Abstract
Cytosolic Ca2+ signaling dynamics are important to pulmonary arterial reactivity, and alterations are implicated in pulmonary vascular disorders. Yet, adaptations in cellular Ca2+ homeostasis and receptor-mediated Ca2+ signaling with maturation from fetal to adult life in pulmonary arterial smooth muscle cells (PASMCs) are not known. The present study tested the hypothesis that cytosolic Ca2+ homeostasis and receptor-generated Ca2+ signaling adapt with maturation in sheep PASMCs. Digitalized fluorescence microscopy was performed using isolated PASMCs from fetal and adult sheep that were loaded with the Ca2+ indicator fura 2. The results show that basal cytosolic and sarcoplasmic reticulum Ca2+ levels are attained before birth. Similarly, Ca2+ efflux pathways from the cytosol and basal as well as capacitative Ca2+ entry (CCE) are also developed before birth. However, receptor-mediated Ca2+ signaling adapts with maturation. Prominently, serotonin stimulation elicited Ca2+ elevations in very few fetal compared with adult PASMCs; in contrast, phenylephrine elevated Ca2+ in a similar percentage of fetal and adult PASMCs. Serotonin and phenylephrine elicited Ca2+ increases of a similar magnitude in reactive cells of fetus and adult, supporting the assertion that inositol trisphosphate signaling is intact. Caffeine and ATP elevated Ca2+ in equivalent numbers of fetal and adult PASMCs. However, the caffeine-induced cytosolic Ca2+ increase was significantly greater in fetal PASMCs, whereas the ATP-elicited increase was greater in adult cells. Overall, the results of this study demonstrate selective adaptations in receptor-mediated Ca2+ signaling, but not in cellular Ca2+ homeostasis.
Keywords: fura 2, plasma membrane Ca2+-ATPase, sarco(endo)plasmic reticulum Ca2+-ATPase, capacitative Ca2+ entry, inositol trisphosphate, ryanodine receptor, ATP, serotonin, caffeine
cytosolic Ca2+ is central to vascular reactivity, with any alteration in the intracellular Ca2+ concentration ([Ca2+]i) affecting arterial tone. In particular, G protein-coupled receptor activation induces arterial contractility through [Ca2+]i elevations. Furthermore, receptor stimulation activates Ca2+-permeable ion channels, which elevates [Ca2+]i, and induces smooth muscle excitation-contraction coupling. Arterial smooth muscle cells maintain their [Ca2+]i within a narrow range not only to maintain basal tone, but also because large fluctuations can alter vascular tone and receptor-generated reactivity.
[Ca2+]i is regulated dynamically with equilibrium involving the activities of influx and efflux pathways across the plasma membrane, sarcoplasmic reticulum (SR), and mitochondria. Changes in Ca2+ influx into or efflux from the cytosol will regulate vascular tone (13, 21, 22). The primary pathways critical to increases in [Ca2+]i include voltage-gated L-type Ca2+ channels and nonselective cation channels (NSCC) on the plasma membrane and inositol trisphosphate (IP3)- and ryanodine-sensitive receptors, which release Ca2+ from the SR (11, 16). The major mechanisms that remove Ca2+ from the cytosol include the plasma membrane Ca2+-ATPase and the Na+/Ca2+ exchanger, through which Ca2+ is extruded into the extracellular space, and the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA), which sequesters Ca2+ into the SR (40). Capacitative Ca2+ entry (CCE), also known as store-operated Ca2+ entry, is important in the regulation of myogenic reactivity (4, 30). Classically, depletion of the SR Ca2+ stores leads to extracellular Ca2+ entry (6). CCE is not only important for the repletion of emptied Ca2+ stores, but it also regulates stimulus-mediated contractility (24) and provides a mechanism to maintain constant Ca2+ elevations in response to multiple stimuli (31).
Various inflammatory, neural, and humoral mediators modulate vascular contractility by altering Ca2+ homeostasis, and a number of reports suggest that these physiological processes adapt with maturation (2, 9, 14, 17, 23, 27, 28). With maturation from fetus to adult, the carotid arteries of sheep become more responsive to serotonin (5-HT) (2, 3). Other studies indicate that prostacyclin synthesis in sheep pulmonary arteries is developmentally regulated (8, 9, 32). Similarly, β2-adrenergic-, nitric oxide-, and atrial natriuretic peptide-mediated relaxation of pulmonary arteries and veins is developmentally regulated in fetal and newborn lambs (14, 20, 36). Thus, despite considerable evidence suggesting changes in Ca2+-dependent signaling with maturation in the pulmonary vasculature, the specific changes that occur are not well defined. We therefore tested the hypothesis that cytosolic Ca2+ homeostasis and receptor-generated Ca2+ signaling adapt with maturation in sheep pulmonary arterial smooth muscle cells (PASMCs). In fetal and adult sheep pulmonary arterial myocytes, we tested this hypothesis by examining Ca2+ elevations in response to the vasoactive substances 5-HT, ATP, phenylephrine (PE), and caffeine, as well as the functionality of capacitative Ca2+ influx pathways, plasma membrane Ca2+ extrusion, and SR Ca2+ uptake pathways.
METHODS
Cell isolation.
Smooth muscle cells were isolated from third- and fourth-generation sheep pulmonary arteries. Adult pulmonary arteries were obtained from healthy nonpregnant sheep (18–24 mo old) of either sex euthanized with pentobarbital sodium (100 mg/kg iv). Fetal pulmonary arteries were obtained from near-term (139–141 days gestation) fetuses (2.5–4.0 kg body wt), which were delivered by hysterotomy and then euthanized with pentobarbital sodium (100 mg/kg iv). The isolations, which were carried out at Loma Linda University, consisted of dissection of arteries at 5°C to decrease cellular metabolic activity in a low-Ca2+ physiological saline solution (PSS) containing (in mM) 125 NaCl, 5.36 KCl, 0.336 Na2HPO4, 0.44 K2HPO4, 11 HEPES, 1.2 MgCl2, 0.05 CaCl2, 10 glucose, and 2.9 sucrose, with pH adjusted to 7.4 with Tris and osmolarity adjusted to 300 mosM with sucrose. These arterial segments were then shipped overnight on ice to the University of Mississippi. All procedures were reviewed and approved by the Institutional Animal Use and Care Committee of Loma Linda University and the University of Mississippi. Tissue was digested with low-Ca2+ PSS containing (in mg/ml) 0.5 collagenase type XI, 0.03 elastase type IV, and 0.5 bovine serum albumin for 14–16 h at 4°C using protocols adapted from our previous studies (29,46). The tissue was then washed several times with 5°C low-Ca2+ PSS and triturated with a fire-polished Pasteur pipette. The resulting dispersed PASMCs were then stored at 5°C for up to 8 h.
