Abstract
The roles of Rho kinase (ROCK) and cGMP-dependent protein kinase (PKG) in cGMP-mediated relaxation of fetal pulmonary veins exposed to chronic hypoxia (CH) were investigated. Fourth generation pulmonary veins were dissected from near-term fetuses (∼140 days of gestation) delivered from ewes exposed to chronic high altitude hypoxia for ∼110 days (CH) and from control ewes. After constriction with endothelin-1, 8-bromoguanosine 3′,5′-cyclic monophosphate (8-Br-cGMP) caused a similar relaxation of both control and CH vessels. Rp-8-Br-PET-cGMPS (a PKG inhibitor) inhibited whereas Y-27632 (a ROCK inhibitor) augmented relaxation of control veins to 8-Br-cGMP. These effects were significantly diminished in CH veins. PKG protein expression and activity were greater whereas ROCK protein expression and activity were less in CH vessels compared with controls. Phosphorylation of threonine 696 (ROCK substrate) and serine 695 (PKG substrate) of the regulatory myosin phosphatase targeting subunit MYPT1 of myosin light chain (MLC) phosphatase was stimulated to a lesser extent in CH than in control veins by endothelin-1 (ROCK stimulant) and 8-Br-cGMP (PKG stimulant), respectively. The phosphorylation and dephosphorylation of MLC caused by endothelin-1 and 8-Br-cGMP, respectively, were less in CH veins than in controls. These results suggest that CH in utero upregulates PKG activity but attenuates PKG action in fetal pulmonary veins. These effects are offset by the diminished ROCK action on MYPT1 and MLC and thus lead to an unaltered response to cGMP.
Keywords: myosin phosphatase targeting subunit 1, myosin light chain phosphatases, vascular smooth muscle, lung
cyclic GMP-dependent protein kinase (PKG) and Rho kinase (ROCK) are critical enzymes involved in the regulation of vascular contractility through opposing actions on myosin light chain phosphatase (MLCP). MLCP is stimulated by PKG, which leads to increased dephosphorylation of myosin light chain (MLC) and vasodilatation. On the other hand, MLCP is inhibited by ROCK, which results in decreased dephosphorylation of MLC and vasoconstriction. Stimulation of MLCP activity induced by PKG occurs through the interaction between the leucine zipper (LZ) motifs of PKG and myosin phosphatase targeting subunit 1 (MYPT1), the regulatory subunit of MLCP. PKG may also stimulate MLCP activity independent of MYPT1 through phosphorylation of telokin (17, 40, 43). As for ROCK, it inhibits MLCP activity by phosphorylating MYPT1 at threonine 696 (Thr696) and threonine 853 (Thr853). Phosphorylation of MYPT1 induced by ROCK can be suppressed by PKG through phosphorylation of MYPT1 at serine 695 (Ser695) and serine 852 (Ser852). In addition, ROCK may also inhibit MLCP activity through PKC-potentiated inhibitor protein of 17 kDa (CPI-17) (31, 40, 41, 49).
PKG is the major enzyme involved in cGMP-mediated vasodilatation of fetal and newborn lungs (9, 10, 12, 32). Impaired PKG-mediated signaling has been found to be responsible for diminished cGMP-mediated pulmonary vasodilatation following acute and chronic hypoxia (CH) (10, 11, 20, 32). Our recent study shows that diminished cGMP-mediated relaxation of chronically hypoxic fetal pulmonary arteries may be in part due to decreased PKG activity and in part due to enhanced ROCK activity. ROCK may counteract PKG actions through its opposing effect on MLCP and thus attenuate cGMP-mediated relaxation (11).
Pulmonary veins of the fetus and the newborn exhibit marked vasoreactivity in response to various stimuli and contribute to a significant portion of the total pulmonary vascular resistance (12). Acute hypoxia has been shown to inhibit cGMP-induced relaxation of fetal pulmonary veins (10, 32). The present study was to determine the effect of CH on the relaxation responses of fetal pulmonary veins to cGMP. We found that CH in utero caused no significant change in relaxation of ovine fetal pulmonary veins to the cyclic nucleotide. This unaltered response, however, is the result of a change in balance between PKG and ROCK-mediated effects in pulmonary vascular smooth muscle. A decreased phosphorylation of MYPT1 at Ser695 by PKG offset by a decreased phosphorylation of the regulatory unit of MLCP at Thr696 contributes to the unaltered functional response to cGMP.
MATERIALS AND METHODS
Animals.
Pregnant ewes carrying single or twin fetuses (∼140 days of gestation; term being 147 days, either sex) were obtained from Nebeker Ranch in Lancaster, CA [altitude: ∼300 m; arterial Po2 (PaO2): 102 ± 2 mmHg]. To induce CH in the fetus, some pregnant sheep were transported to Barcroft Laboratory, White Mountain Research Station, Bishop, CA (altitude: 3,801 m; PaO2: 60 ± 2 mmHg) at 30 days of gestation and kept there for ∼110 days. The ewes were brought down to sea level immediately before delivery. They were anesthetized with thiamylal (10 mg/kg iv), and anesthesia was maintained on 1.5–2.0% halothane in oxygen throughout surgery. The fetus was delivered by cesarean section and killed by a lethal dose of pentobarbital (100 mg/kg) via the umbilical vein. After the fetuses were delivered, the ewe was then euthanized with T-61 (euthanasia solution; Hoechst-Roussel, Somerville, NJ). All procedures and protocols used in the present study were approved by the Animal Research Committees of Loma Linda University (11, 28) and Los Angeles Biomedical Research Institute at Harbor-UCLA.
Tissue preparation.
Fourth generation pulmonary veins (outside diameter: 1.5–2.5 mm) were dissected free of parenchyma and cut into rings (length: 5 mm) in ice-cold modified Krebs-Ringer bicarbonate buffer (composition in mM: 118.3 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25.0 NaHCO3, and 11.1 glucose).
Organ chamber study.
Rings of pulmonary veins were suspended in organ chambers filled with 10 ml of modified Krebs-Ringer bicarbonate solution maintained at 37°C and aerated with 95% O2-5% CO2 (pH 7.4). Each ring was suspended by two stirrups passed through the lumen. One stirrup was anchored to the bottom of the organ chamber; the other one connected to a strain gauge (model FT03C; Grass Instrument, Quincy, MA) for the measurement of isometric force (11). In studies with isolated vessels, oxygen reaches the cells by physical diffusion. To ensure that the cells located farthest from the surface of the blood vessel walls are adequately oxygenated, much higher oxygen tension is needed. Therefore, 95% O2-5% CO2 was chosen in isolated vessel studies (36).
