Abstract
Cardiac defects associated with increased pulmonary blood flow result in pulmonary vascular dysfunction that may relate to a decrease in bioavailable nitric oxide (NO). An 8-mm graft (shunt) was placed between the aorta and pulmonary artery in 30 late gestation fetal lambs; 27 fetal lambs underwent a sham procedure. Hemodynamic responses to ACh (1 μg/kg) and inhaled NO (40 ppm) were assessed at 2, 4, and 8 wk of age. Lung tissue nitric oxide synthase (NOS) activity, endothelial NOS (eNOS), neuronal NOS (nNOS), inducible NOS (iNOS), and heat shock protein 90 (HSP90), lung tissue and plasma nitrate and nitrite (NOx), and lung tissue superoxide anion and nitrated eNOS levels were determined. In shunted lambs, ACh decreased pulmonary artery pressure at 2 wk (P < 0.05) but not at 4 and 8 wk. Inhaled NO decreased pulmonary artery pressure at each age (P < 0.05). In control lambs, ACh and inhaled NO decreased pulmonary artery pressure at each age (P < 0.05). Total NOS activity did not change from 2 to 8 wk in control lambs but increased in shunted lambs (ANOVA, P < 0.05). Conversely, NOx levels relative to NOS activity were lower in shunted lambs than controls at 4 and 8 wk (P < 0.05). eNOS protein levels were greater in shunted lambs than controls at 4 wk of age (P < 0.05). Superoxide levels increased from 2 to 8 wk in control and shunted lambs (ANOVA, P < 0.05) and were greater in shunted lambs than controls at all ages (P < 0.05). Nitrated eNOS levels were greater in shunted lambs than controls at each age (P < 0.05). We conclude that increased pulmonary blood flow results in progressive impairment of basal and agonist-induced NOS function, in part secondary to oxidative stress that decreases bioavailable NO.
Keywords: pulmonary circulation, oxidant stress, congenital heart disease, reactive oxygen species
infants and children with congenital cardiac defects that cause significantly increased pulmonary blood flow suffer morbidity due to early aberrations in pulmonary vascular function (17). In fact, this early pulmonary vascular dysfunction is often exacerbated in the immediate postoperative period, manifesting as increased vascular reactivity that may produce severe hypoxemia, acidosis, low cardiac output, and death if not treated immediately (7, 11, 31). A complete understanding of the mechanisms responsible for this pulmonary vascular dysfunction is lacking, but evidence suggests that aberrant nitric oxide (NO)-cGMP signaling and oxidative stress may participate (1, 3, 6, 11, 15, 33, 39, 41).
Basal NO production by the vascular endothelium is integral to the maintenance of the normal low resistance state of the pulmonary vasculature, and dynamic alterations in NO production modulate vascular relaxation and constriction in response to various stimuli. NO is produced in vascular endothelial cells by nitric oxide synthase (NOS) from the oxidation of the guanidino nitrogen moiety of l-arginine. Once formed, NO diffuses into vascular smooth muscle cells where it activates soluble guanylate cyclase (sGC), which produces cGMP from GTP, resulting in smooth muscle relaxation through activation of a cGMP-dependent protein kinase (19, 25). Both animal and human studies indicate that abnormally increased pulmonary blood flow results in an early selective impairment of endothelium-dependent pulmonary vascular relaxation, suggestive of decreased NO bioavailability (11, 33).
Impaired bioavailable NO has been implicated in a number of cardiovascular diseases and has been associated with oxidative stress resulting from the production of reactive oxygen species (ROS) (10, 15). Although multiple pathological mechanisms have been proposed, the reaction of superoxide anion with NO may be particularly important. Both NO and superoxide possess unpaired electrons and thus react avidly to form peroxynitrite. In the setting of increased superoxide generation, this rapid reaction may scavenge NO, decreasing its bioavailability. In addition, peroxynitrite may directly disrupt cellular function, in part through the nitration of tyrosine residues on target proteins.