Global Ca2+ measurements.
Cytosolic Ca2+ was measured in PASMCs loaded with the ratiometric Ca2+-sensitive dye fura 2-AM (Molecular Probes, Eugene, OR) using a dual-excitation digital Ca2+-imaging system (IonOptix, Milton, MA) equipped with an intensified charged-coupled device, as previously described (46, 61). Briefly, cells were loaded with 10 μM fura 2-AM for 20–25 min at room temperature in the dark and then washed for 30 min to allow for dye esterification at 1 ml/min with a balanced salt solution consisting of (in mM) 126 NaCl, 5 KCl, 0.3 NaH2PO4, 10 HEPES, 1 MgCl2, 2 CaCl2, and 10 glucose (with pH adjusted to 7.4 with NaOH) and osmolarity of 300 mosM. Cells were illuminated with a xenon arc lamp at 340 ± 15 and 380 ± 15 nm (Chroma Technology, Rockingham, VT), and emitted light was collected from regions that encompassed single cells with a charge-coupled device at 510 nm at an acquisition rate of 1 Hz. [Ca2+]i was estimated from the ratio of fluorescence excited at 340 nm to fluorescence excited at 380 nm, as described previously (23) using in situ calibration solutions and protocols as previously described (38). The amplitudes of the increase in cytosolic Ca2+ due to depletion of the SR Ca2+ stores are expressed relative to baseline values. Background fluorescence was collected automatically and subtracted from the acquired fluorescence video images during each experiment. The Ca2+-free balanced salt solution was prepared by substitution of MgCl2 for CaCl2 and addition of 1 mM EGTA. All experiments were performed at room temperature (22–25°C).
Chemicals and drugs.
Ionomycin-free acid was purchased from Calbiochem (San Diego, CA), and all other chemicals were purchased from Sigma (St. Louis, MO).
Statistical analysis.
All curve-fitting and integration routines were performed with IGOR pro 3.0 (Wavemetrics, Lake Oswego, OR). Values are means ± SE. Statistical difference within groups was determined with a two-tailed paired Student's t-test for parametric data and χ2 test for nonparametric data. P < 0.05 was accepted as statistically significant. The n values reflect the total number of cells tested, as well as the total number of animals. Multiple trials were performed, and cells were isolated from multiple sheep for each experimental paradigm.
RESULTS
Maturation, cytosolic Ca2+ homeostasis, and plasma membrane Ca2+ clearance.
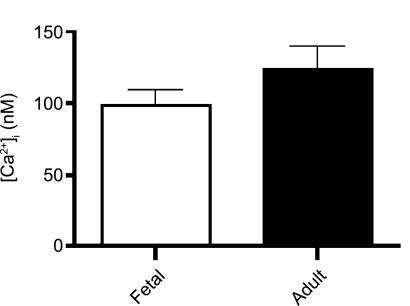
Figure 1 shows the average basal [Ca2+]i recorded in PASMCs from fetal and adult sheep at room temperature. In cells from the fetus, basal [Ca2+]i was 99 ± 11 nM (n = 85 cells and 16 animals), which did not differ significantly from basal [Ca2+]i measured in cells from adult sheep (124 ± 15 nM, n = 49 cells and 10 animals). This suggests that [Ca2+]i in PASMCs is maintained even before birth.
Fig. 1.
Basal intracellular Ca2+ concentration ([Ca2+]i) is unchanged in pulmonary arterial smooth muscle cells (PASMCs) with maturation. Values are means ± SE. No significant difference was observed (P = 0.16 by unpaired t-test).
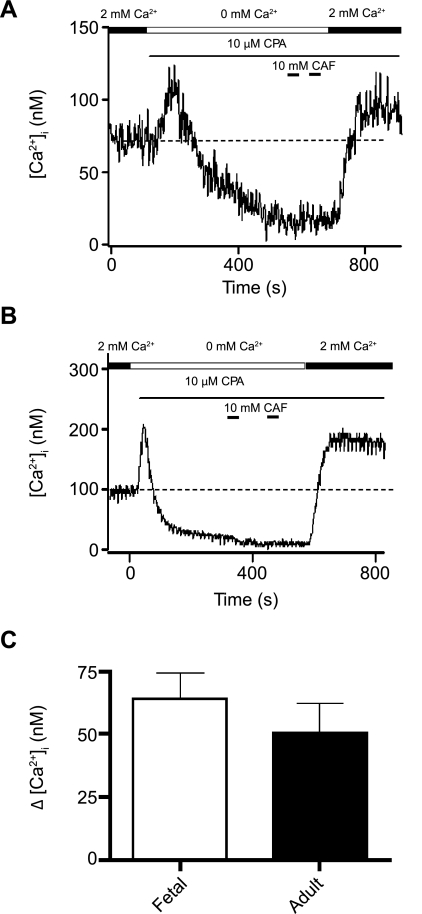
We then performed experiments to examine whether there might be changes in CCE, which has been shown to be important in proliferation of pulmonary arterial myocytes in culture (41, 42) and, therefore, might be important in the developing lung. The experimental protocol was similar to protocols used previously to examine CCE in pulmonary and renal arterial smooth muscle cells (39, 42). Figure 2 shows that depletion of the intracellular Ca2+ stores results in sustained elevations in [Ca2+]i consistent with activation of CCE. As shown in Fig. 2, in a fetal and adult PASMC, the SR Ca2+ stores were fully depleted by perfusion with a Ca2+-free bathing solution in the continuous presence of 10 μM cyclopiazonic acid (CPA) and two 30-s exposures to 10 mM caffeine. Inhibition of SERCA in these cells caused marked increases in [Ca2+]i due to passive leak of Ca2+ from the SR, which then relaxed due to the unidirectional flux of Ca2+ through the cytosol and across the plasma membrane. For the fetal cell shown in Fig. 2A, subsequent addition of extracellular Ca2+, in the continued presence of 10 μM CPA, caused [Ca2+]i to increase a modest 27 nM above basal values; for the adult cell in Fig. 2B, [Ca2+]i increased 96 nM above basal values, indicative of activation of CCE (39). Average data in Fig. 2C show that CCE is well developed in fetal PASMCs. The average increase in [Ca2+]i above basal levels was 64 ± 10 nM (n = 8 cells and 4 animals) in PASMCs isolated from fetal sheep and 50 ± 12 nM (n = 5 cells and 3 animals) in PASMCs from adult sheep. These results are qualitatively similar to those in canine pulmonary and renal arterial smooth muscle cells (39).