At the beginning of each experiment, vessel rings were brought to their optimal tension by stretching the vessels progressively until the contractile responses to 100 mM potassium chloride were maximal. The optimal resting tension ranged 0.40 ± 0.09 to 0.46 ± 0.07 g among different experiment groups (n = 6–8 for each group; P > 0.05). One hour of equilibration was allowed after the vessels were brought to their optimal tension.
The effect of 8-bromoguanosine 3′,5′-cyclic monophosphate (8-Br-cGMP), a cell-permeable cGMP analog (29), was determined after the vessels were constricted to a similar tension level with endothelin-1 (3 × 10−9 to 10−8 M). It has been found that endothelin-1 may induce reactive oxygen species (ROS) production in pulmonary vascular smooth muscle at concentrations as low as 10−7 M (46). Using 2′,7′-dichlorofluorescein diacetate as a probe (32), we found that endothelin-1 at concentrations up to 10-fold higher than that used in our present study had no effect on ROS production in fetal ovine pulmonary venous smooth muscle cells, but endothelin-1 at 10−6 M increased ROS production by 34.8% ± 16.6% (n = 4 for each group; P < 0.05). In some experiments, Rp-8-Br-PET-cGMPS (a PKG inhibitor; Ref. 4) or Y-27632 (an inhibitor of ROCK; Ref. 39) was present. The inhibitors were administrated at least 30 min before testing their effects.
PKG activity assay.
Isolated pulmonary veins of fetal lambs were homogenized in a buffer containing 50 mM Tris·HCl (pH 7.4), 10 mM EDTA, 2 mM dithiothreitol, 1 mM isobutylmethylxanthine, 100 μM nitro-l-arginine, and 10 μM indomethacin. The homogenate was sonicated and centrifuged at 13,000 g for 10 min at 4°C. Supernatants were assayed for PKG activity by measuring the incorporation of 32P from [γ-32P]ATP into a specific PKG substrate, BPDEtide (Biomol Research Laboratories, Plymouth Meeting, PA). Aliquots (20 μl) of supernatant were added to a mixture (total volume: 50 μl) containing 50 mM Tris·HCl (pH 7.4), 20 mM MgCl2, 0.1 mM isobutylmethylxanthine, 10 μM indomethacin, 100 μM nitro-l-arginine, 150 μM BPDEtide, 1 μM PKI (a synthetic PKA inhibitor; Peninsula Laboratories, Belmont, CA), and 0.2 mM [γ-32P]ATP (specific activity: 3,000 Ci/mmol). The reaction mixture was incubated at 30°C for 10 min in the presence or absence of 3 μM exogenous cGMP. Reaction was terminated by spotting 40-μl aliquots of mixture onto phosphocellulose papers (2 × 2 cm; P81 Whatman) and placing them in ice-cold 75 mM phosphoric acid. The filter papers were washed, dried, and counted in a liquid scintillation counter. Assays were performed in triplicate with appropriate controls. After subtracting control counts, counts obtained indicate PKG activity, which is expressed as picomoles of 32P incorporated into PKG substrate per minute per milligram of protein. Protein content in supernatant was measured by Bradford procedure, using bovine serum albumin as a standard. Preliminary experiments confirmed the linearity of PKG activity at the protein concentration used within the incubation time. In the present study, sodium orthovanadate was used in ROCK activity assay and Western analysis but not in PKG activity assay. In preliminary studies, we have found that PKG activity was not affected by this phosphatase inhibitor (2 mM; n = 5 for each group, data not shown).
ROCK activity assay.
ROCK activity was measured using a CycLex Rho-kinase Assay Kit (MBL International, Nagano, Japan) according to the manufacturer's instructions. Briefly, tissues were homogenized in a buffer containing 50 mM Tris·HCl (pH 8.0), 0.1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 0.5 mM PMSF, 1 μg/ml pepstatin, 0.5 μg/ml leupeptin, 10 mM NaF, 2 mM Na3VO4, 10 mM 2-mercaptoethanol, 100 μM nitro-l-arginine, and 10 μM indomethacin. The homogenate was sonicated and centrifuged at 13,000 g for 10 min at 4°C. Aliquots (20 μg protein/25 μl) of supernatant were mixed with 60 μl of kinase reaction buffer provided by the kit and 15 μl solvent, 30 μM or 60 μM arachidonic acid, 100 μM ATP, and/or Y-27632 (10 μM). The mixture was added into the wells of a 96-well plate coated with peroxidase coupled with a monoclonal antibody [anti-phosphorylated MYPT1 (anti-p-MYPT1) at Thr696] and incubated at 30°C for 30 min (volume: 100 μl per well). The wells were then washed five times, incubated with 100 μl of horseradish peroxidase-conjugated detection antibody AF20 for 1 h at room temperature, washed five times, and then incubated for 15 min with 100 μl of substrate reagent at room temperature. Afterward, 100 μl of stop solution was added to each well, and the absorbance in each well was measured (450 nm). The activity of ROCK was expressed in arbitrary units relative to the absorbance of normoxic control.
cGMP assay.
Vessel rings were incubated in modified Krebs-Ringer bicarbonate solution (37°C 95% O2-5% CO2) in the presence and absence of 10−4 M Nω-nitro-l-arginine. Forty-five minutes after the inhibitors were added, vessel rings were rapidly freeze-clamped, thawed in trichloroacetic acid (6%), homogenized, sonicated for 5 s, and centrifuged (13,000 g for 15 min). The supernatant was extracted with four volumes of water-saturated diethyl ether and lyophilized; the protein content of the pellets was weighed. The lyophilized samples were resuspended in 1 ml of sodium acetate buffer (0.05 M, pH 6.2), and the content of cGMP was determined using a cGMP kit (Biomedical Technologies, Stoughton, MA). The content of cyclic nucleotide is expressed as picomoles per milligram of protein (13).
Western blots for PKG type I, ROCK isoforms, MYPT1, and MLC.