We (32) have previously developed a clinically relevant animal model of a congenital cardiac defect with abnormally increased pulmonary blood flow, created by the placement of a vascular graft (shunt) between the aorta and pulmonary artery in the late gestation fetal lamb, with which early mechanisms of disease may be explored. The purpose of this study was to test the hypothesis that bioavailable NO decreases over time in shunted lambs due to progressive NOS dysfunction and increased oxidative stress. To evaluate NOS function over time, we assessed pulmonary vascular relaxation in response to the NOS-dependent vasodilator ACh and the NOS-independent selective pulmonary vasodilator inhaled NO (iNO) in sham-operated and shunted lambs at 2, 4, and 8 wk of age. In addition, we performed peripheral lung tissue assays of NOS activity, assessed nitrate and nitrite (NOx) levels (an indirect determinant of bioavailable NO) in plasma and peripheral lung tissue, determined lung tissue endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS) protein levels, and localized iNOS protein using immunohistochemistry in sham-operated control and shunted lambs at 2, 4, and 8 wk of age. In addition, since interactions between heat shock protein 90 (HSP90) and eNOS are known to be important for normal NOS function, we determined lung tissue protein levels of HSP90 in sham-operated and shunted lambs at each age (40). Finally, as a measure of oxidative stress, we determined lung tissue superoxide anion levels by electron paramagnetic resonance (EPR) and eNOS nitration, an indirect measure of peroxynitrite production, in sham-operated and shunted lambs at each age.
METHODS
Surgical preparation.
Thirty mixed-breed Western pregnant ewes (137–141 days gestation, term = 145 days) were anesthetized with the use of local anesthesia (2% lidocaine hydrochloride) and inhaled anesthesia (1–3% isoflurane). The pregnant horn of the uterus was exposed, followed by the left fetal chest. With the use of side-biting vascular clamps, an 8.0-mm Gore-tex vascular graft (∼2-mm length) (W. L. Gore and Assos., Milpitas, CA) was anastomosed between the ascending aorta and main pulmonary artery with 7.0 proline (Ethicon, Somerville, NJ) using a continuous suture technique. This procedure was previously described in detail (28). An additional group of 27 fetal lambs underwent an identical procedure but did not have a graft placed (sham-operated lambs).
At 2, 4, or 8 wk after spontaneous delivery, sham-operated (n = 4 for each age group) and shunted lambs (n = 5 for each age group) were anesthetized with ketamine hydrochloride (15 mg/kg im). Polyurethane catheters were placed in an artery and vein of a hind leg and advanced to the descending aorta and the inferior vena cava, respectively. The lambs were then anesthetized with ketamine hydrochloride (∼0.3 mg·kg−1·min−1), diazepam (0.002 mg·kg−1·min−1), and fentanyl citrate (1.0 μg·kg−1·h−1), intubated with a 7.0-mm outer diameter cuffed endotracheal tube, and mechanically ventilated with a V.I.P. Bird Sterling (Palm Springs, CA) time-cycled, pressure-limited ventilator. The lambs were maintained normothermic (39°C) with a heating blanket. With strict aseptic technique, a midsternotomy incision was then performed. Using a purse-string suture technique, polyurethane catheters were placed directly into the right and left atrium and main pulmonary artery. An ultrasonic flow probe (Transonic Systems, Ithaca, NY) was placed around the left pulmonary artery to measure pulmonary blood flow.
An additional group of 2-, 4-, and 8-wk sham-operated control and shunted lambs (n = 5 for each group) were anesthetized as above, and the lungs were harvested for study. Lung pieces were snap-frozen in liquid nitrogen and stored at −80°C. In addition, 30 ml of arterial blood was collected in iced BD Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ) containing EDTA (7.5 mmol/l). The samples were centrifuged (4,000 g for 15 min) at 4°C, and the resulting plasma was snap-frozen in liquid nitrogen and then stored in polypropylene storage tubes at −80°C.
At the end of the protocol, all lambs were killed with a lethal injection of sodium pentobarbital followed by bilateral thoracotomy as described in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. All protocols and procedures were approved by the Committee on Animal Research of the University of California, San Francisco.
Measurements.