Fig. 2.
Activation of capacitative Ca2+ entry (CCE) in fetal and adult PASMCs. Representative traces of [Ca2+]i of myocytes from fetal (A) and adult (B) sheep show effect of extracellular Ca2+ removal, addition of 10 μM cyclopiazonic acid (CPA), and sequential exposure to 10 mM caffeine (Caf) and readdition of extracellular Ca2+ on cytosolic Ca2+. Dashed horizontal line shows basal [Ca2+]i. C: average change in [Ca2+]i from basal levels during readdition of Ca2+ in cells from fetal and adult sheep. Values are means ± SE. No significant difference was observed (P = 0.4 by unpaired t-test).
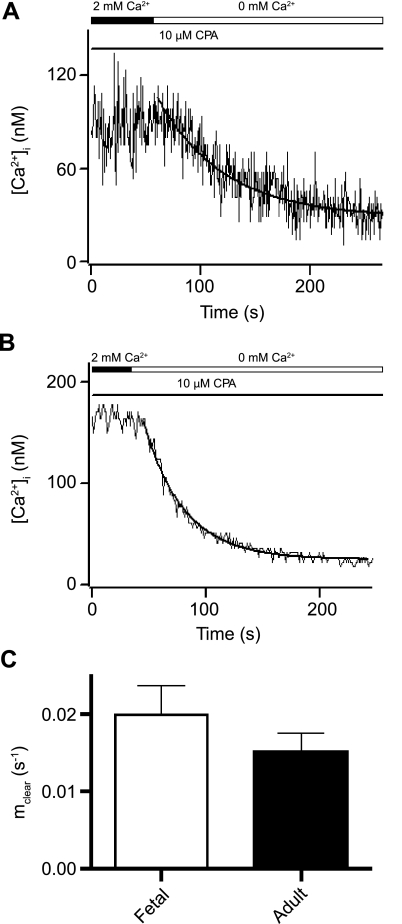
Using experimental and mathematical approaches developed by Bergling et al. (5), we performed experiments to determine whether Ca2+ clearance across the plasma membrane (i.e., cytosolic Ca2+ clearance) was altered with maturation. These analytic techniques do not account for any potential contribution of the mitochondria to the Ca2+ clearance under the experimental conditions performed. This is a reasonable assumption, inasmuch as the data were well fit by a single-exponential curve-fit analysis. These analysis techniques are well suited to our studies, inasmuch as the measurements are expressed in cytosolic volume equivalents (i.e., [Ca2+]i), which allows for comparisons between cells of different sizes. Figure 3 shows Ca2+ clearance from the cytosol in fetal and adult myocytes from sheep pulmonary arteries. The experimental design entails depletion of the SR Ca2+ stores, activation and measurement of CCE (Fig. 2), and, finally, removal of extracellular Ca2+. The rate constant of plasma membrane Ca2+ clearance (mclear) was determined by fitting the data with an exponential function
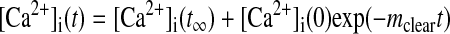 |
(1) |
The Ca2+ decay curves used to compute mclear (Fig. 3, A and B) were well fit by Eq. 1 and similar to those published for T lymphocytes (5), with mclear of 0.014 and 0.031 s−1 for the fetus and adult, respectively. Figure 3C provides averaged data for the mclear rates calculated for fetal and adult myocytes. It was not significantly changed with maturation: mclear was 0.02 ± 0.004 s−1 (n = 18 cells and 5 animals) in cells isolated from the fetus and 0.016 ± 0.002 s−1 (n = 11 cells and 5 animals) in cells isolated from the adult.
Fig. 3.
Rate of plasma membrane Ca2+ extrusion is unchanged in PASMCs with maturation. Representative [Ca2+]i recordings from myocytes of fetal (A) and adult (B) sheep show effect of extracellular Ca2+ removal in the presence of 10 μM CPA upon depletion of sarcoplasmic reticulum (SR) Ca2+ stores using the protocol described in Fig. 2. Solid curves show exponential curve-fit analyses. C: average plasma membrane clearance rate constant (mclear) for cells from fetal and adult sheep. Values are means ± SE. No significant difference was observed (P = 0.37 by unpaired t-test).
Maturation, SR Ca2+ uptake, and storage.
Once the rate constant of Ca2+ clearance is known, the SR Ca2+ store content can be calculated when SR Ca2+ uptake is inhibited and extracellular Ca2+ influx is abolished (5) using the following relationship
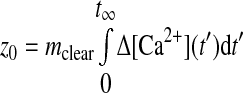 |
(2) |
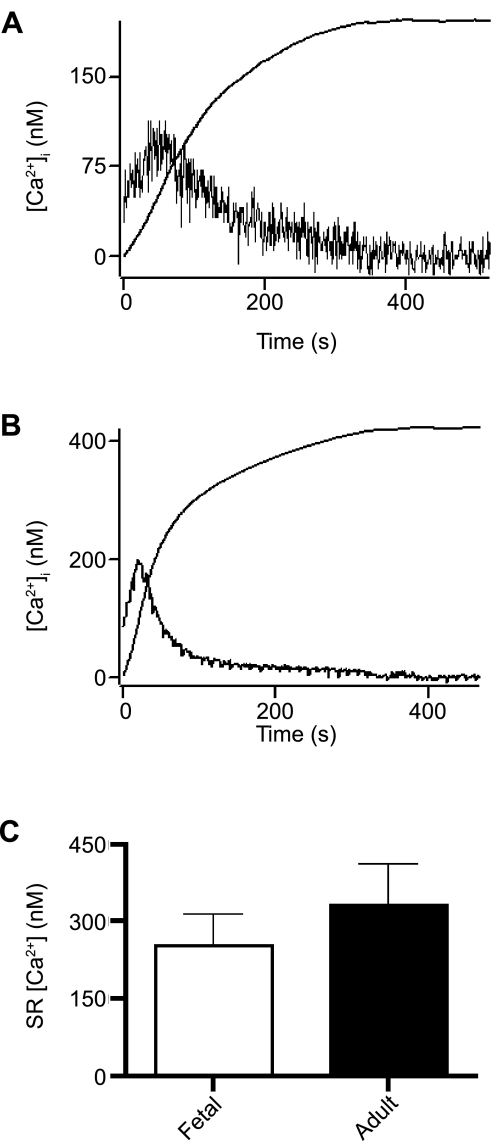
On the basis of this relationship in cells where mclear was measured, we estimated SR releasable store [Ca2+] content (z0). This value is equivalent to the [Ca2+]i that would be obtained if the SR Ca2+ stores were released and distributed instantaneously throughout the cytosol. This computation was achieved through integration of the Ca2+ release curve (Eq. 2) at the time points illustrated in Fig. 4A (fetal) and 4B (adult), where extracellular Ca2+ was removed and SERCA was inhibited with 10 μM CPA; z0 was 198 nM in the fetal cell (Fig. 4A) and 423 nM in the adult cell (Fig. 4B). Figure 4C shows the mean z0, which was 330 ± 80 nM in fetal (n = 18 cells and 5 animals) and 253 ± 60 nM in adult (n = 11 cells and 5 animals) PASMCs.
