3-mm slices. The remaining right lobes (apical/cranial and middle) were bisected through the main stem bronchus and parallel to the broad surface of the lobes. The azygous lobe was bisected lengthwise through the main stem bronchus. The tissues were processed routinely for paraffin sectioning, sectioned at 5 μm, and stained with hematoxylin and eosin. Slides were examined by one of the coauthors (T. R. Schoeb) without knowledge of experimental treatments.
Lung wet-to-dry weight ratios.
These measurements were performed as previously described (12). Briefly, rats were euthanized and exsanguinated, their lungs were removed and washed, and extraneous tissue and extrapulmonary airways were removed. Lungs were then weighed and placed in an oven at 55°C for 7 days. After drying, the lungs were weighed again. The wet-to-dry weight ratio was then calculated as an index of intrapulmonary fluid accumulation. No correction for blood content was made.
Administration of antioxidants.
A bolus of endotoxin-free sterile saline (0.5 ml) containing 1) AA (ascorbic acid injection, American Regent, Shirley, NY; 200 mg/kg), 2) NAC (Acetadote, Cumberland Pharmaceuticals, Nashville, TN; 150 mg/kg), and 3) Def (deferoxamine mesylate, Hospira; 15 mg/kg) was injected intraperitoneally into rats 18 h before exposure to Cl2. A second injection was administered via the tail vein 1 h before initiation of Cl2 exposures. Care was taken to correct for the salt content of Def when calculating the amount to be injected. Control animals received equal volumes of saline.
One hour after the second injection the rats were exposed to Cl2 (184 ppm for 30 min) as described above. They were then returned to room air and were killed at either 1 or 24 h after Cl2 exposure. Control animals received an equivalent amount of saline. Arterial blood gases, BALF, and tissue antioxidant levels were measured as described above.
Statistical analysis.
All values are expressed as means ± SE. Data were analyzed by one-way analysis of variance (ANOVA) with Bonferroni's test or Student's paired test, as appropriate. P values of < 0.05 were considered significant.
RESULTS
Exposure to Cl2 causes hypoxemia and respiratory acidosis.
At exposure onset, chamber Cl2 concentrations rose rapidly to near target values and remained stable throughout the exposure period (Fig. 1B). All rats survived the 30-min exposure to Cl2 gas. However, after 20–25 min of exposure their breathing became labored, with flaring of the nares. Noticeable expiratory grunting was observed. These effects were much more noticeable in rats exposed to 400 ppm. When returned to room air, rats exhibited labored breathing for 1–4 h after exposure, which improved during the next 24 h in air.
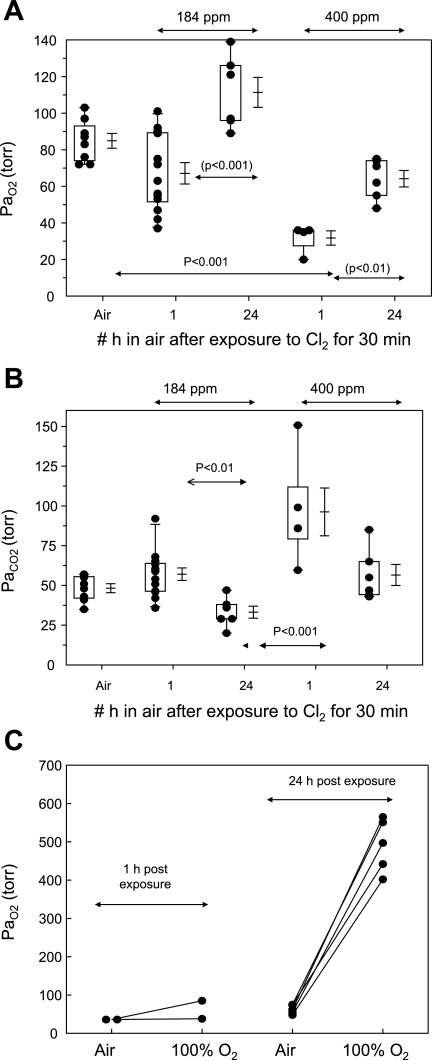
As shown in Fig. 2A and summarized in Table 1, Cl2 exposure resulted in arterial hypoxemia in a Cl2-concentration dependent fashion: arterial Po2 (PaO2) values of rats exposed to either 184 or 400 ppm and returned to room air breathing for 1 h were 67 ± 6 and 36 ± 0.3 Torr, respectively, which were significantly different from the corresponding values of air-exposed rats (85 ± 4 Torr, means ± SE; P < 0.01 compared with either Cl2 group). The severe hypoxemia in the 400 ppm group was most likely the result of right-to-left shunting, as shown by the fact that breathing 100% O2 for 15 min increased PaO2 marginally (Fig. 2C). Rats exposed to 184 ppm had normal arterial blood gases 24 h after exposure. On the other hand, the mean PaO2 of the 400 ppm group 24 h after exposure was 64 ± 4 Torr, which was significantly lower than air-breathing controls. When rats exposed to 400 ppm and returned to room air for 24 h breathed 100% O2 for 15 min, the PaO2 rose to 491 ± 71 Torr, indicating that the most likely cause of arterial hypoxemia in this group was ventilation perfusion mismatching instead of right-to-left shunting.
Fig. 2.
Arterial Po2 (PaO2) of air- and Cl2-exposed rats. Rats were exposed to either 184 or 400 ppm Cl2, after which they resumed breathing room air. They were anesthetized after 1 or 24 h postexposure, and an arterial blood sample was obtained from the right carotid artery while they breathed air for the measurement of PaO2 (A) and arterial Pco2 (PaCO2; B). Box-whisker plots show individual points (each dot is a different rat), boxes (25–75% percentile of the data), whiskers (lower 5th and upper 95% percentile of the data) as well as means ± SE. Statistical analysis of mean values was performed by 1-way analysis of variance followed by the Bonferroni modification of the t-test. C: PaO2 values of rats exposed to 400 ppm Cl2 and breathing room air for either 1 or 24 h. PaO2 values were measured in arterial blood samples while rats breathed air or 100% O2 for 15 min. Each point represents a rat.
Table 1.