Tissue lysates prepared from whole tissue homogenate of fetal ovine pulmonary veins were solubilized in 2× Laemmli sample buffer and clarified (800 g, 10 min) before SDS-PAGE. The lysates, each containing 20 μg of protein, were subjected to SDS-PAGE and then electrotransferred to nitrocellulose. Nonspecific binding of antibody was blocked by washing with TBS buffer containing 10% milk for 1 h. The blot was then subjected to two brief washes with TBS plus 0.5% Tween-20 and incubated overnight in TBS plus 0.1% Tween-20 and the primary antibody with appropriate dilution. After two more washes in TBS plus 0.1% Tween-20, the blot was incubated for 1 h in secondary antibody, washed, and developed using the chemiluminescent detection method (SuperSignal West Pico Chemiluminescent Substrate; Pierce, Rockford, IL). The amount of the specific protein present in blots was quantified by densitometry using an Eagle Eye II still video system (Stratagene, La Jolla, CA). The protein levels of PKG and ROCK are normalized to scanning signals of actin (Oncogene, La Jolla, CA). The phosphorylation of MYPT1 and MLC are expressed as the ratio of phosphorylated to unphosphorylated scanning signals of the respective proteins. The primary antibodies used were anti-PKG type I (cat. no. KAS-PK005, polyclonal, detects an ∼75-kDa protein, corresponding to PKG type I; dilution 1:5,000; Stressgen, Victoria, Canada), anti-ROCK I (cat. no. 611137, monoclonal antibody raised against the amino acid sequence 906–1012 of mouse ROCK-I; dilution 1:1,000; BD Biosciences, San Jose, CA), anti-ROCK II (cat. no. 610624, monoclonal antibody raised against the amino acid sequence 567–718 of rat ROCK II; dilution 1:1,000; BD Biosciences), anti-MYPT1 (cat. no. 612165, monoclonal antibody raised against the amino acid sequence 723–840 of rat MYPT1; dilution 1:1,000; BD Biosciences), anti-p-MYPT1 at Thr696 {cat. no. 07–251, polyclonal antibody raised against keyhole limpet hemocyanin-conjugated synthetic peptide [CQSRRS(pT)QGVTL] corresponding to amino acids 691–701 of human MYPT1; dilution 1:1,000; Upstate Biotechnology, Lake Placid, NY}, anti-p-MYPT1 at Ser695 (cat. no. sc-33360-R, polyclonal antibody raised against the amino acid sequence containing phosphorylated Ser695 of MYPT1 of human origin; dilution 1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA), anti-MLC2 (cat. no. sc-15370, polyclonal antibody raised against amino acids 1–172 representing full-length MLC2 of human origin; dilution 1:1,000; Santa Cruz Biotechnology), and anti-p-MLC at Thr18/Ser19 (cat. no. sc-12896, polyclonal antibody raised against a short amino acid sequence containing phosphorylated Thr18 and Ser19 of MLC of human origin; dilution 1:1,000; Santa Cruz Biotechnology).
For Western analysis of phosphorylated and nonphosphorylated MYPT1 and MLC, pulmonary veins were first incubated in the modified Krebs-Ringer bicarbonate solution (37°C, 95% O2-5% CO2, pH 7.4) for 30 min in the presence of solvent, endothelin-1 (3 × 10−9 M), and endothelin-1 (3 × 10−9 M) plus 8-Br-cGMP (10−4 M). In some vessels, Rp-8-Br-PET-cGMPS (3 × 10−5 M) or Y-27632 (10−5 M) was included in the incubation buffer and was administered at least 30 min before the addition of endothelin-1. At the end of the incubation period, the tissues were quick-frozen with liquid nitrogen and subsequently sonicated on ice (5 s, 3×) in a buffer containing 50 mM Tris·HCl (pH 7.4), 100 μM EGTA, 1 mM sodium orthovanadate, 1% 2-mercaptoethanol, and 10% SDS. The homogenate was then centrifuged (1,000 g, 10 min, 4°C), and solubilized in 2× Laemmli sample buffer before SDS-PAGE. Western analysis of PKG and ROCKs was conducted using tissues without pretreatment.
Drugs.
The following drugs were used (unless otherwise specified, all were obtained from Sigma, St. Louis, MO): 8-Br-cGMP; β-phenyl-1,N2-etheno-8-bromoguanosine 3′,5′-cyclic monophosphorothioate, Rp isomer (Rp-8-Br-PET-cGMPS; Biolog Life Science Institute, La Jolla, CA); endothelin-1 (American Peptide, Sunnyvale, CA); indomethacin; nitro-l-arginine; and Y-27632 (Biomol). Indomethacin (10−5 M) was prepared in equimolar Na2CO3. This concentration of Na2CO3 did not significantly affect the pH of the solution in the organ chamber. The other drugs were prepared using distilled water.
Data analyses.
Contractions are expressed in grams. Relaxations are expressed as percent of contraction of vessels to endothelin-1. Data are shown as means ± SE. When mean values of two groups were compared, Student's t-test for unpaired observations was used. When the mean values of the same group before and after stimulation were compared, Student's t-test for paired observations was used. Comparison of mean values of more than two groups was made with one-way ANOVA test with Student-Newman-Keuls test for post hoc testing of multiple comparisons. All these analyses were performed using a commercially available statistics package (SigmaStat; Jandel Scientific, San Rafael, CA). Statistical significance was accepted when the P value (2-tailed) was less than 0.05. In all experiments, n represents the number of lambs.
RESULTS
Vessel tension study.
Relaxation of fetal pulmonary veins to 8-Br-cGMP (a cell-permeable analog of cGMP; Ref. 29) was determined after the vessel tension was raised to a similar level with endothelin-1 (3 × 10−9 to 10−8 M; vessel tension: 2.45 ± 0.32 to 2.88 ± 0.43 g; n = 6–8 for each group; P > 0.05) in the presence of nitro-l-arginine (10−4 M) and indomethacin (10−5 M) to exclude the involvement of endothelium-derived nitric oxide (EDNO) and cyclooxygenase products (13). These inhibitors increased the tension of control and chronic hypoxic (CH) pulmonary veins by 0.71 ± 0.10 and 0.80 ± 0.13 g, respectively (n = 8 for each group; P > 0.05). The endogenous cGMP level, an indicator of the activity of EDNO (13), was significantly less in CH veins (2.26 ± 0.13 pmol/mg protein, n = 9) than that in control veins (16.90 ± 1.71 pmol/mg protein, n = 9; P < 0.05). In the presence of nitro-l-arginine (10−4 M), the cGMP levels of control and CH veins were similar (1.04 ± 0.11 vs. 1.10 ± 0.12 pmol/mg protein, n = 8–9; P > 0.05) and significantly less than those not treated with the inhibitor of nitric oxide synthase (n = 8–9; P < 0.05).
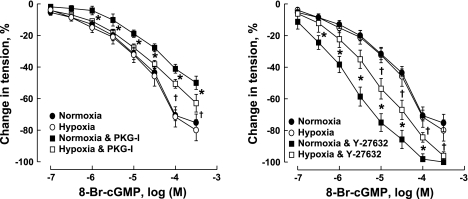
As shown in Fig. 1, 8-Br-cGMP caused a concentration-dependent relaxation of the CH veins that was not significantly different from control veins. Rp-8-Br-PET-cGMPS (3 × 10−5 M), a selective inhibitor of PKG (4), resulted in significantly less attenuation of relaxation of CH veins to the cGMP analog compared with control veins. Y-27632 (10−5 M), a specific inhibitor of ROCK (39), augmented relaxation of CH veins to the cGMP analog to a lesser extent in CH veins than in controls (Fig. 1).
Fig. 1.