Pulmonary and systemic arterial and right and left atrial pressures were measured using Sorenson Neonatal Transducers (Abbott Critical Care Systems, North Chicago, IL). Mean pressures were obtained by electrical integration. Heart rate was measured by a cardiotachometer triggered from the phasic systemic arterial pressure pulse wave. Left pulmonary blood flow was measured on an ultrasonic flow meter (Transonic Systems). All hemodynamic variables were measured continuously using the Gould Ponemah Physiology Platform (version 4.2) and Acquisition Interface (Model ACG-16; Gould, Cleveland, OH) and recorded with a Dell Inspiron 5160 computer (Dell, Round Rock, TX). Blood gases and pH were measured on a Radiometer ABL 5 pH/blood gas analyzer (Radiometer, Copenhagen, Denmark). Hemoglobin concentration and oxyhemoglobin saturation were measured by a co-oximeter (model 682; Instrumentation Laboratory, Lexington, MA). Pulmonary vascular resistance was calculated using standard formulas. Shunt fraction (Qp/Qs) was determined using the Fick principle. Body temperature was monitored continuously with a rectal temperature probe.
Pulmonary vascular reactivity.
Pulmonary vascular responses were assessed in response to ACh and iNO. Lambs were instrumented as above and allowed to rest for a minimum of 30 min. Mechanical ventilation was adjusted to maintain Pco2 between 35 and 40 mmHg and pH between 7.35 and 7.40. Sodium bicarbonate was administered to correct metabolic acidosis if it occurred. The inspired oxygen concentration was maintained at 21%. Baseline hemodynamics and pulmonary blood flow were recorded. In random order, ACh (1 μg/kg) or iNO (40 ppm) was administered. ACh chloride (IOLAB, Claremont, CA) was diluted in sterile 0.9% saline and delivered by rapid injection into the pulmonary artery. iNO was delivered to the inspiratory limb of the respiratory circuit (INOvent; Ohmeda, Liberty, NJ) and continued for 15 min. The inspired concentrations of NO and nitrogen dioxide were continuously quantified by electrochemical methodology (INOvent, Ohmeda). The hemodynamic variables were monitored and recorded continuously. A minimum of 30 min separated the administration of ACh and iNO, and the second agent was not given until baseline hemodynamics returned.
We (3, 27, 33) demonstrated previously intact pulmonary vascular responses to endothelium-dependent and -independent vasodilating agents in normal lambs at 1, 4, and 8 wk of age. Since pulmonary vasodilating responses to many vasoactive agents are dependent on baseline pulmonary vascular tone and are minimal in the normal resting state, responses to ACh and iNO were assessed after the pulmonary vasculature was constricted by an intravenous infusion of U-46619 (a thromboxane A2 mimic) (34). To confirm these findings in the present study, pulmonary vascular responses to ACh and iNO were assessed in 12 sham-operated lambs at 2, 4, and 8 wk of age (n = 4 for each age group). Lambs were instrumented and allowed to recover as above. Baseline hemodynamics were recorded. An infusion of U-46619 was initiated through a dedicated catheter into the inferior vena cava. The dose (approximately 1–2 μg·kg−1·min−1) was titrated to increase mean pulmonary artery pressure to 1.5 times baseline. After a period of 15 min at a steady state, pulmonary vascular responses to ACh and iNO were assessed as above.
Preparation of protein extracts and Western blot analysis.
Lung protein extracts were prepared by homogenizing peripheral lung tissues in Triton lysis buffer (50 mM Tris·HCl, pH 7.6, 0.5% Triton X-100, 20% glycerol) containing a protease inhibitor cocktail. Extracts were then clarified by centrifugation (15,000 g × 10 min at 4°C). Supernatant fractions were then assayed for protein concentration using the Bradford reagent (Bio-Rad) and used for Western blot analysis. Western blot analysis was performed as previously described (2, 23, 44). Briefly, protein extracts (25 μg) were separated on 7.5% denaturing polyacrylamide gels for eNOS, iNOS, nNOS, and HSP90. All gels were electrophoretically transferred to Hybond-PVDF membranes (Amersham, Arlington Heights, IL). The membranes were blocked with 5% nonfat dry milk in TBS containing 0.1% Tween (TBS-T). After blocking, the membranes were incubated at room temperature with a 1:2,500 dilution of the NOS antiserum of interest, 1:1,000 dilution of HSP90, or a 1:10,000 dilution of β-actin, washed with TBS containing 0.1% Tween, and then incubated with a goat anti-mouse IgG-horseradish peroxidase. After washing, the protein bands were visualized with chemiluminescence using a Kodak Digital Science Image Station (NEN) and analyzed using the KED-1 software. All captured and analyzed images were determined to be in the dynamic range of the system.