Effects of Cl2 exposure
| Air | Cl2 184 ppm, 1 h in Air | Cl2 184 ppm, 24 h in Air | Cl2 400 ppm, 1 h in Air | Cl2 400 ppm, 24 h in Air | |
|---|---|---|---|---|---|
| PaO2, Torr | 85±4 (8) | 67±6* (13) | 111±8 (6) | 36±0.3* (4) | 64±4* (6) |
| PaCO2, Torr | 48±3 (8) | 57±4† (13) | 33±4 (6) | 92±27† (4) | 56±6† (6) |
| pH | 7.29±0.02 (8) | 7.28±0.02 (13) | 7.35±0.05 (6) | 7.05±0.050* (4) | 7.32±0.03* (6) |
| Breathing frequency, breaths/min | 109±4 (4) | 116±8 (4) | 104±3 (4) | 97±6 (4) | 93±7 (4) |
| Lung wet wt, g | 1.1±0.0 (3) | 1.05±0.05 (3) | 1.26±0.07 (3) | 1.23±0.1 (4) | 1.2±0.06 (4) |
| Lung dry wt, g | 0.23±0.0 (3) | 0.22±0.01 (3) | 0.26±0.01 (3) | 0.24±0.1 (4) | 0.24±0.02 (4) |
| Lung wet-to-dry wt ratio | 4.85±0.06 (3) | 4.76±0.03 (3) | 4.93±0.05 (3) | 5.03±0.14 (4) | 4.82±0.8 (4) |
Values are means ± SE for no. of rats in parentheses. Rats were exposed to the indicated levels of Cl2 gas for 30 min as discussed in materials and methods and then returned to room air. Arterial blood was drawn from the common carotid artery under anesthesia. Breathing frequency was measured by direct observation in conscious rats. PaO2, arterial Po2; PaCO2, arterial Pco2. At least P < 0.05:
vs. corresponding control values;
vs. corresponding 24 h in air.
Rats exposed to either 184 or 400 ppm of Cl2 also developed significant hypercapnia (PaCO2 57 ± 4 and 92 ± 27 Torr), and these values improved considerably 24 h later (Fig. 2B). Similar values for arterial blood gases and pH were obtained in some unanesthetized, chronically instrumented rats exposed to Cl2 and returned to room air (data not shown). Despite the significant respiratory acidosis, breathing frequency remained at near-control levels for all levels of exposure (see Table 1). However, during exposure to Cl2 and on return to room air, rats switched from nose to mouth breathing, an event associated with hypoxemia and respiratory acidosis (19).
Cl2 exposure damages the alveolar epithelium.
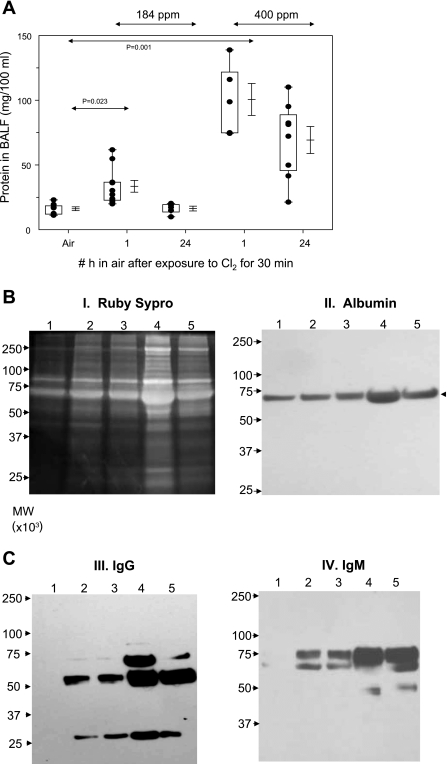
Exposure to Cl2 caused a dose-dependent increase in protein concentration in the BALF (Fig. 3A), an index of alveolar epithelial injury. The presence of protein-rich edema in the alveolar spaces indicates severe injury to the alveolar epithelium by the chlorine gas and its reactive intermediates consistent with the presence of severe arterial hypoxemia in these rats. Twenty-four hours after exposure, BALF protein levels decreased significantly but were still higher than the control values. SDS-PAGE studies (Fig. 3B) showed that exposure to Cl2 resulted in increased levels of proteins with approximate molecular weights of 25,000, 55,000, 70,000, and 250,000. Western blotting studies (Fig. 3BII) showed that these proteins correspond to the light and heavy chains of IgG (25,000 and 55,000), albumin (69,000), and IgM (75,000). The higher-molecular-weight protein was not identified. Interestingly, neither IgG nor IgM could be detected in control BALF. Despite the noted increases of BALF protein concentrations of Cl2-exposed rats, their lung wet and dry weights remained unchanged from the corresponding control values (Table 1).
Fig. 3.
Protein values in bronchoalveolar lavage fluid (BALF) after Cl2 exposure. Rats were exposed to either 184 or 400 ppm Cl2, after which they resumed breathing room air. They were killed 1 or 24 h after exposure, and their lungs were lavaged as described in materials and methods. A: protein values in BALF. Box-whisker plot showing individual points (each dot is a different rat), boxes (25–75% percentile of the data), whiskers (lower 5th and upper 95% percentile of the data), as well as means ± SE. Statistical analysis of mean values was performed by 1-way analysis of variance followed by the Bonferroni modification of the t-test. B and C: equal volumes of BALF proteins (40 μl) were separated by SDS-PAGE (10%) and visualized with Sypro Ruby staining (I) or transferred to nitrocellulose and immunoblotted for albumin [molecular weight (MW) ∼69,000; II], IgG (MW ∼55,000 for heavy chain and ∼25,000 for light chain; III), and IgM (MW ∼75,000; IV). Lane 1, control (air); lanes 2 and 3, 184 ppm Cl2 for 30 min followed by 1 or 24 h in air; lanes 4 and 5, 400 ppm Cl2 for 30 min followed by 1 or 24 h in air. Typical records shown were repeated 2 times with BALF from different rats with identical results.