Relaxation of pulmonary veins of normoxic and chronically hypoxic fetal lambs induced by 8-bromoguanosine 3′,5′-cyclic monophosphate (8-Br-cGMP). Vessels were preconstricted with endothelin-1 (6 × 10−9 to 10−8 M). PKG-I, Rp-8-Br-PET-cGMPS, β-phenyl-1,N2-etheno-8-bromoguanosine 3′,5′-cyclic monophosphorothioate, Rp isomer, 3 × 10−5 M. Y-27632, 10−5 M. Data are shown as means ± SE; n = 6–8 for each group. *Significant difference between normoxic and chronically hypoxic vessels; †significant difference between control and vessels treated with PKG-I (left) or Y-27632 (right) (P < 0.05).
PKG protein expression and activity.
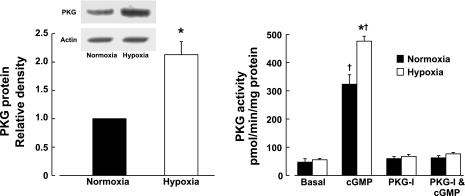
Expression of PKG protein in CH veins was significantly greater than in controls (Fig. 2, left). Basal PKG activity was similar in CH and control veins. However, cGMP (3 × 10−6 M) caused greater stimulation of PKG activity in CH veins than in controls. In all vessels, PKG activity stimulated with cGMP was inhibited by Rp-8-Br-PET-cGMPS (3 × 10−5 M) (Fig. 2, right).
Fig. 2.
Effect of chronic hypoxia on PKG protein expression and activity in pulmonary veins of fetal lambs. Left: Western blots and densitometric scanning of PKG protein normalized to actin. Right: PKG activity at basal conditions and stimulated with cGMP (3 × 10−6 M) of pulmonary veins of normoxic and chronically hypoxic fetal lambs. PKG-I, Rp-8-Br-PET-cGMPS (3 × 10−5 M). Data are shown as means ± SE; n = 6 -8 for each group. *Significantly different from normoxic vessels. †Significantly different from basal (P < 0.05).
ROCK protein expression and activity.
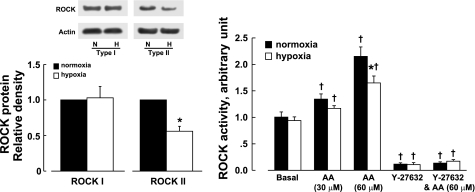
Expression of type I ROCK protein was not significantly different between control and CH veins, whereas the expression of type II ROCK protein was significantly less compared with that in controls (Fig. 3, left). Basal ROCK activity was similar in CH and control veins. When stimulated with arachidonic acid at 60 μM, ROCK activity in CH veins was significantly less than in controls. ROCK activity in both CH and control veins was reduced below basal levels by Y-27632 (10−5 M) (Fig. 3, right).
Fig. 3.
Effect of chronic hypoxia on Rho kinase (ROCK) protein expression and activity in pulmonary veins of fetal lambs. Left: Western blots and densitometric scanning of ROCK type I and II protein normalized to actin. Right: ROCK activity at basal conditions and stimulated with arachidonic acid (AA) of pulmonary veins of normoxic and chronically hypoxic fetal lambs. Y-27632, 10−5 M. Data are shown as means ± SE; n = 6 for each group. *Significantly different from normoxic vessels. †Significantly different from basal (P < 0.05). N, normoxia; H, hypoxia.
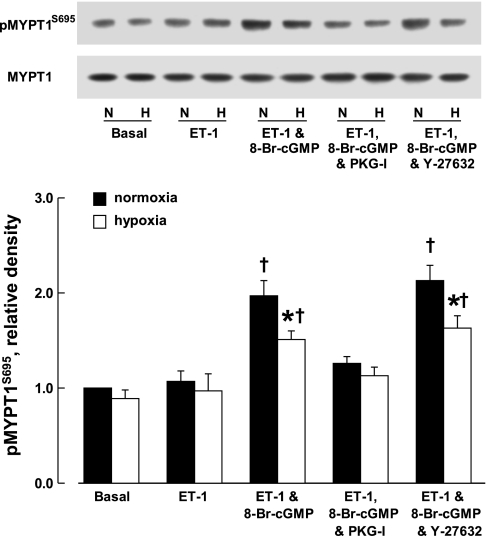
Phosphorylation of MYPT1 at Thr696 and Ser695.
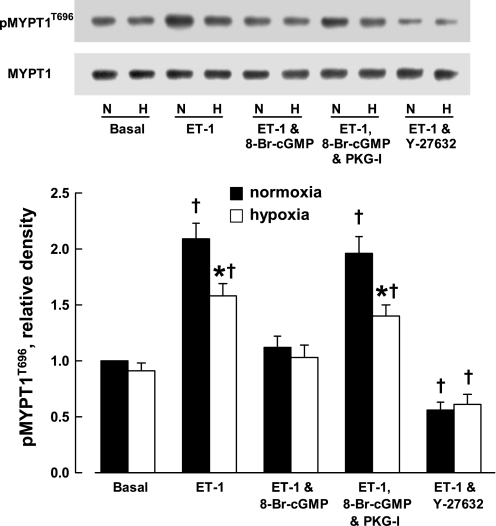
MYPT1, the regulatory subunit of MLCP, can be phosphorylated at Thr696 and Ser695 by ROCKs and PKG, respectively. Phosphorylation at Thr696 suppresses MLCP activity and thus results in vasoconstriction. In contrast, phosphorylation of MYPT1 at Ser695 counters the effect caused by phosphorylation at Thr696 (31, 49). The basal level of phosphorylation of MYPT1 at Thr696 and Ser695 were comparable between CH and control veins (Figs. 4 and 5). Endothelin-1 (3 × 10−9 M) caused less phosphorylation of MYPT1 at Thr696 in CH veins compared with that in controls. This difference was eliminated by 8-Br-cGMP (10−4 M). The effect of the cGMP analog was blocked by Rp-8-Br-PET-cGMPS (3 × 10−5 M). When vessels were treated with Y-27632 (10−5 M), the phosphorylation of MYPT1 at Thr696 induced by endothelin-1 in both CH and control veins was reduced to a similar level below basal values (Fig. 4).
Fig. 4.
Effect of chronic hypoxia on phosphorylation (p) of myosin phosphatase targeting subunit 1 (MYPT1) at Thr696 (T696). Top: Western blots. Bottom: densitometric scanning of phosphorylated MYPT1 at Thr696 normalized to nonphosphorylated MYPT1. ET-1, endothelin-1, 3 × 10−9 M; 8-Br-cGMP, 10−4 M; PKG-I, Rp-8-Br-PET-cGMPS, 3 × 10−5 M; Y-27632, 10−5 M. Data are shown as means ± SE; n = 8 for each group. *Significantly different from normoxia; †significantly different from basal (P < 0.05).
Fig. 5.