The eNOS, iNOS, nNOS, and HSP90 antiserum was obtained from BD Transduction Laboratories (Lexington, KY). Positive controls were run to demonstrate antibody specificity. To normalize for protein loading, blots were reprobed with the housekeeping protein, β-actin. The methodology and exposure times used were those that we have previously demonstrated to be within the linear range able to detect changes in lung protein expression.
Confocal immunohistochemistry.
Snap-frozen lung tissue samples were embedded in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA), cryosectioned at 5 μm, collected onto Superfrost Plus slides (VWR Scientific, West Chester, PA), allowed to air-dry at room temperature, and stored at −80°C until needed. Double-labeling immunofluorescence was performed on serial lung sections using rabbit anti-iNOS (BD Transduction Laboratories), mouse anti-eNOS (BD Transductions Laboratories), and mouse anti-caldesmon (Sigma Chemical, St. Louis, MO). Fresh-frozen tissue sections were allowed to come to room temperature, washed in PBS, and fixed in ice-cold acetone for 10 min. Sections were air-dried for 1 h, permeabilized in PBS with 0.1% Triton X-100 for 10 min, blocked in 10% normal goat serum overnight at 4°C, and then incubated in primary antibody for 1 h at room temperature. Alexa Fluor 488 goat anti-rabbit and Alexa Fluor 546 goat anti-mouse antibodies (Molecular Probes) were used for detection of iNOS and eNOS/smooth muscle caldesmon, respectively. Sections were washed several times in PBS, mounted, and coverslipped in antifading aqueous mounting medium.
The confocal images were taken on a Zeiss LSM 510 Meta confocal laser scanning microscope using Plan Neofluar ×40/1.3 oil differential interference contrast (DIC) objective and Argon 488 and HeNe 543 lasers. A series of images at 1-μm interval focal planes were collected into a single file or z-stack to determine actual colocalization. Recorded images were processed using Adobe Photoshop software. The images were imported to the Zeiss LSM Image Browser software. Region of interest was selected for analysis using a freehand selection tool. To minimize the noise levels in the fluorescence quantification, minimum threshold value was set to 1,800. Mean intensity of the signal above this threshold was recorded.
Assay for NOS activity.
NOS activity was determined using the conversion of [3H]l-arginine to [3H]l-citrulline as described by Bush et al. (8). Briefly, peripheral lung tissues were homogenized in NOS assay buffer (50 mM Tris·HCl, pH 7.5, containing 0.1 mM EDTA and 0.1 mM EGTA) with a protease inhibitor cocktail. Enzyme reactions were carried out at 37°C in the presence of total lung protein extracts (500 μg), 1 mM NADPH, 14 μM tetrahydrobiopterin, 100 μM FAD, 1 mM MgCl2, 5 μM unlabeled l-arginine, 15 nM [3H]l-arginine, calmodulin (25 units), and 5 mM calcium to produce conditions that drive the reaction at maximal velocity. Duplicate assays were run in the presence of the NOS inhibitor nitro-l-arginine methyl ester (l-NAME) to detect nonspecific production of [3H]citrulline. This value was then subtracted to obtain the final activity value. Assays were incubated for 60 min at 37°C such that no more than 20% of the [3H]arginine was metabolized to insure that the substrate was not limiting. The reactions were stopped by the addition of iced stop buffer (20 mM sodium acetate, pH 5, 1 mM l-citrulline, 2 mM EDTA, and 0.2 mM EGTA) and then applied to columns containing 1 ml of Dowex AG 50W-X8 resin, Na+ form, preequilibrated with 1 N NaOH. [3H]l-citrulline was then quantitated by scintillation counting. All activities were normalized to the amount of protein in each lysate. To determine the potential contribution of iNOS to total NOS activity, assays were repeated without calcium supplementation.