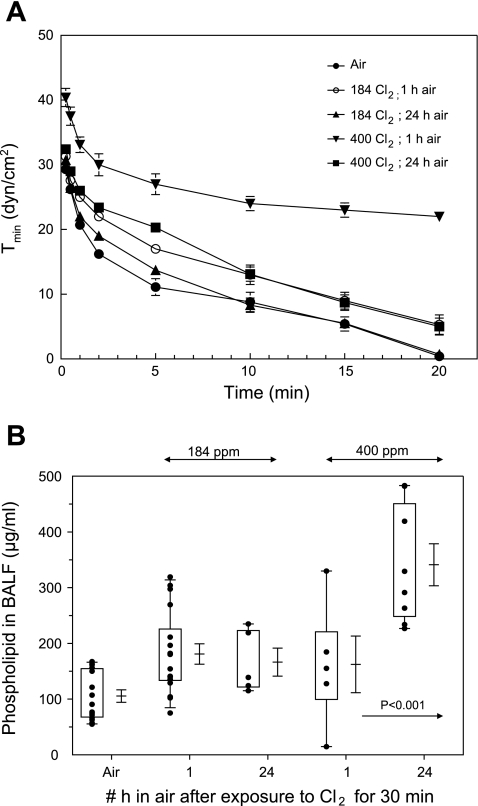
Exposure to Cl2 also decreased the ability of pulmonary surfactant to reach a minimum surface tension during dynamic compression as measured in an oscillating bubble surfactometer (Fig. 4A). Phospholipid levels in BALF increased significantly 24 h after exposure to 400 ppm Cl2 (Fig. 4B), consistent with the higher numbers of alveolar type II cells seen with the onset of repair after severe injury to the alveolar epithelium (29, 30). TLC showed that the majority of phospholipid was phosphatidylcholine (85%). The remaining phospholipids consisted of phosphatidylglycerol (5%), phosphatidylinositol (3%), sphingomyelin (3%), and phosphatidylethanolamine (2%). No changes in the composition of phospholipids were noted for any of the experimental groups (data not shown).
Fig. 4.
Surfactant minimum surface tension and BALF phospholipid levels. A: minimum surface tension [Tmin (dyn/cm2)] of cell-free BALF, reconstituted at 1 mg/ml, measured in a bubble surfactometer during dynamic compression of a bubble to 50% of its original area as described in materials and methods. x-Axis shows the accumulative time that the bubble was oscillated. Values are means ± SE; n = 3 for each value. Note very high values of Tmin in BALF of rats exposed to 400 ppm Cl2 and returned to room air for 1 h. Values of BALF from 400 ppm Cl2-exposed rats as well as 184 ppm Cl2 and 1 h in air were significantly different from the corresponding air values (with t-test; P < 0.05). B: phospholipids in BALF: total phospholipid levels in BALF, pelleted by centrifugation at 12,500 g as described in the text. Box-whisker plot shows individual points (each dot is a different rat), boxes (25–75% percentile of the data), whiskers (5th and 95th percentiles of the data), as well as means ± SE.
Histological assessment of injury to the airway and alveolar epithelia.
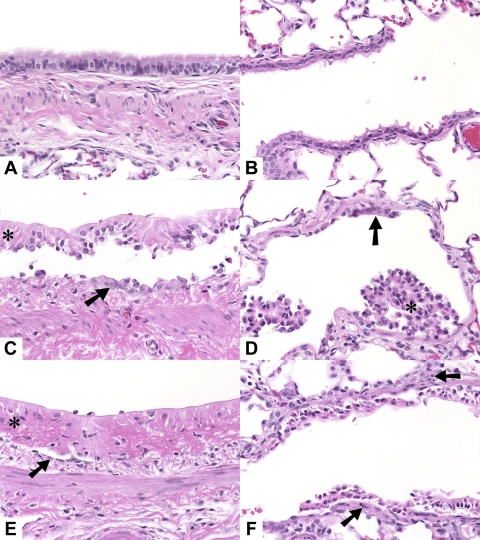
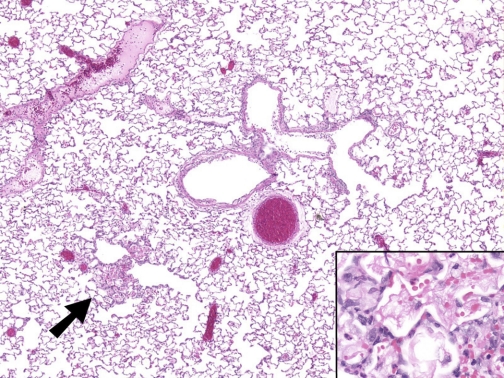
Exposure of rats to 400 ppm for 30 min resulted in extensive injury to their airway and alveolar epithelia (Fig. 5). The most prominent change was multifocal to diffuse coagulative necrosis and sloughing of bronchial and bronchiolar epithelia accompanied by sparse neutrophil accumulation. Main stem bronchi and intermediate airways were most severely affected. Small and terminal bronchioles were largely spared in 9 of the 18 Cl2-exposed rats; in the remainder, changes were similar to those in the larger airways. In a few rats examined 24 h after exposure, there was less extensive airway epithelial necrosis and more epithelium remained, although it was flattened, indicating partial loss. In these rats the small and terminal bronchioles were spared. A few rats had slight to mild alveolitis characterized by intra-alveolar hemorrhage and fibrin, alveolar epithelial swelling, and mild accumulation of neutrophils and macrophages (Fig. 6).
Fig. 5.
Hematoxylin and eosin (H & E) sections of lungs of rats exposed to either air or Cl2: control (A and B), 1 h after exposure to 400 ppm Cl2 for 30 min (C and D), and 24 h after exposure to 400 ppm Cl2 for 30 min (E and F). A, C, and E: main stem bronchus. B, D, and F: terminal bronchiole and adjacent alveoli. C, D, and E: necrotic epithelium (*), flattened remnant epithelial cells (arrow). F: necrotic epithelium, sparse neutrophil accumulation in wall (arrows). Original magnification ×40, H & E stain.
Fig. 6.
H & E section of lung of rat exposed to Cl2. Mild focal alveolitis (arrow) characterized by alveolar hemorrhage and fibrin and sparse neutrophils (inset). Original magnifications ×4 and ×40 (inset), H & E stain.