Fig. 4.
SR Ca2+ storage undergoes early maturation. Representative [Ca2+]i recordings and integrated Ca2+ response (dashed curve) of myocytes from fetal (A) and adult (B) sheep show response to extracellular Ca2+ removal and addition of 10 μM CPA. C: average of integrated Ca2+ concentration, which is equivalent to Ca2+ content of SR for cells from fetal and adult sheep. Values are means ± SE. No significant difference was observed (P = 0.49 by unpaired t-test).
On the basis of the computations of mclear and z0, the SR Ca2+ leak rate [Q(t)] can be computed using the following two relationships (5)
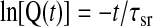 |
(3) |
with
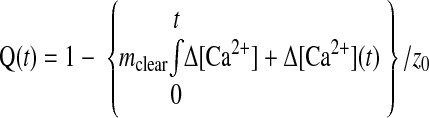 |
(4) |
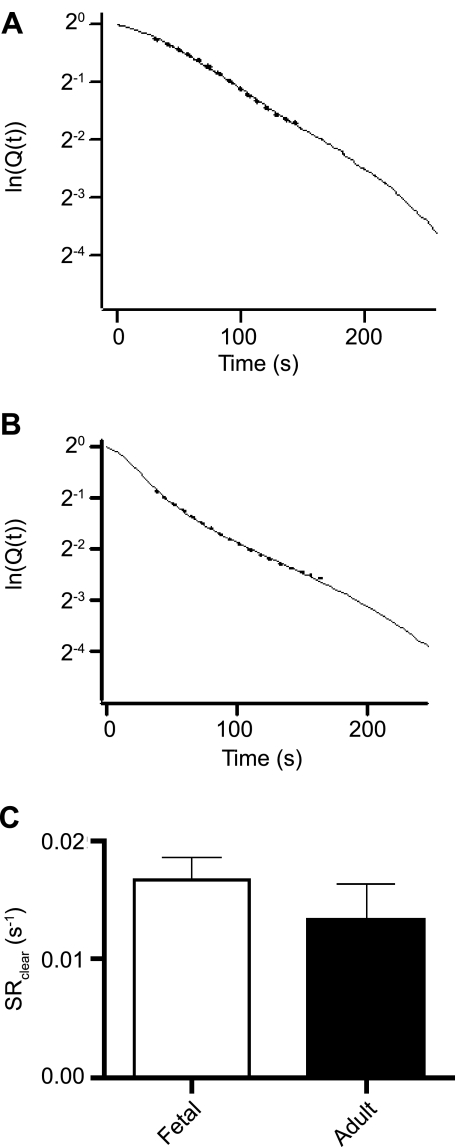
Figure 5 shows representations based on the analysis of Q(t). Semilogarithmic plots of Q(t) for cells from fetal (Fig. 5A) and adult (Fig. 5B) PASMCs are shown following exposure to 10 μM CPA and removal of extracellular Ca2+. The decline in fractional Ca2+ release is monoexponential for <90% of the Ca2+ released from the store, as illustrated by the single-exponential curve fit to ln[Q(t)]. The deviations in [Ca2+]i decay at very low values were expected, inasmuch as these nonexponential changes in Ca2+ were observed previously in T lymphocytes (5). Average data for these curve-fitting analyses are shown in Fig. 5C, which illustrates that the rate constants for the Ca2+ leak from the SR [0.017 ± 0.002 s−1 (n = 18 cells and 5 animals) for fetal sheep and 0.014 ± 0.003 s−1 (n = 11 cells and 5 animals) for adult sheep] are not altered significantly by maturation.
Fig. 5.
Rate constant for Ca2+ leak from SR is unchanged in PASMCs with maturation. SR Ca2+ leak rate [Q(t)] for myocytes from fetal (A) and adult (B) sheep is shown as semilogarithmic plots. C: mean clearance rate for passive Ca2+ leak from SR (1/τSR) for cells from fetal and adult sheep. Values are means ± SE. No significant differences were found (P = 0.34 by unpaired t-test).
At rest, the Ca2+ uptake and release rates of the mitochondria, SR, and other intracellular compartments are balanced. Thus, under basal conditions, the rate of SR Ca2+ release is equal to the rate of SERCA-dependent Ca2+ uptake. Therefore, ln[Q(t)] can be used under these circumstances to evaluate SERCA function. On the basis of this analysis, it is unlikely that SERCA function was altered with maturation in the cells examined in the present studies.
Ca2+ influx rates (Jin) can be determined when the [Ca2+]i is at steady state once mclear is known
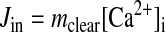 |
(5) |
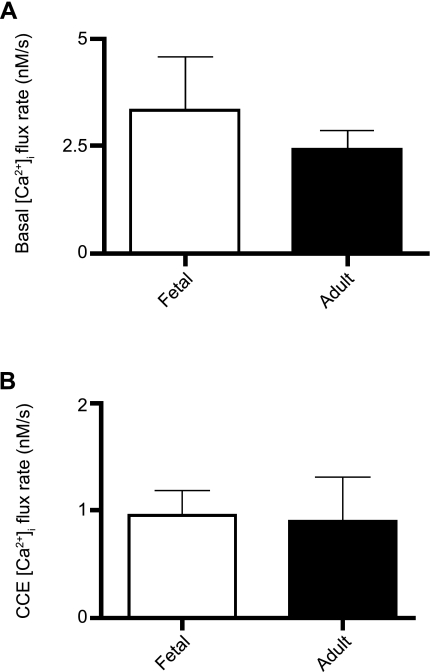
In the present experiments, the Ca2+ influx rates can be evaluated during resting conditions and once CCE is activated. Results of these calculations are shown in Fig. 6. Figure 6A shows no significant differences between the influx rates under resting conditions: 3.35 ± 1.24 nM/s (n = 16 cells and 5 animals) in cells from fetal sheep and 2.4 ± 0.45 nM/s (n = 11 cells and 5 animals) in cells from adult sheep. In Fig. 6B, which depicts the average influx rate during CCE, the influx rate in fetal cells [0.95 ± 0.22 nM/s (n = 18 cells and 5 animals)] is not different from that in cells from adult sheep [0.90 ± 0.42 nM/s (n = 11 cells and 5 animals)]. These findings are intriguing, inasmuch as they indicate that Ca2+ flux rates are relatively high under resting conditions in pulmonary arterial myocytes.