Effect of chronic hypoxia on phosphorylation of MYPT1 at Ser695 (S695). Top: Western blots. Bottom: densitometric scanning of phosphorylated MYPT1 at Ser695 normalized to nonphosphorylated MYPT1. Endothelin-1, 3 × 10−9 M; 8-Br-cGMP, 10−4 M; PKG-I, Rp-8-Br-PET-cGMPS, 3 × 10−5 M; Y-27632, 10−5 M. Data are shown as means ± SE; n = 8 for each group. *Significantly different from normoxia; †significantly different from basal (P < 0.05).
Phosphorylation of MYPT1 at Ser695 of the veins caused by 8-Br-cGMP was determined in the presence of endothelin-1, a condition similar to that when the relaxant effect of the cyclic nucleotide was examined. Endothelin-1 (3 × 10−9 M) alone had no significant effect on MYPT1 phosphorylation at Ser695. In the presence of endothelin-1, 8-Br-cGMP (10−4 M) caused less phosphorylation of MYPT1 at Ser695 of CH veins compared with that in controls. This difference was not affected by Y-27632 (10−5 M) but was blocked by Rp-8-Br-PET-cGMPS (3 × 10−5 M) (Fig. 5).
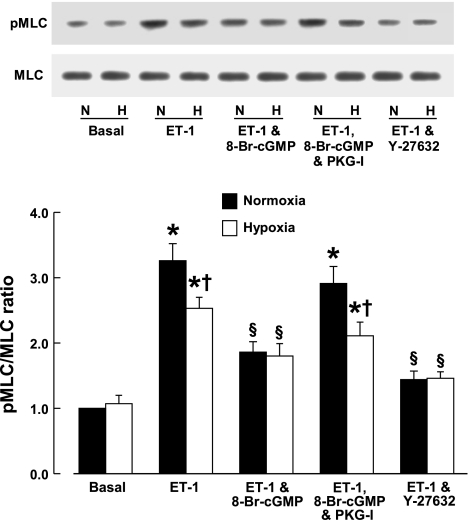
Phosphorylation of MLC at Thr18/Ser19.
Phosphorylation of MLC at Thr18 and Ser19 increases actin-activated myosin ATPase activity and smooth muscle contractility (38). Basal levels of MLC phosphorylation at these residues were not significantly different between CH and control veins. Endothelin-1 (3 × 10−9 M) increased the phosphorylation of MLC to a greater extent in control than in CH veins. Phosphorylation of MLC caused by endothelin-1 was reduced by Y-27632 (10−5 M) to a similar level in both CH and control veins. The increase in MLC phosphorylation induced by endothelin-1 was also inhibited by 8-Br-cGMP (10−5 M) to a similar extent in both CH and control veins. The effect of 8-Br-cGMP was blocked by Rp-8-Br-PET-cGMPS (3 × 10−5 M) (Fig. 6).
Fig. 6.
Effect of chronic hypoxia on the ratio of phosphorylation of myosin light chain (pMLC) at Thr18/Ser19 to unphosphorylated MLC. Top: Western blots. Bottom: pMLC/MLC ratio determined by densitometric scanning of the blots. Endothelin-1, 3 × 10−9 M; 8-Br-cGMP, 10−4 M; PKG-I, Rp-8-Br-PET-cGMPS, 3 × 10−5 M; Y-27632, 10−5 M. Data are shown as means ± SE; n = 6 for each group. *Significantly different from basal; †significantly different from normoxia; §significantly different from basal and from vessels treated with endothelin-1 (P < 0.05).
DISCUSSION
In perinatal lambs, chronic high altitude hypoxia induces sustained pulmonary hypertension (16). The arterial Po2 of the fetuses exposed to high altitude hypoxia is significantly lower than that of controls (22, 23), and the percentage medial wall thickness of pulmonary arteries is significantly increased (3). Furthermore, in pulmonary arteries, cGMP and PKG-dependent relaxation is attenuated, in part due to a reduced specific PKG activity and an enhanced ROCK activity (11). Substantial evidence shows abnormal vasoconstriction in resistance pulmonary arteries of chronically hypoxic (CH) lungs, whereas there appears to be little evidence of significant abnormalities in pulmonary veins (1, 12, 37). Therefore, the results of this study that there was no change in cGMP-induced relaxation of fetal pulmonary veins exposed to CH were not surprising. However, the unaltered responses to cGMP do not indicate that veins were unaffected by CH but rather represent a dynamic adaptation of the effects of two counteracting enzymes, PKG and ROCK, on contractility and relaxation.
The present study shows that 8-Br-cGMP-induced relaxation of normoxic veins was inhibited by Rp-8-Br-PET-cGMPS, a specific PKG inhibitor (4). The relaxation of CH veins to the cGMP analog at lower concentrations ≤30 μM, however, was not affected by the PKG inhibitor. Vasodilatation caused by cGMP is mediated by both PKG-dependent and -independent mechanisms (17). It appears that the PKG-dependent portion of the relaxation of pulmonary veins is selectively impaired by CH. In the presence of Y-27632, an inhibitor of ROCK (39), the relaxation of CH veins to cGMP was less augmented than that of normoxic veins, indicating that ROCK was downregulated in CH veins. Taken together, the results of the vessel tension studies suggest that the reduced role of PKG may be compensated by the diminished activity of ROCK, resulting in unchanged relaxation response of CH veins to cGMP. The present study was focused on the downstream mechanism of cGMP-mediated relaxation, and the influence of endogenous cGMP was largely prevented by reducing the cGMP content of the vessels to a very low level using nitro-l-arginine (13). However, our results show that, in the absence of nitro-l-arginine, the endogenous cGMP level of CH veins was substantially less than that of control veins. CH has been found to reduce relaxation of fetal ovine pulmonary veins to nitric oxide but does not affect the expression of endothelial nitric oxide synthase (50). Thus the low cGMP level of CH veins may result from a decreased EDNO bioavailability or suppressed soluble guanylyl cyclase (13).