Superoxide quantitation.
Superoxide levels in lung tissue taken from shunted lambs were estimated by EPR assay using the spin-trap compound 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine·HCl (CMH) in the presence and absence of polyethylene glycol-SOD (PEG-SOD), as we (45, 46) have previously described.
Approximately 0.1 g of tissue was sectioned from fresh-frozen biopsies of lung tissue and immediately immersed, while still frozen, in 200 μl of EPR buffer [PBS supplemented with 5 μM diethyldithiocarbamate (DETC; Sigma-Aldrich) and 25 μM desferrioxamine (Def MOS; Sigma-Aldrich)]. To determine the specificity of detected EPR signal, additional sample groups were immersed in EPR buffer supplemented with 100 U/ml membrane permeable form of SOD (PEG-SOD). In addition, to determine the relative contribution of uncoupled NOS activity to superoxide production, equivalent samples were preincubated in 200 μM S-ethylisothiourea hydrobromide (ETU; Sigma), a nonspecific inhibitor of NOS isoforms (16). All samples were then incubated for 30 min on ice and homogenized for 30 s with a VWR PowerMAX AHS 200 tissue homogenizer. Following incubation, samples were analyzed for protein content using Bradford analysis (Bio-Rad). Sample volumes were then adjusted with EPR buffer and 25 mg/ml CMH hydrochloride to achieve equal protein content and a final CMH concentration of 5 mg/ml. Samples were further incubated for 60 min on ice and then centrifuged at 14,000 g for 15 min at room temperature. Supernatant (35 μl) from each sample was loaded into a 50-μl capillary tube and analyzed with a MiniScope MS200 ESR (Magnettech, Berlin, Germany) at a microwave power of 40 mW, modulation amplitude of 3,000 mG, and modulation frequency of 100 kHz, with a magnetic strength of 333.95–3,339.94 mT. Resulting EPR spectra were analyzed using ANALYSIS v.2.02 software (Magnettech), whereby the EPR maximum and minimum spectral amplitudes for the CM·superoxide spin-trap product waveform were quantified.
To quantitate the amount of superoxide per milligram of protein, we also performed a standard reaction of the superoxide-generating enzyme xanthine oxidase in the presence of xanthine and CMH. A reaction curve was generated by adding 1 U/ml xanthine oxidase (0.5 U/mg; Sigma) into 500 μM xanthine (Sigma) solution in PBS, pH 7.4, in the EPR reaction buffer described above. Reactions were allowed to proceed at 25°C for up to 20 min. Following incubation, 35 μl of each reaction mixture were loaded, and the EPR spectra were analyzed as described above. Given that 1 unit of xanthine oxidase, under standard reaction conditions, converts 1 μmol of xanthine per minute at 25°C, on the basis of this standard curve, we calculated the coefficient of 840.6 EPR amplitude units/μmol of superoxide produced in the reaction. We then utilized this to convert the waveform amplitude generated by each sample into micromole of superoxide per milligram of protein. Experimental groups were then compared for differences in superoxide concentration using statistical analysis.
Measurement of NOx.
To quantify bioavailable NO, NO and its metabolites were determined in tissue and plasma from shunted lambs. In solution, NO reacts with molecular oxygen to form nitrite and with oxyhemoglobin and superoxide anion to form nitrate. Nitrite and nitrate are reduced using vanadium(III) and hydrochloric acid at 90°C. NO is purged from solution resulting in a peak of NO for subsequent detection by chemiluminescence (NOA 280; Sievers Instruments, Boulder, CO), as we (5, 24) have previously described. The sensitivity is 1 × 10−12 mol, with a concentration range of 1 × 10−9 to 1 × 10−3 molar of nitrate.