Fig. 6.
Ca2+ influx rate at rest and during CCE is unchanged in PASMCs with maturation. Average Ca2+ influx rates under basal conditions (A) and during CCE (B) are shown for cells from fetal and adult sheep. Values are means ± SE. No significant difference was observed for basal (P = 0.55 by unpaired t-test) or CCE (P = 0.27 by unpaired t-test) Ca2+ influx.
Maturational adaptations in receptor-generated Ca2+ signals.
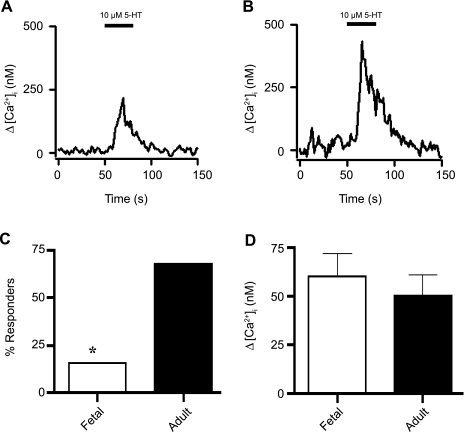
The activities of various neural, humoral, and inflammatory signaling are known to change with postnatal maturation. To evaluate maturational adaptations in inflammatory-mediated processes, we measured the responses to 5-HT. Recordings from fetal and adult PASMCs in Fig. 7 show cytosolic Ca2+ elevations in response to to 10 μM 5-HT. Moreover, the results show that 10 μM 5-HT elicited Ca2+ increases in far fewer fetal than adult PASMCs (Fig. 7C). Of 78 PASMCs from 17 fetuses, 5-HT-mediated Ca2+ increases were observed in only 12 cells (15%), whereas 29 of 43 cells (67.5%) from 10 adults showed such elevations. Nevertheless, the [Ca2+]i elevations due to 5-HT were unaffected by maturation in those cells that did respond to the agonist. The mean [Ca2+]i increase due to 5-HT in responsive fetal [60 ± 12 nM (n = 12 cells and 17 animals)] and adult [51 ± 10 nM (n = 29 cells and 10 animals)] PASMCs is shown in Fig. 7D.
Fig. 7.
Serotonin (5-HT)-mediated Ca2+ signaling increases with maturation in PASMCs. Representative traces show 5-HT-mediated [Ca2+]i elevation in myocytes from fetal (A) and adult (B) sheep. C: percentage of PASMCs from fetal and adult sheep showing Ca2+ elevation in response to 10 μM 5-HT. Responses of 78 cells from fetal and 43 cells from adult sheep were recorded. D: average increase in [Ca2+]i due to 10 μM 5-HT in cells from fetal and adult sheep. Values are means ± SE. No significant difference was observed (P = 0.6 by unpaired t-test). *P < 0.001 (by χ2 test).
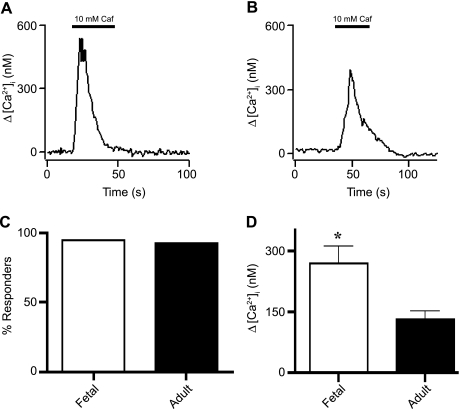
To evaluate changes in ryanodine receptor function, we stimulated cells with 10 mM caffeine, a selective ryanodine receptor activator (10, 38, 39). In Fig. 8, cytosolic Ca2+ elevations upon exposure to 10 mM caffeine is shown for fetal and adult PASMCs. As shown in Fig. 8C, there was no significant difference in the percentage of cells that exhibited caffeine-mediated increases in Ca2+ between fetal and adult PASMCs: 53 of 55 (95%) PASMCs from 17 fetuses and 37 of 41 (93%) PASMCs from 10 adults responded to caffeine. Yet, as shown in Fig. 8D, the mean [Ca2+]i increase was twofold greater in fetal than adult PASMCs: 269 ± 43 nM (n = 55 cells and 17 animals) in PASMCs from fetus and 132 ± 19 nM (n = 37 cells and 10 animals) in cells from adult.
Fig. 8.
Caffeine (Caf)-dependent Ca2+ release is augmented in PASMCs from fetus. Representative traces show caffeine-mediated [Ca2+]i elevation in myocytes from fetal (A) and adult (B) sheep. C: percentage of PASMCs from fetal and adult sheep showing Ca2+ elevation in response to 10 mM caffeine. No significant difference was observed (P = 0.7 by χ2 test). Responses of 55 cells from fetal and 41 cells from adult sheep were recorded. D: mean caffeine-elicited increase in [Ca2+]i in cells from fetal and adult sheep. Values are means ± SE. *P = 0.01 (by unpaired t-test).
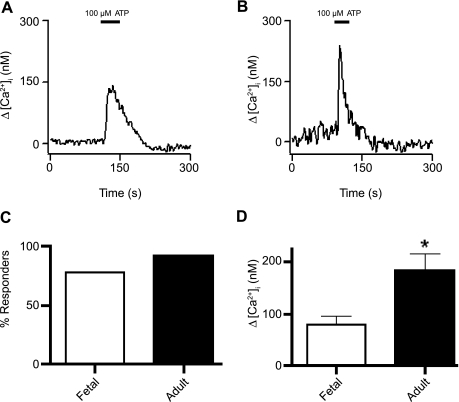
We evaluated the potential for maturational-related changes in neural stimulation by measuring the responses to P2 puringeric and α-adrenergic stimulation. Figure 9 shows recordings from fetal and adult PASMCs where cytosolic Ca2+ was elevated upon exposure to 100 μM ATP. Figure 9C shows that the percentage of PASMCs that responded to 100 μM ATP with an increase in Ca2+ was similar in the fetus and the adult: 40 of 51 cells (78%) from 9 fetuses and 35 of 38 cells (92%) from 5 adults showed cytosolic Ca2+ elevations in response to ATP. In contrast to the effects of caffeine, the mean [Ca2+]i increase was significantly greater for PASMCs isolated from adults than from fetuses. Figure 9D shows the mean [Ca2+]i increase due to ATP stimulation: 79 ± 17 nM (n = 51 cells and 9 animals) and 159 ± 28 nM (n = 35 cells and 5 animals) in PASMCs from fetuses and adults, respectively.