The ineffectiveness of the PKG inhibitor at attenuating relaxation of CH veins to 8-Br-cGMP would suggest that the expression and activity of PKG is downregulated by CH. However, this was not the case in our study. In rat lungs, it has also been found that CH attenuates pulmonary vasodilatation to cGMP analogs but augments the expression of PKG protein (21). These results indicate that the diminished relaxation of pulmonary vessels to cGMP following CH is not due to decreased PKG activity per se but rather due to impaired actions of PKG. Phosphorylation of MYPT1 at Ser695 and Ser852 by PKG inhibits phosphorylation of MYPT1 at Thr696 and Thr853 by ROCK and leads to decreased calcium sensitivity of myofilament and vasodilatation (31, 41, 49). In our study, phosphorylation of MYPT1 at Ser695 caused by 8-Br-cGMP was prevented by Rp-8-Br-PET-cGMPS, suggesting that phosphorylation at this site is indeed mediated by PKG. Phosphorylation of MYPT1 at Ser695 caused by the cGMP analog was significantly less in CH veins than in controls, indicating impaired PKG-mediated action in CH veins. PKG-mediated MYPT1 phosphorylation may depend on the expression of the LZ motif of the MLCP subunit (18, 25). If the LZ motif of MYPT1 is downregulated by CH, as occurs under certain pathophysiological conditions (6, 24), PKG-mediated phosphorylation of MYPT1 could be impaired. Hypoxia may also cause covalent modification of MYPT1 and thus impair normal interaction with PKG and PKG-mediated phosphorylation (32). Since PKG may interfere with RhoA-ROCK signaling through inhibition of RhoA by phosphorylating the enzyme at Ser188 (27), the diminished PKG action may result from an impairment of the interaction between PKG and RhoA by PKG. In smooth muscle cells, PKG is mainly located in cytosol (17). We found that the expression of PKG in both normoxic and CH veins is predominantly in cytosol and negligible in cell membrane preparations (Y. Gao and J. U. Raj, unpublished observation). Therefore, it is unlikely that the decreased PKG effect results from a redistribution of the enzyme between cytosol and membrane following CH. PKG causes vasodilation by acting on various effectors located in different subcellular compartments (17). Whether CH may selectively affect certain PKG effectors and their subcellular locations remains to be determined.
ROCK and PKG exert opposing effects on MLCP activity (17, 40, 41). Therefore, it is expected that inhibition of ROCK will potentiate PKG-mediated vasodilatation. We found that cGMP-induced relaxation of CH veins was potentiated by Y-27632 to a lesser extent than in controls, suggesting that ROCK activity is downregulated following CH. Indeed, ROCK activity stimulated with arachidonic acid, a direct activator for the enzyme (7), was significantly less in CH veins than in controls. Arachidonic acid-stimulated ROCK activity was fully eliminated by Y-27632, indicating that the activity measured was indeed ROCK activity.
ROCKs are present in two isoforms, type I and II. Both of the isoforms are present in vascular smooth muscle, but their functional distinction is not clear yet (30). In our study, protein expression of type I ROCK was not different between CH and control veins, but type II ROCK protein expression was significantly less in CH than in control veins. Thus it appears that the reduced ROCK activity following CH is due to decreased expression of ROCK type II protein. Vasoconstrictors such as endothelin-1 may stimulate ROCK activity by activating RhoA via G protein (41). RhoA-activated ROCK activity leads to phosphorylation of MYPT1 at Thr696, which has been shown to be associated with inhibition of the catalytic activity of MLCP (40). In the present study, endothelin-1 induced less phosphorylation of MYPT1 at Thr696 in CH veins than in controls. The phosphorylation was inhibited by Y-27532 to below basal levels in both CH and control veins, suggesting that phosphorylation at this site is mediated by ROCK. The difference in phosphorylation of MYPT1 at Thr696 between CH and control veins was eliminated by inhibition of ROCK. Therefore, the decreased phosphorylation of MYPT1 at Thr696 following CH is likely to be due to reduced ROCK activity.
In permeabilized rabbit ileum (49) and femoral arteries (31) as well as in intact fetal ovine pulmonary arteries (11), 8-Br-cGMP inhibits Thr696 phosphorylation of MYPT1 and contraction. Consistent with these findings, Thr696 phosphorylation of both CH and control veins of the present study was inhibited by the cGMP analog accompanied with reduced contraction to endothelin-1. It appears that these effects were mediated by PKG as they were reversed by the inhibition of PKG with Rp-8-Br-PET-cGMPS. Although the expression and activity of PKG in CH veins were greater, inhibition of Thr696 phosphorylation caused by 8-Br-cGMP in CH veins was not greater than that in controls. The ineffectiveness of PKG at inhibiting Thr696 phosphorylation of CH veins may be due to the reduced Ser695 phosphorylation in CH veins as evident in the present study. Ser695 phosphorylation has been shown to prevent phosphorylation of MYPT1 at Thr696, which is immediately adjacent to Ser695 (31, 40, 49). Secondly, it has been proposed that cGMP can promote dephosphorylation of Thr696 via an unknown phosphatase (31). If this is true, a reduced PKG-mediated activation of this unknown phosphatase in CH may diminish cGMP-induced inhibition of Thr696 phosphorylation. Alternatively, downregulation of the expression of the LZ of MYPT1 may reduce PKG-mediated inhibition of Thr696 phosphorylation (6, 24, 35). Studies show that the MYPT1 LZ may be required for PKG action on MYPT1 (18, 41) and that MYPT1 LZ expression is downregulated under some pathophysiological conditions (6, 24, 35).
Smooth muscle contractility is modulated by the ratio of phosphorylated MLC to unphosphorylated MLC (p-MLC/MLC). An increase in the p-MLC/MLC ratio leads to increased activity of myosin ATPase and smooth muscle contraction, whereas a decrease in the ratio is associated with reduced myosin ATPase activity and relaxation. The dephosphorylation of MLC is catalyzed by MLCP. By their opposing actions on MLCP, PKG and ROCK have opposite effects on p-MLC/MLC ratio and therefore different effects on the contractility of smooth muscle (40). In the present study, the ratio of p-MLC at Ser18/Thr19, the dominant phosphorylation sites in vitro (38), to unphosphorylated MLC was increased by endothelin-1 to a lesser extent in CH veins than in control veins. Since the changes caused by endothelin-1 were eliminated by Y-27632, the smaller increase in the p-MLC/MLC ratio of CH veins compared with the controls is likely to result from the decreased ROCK activity. The increases in the p-MLC/MLC ratio caused by endothelin-1 of the vessels were inhibited by 8-Br-cGMP. The effect of the cGMP analog was blocked by the PKG inhibitor in the control but not in CH veins. This suggests that the effect of cGMP on MLC phosphorylation is mainly PKG-dependent in normoxic veins but PKG-independent in CH veins. Cyclic GMP may affect MLC phosphorylation independent of PKG by a number of mechanisms such as cross-activating PKA, activating cGMP-inhibited phosphodiesterase, and activating cGMP-gated potassium channel (2, 17, 26, 40). Whether these mechanisms are responsible for cGMP-mediated effects on CH veins remains to be determined. In the present study, the contraction level of CH veins caused by ET-1 was similar to that of normoxic veins. However, phosphorylation of MYPT1 at Thr696 and phosphorylation of MLC of CH veins stimulated by ET-1 were less than that of normoxic veins. Although MYPT1, MLCP, and MLC play important roles in regulation of smooth muscle contraction, other mechanisms may also be involved (14, 15, 33, 40, 42, 44). For instance, vasocontractility can be affected by a direct action of caldesmon on actin-activated myosin ATPase (15).