Exposure to Cl2 depletes key BALF and lung tissue antioxidant levels.
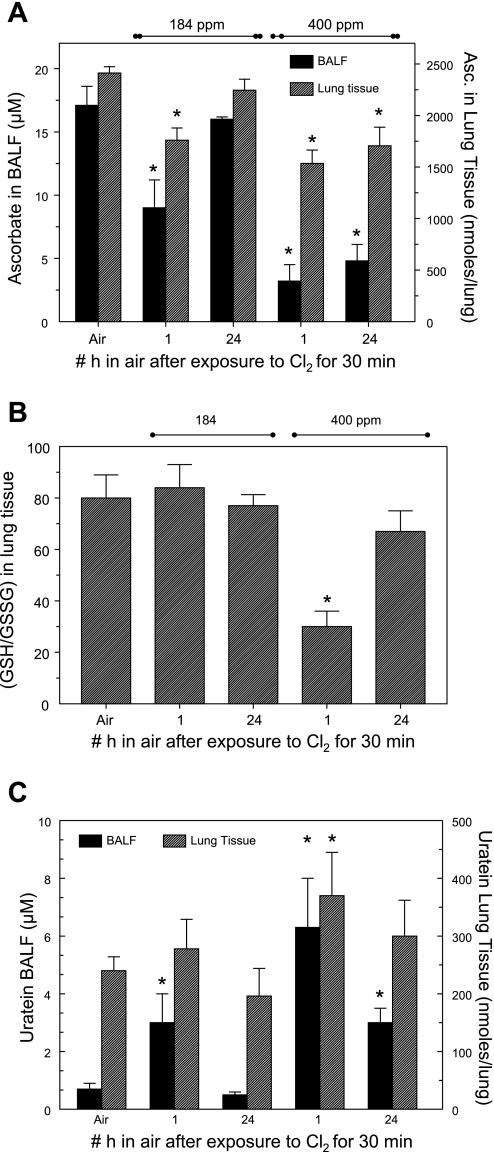
At the end of the lavage procedure, the heart and lungs were removed en bloc. Levels of AA, GSH, GSSG, and urate were measured by HPLC as described in materials and methods. As shown in Fig. 7A, exposure to Cl2 depleted both BALF and lung tissue AA. Importantly, 24 h after exposure, BALF AA levels in rats exposed to 400 ppm for 30 min were only 30% of control. In contrast, AA levels in heart tissue of Cl2-exposed rats remained at control levels (data not shown). Measurements in lung tissue of animals exposed to 184 ppm of Cl2 also showed a small (but not significant) decrease in GSH/GSSG levels (Fig. 7B). However, exposure to 400 ppm resulted in a significant decrease of lung tissue GSH/GSSG ratios 1 h after exposure that returned to baseline 24 h later. In contrast, exposure to Cl2 resulted in reversible increases of BALF urate (Fig. 7C) in a manner that paralleled changes in BALF protein levels. No significant changes were detected in BALF GSH, while BALF GSSG was barely above background levels. Thus these results are not shown.
Fig. 7.
Antioxidant levels in BALF and lung tissue of Cl2-exposed rats. A: ascorbate in cell-free BALF (left y-axis) and lung tissue (right y-axis) of air- and Cl2-exposed rats measured by HPLC as described in materials and methods. Values are means ± SE; no. of rats for BALF and lung tissue in each group: air, 9 and 7; 184 ppm Cl2 and 1 h in air, 12 and 11; 184 ppm Cl2 and 24 h in air, 4 and 5; 400 ppm Cl2 and 1 h in air, 6 and 7; 400 ppm Cl2 and 24 h in air, 8 and 8. *P < 0.05 vs. corresponding control (air-only value). B: reduced glutathione (GSH)-to-oxidized glutathione (GSSG) ratio values in lung of Cl2-exposed rats. Values are means ± SE; no. of rats in each group: air, 10; 184 ppm Cl2 and 1 h in air, 11; 184 ppm Cl2 and 24 h in air, 5; 400 ppm Cl2 and 1 h in air, 7; 400 ppm Cl2 and 24 h in air, 8. *P < 0.05 vs. corresponding control (air-only value). C: urate in cell-free BALF (left y-axis) and lung tissue (right y-axis) of air- and Cl2-exposed measured by HPLC as described in materials and methods. Values are means ± SE; no. of rats for BALF and lung tissue in each group: Air, 9 and 7; 184 ppm Cl2 and 1 h in air, 12 and 11; 184 ppm Cl2 and 24 h in air, 4 and 5; 400 ppm Cl2 and 1 h in air, 6 and 7; 400 ppm Cl2 and 24 h in air, 8 and 8. *P < 0.05 vs. corresponding control (air-only value).
Administration of antioxidants mitigates Cl2 lung injury.
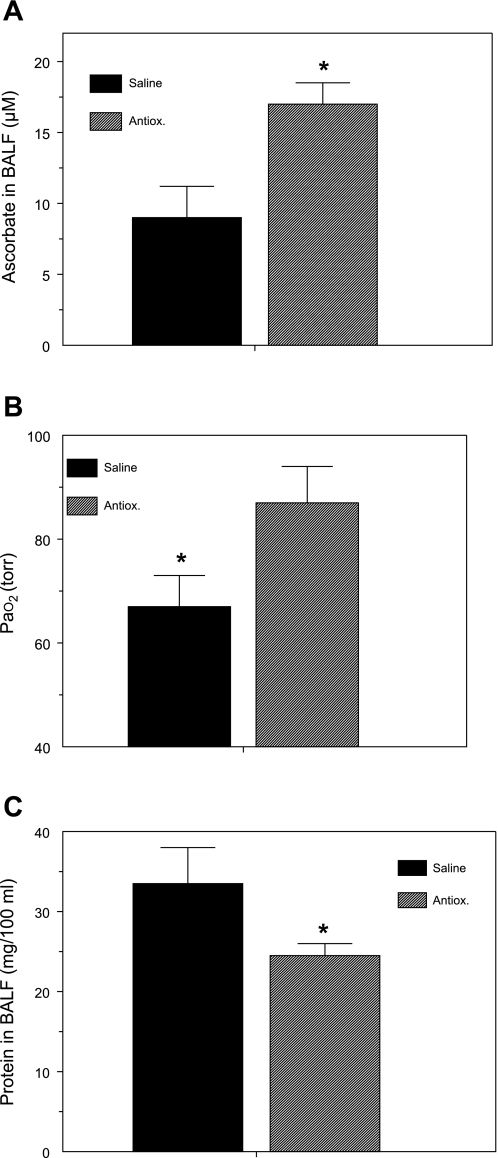
Because our findings showed that exposure of rats to Cl2 gas (184 or 400 ppm) for 30 min depleted AA levels in BALF and lung tissue, we designed a series of experiments to assess whether systemic administration of a mixture of AA, Def, and NAC would mitigate Cl2-induced lung injury. As shown in Fig. 8, administration of antioxidants in rats exposed to 184 ppm of Cl2 restored BALF AA to normal levels, increased PaO2 by ∼20 Torr, and decreased BALF albumin concentration (an index of lung injury) by 30%. Administration of antioxidants also decreased BALF urate concentrations from 3 ± 1 to 2 ± 0.4 (means ± SE; n = 14 each), although these values were not significantly different from each other.