Fig. 9.
P2 receptor-dependent Ca2+ elevations are higher in adult PASMCs. Representative traces show 100 μM ATP-mediated [Ca2+]i elevation in myocytes from fetal (A) and adult (B) sheep. C: percentage of PASMCs from fetal and adult sheep showing Ca2+ elevation in response to 100 μM ATP. No significant difference was observed (P = 0.15 by χ2 test). D: ATP-mediated increases in [Ca2+]i in cells from fetal and adult sheep. Values are means ± SE. *P = 0.02 (by unpaired t-test).
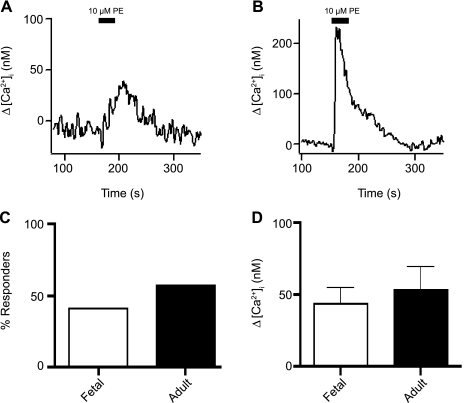
We used the sympathomimetic phenylephrine (PE) to determine whether there were any maturational-related changes in the Ca2+ responses to α-adrenergic receptor stimulation. Figure 10 shows recordings from fetal and adult PASMCs upon exposure to 10 μM PE. As shown in Fig. 10C, the percentage of PASMCs showing cytosolic Ca2+ elevations in response to 10 μM PE was similar in the fetus and the adult: of 46 PASMCs, 19 (41%) cells from 7 fetal sheep showed cytosolic Ca2+ increases to PE, whereas 16 of 28 (57%) cells from 3 adults responded. Figure 10D shows no significant differences in the mean [Ca2+]i increase: 44 ± 11 nM (n = 19 cells and 7 animals) and 53 ± 16 nM (n = 16 cells and 3 animals) in fetal and adult PASMCs, respectively.
Fig. 10.
α-Adrenergic receptor-dependent Ca2+ responses are unchanged with maturation in PASMCs. Representative traces show 10 μM phenylephrine (PE)-mediated [Ca2+]i elevations in myocytes from fetal (A) and adult (B) sheep. C: percentage of PASMCs from fetal and adult sheep showing Ca2+ elevation in response to 10 μM PE. No significant difference was observed (P = 0.2 by χ2 test). D: average PE-mediated increase in [Ca2+]i in cells from fetal and adult sheep. Values are means ± SE. No significant difference was observed (P = 0.6 by unpaired Student's t-test).
DISCUSSION
This is the first report to examine regulation of Ca2+ homeostasis with maturation and changes in agonist-mediated Ca2+ signaling in sheep pulmonary arterial myocytes. The findings suggest selective maturational adaptations in 5-HT-, caffeine-, and ATP-mediated Ca2+ signaling in these cells. The data also support the premise that general mechanisms important to regulating cytosolic Ca2+, including plasma membrane Ca2+ extrusion, SERCA function, SR storage, and CCE, are well developed before birth.
The basal (resting) [Ca2+]i provides a generalized measure of the tension that can be developed by Ca2+ under unstimulated conditions and represents the balance of plasma membrane Ca2+ influx relative to Ca2+ extrusion (19, 35, 39). The similar levels of basal Ca2+ in fetal and adult myocytes suggest similar roles for Ca2+ in resting tone (15).
Apart from [Ca2+]i, the Ca2+ stored in the SR plays an important role in the maintenance of basal pulmonary arterial tone and agonist-mediated contractile responsiveness (33, 34, 40). This is because cytosolic Ca2+ is in dynamic equilibrium with SR Ca2+, where the continuous leak of Ca2+ from the SR stores is counterbalanced by SERCA-dependent Ca2+ uptake. Previous studies indicate that the functionality of the SR Ca2+ stores can modulate agonist-evoked Ca2+ elevations and, thus, affect arterial tone (34). Our data (Fig. 4) show that the SR Ca2+ stores are mature before birth, being equivalent in myocytes of fetus and adult. This indicates that any difference in the amplitude of Ca2+ elevations due to agonist stimulation, such as that observed for caffeine (Fig. 8) as well as ATP (Fig. 9), is likely mediated by changes in the density or subtypes of plasma membrane, as well as intracellular receptors that are expressed, the second messengers generated by stimulated receptors, or the ability of the second messengers to elicit Ca2+ release from the SR stores.
In parallel with the ability of the SR to store Ca2+ before birth, their ability to release Ca2+ is also mature. The findings that similar proportions of fetal and adult cells respond to caffeine (Fig. 8) indicate that ryanodine receptors are developed before birth. The studies also suggest that maturation does not cause any generalized changes in IP3-related signaling. Specifically, PE-generated cytosolic Ca2+ increases were unchanged with maturation (Fig. 10), suggesting that the functional expression of IP3 receptors or their open probability is unchanged with maturation and that α-adrenergic receptor expression is also unaffected by postnatal maturation. Together, this evidence indicates that neural-dependent regulation of Ca2+ signaling through adrenergic pathways occurs before birth. This suggests that adrenergic-mediated pulmonary arterial tone may also be developed before birth.
The present study demonstrated that caffeine caused substantially greater Ca2+ elevations in fetal than in adult pulmonary arterial myocytes (Fig. 8). The simplest explanation for this finding is that there are a greater number of ryanodine receptors on the SR of fetal myocytes; however, there are other potential explanations. Ryanodine receptors in the fetus could be more readily activated by caffeine than those in the adult, possibly through enhancement of channel open probability. Also, there could be alterations in the relative expression of the various ryanodine receptor subtypes (i.e., RyR1, RyR2, or RyR3). Similar to our finding, the release of Ca2+ through ryanodine receptors on the SR Ca2+ stores was shown to be greater in PASMCs of the newborn than the adult rabbit (29). Regardless of the exact changes in ryanodine receptor functionality or expression, these changes may represent important adaptive processes from fetal to adult life.