Immunoprecipitation: Western blot analysis for eNOS nitration.
To confirm that changes in eNOS uncoupling and increased superoxide generation were associated with eNOS nitration, we determined the level of eNOS protein nitration in 2- (n = 4), 4- (n = 5), and 8-wk-old (n = 4) shunt and age matched control lambs utilizing an immunoprecipitation-Western blot technique we (44) have previously described. Frozen lung tissue from shunted and control lambs was homogenized in 3× volume per tissue weight of immunoprecipitation buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 10 mM MgCl2, 1 mM EDTA, and 2% glycerol supplemented with protease inhibitors), centrifuged at 14,000 rpm at 4°C for 10 min, the supernatant was collected, and the protein concentration was quantified by the Bio-Rad DC Protein Assay. To 1,000 μg of total protein, 20 μl of Protein G-Agarose (EMD/Calbiochem, San Diego, CA) was added and then nutated at 4°C for 1 h to preclean the samples. Samples were then centrifuged at 12,800 rpm for 30 s. Two micrograms of anti-eNOS antibody was then added to the supernatants, the volume was brought to 1 ml with immunoprecipitation buffer, and the mixture was nutated at 4°C overnight. To precipitate the bound eNOS, 30 μl of Protein G-Agarose (EMD/Calbiochem) was added, and the samples were nutated for 1 h at 4°C. To collect the bead-bound antibody, the samples were centrifuged at 12,800 rpm for 30 s, the supernatant removed, and the beads washed with 500 μl of immunoprecipitation buffer. The wash step was repeated two additional times, and 30 μl of 2× Laemmli sample buffer was added to the samples and boiled for 5 min. The samples were then divided equally and loaded onto duplicate Life 4–20% Tris-SDS-HEPES gels (Gradipore, Frenchs Forest, Australia) and run to completion according to the manufacturers' instructions. The proteins were transferred to Immun-Blot PVDF membrane (Bio-Rad), and the membrane was blocked with 5% skim milk in TBS containing 0.1% Tween for 1 h to overnight. The membranes were probed with antibodies to either eNOS (to normalize for the immunoprecipitation efficiency) or 3- nitrotyrosine (EMD/Calbiochem), and reactive bands were visualized using the SuperSignal West Femto Maximum Sensitivity Substrate Kit (Pierce, Rockford, IL) and Kodak 440CF image station. Relative nitrated eNOS was then determined as a ratio of the 3-nitrotyrosine-eNOS-to-total eNOS signals with the controls set to 1.0 for each age examined.
Statistical analysis.
The means ± SD were calculated for the Western blot analyses, hemodynamic variables, NOS activities, NOx levels, fluorescence intensity, and EPR amplitudes. Differences over time were determined by ANOVA for repeated measures, and Student-Newman-Keuls post hoc testing was performed. Values between groups were compared by unpaired t-test. A P value of <0.05 was considered statistically significant.
RESULTS
Hemodynamics.
The hemodynamic data for sham-operated control and shunted lambs at 2, 4, and 8 wk of age are shown in Table 1. There were no differences in any of the hemodynamic indices from 2 to 8 wk in control lambs. In shunted lambs, there were no differences in any of the hemodynamic indices or the pulmonary to systemic blood flow ratios (Qp/Qs) from 2 to 8 wk of age.
Table 1.