Fig. 8.
Systemic administration of antioxidants decreases lung injury. Rats were injected with a mixture of antioxidants [Antiox; ascorbate, N-acetyl-l-cysteine (NAC), and deferoxamine] or an equivalent amount of vehicle (saline) 18 h and 1 h before being exposed to 184 ppm Cl2 for 30 min, returned to room air, and killed 1 h later, as described in materials and methods. Values are means ± SE. A: BALF ascorbate; no. of rats: saline, 5; Antiox, 16. B: PaO2 (from samples from carotid artery drawn during anesthesia); no. of rats: saline, 13; Antiox, 13. C: BALF protein; no. of rats: saline, 10; Antiox, 15; *P < 0.05 vs. corresponding saline values.
DISCUSSION
Here we assessed Cl2-induced injury to both airway and alveolar epithelia in conscious rats with physiological, biochemical, biophysical, and histological techniques. Our results show that exposure of rats to Cl2 concentrations likely to be encountered in industrial accidents or during deliberate release of Cl2 into the atmosphere compromised gas exchange by damaging seminal functions of the respiratory and alveolar epithelia in a Cl2 concentration-dependent fashion. Rats exposed to 184 ppm developed moderate hypoxemia and hypercapnia and had increased levels of albumin, IgG, and IgM in their BALF 1 h after exposure. However, all variables returned to normal by 24 h after exposure. In contrast, rats exposed to 400 ppm Cl2 developed respiratory distress characterized by very high levels of albumin, IgG, and IgM in their BALF, abnormalities in pulmonary surfactant function, severe morphological injury, and life-threatening hypoxemia refractive to oxygen breathing. More importantly, these changes persisted 24 h after exposure.
It is interesting to note that despite clear evidence of alveolar epithelial dysfunction, histological examination of lung tissues of rats exposed to 400 ppm Cl2 showed extensive airway epithelial loss but relatively little alveolar injury (Figs. 5 and 6) and no increase in lung wet/dry weights. These findings are consistent with those of previous studies using rats and mice, in which alveolar lesions were not observed except at very high Cl2 concentrations (14, 16, 37). Furthermore, functional changes of alveolar epithelial function are more sensitive indexes of injury and precede the appearance of morphological changes (44, 45) as well as of lung wet/dry weights (30). The presence of lymph flow as well as alveolar fluid clearance secondary to active vectorial Na+ transport (42) helps to maintain the interstitial and alveolar spaces free of fluid until fluid flux overwhelms the capacity of lymph to remove fluid or there is significant breakdown of the alveolar barrier.
The presence of albumin in the normal alveolar space may be due to the movement of this molecule by transcytosis secondary to its binding to a 60-kDa glycoprotein located in the caveolae in addition to its movement through the paracellular junctions (31, 54). On the other hand, both the light and heavy chains of IgG as well as IgM, which presumably enter the alveolar spaces from the lung interstitial spaces through paracellular junctions, were not detected by Western blotting in normal BALF. These data are consistent with previous reports indicating that normal but not oxidant-injured rabbit alveolar epithelia prevented the egress of cytochrome c (a lipid-insoluble molecule with an effective radius of 17 μ) from the interstitial to the alveolar spaces (38, 39, 45). Thus the detection of IgG and IgM may be considered specific indexes of epithelial injury.
Exposure of rats to 184 ppm caused significant but transient injury to the alveolar and airway epithelia. The observed hypoxemia was partly due to hypoventilation and partly due to development of lung regions with low ventilation/perfusion (V/Q). Batchinsky et. al. (3) demonstrated the presence of regions with low V/Q in the lungs of anesthetized sheep exposed to 120 ppm Cl2. Previous studies reported that inhalation of Cl2 caused a decrease in respiratory rate due to stimulation of TRPV1-expressing sensory nerve C fibers (43). However, in our studies breathing rate remained unchanged both during exposure to Cl2 and on return to room air and thus cannot account for the observed hypoventilation. Instead, hypoventilation resulted from decreased alveolar volume due to 1) the switch from nose to mouse breathing because of increased airway resistance (14) and/or 2) decreased lung compliance.
On the other hand, reactive intermediates generated after exposure to 400 ppm Cl2 overwhelmed antioxidant defenses (as seen by significant decreases of AA and GSH-to-GSSG ratio) in the BALF and lung tissue and caused extensive injury to both airway and alveolar epithelia as seen by significantly higher levels of albumin in BALF that remained elevated even after 24 h. As we have previously demonstrated (30, 36, 41) in rabbits exposed to normobaric hyperoxia, increased BALF protein-to-lipid ratios contribute to surfactant inactivation, which in turn will result in atelectasis and the development of right-to-left shunt, as indeed was the case in this group of rats. At 24 h after exposure, as protein concentration decreased, surfactant function returned to normal and right-to-left shunt progressed to low V/Q as shown by the robust response of PaO2 to 100% O2 breathing. Batchinsky et al. (3) used the multiple inert gas elimination technique and computer tomography to show progression of low V/Q to right-to-left shunt in anesthetized, mechanically ventilated sheep breathing increasing concentrations of Cl2 for 30 min. Severe hypoxemia secondary to low V/Q was also seen in mechanically ventilated pigs exposed to Cl2 (23). The confounding effects of surgical anesthesia and mechanical ventilation may alter responses of the cardiorespiratory system to oxidant gases. For example, mechanical ventilation with positive end-expiratory pressure will probably prevent the onset of alveolar collapse and may also explain the much higher sensitivity of animals in both of these studies to Cl2: in the study of Gunnarsson et al. (23) five of six pigs died within 6 h of breathing 140 ppm for 30 min. In contrast, >90% of our rats were alive with oxygen saturations near 90% 24 h after exposure to 400 ppm Cl2 for 30 min.