Depletion of the SR Ca2+ stores, with the resultant increase in intracellular Ca2+, i.e., CCE, provides an estimate of store-operated channel activity, which often is mediated through activation of NSCC (38, 39). The results show that CCE is similar in fetal and adult myocytes (Fig. 2), with the CCE magnitude being similar to that found in canine PASMCs (39). Development of CCE before birth illustrates that any changes in agonist-elicited Ca2+ elevations due to NSCC during maturation may be due to altered expression or activity of receptor- and not store-operated channels.
Serotonergic-mediated Ca2+ signaling develops after birth in sheep PASMCs, inasmuch as there were marked increases in the proportion of PASMCs that exhibit 5-HT-elicited Ca2+ increases in the adult, compared with the fetus. Interestingly, Ca2+ elevations in the responsive fetal cells were similar to those in adult myocytes (Fig. 7). This was not entirely surprising, inasmuch as there were no differences in the magnitude of the Ca2+ increases due to PE, suggesting that if the 5-HT receptors were fully functional, then the Ca2+ responses were also developed. Similar to the finding of the present study, 5-HT-induced contractility was greater in arteries from adult sheep than from newborn lambs (12) and in adult than in perinatal rabbit pulmonary arteries (25). In combination, these studies illustrate that changes in 5-HT responsiveness may be a general maturational adaptation that is independent of the species examined. One possible explanation for this phenomenon is that, with maturation, there is an increase in the selective expression of the 5-HT receptors responsible for Ca2+ release. 5-HT-mediated contractility and Ca2+ signaling of pulmonary arteries in the dog is predominantly due to 5-HT2A receptors (38). This suggests that, with postnatal maturation, there could be an increase in 5-HT2A receptor expression in sheep pulmonary arteries, which then leads to a greater proportion of responsive myocytes from adult. It also is possible that postreceptor signaling pathways connected to IP3 generation are not properly developed before birth, which reduces cytosolic Ca2+ responsiveness.
P2 purinergic-mediated signaling is known to undergo postnatal maturation (18, 37), and ATP is a well-described vasoactive compound in the lung. ATP is released by sympathetic nerve terminals or by mast cells during inflammatory events (1). In model vascular systems, ATP causes cytosolic Ca2+ elevations through activation of P2X ionotropic receptors and concomitant Ca2+ influx, as well as by stimulation of P2Y metabotropic receptors, which induces IP3-generated Ca2+ responses (7, 26). Given that ATP can activate P2X or P2Y receptors, the present data (Fig. 9) cannot distinguish whether the postnatal maturation of the Ca2+ elevations due to ATP is mediated through changes in the function or expression of either receptor subtype.
The present study provides important details regarding the maturational adaptations in agonist-coupled Ca2+ signaling events in PASMCs. The changes indicate maturation of vasodilatory and vasoconstrictor signaling in the lung, providing information that begins to fill a large gap in our knowledge about the manner in which Ca2+ signaling matures in the pulmonary vasculature and the regulation of pulmonary arterial tone during early development. Collectively, the data provide compelling evidence that maturation results in multifaceted adaptations in the function of PASMCs, which is predicted to have implications for the adaptive processes that occur in response to life outside the womb.
GRANTS
This work was supported by National Science Foundation Grant MRI 0619774 (S. M. Wilson) and Career Grant 0133132 (G. D. Smith) and National Institute of Child Health and Human Development Grants P01 HD-031226 and R01 HD-003807 (L. D. Longo), as well as a University of Mississippi graduate student fellowship and a Sigma Xi research fellowship (to R. Goyal) and research experience opportunity programs funded by National Institutes of Health National Center for Research Resources Grant P20 RR-016476 (K. D. Creel) and National Science Foundation Grant 0450362 (E. Chavis).
Acknowledgments
We thank Stacy George, Douglas Hatran, and Matthew Loftin for technical assistance.
Present address of S. M. Wilson and R. Goyal: Center for Perinatal Biology, Loma Linda University School of Medicine, Loma Linda, CA 92350.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahmad S, Ahmad A, White CW. Purinergic signaling and kinase activation for survival in pulmonary oxidative stress and disease. Free Radic Biol Med 41: 29–40, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Angeles DM, Williams J, Purdy RE, Zhang L, Pearce WJ. Effects of maturation and acute hypoxia on receptor-IP3 coupling in ovine common carotid arteries. Am J Physiol Regul Integr Comp Physiol 280: R410–R417, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Angeles DM, Williams J, Zhang L, Pearce WJ. Acute hypoxia modulates 5-HT receptor density and agonist affinity in fetal and adult ovine carotid arteries. Am J Physiol Heart Circ Physiol 279: H502–H510, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Beech DJ Emerging functions of 10 types of TRP cationic channel in vascular smooth muscle. Clin Exp Pharmacol Physiol 32: 597–603, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergling S, Dolmetsch R, Lewis RS, Keizer J. A fluorometric method for estimating the calcium content of internal stores. Cell Calcium 23: 251–259, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Birnbaumer L, Boulay G, Brown D, Jiang M, Dietrich A, Mikoshiba K, Zhu X, Qin N. Mechanism of capacitative Ca2+ entry (CCE): interaction between IP3 receptor and TRP links the internal calcium storage compartment to plasma membrane CCE channels. Recent Prog Horm Res 55: 127–161, 2000. [PubMed] [Google Scholar]