Hemodynamic data for sham-operated control and shunted lambs at 2, 4, and 8 wk of age
|
Sham-Operated Control Lambs |
|||
|---|---|---|---|
| 2 wk | 4 wk | 8 wk | |
| PAP mean, mmHg | 15.4±2.9 | 16.7±2.6 | 15.7±5.1 |
| LAP mean, mmHg | 3.0±1.8 | 5.4±2.1 | 4.2±1.0 |
| RAP mean, mmHg | 2.3±1.4 | 4.6±2.6 | 2.2±0.5 |
| Systolic BP, mmHg | 88.3±12.9 | 89.0±17.9 | 105.8±12.5 |
| Diastolic BP, mmHg | 53.5±8.8 | 57.3±14.1 | 61.1±17.5 |
| Mean BP, mmHg | 66.2±7.0 | 75.3±16.8 | 81.3±7.61 |
| Flow, mg·min−1·kg−1 | 54.3±14.5 | 56.5±27.6 | 51.9±12.2 |
| Heart rate, beats/min | 173.8±25.5 | 146.4±33.0 | 169.8±21.8 |
| LPVR, mmHg/ml/min/kg | 0.24±0.08 | 0.23±0.09 | 0.22±0.09 |
| pH | 7.37±0.04 | 7.37±0.03 | 7.35±0.06 |
| Paco2 | 37±5.6 | 33.2±8.2 | 39.3±2.6 |
| Pao2 | 67.2±17.4 | 87±25.8 | 73.5±32.6 |
|
Shunted Lambs |
|||
|---|---|---|---|
| 2 wk | 4 wk | 8 wk | |
| PAP mean, mmHg | 21.6±3.8* | 22.2±2.6* | 20.6±8.4* |
| LAP mean, mmHg | 7.0±3.8* | 6.4±1.2 | 8.2±4.9 |
| RAP mean, mmHg | 4.8±3.5 | 2.9±0.8 | 5.0±4.4 |
| Systolic BP, mmHg | 93.1±28.4* | 108.8±45.5 | 114.8±13.3 |
| Diastolic BP, mmHg | 33.9±4.1* | 42.4±12.0* | 47.2±7.0 |
| Mean BP, mmHg | 71.4±29.0 | 70.4±8.9 | 70.6±1.7 |
| Flow, mg·min−1·kg−1 | 155.1±27.8* | 147.1±78.7* | 115.8±49.9* |
| Heart rate, beats/min | 171.6±32.9 | 173.7±23.4 | 139.4±24.0 |
| Qp/Qs | 3.1±1.4 | 2.9±0.9 | 2.0±0.4 |
| LPVR, mmHg/ml/min/kg | 0.10±0.03* | 0.13±0.05 | 0.11±0.02 |
| pH | 7.41±0.05 | 7.35±0.13 | 7.35±0.14 |
| Paco2 | 34.4±9.1 | 43.2±8.9 | 40.3±9.5 |
| Pao2 | 74.8±12.7 | 56.3±12.6* | 57.8±12.7 |
Values are means ± SD. PAP, pulmonary arterial pressure; LAP, left atrial pressure; RAP, right atrial pressure; BP, systemic blood pressure; Qp/Qs, pulmonary-to-systemic blood flow ratio; LPVR, left pulmonary vascular resistance; Paco2, arterial Pco2.
P < 0.05 compared with sham-operated control lambs at the corresponding age.
Pulmonary blood flow and pulmonary arterial pressures were greater in shunted lambs than sham-operated controls at each age (Table 1). In addition, at 2 wk of age, left atrial pressure and systolic systemic arterial pressure were greater, and diastolic systemic arterial pressure and calculated left pulmonary vascular resistance were lower in shunted lambs compared with control lambs (Table 1). Finally, diastolic systemic arterial pressure and arterial Po2 (PaO2) were lower in shunted lambs than control lambs at 4 wk of age (Table 1).
Pulmonary vascular reactivity.
At 2 wk of age, shunted lambs demonstrated a significant decrease from baseline in pulmonary artery pressure (Fig. 1; P < 0.05) and calculated pulmonary vascular resistance (−14.9% ± 11.4%; P < 0.05) in response to ACh. However, at 4 and 8 wk of age, neither pulmonary artery pressure (Fig. 1) nor pulmonary vascular resistance (−2.2% ± 4.8% at 4 wk and +13.3% ± 12.0% at 8 wk) changed significantly from baseline in response to ACh. In contrast, pulmonary artery pressure (Fig. 1; P < 0.05) and calculated pulmonary vascular resistance [−17.9% ± 16.9% (at 2 wk), −25.1% ± 9.7% (at 4 wk), and −17.6% ± 9.7% (at 8 wk); P < 0.05] decreased significantly in response to iNO in all age groups.
Fig. 1.