During normal metabolism, steady-state levels of reactive oxygen and nitrogen species are kept at very low levels by the existence of various antioxidant enzymes [such as superoxide dismutase (SOD), glutathione peroxidase, glutathione S-transferase, catalase, and thioredoxin] as well as low-molecular-weight antioxidants (such as glutathione, urate, AA, vitamin E, etc.) (8, 22, 34). Our previous work (40) and that of others (8) indicate that lung ELF, pulmonary surfactant, as well as lung tissues contain significant amount of three forms of SODs, catalase, and glutathione peroxidase. In addition to enzymatic antioxidants, lung ELF contains a number of low-molecular-weight scavengers including AA, vitamin E, glutathione, and urate that decrease steady-state concentrations of reactive oxygen-nitrogen intermediates in conjunction with or independent of the antioxidant enzymes (34). Furthermore, a variety of iron chelators, such as ceruloplasmin, transferrin, ferritin, and bilirubin, prevent the formation of hydroxyl radicals and secondary self-propagating reactions by maintaining levels of free iron at very low levels.
In this study we investigated the contributions of low-molecular-weight antioxidants in Cl2-induced toxicity for two reasons: first, our data showed significant depletion of AA and GSH in BALF and lung tissues after exposure to Cl2, lasting well after the cessation of exposure. Second, depleted levels can be augmented prior to exposure via simple techniques.
Vitamin C or l-ascorbic acid is the l-enantiomer of AA. Ascorbic acid is a strong reducing agent and very important hydrophilic antioxidant. At physiological pH most of the ascorbic acid is present as AA, which acts as an antioxidant by donating one electron to oxidants or radicals. The two-electron reduction of HOCl by AA will yield Cl− and H2O. Nishikimi (46) demonstrated that AA reacts with superoxide with a second rate constant of 3 × 105 M−1s−1. Although this rate constant is significantly lower than the SOD-catalyzed dismutation of superoxide (>109 M−1s−1), the relatively high levels of AA in BALF (17 μM; Fig. 7) compared with BALF SOD levels (1 nM; Ref. 55) suggest that AA may play a significant role in reducing superoxide, which would also diminish steady-state levels of hydroxyl radicals (through Fenton reactions) and peroxynitrite [via the reaction of superoxide with nitric oxide (4)]. AA will also react directly with hydroxyl radical and participates in the reduction of vitamin E radical (produced during the reduction of polyunsaturated fatty acids by vitamin E) (2).
However, as recently demonstrated, AA may increase oxidant stress by 1) increasing levels of hydrogen peroxide during scavenging of hydrogen peroxide and 2) reduction of Fe3+ to Fe2+, therefore enhancing the generation of hydroxyl radicals through the Fenton reaction (10). For these reasons we administered AA along with NAC (a precursor of glutathione that will regenerate l-ascorbate by reducing dehydroascorbate) and Def, an iron chelator that can also repair tyrosyl radicals and decrease formation of nitrotyrosine (51). GSH is the most abundant intracellular free thiol, and a decrease of the GSH-to-GSSG ratio is often considered to be an index of increased steady-state levels or reactive intermediates. NAC has been shown to be effective in correcting the pathological manifestations of oxidative stress in a number of organs (reviewed in Refs. 1, 25). Our data clearly indicate that glutathione exists mainly in the reduced state in lung tissue, and our control values of GSH/GSSG ratios are in good agreement with those of others (13, 32, 35). Furthermore, the much lower GSH/GSSG ratio in Cl2-exposed rats as well as the restoration of this ratio to its air control value after NAC administration provide strong evidence for both the existence of oxidative stress and the effectiveness of NAC administration as a precursor of glutathione synthesis.
A very interesting finding in our studies is the noted transient increase of urate in BALF of Cl2-exposed rats (Fig. 7C). Urate is the most abundant antioxidant in both the upper and lower respiratory tracts in human lung ELF (48, 56). It is thought to be secreted by submucosal glands into the nasal epithelium fluid (48) and generated by purine metabolism (56). Our data in rats show that under baseline conditions urate concentration in BALF is considerably smaller than that of AA. On the other hand, urate does become the most abundant antioxidant in the BALF of rats exposed to 400 ppm Cl2 and may help limit injury to the alveolar epithelium by reactive intermediates. For example, urate prevented nitration of surfactant protein A (and consequent loss of function) by peroxynitrite (24).
One may ask whether the levels of exposure used in this study (up to 400 ppm Cl2 for 30 min) mimic the concentrations likely to be encountered in the vicinity of industrial accidents or acts of terrorism involving the use of Cl2. Weill et al. (60) reported levels of 400 ppm of Cl2 within 75 yards of an accident involving spill of Cl2 from rail cars. Ten exposed individuals were hospitalized with pulmonary edema. In another report, 23 of 418 patients exposed to Cl2 during a chemical spill were hospitalized with pulmonary edema (20). Presently, treatment of Cl2-exposed individuals consists of administration of oxygen to alleviate hypoxemia, β2 agonists to alleviate bronchoconstriction and enhance alveolar fluid clearance, and corticosteroids to reduce the inflammatory response (5, 57–59, 61). Data presented in the present study establish the central role of low-molecular-weight antioxidants in the prevention of Cl2-induced injury to seminal functions of the alveolar epithelium.
GRANTS
This work was supported by National Institute of Environmental Health Sciences Grant 5U01-ES-015676-02.