- 7.Boeynaems JM, Communi D, Gonzalez NS, Robaye B. Overview of the P2 receptors. Semin Thromb Hemost 31: 139–149, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Brannon TS, MacRitchie AN, Jaramillo MA, Sherman TS, Yuhanna IS, Margraf LR, Shaul PW. Ontogeny of cyclooxygenase-1 and cyclooxygenase-2 gene expression in ovine lung. Am J Physiol Lung Cell Mol Physiol 274: L66–L71, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Brannon TS, North AJ, Wells LB, Shaul PW. Prostacyclin synthesis in ovine pulmonary artery is developmentally regulated by changes in cyclooxygenase-1 gene expression. J Clin Invest 93: 2230–2235, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corsso CD, Ostrovskaya O, McAllister CE, Murray K, Hatton WJ, Gurney AM, Spencer NJ, Wilson SM. Effects of aging on Ca2+ signaling in murine mesenteric arterial smooth muscle cells. Mech Ageing Dev 127: 315–323, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Drummond RM, Tuft RA. Release of Ca2+ from the sarcoplasmic reticulum increases mitochondrial [Ca2+] in rat pulmonary artery smooth muscle cells. J Physiol 516: 139–147, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn JA, Lorch V, Sinha SN. Responses of small intrapulmonary arteries to vasoactive compounds in the fetal and neonatal lamb: norepinephrine, epinephrine, serotonin, and potassium chloride. Pediatr Res 25: 360–363, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Floyd R, Wray S. Calcium transporters and signalling in smooth muscles. Cell Calcium 42: 467–476, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Gao Y, Tolsa JF, Botello M, Raj JU. Developmental change in isoproterenol-mediated relaxation of pulmonary veins of fetal and newborn lambs. J Appl Physiol 84: 1535–1539, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Geary GG, Osol GJ, Longo LD. Development affects in vitro vascular tone and calcium sensitivity in ovine cerebral arteries. J Physiol 558: 883–896, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurney AM, Drummond RM, Fay FS. Calcium signalling in sarcoplasmic reticulum, cytoplasm and mitochondria during activation of rabbit aorta myocytes. Cell Calcium 27: 339–351, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Harvey LM, Gilbert RD, Longo LD, Ducsay CA. Changes in ovine fetal adrenocortical responsiveness after long-term hypoxemia. Am J Physiol Endocrinol Metab 264: E741–E747, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Haworth SG Development of the normal and hypertensive pulmonary vasculature. Exp Physiol 80: 843–853, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Keizer J, De Young G. Effect of voltage-gated plasma membrane Ca2+ fluxes on IP3-linked Ca2+ oscillations. Cell Calcium 14: 397–410, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Kolber KA, Gao Y, Raj JU. Maturational changes in endothelium-derived nitric oxide-mediated relaxation of ovine pulmonary arteries. Biol Neonate 77: 123–130, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Kuriyama H, Kitamura K, Itoh T, Inoue R. Physiological features of visceral smooth muscle cells, with special reference to receptors and ion channels. Physiol Rev 78: 811–920, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Kuriyama H, Kitamura K, Nabata H. Pharmacological and physiological significance of ion channels and factors that modulate them in vascular tissues. Pharmacol Rev 47: 387–573, 1995. [PubMed] [Google Scholar]
- 23.Long W, Zhang L, Longo LD. Cerebral artery sarcoplasmic reticulum Ca2+ stores and contractility: changes with development. Am J Physiol Regul Integr Comp Physiol 279: R860–R873, 2000. [DOI] [PubMed] [Google Scholar]
- 24.McDaniel SS, Platoshyn O, Wang J, Yu Y, Sweeney M, Krick S, Rubin LJ, Yuan JXJ. Capacitative Ca2+ entry in agonist-induced pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol 280: L870–L880, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Morecroft I, MacLean MR. 5-Hydroxytryptamine receptors mediating vasoconstriction and vasodilation in perinatal and adult rabbit small pulmonary arteries. Br J Pharmacol 125: 69–78, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motte S, Communi D, Pirotton S, Boeynaems JM. Involvement of multiple receptors in the actions of extracellular ATP: the example of vascular endothelial cells. Int J Biochem Cell Biol 27: 1–7, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Nauli SM, Williams JM, Akopov SE, Zhang L, Pearce WJ. Developmental changes in ryanodine- and IP3-sensitive Ca2+ pools in ovine basilar artery. Am J Physiol Cell Physiol 281: C1785–C1796, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Nebigil CG, Etienne N, Schaerlinger B, Hickel P, Launay JM, Maroteaux L. Developmentally regulated serotonin 5-HT2B receptors. Int J Dev Neurosci 19: 365–372, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Porter VA, Reeve HL, Cornfield DN. Fetal rabbit pulmonary artery smooth muscle cell response to ryanodine is developmentally regulated. Am J Physiol Lung Cell Mol Physiol 279: L751–L757, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Putney JW Pharmacology of capacitative calcium entry. Mol Interv 1: 84–94, 2001. [PubMed] [Google Scholar]
- 31.Putney JW Capacitative calcium entry: sensing the calcium stores. J Cell Biol 169: 381–382, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaul PW, Pace MC, Chen Z, Brannon TS. Developmental changes in prostacyclin synthesis are conserved in cultured pulmonary endothelium and vascular smooth muscle. Am J Respir Cell Mol Biol 20: 113–121, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Shmigol AV, Eisner DA, Wray S. The role of the sarcoplasmic reticulum as a Ca2+ sink in rat uterine smooth muscle cells. J Physiol 520: 153–163, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shmygol A, Wray S. Modulation of agonist-induced Ca2+ release by SR Ca2+ load: direct SR and cytosolic Ca2+ measurements in rat uterine myocytes. Cell Calcium 37: 215–223, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Smith GD, Lee RJ, Oliver JM, Keizer J. Effect of Ca2+ influx on intracellular free Ca2+ responses in antigen-stimulated RBL-2H3 cells. Am J Physiol Cell Physiol 270: C939–C952, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Tolsa JF, Gao Y, Sander FC, Souici AC, Moessinger A, Raj JU. Differential responses of newborn pulmonary arteries and veins to atrial and C-type natriuretic peptides. Am J Physiol Heart Circ Physiol 282: H273–H280, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Wallace A, Knight GE, Cowen T, Burnstock G. Changes in purinergic signalling in developing and ageing rat tail artery: importance for temperature control. Neuropharmacology 50: 191–208, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Wilson SM, Mason HS, Ng LC, Montague S, Johnston L, Nicholson N, Mansfield S, Hume JR. Role of basal extracellular Ca2+ entry during 5-HT-induced vasoconstriction of canine pulmonary arteries. Br J Pharmacol 144: 252–264, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson SM, Mason HS, Smith GD, Nicholson N, Johnston L, Janiak R, Hume JR. Comparative capacitative calcium entry mechanisms in canine pulmonary and renal arterial smooth muscle cells. J Physiol 543: 917–931, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wray S, Burdyga T, Noble K. Calcium signalling in smooth muscle. Cell Calcium 38: 397–407, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Zhang S, Remillard CV, Fantozzi I, Yuan JX. ATP-induced mitogenesis is mediated by cyclic AMP response element-binding protein-enhanced TRPC4 expression and activity in human pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 287: C1192–C1201, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Zhang S, Yuan JX, Barrett KE, Dong H. Role of Na+-Ca2+ exchange in regulating cytosolic Ca2+ in cultured human pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 288: C245–C252, 2005. [DOI] [PubMed] [Google Scholar]