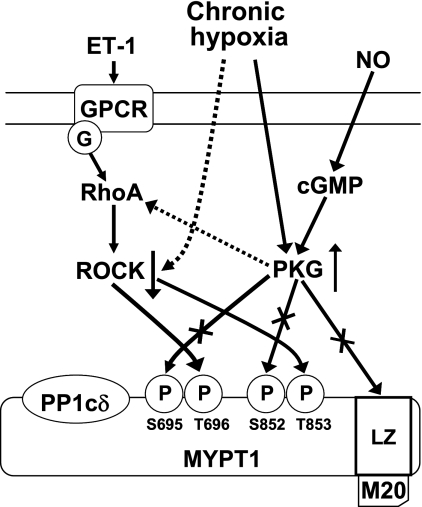
Impaired PKG (10, 11, 20, 32) and ROCK (11, 19, 34, 39, 45, 47) signaling are implicated in a number of vascular diseases including pulmonary hypertension. Our previous study shows that relaxation response to 8-Br-cGMP of pulmonary arteries of chronically hypoxic fetal lambs is attenuated, in part due to reduced PKG-specific activity and in part due to augmented ROCK activity (11). The present study shows that the cGMP-induced relaxation of pulmonary veins of fetal lambs was not altered following CH. It seems that the unchanged response to cGMP is due to a dynamic adaptation, in which the impaired PKG action is offset by a downregulated ROCK activity (Fig. 7). The expression and activity of ROCK can be upregulated or downregulated at various physiological and pathophysiological conditions (5, 11, 39, 48). The mechanisms responsible for the opposite effects of CH on ROCK between pulmonary arteries and veins are not clear. In recent years, inhibition of ROCK is being recognized as an effective way to treat pulmonary hypertension (8, 39). Our results suggest that some compensatory mechanisms may exist to downregulate ROCK. It may be of therapeutic significance to unveil the mechanisms underlying these adaptive changes in pulmonary veins exposed to CH.
Fig. 7.
Possible mechanisms of the role of cGMP-dependent protein kinase (PKG) and ROCK in the preservation of cGMP-induced relaxation of fetal pulmonary veins after chronic hypoxia. PKG and ROCK may affect the vessel tension through their opposing action on myosin light chain phosphatase (MLCP). PKG is activated by cGMP following the stimulation of soluble guanylyl cyclase by nitric oxide (NO). PKG stimulates MLCP activity through interaction with the leucine zipper (LZ) motif of the regulatory subunit of MLCP (MYPT1), which leads to increased dephosphorylation of MLC and vasodilatation. ROCK is activated by RhoA after stimulation with vasoconstrictors such as endothelin-1 and activation of G protein-coupled receptors (GPCR). ROCK inhibits MLCP activity through phosphorylating MYPT1 at Thr696 and Thr853 (human sequence), which leads to increased phosphorylation of MLC and vasoconstriction. PKG may interfere with the RhoA-ROCK signaling through phosphorylating RhoA at Ser188 and phosphorylating MYPT1 at Ser695 and Ser852. Chronic hypoxia upregulates PKG activity of PKG but impairs PKG-mediated phosphorylation of MYPT1 at Ser695 and Ser852. Chronic hypoxia may also downregulate the expression of the LZ motif of MYPT1, which leads to reduced stimulatory action of PKG on MLCP. ROCK is downregulated by chronic hypoxia, which results in reduced phosphorylation of MYPT1 at Thr696 and Thr853 and thus decreased inhibitory effect of ROCK on MLCP. The impaired PKG actions may be compensated by decreased ROCK signaling and thus preserves cGMP-mediated vasodilatation. G, G protein; P, phosphate; PP1cδ, the catalytic unit of MLCP; M20, a 20-kDa subunit of MLCP with unknown function. The dotted line indicates inhibitory action; X, impaired interactions.
GRANTS
This study was supported in part by the National Heart, Lung, and Blood Institute Grants HL-059435 and HL-075187, National Institute of Child Health and Human Development Grant P01-HD-31226, the National Natural Science Foundation of China Grant 30770789, and the Major National Basic Research Program of China Grant 2006CB503802.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abman SH Recent advances in the pathogenesis and treatment of persistent pulmonary hypertension of the newborn. Neonatology 91: 283–290, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58: 488–520, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bixby CE, Ibe BO, Abdallah MF, Zhou W, Hislop AA, Longo LD, Raj JU. Role of platelet-activating factor in pulmonary vascular remodeling associated with chronic high altitude hypoxia in ovine fetal lambs. Am J Physiol Lung Cell Mol Physiol 293: L1475–L1482, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Butt E, Pöhler D, Genieser HG, Huggins JP, Bucher B. Inhibition of cyclic GMP-dependent protein kinase-mediated effects by (Rp)-8-bromo-PET-cyclic GMPS. Br J Pharmacol 116: 3110–3116, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cario-Toumaniantz C, Evellin S, Maury S, Baron O, Pacaud P, Loirand G. Role of Rho kinase signaling in healthy and varicose human saphenous veins. Br J Pharmacol 137: 205–212, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen FC, Ogut O, Rhee AY, Hoit BD, Brozovich FV. Captopril prevents myosin light chain phosphatase isoform switching to preserve normal cGMP-mediated vasodilatation. J Mol Cell Cardiol 41: 488–495, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Fu X, Gong MC, Jia T, Somlyo AV, Somlyo AP. The effects of the Rho-kinase inhibitor Y-27632 on arachidonic acid-, GTPγS-, and phorbol ester-induced Ca2+-sensitization of smooth muscle. FEBS Lett 440: 183–187, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Fukumoto Y, Matoba T, Ito A, Tanaka H, Kishi T, Hayashidani S, Abe K, Takeshita A, Shimokawa H. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart 91: 391–392, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Y, Dhanakoti SR, Tolsa JF, Raj JU. Role of protein kinase G in nitric oxide- and cGMP-induced relaxation of newborn ovine pulmonary veins. J Appl Physiol 87: 993–998, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Gao Y, Dhanakoti S, Trevino EM, Sander FC, Portugal AM, Raj JU. Effect of oxygen on cyclic GMP-dependent protein kinase-mediated relaxation in ovine fetal pulmonary arteries and veins. Am J Physiol Lung Cell Mol Physiol 285: L611–L618, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Portugal AD, Negash S, Zhou W, Longo LD, Usha Raj J. Role of Rho kinases in PKG-mediated relaxation of pulmonary arteries of fetal lambs exposed to chronic high altitude hypoxia. Am J Physiol Lung Cell Mol Physiol 292: L678–L684, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Raj JU. Role of veins in regulation of the pulmonary circulation. Am J Physiol Lung Cell Mol Physiol 288: L213–L226, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y, Zhou H, Raj JU. Heterogeneity in role of endothelium-derived NO in pulmonary arteries and veins of full-term fetal lambs. Am J Physiol Heart Circ Physiol 268: H1586–H1592, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg JM, Wolpin ES, Bosgraaf L, Clarkson BK, Van Haastert PJ, Smith JL. Myosin light chain kinase A is activated by cGMP-dependent and cGMP-independent pathways. FEBS Lett 580: 2059–2064, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Gorenne I, Su X, Moreland RS. Caldesmon phosphorylation is catalyzed by two kinases in permeabilized and intact vascular smooth muscle. J Cell Physiol 198: 461–469, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Herrera EA, Pulgar VM, Riquelme RA, Sanhueza EM, Reyes RV, Ebensperger G, Parer JT, Valdéz EA, Giussani DA, Blanco CE, Hanson MA, Llanos AJ. High-altitude chronic hypoxia during gestation and after birth modifies cardiovascular responses in newborn sheep. Am J Physiol Regul Integr Comp Physiol 292: R2234–R2240, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev 86: 1–23, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Huang QQ, Fisher SA, Brozovich FV. Unzipping the role of myosin light chain phosphatase in smooth muscle cell relaxation. J Biol Chem 279: 597–603, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Hyvelin JM, Howell K, Nichol A, Costello CM, Preston RJ, McLoughlin P. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ Res 97: 185–191, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Jernigan NL, Resta TC. Chronic hypoxia attenuates cGMP-dependent pulmonary vasodilation. Am J Physiol Lung Cell Mol Physiol 282: L1366–L1375, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Jernigan NL, Walker BR, Resta TC. Pulmonary PKG-1 is upregulated following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 285: L634–L642, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Kamitomo M, Alonso JG, Okai T, Longo LD, Gilbert RD. Effects of long-term, high-altitude hypoxemia on ovine fetal cardiac output and blood flow distribution. Am J Obstet Gynecol 169: 701–707, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Kamitomo M, Longo LD, Gilbert RD. Right and left ventricular function in fetal sheep exposed to long-term high-altitude hypoxemia. Am J Physiol Heart Circ Physiol 262: H399–H405, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Karim SM, Rhee AY, Given AM, Faulx MD, Hoit BD, Brozovich FV. Vascular reactivity in heart failure: role of myosin light chain phosphatase. Circ Res 95: 612–618, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Khatri JJ, Joyce KM, Brozovich FV, Fisher SA. Role of myosin phosphatase isoforms in cGMP-mediated smooth muscle relaxation. J Biol Chem 276: 37250–37257, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Lang R, Lee G, Liu W, Tian S, Rafi H, Orias M, Segal AS, Desir GV. KCNA10: a novel ion channel functionally related to both voltage-gated potassium and CNG cation channels. Am J Physiol Renal Physiol 278: F1013–F1021, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Loirand G, Guérin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res 98: 322–334, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Long W, Zhang L, Longo LD. Fetal and adult cerebral artery KATP and KCa channel responses to long-term hypoxia. J Appl Physiol 92: 1692–1701, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Meyer RB, Miller JP. Analogs of cyclic AMP and cyclic GMP: general methods of synthesis and the relationship of structure to enzymic activity. Life Sci 14: 1019–1040, 1974. [DOI] [PubMed] [Google Scholar]
- 30.Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov 4: 387–398, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura K, Koga Y, Sakai H, Homma K, Ikebe M. cGMP-dependent relaxation of smooth muscle is coupled with the change in the phosphorylation of myosin phosphatase. Circ Res 101: 712–722, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Negash S, Gao Y, Zhou W, Liu J, Chinta S, Raj JU. Regulation of cGMP-dependent protein kinase (PKG1) mediated vasodilation by hypoxia-induced reactive species in ovine fetal pulmonary veins. Am J Physiol Lung Cell Mol Physiol 293: L1012–L1020, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Ogut O, Yuen SL, Brozovich FV. Regulation of the smooth muscle contractile phenotype by nonmuscle myosin. J Muscle Res Cell Motil 28: 409–414, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100: 923–929, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Payne MC, Zhang HY, Shirasawa Y, Koga Y, Ikebe M, Benoit JN, Fisher SA. Dynamic changes in expression of myosin phosphatase in a model of portal hypertension. Am J Physiol Heart Circ Physiol 286: H1801–H1810, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Pittman RN, Duling BR. Oxygen sensitivity of vascular smooth muscle. I. In vitro studies. Microvasc Res 6: 202–211, 1973. [DOI] [PubMed] [Google Scholar]
- 37.Rhodes J Comparative physiology of hypoxic pulmonary hypertension: historical clues from brisket disease. J Appl Physiol 98: 1092–1100, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Sakurada K, Seto M, Sasaki Y. Dynamics of myosin light chain phosphorylation at Ser19 and Thr18/Ser19 in smooth muscle cells in culture. Am J Physiol Cell Physiol 274: C1563–C1572, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol 25: 1767–1775, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Somlyo AV Cyclic GMP regulation of myosin phosphatase: a new piece for the puzzle? Circ Res 101: 645–647, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Su X, Smolock EM, Marcel KN, Moreland RS. Phosphatidylinositol 3-kinase modulates vascular smooth muscle contraction by calcium and myosin light chain phosphorylation-independent and -dependent pathways. Am J Physiol Heart Circ Physiol 286: H657–H666, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP-dependent protein kinase Iα. Science 286: 1583–1587, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Walsh MP, Horowitz A, Clément-Chomienne O, Andrea JE, Allen BG, Morgan KG. Protein kinase C mediation of Ca2+-independent contractions of vascular smooth muscle. Biochem Cell Biol 74: 485–502, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Jin N, Ganguli S, Swartz DR, Li L, Rhoades RA. Rho-kinase activation is involved in hypoxia-induced pulmonary vasoconstriction. Am J Respir Cell Mol Biol 25: 628–635, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Wedgwood S, Black S. Endothelin-1 decreases endothelial NOS expression and activity through ETA receptor-mediated generation of hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol 288: L480–L487, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Weigand L, Sylvester JT, Shimoda LA. Mechanisms of endothelin-1-induced contraction in pulmonary arteries from chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 290: L284–L290, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Wesselman JP, Kuijs R, Hermans JJ, Janssen GM, Fazzi GE, van Essen H, Evelo CT, Struijker-Boudier HA, De Mey JG. Role of the RhoA/Rho kinase system in flow-related remodeling of rat mesenteric small arteries in vivo. J Vasc Res 41: 277–290, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of serine 695 in response to cyclic nucleotides. J Biol Chem 279: 34496–34504, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Xue Q, Ducsay CA, Longo LD, Zhang L. Effect of long-term high-altitude hypoxia on fetal pulmonary vascular contractility. J Appl Physiol 104: 1786–1792. [DOI] [PubMed]