Acknowledgments
The authors acknowledge the excellent technical assistance of Drs. Robert Notter and Zhengdong Wang (University of Rochester College of Medicine) with the measurements of minimum surface tension and Dr. James Johnson (University of Alabama at Birmingham) for valuable discussions on the interpretation of arterial blood gases. The editorial assistance of Teri Potter was also appreciated.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Atkuri KR, Mantovani JJ, Herzenberg LA, Herzenberg LA. N-acetylcysteine—a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol 7: 355–359, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballinger CA, Cueto R, Squadrito G, Coffin JF, Velsor LW, Pryor WA, Postlethwait EM. Antioxidant-mediated augmentation of ozone-induced membrane oxidation. Free Radic Biol Med 38: 515–526, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Batchinsky AI, Martini DK, Jordan BS, Dick EJ, Fudge J, Baird CA, Hardin DE, Cancio LC. Acute respiratory distress syndrome secondary to inhalation of chlorine gas in sheep. J Trauma 60: 944–956, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87: 1620–1624, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell DG Management of acute respiratory distress syndrome (ARDS) following chlorine exposure (Abstract). Am J Respir Crit Care Med 176: A314, 2008. [Google Scholar]
- 6.Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci USA 104: 17977–17982, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonetto G, Corradi M, Carraro S, Zanconato S, Alinovi R, Folesani G, Da Dalt L, Mutti A, Baraldi E. Longitudinal monitoring of lung injury in children after acute chlorine exposure in a swimming pool. Am J Respir Crit Care Med 174: 545–549, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowler RP, Crapo JD. Oxidative stress in airways: is there a role for extracellular superoxide dismutase? Am J Respir Crit Care Med 166: S38–S43, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Carr AC, Tijerina T, Frei B. Vitamin C protects against and reverses specific hypochlorous acid- and chloramine-dependent modifications of low-density lipoprotein. Biochem J 346: 491–499, 2000. [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR, Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci USA 104: 8749–8754, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Alessandro A, Kuschner W, Wong H, Boushey HA, Blanc PD. Exaggerated responses to chlorine inhalation among persons with nonspecific airway hyperreactivity. Chest 109: 331–337, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Davis IC, Lazarowski ER, Hickman-Davis JM, Fortenberry JA, Chen FP, Zhao X, Sorscher E, Graves LM, Sullender WM, Matalon S. Leflunomide prevents alveolar fluid clearance inhibition by respiratory syncytial virus. Am J Respir Crit Care Med 173: 673–682, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day BJ Glutathione: a radical treatment for cystic fibrosis lung disease? Chest 127: 12–14, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Demnati R, Fraser R, Ghezzo H, Martin JG, Plaa G, Malo JL. Time-course of functional and pathological changes after a single high acute inhalation of chlorine in rats. Eur Respir J 11: 922–928, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Demnati R, Fraser R, Martin JG, Plaa G, Malo JL. Effects of dexamethasone on functional and pathological changes in rat bronchi caused by high acute exposure to chlorine. Toxicol Sci 45: 242–246, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Demnati R, Fraser R, Plaa G, Malo JL. Histopathological effects of acute exposure to chlorine gas on Sprague-Dawley rat lungs. J Environ Pathol Toxicol Oncol 14: 15–19, 1995. [PubMed] [Google Scholar]
- 17.den Hartog GJ, Haenen GR, Vegt E, van der Vijgh WJ, Bast A. Efficacy of HOCl scavenging by sulfur-containing compounds: antioxidant activity of glutathione disulfide? Biol Chem 383: 709–713, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Dugan JM, Knee G, Strauss JF III, Touchstone JC. A simplified procedure for separating amniotic fluid phospholipids with thin-layer chromatography. J Reprod Med 29: 245–247, 1984. [PubMed] [Google Scholar]
- 19.Erkan M, Erhan E, Saglam A, Arslan S. Compensatory mechanisms in rats with nasal obstructions. Tokai J Exp Clin Med 19: 67–71, 1994. [PubMed] [Google Scholar]
- 20.Evans RB Chlorine: state of the art. Lung 183: 151–167, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Folkes LK, Candeias LP, Wardman P. Kinetics and mechanisms of hypochlorous acid reactions. Arch Biochem Biophys 323: 120–126, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Lab Invest 47: 412–426, 1982. [PubMed] [Google Scholar]
- 23.Gunnarsson M, Walther SM, Seidal T, Bloom GD, Lennquist S. Exposure to chlorine gas: effects on pulmonary function and morphology in anaesthetised and mechanically ventilated pigs. J Appl Toxicol 18: 249–255, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Haddad IY, Crow JP, Hu P, Ye Y, Beckman J, Matalon S. Concurrent generation of nitric oxide and superoxide damages surfactant protein A. Am J Physiol Lung Cell Mol Physiol 267: L242–L249, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Hagiwara SI, Ishii Y, Kitamura S. Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am J Respir Crit Care Med 162: 225–231, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Hardiman KM, Lindsey JR, Matalon S. Lack of amiloride-sensitive transport across alveolar and respiratory epithelium of iNOS(−/−) mice in vivo. Am J Physiol Lung Cell Mol Physiol 281: L722–L731, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Hawkins CL, Pattison DI, Davies MJ. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids 25: 259–274, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Hickman-Davis JM, Wang Z, Fierro-Perez GA, Chess PR, Page GP, Matalon S, Notter RH. Surfactant dysfunction in SP-A−/− and iNOS−/− mice with mycoplasma infection. Am J Respir Cell Mol Biol 36: 103–113, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holm BA, Notter RH, Leary JF, Matalon S. Alveolar epithelial changes in rabbits after a 21-day exposure to 60% O2. J Appl Physiol 62: 2230–2236, 1987. [DOI] [PubMed] [Google Scholar]
- 30.Holm BA, Notter RH, Siegle J, Matalon S. Pulmonary physiological and surfactant changes during injury and recovery from hyperoxia. J Appl Physiol 59: 1402–1409, 1985. [DOI] [PubMed] [Google Scholar]
- 31.John TA, Vogel SM, Minshall RD, Ridge K, Tiruppathi C, Malik AB. Evidence for the role of alveolar epithelial gp60 in active transalveolar albumin transport in the rat lung. J Physiol 533: 547–559, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kachadourian R, Day BJ. Flavonoid-induced glutathione depletion: potential implications for cancer treatment. Free Radic Biol Med 41: 65–76, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy SM, Enarson DA, Janssen RG, Chan-Yeung M. Lung health consequences of reported accidental chlorine gas exposures among pulpmill workers. Am Rev Respir Dis 143: 74–79, 1991. [DOI] [PubMed] [Google Scholar]
- 34.Lang JD, McArdle PJ, O'Reilly PJ, Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest 122: 314S–320S, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Leff JA, Parsons PE, Day CE, Taniguchi N, Jochum M, Fritz H, Moore FA, Moore EE, McCord JM, Repine JE. Serum antioxidants as predictors of adult respiratory distress syndrome in patients with sepsis. Lancet 341: 777–780, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Loewen GM, Holm BA, Milanowski L, Wild LM, Notter RH, Matalon S. Alveolar hyperoxic injury in rabbits receiving exogenous surfactant. J Appl Physiol 66: 1087–1092, 1989. [DOI] [PubMed] [Google Scholar]
- 37.Martin JG, Campbell HR, Iijima H, Gautrin D, Malo JL, Eidelman DH, Hamid Q, Maghni K. Chlorine-induced injury to the airways in mice. Am J Respir Crit Care Med 168: 568–574, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Matalon S, Egan EA. Effects of 100% O2 breathing on permeability of alveolar epithelium to solute. J Appl Physiol 50: 859–863, 1981. [DOI] [PubMed] [Google Scholar]
- 39.Matalon S, Egan EA. Interstitial fluid volumes and albumin spaces in pulmonary oxygen toxicity. J Appl Physiol 57: 1767–1772, 1984. [DOI] [PubMed] [Google Scholar]
- 40.Matalon S, Holm BA, Baker RR, Whitfield MK, Freeman BA. Characterization of antioxidant activities of pulmonary surfactant mixtures. Biochim Biophys Acta 1035: 121–127, 1990. [DOI] [PubMed] [Google Scholar]
- 41.Matalon S, Holm BA, Notter RH. Mitigation of pulmonary hyperoxic injury by administration of exogenous surfactant. J Appl Physiol 62: 756–761, 1987. [DOI] [PubMed] [Google Scholar]
- 42.Matalon S, O'Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu Rev Physiol 61: 627–661, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Morris JB, Wilkie WS, Shusterman DJ. Acute respiratory responses of the mouse to chlorine. Toxicol Sci 83: 380–387, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Nickerson PA, Matalon S. Quantitative ultrastructural study of the rabbit lung: exposure to 60% oxygen for 21 days. Undersea Biomed Res 17: 323–331, 1990. [PubMed] [Google Scholar]
- 45.Nickerson PA, Matalon S, Farhi LE. An ultrastructural study of alveolar permeability to cytochrome C in the rabbit lung: effect of exposure to 100% oxygen at one atmosphere. Am J Pathol 102: 1–9, 1981. [PMC free article] [PubMed] [Google Scholar]
- 46.Nishikimi M Oxidation of ascorbic acid with superoxide anion generated by the xanthine-xanthine oxidase system. Biochem Biophys Res Commun 63: 463–468, 1975. [DOI] [PubMed] [Google Scholar]
- 47.Nodelman V, Ultman JS. Longitudinal distribution of chlorine absorption in human airways: comparison of nasal and oral quiet breathing. J Appl Physiol 86: 1984–1993, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Peden DB, Hohman R, Brown ME, Mason RT, Berkebile C, Fales HM, Kaliner MA. Uric acid is a major antioxidant in human nasal airway secretions. Proc Natl Acad Sci USA 87: 7638–7642, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinho RA, Silveira PC, Silva LA, Luiz SE, Dal-Pizzol F, Moreira JC. N-acetylcysteine and deferoxamine reduce pulmonary oxidative stress and inflammation in rats after coal dust exposure. Environ Res 99: 355–360, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Prutz WA Reactions of hypochlorous acid with biological substrates are activated catalytically by tertiary amines. Arch Biochem Biophys 357: 265–273, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies KJ. Free radical biology and medicine: it's a gas, man! Am J Physiol Regul Integr Comp Physiol 291: R491–R511, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Sander R Compilation of Henry's Law Constants for Inorganic and Organic Species of Potential Importance in Environmental Chemistry (Online). 1999.
- 53.Sexton JD, Pronchik DJ. Chlorine inhalation: the big picture. J Toxicol Clin Toxicol 36: 87–93, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem 272: 25968–25975, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Vallyathan V, Goins M, Lapp LN, Pack D, Leonard S, Shi X, Castranova V. Changes in bronchoalveolar lavage indices associated with radiographic classification in coal miners. Am J Respir Crit Care Med 162: 958–965, 2000. [DOI] [PubMed] [Google Scholar]
- 56.van der Vliet A, O'Neill CA, Cross CE, Koostra JM, Volz WG, Halliwell B, Louie S. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am J Physiol Lung Cell Mol Physiol 276: L289–L296, 1999. [DOI] [PubMed] [Google Scholar]
- 57.Wang J, Winskog C, Edston E, Walther SM. Inhaled and intravenous corticosteroids both attenuate chlorine gas-induced lung injury in pigs. Acta Anaesthesiol Scand 49: 183–190, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Wang J, Zhang L, Walther SM. Inhaled budesonide in experimental chlorine gas lung injury: influence of time interval between injury and treatment. Intensive Care Med 28: 352–357, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Zhang L, Walther SM. Administration of aerosolized terbutaline and budesonide reduces chlorine gas-induced acute lung injury. J Trauma 56: 850–862, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Weill H, George R, Schwarz M, Ziskind M. Late evaluation of pulmonary function after acute exposure to chlorine gas. Am Rev Respir Dis 99: 374–379, 1969. [PubMed] [Google Scholar]
- 61.Winder C The toxicology of chlorine. Environ Res 85: 105–114, 2001. [DOI] [PubMed] [Google Scholar]