Changes in pulmonary artery pressure (PAP), expressed as percent change from baseline, in response to ACh (1 μg/kg), an endothelium-dependent agent, and inhaled nitric oxide (NO; 40 ppm), an endothelium-independent agent, in shunted lambs at 2, 4, and 8 wk of age. At 2 wk of age, PAP decreased significantly from baseline in response to ACh and inhaled NO. At 4 and 8 wk of age, PAP decreased significantly in response to inhaled NO but not to ACh. n = 5 for each group. Values are means ± SD. *P < 0.05 compared with baseline.
As expected, sham-operated lambs demonstrated a decrease in pulmonary artery pressure and calculated pulmonary vascular resistance in response to both ACh and iNO at each age group. At 2 wk, pulmonary artery pressure decreased 11.4% ± 4.5% and 26.3% ± 7.9%, and calculated pulmonary vascular resistance decreased 24.7% ± 12.7% and 37.3% ± 16.5% in response to ACh and iNO, respectively (P < 0.05). At 4 wk, pulmonary artery pressure decreased 12.3% ± 5.1% and 15.9% ± 8.5%, and calculated pulmonary vascular resistance decreased 25.8% ± 16.8% and 23.2% ± 6.7% in response to ACh and iNO, respectively (P < 0.05). At 8 wk, pulmonary artery pressure decreased 9.8% ± 4.0% and 22.4% ± 5.2%, and calculated pulmonary vascular resistance decreased 32.3% ± 9.2% and 23.4% ± 9.9% in response to ACh and iNO, respectively (P < 0.05).
NOS protein levels.
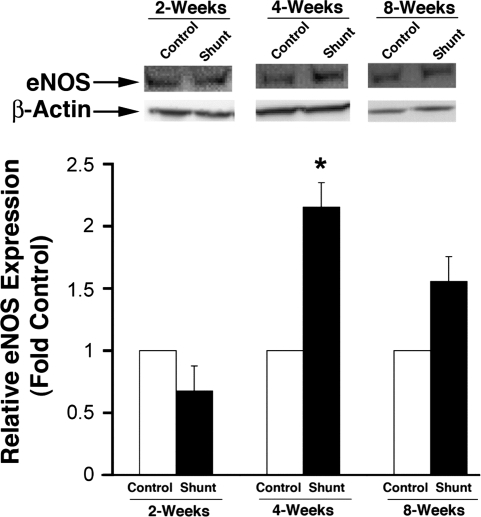
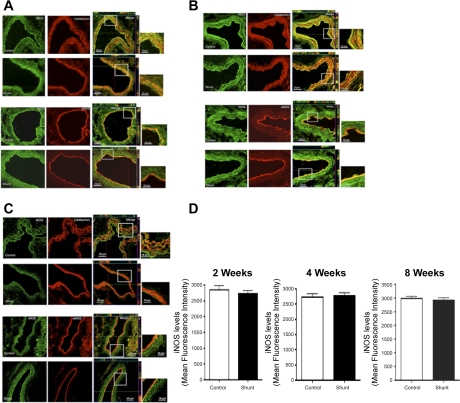
eNOS protein expression as determined by Western blot analysis on peripheral lung tissue taken from sham-operated control lambs and shunted lambs at 2, 4, and 8 wk of age are shown in Fig. 2. eNOS protein levels were greater in shunted lambs than controls at 4 wk but not at 2 or 8 wk (P = 0.067). iNOS and nNOS protein levels were minimally detectable and not different between control lambs and shunted lambs at 2, 4, and 8 wk of age (data not shown). To confirm further that inflammation due to abnormal pulmonary blood flow did not alter iNOS expression, immunohistochemistry was also performed on peripheral lung tissue taken from control and shunted lambs at 2, 4, and 8 wk of age (Fig. 3). Immunohistochemistry did not detect changes in iNOS protein between shunt and control lambs at any age and demonstrated iNOS localization to the pulmonary vasculature in both the endothelial and smooth muscle cell layers (Fig. 3). As expected, eNOS localized to the endothelial cell layer in each age group (Fig. 3).
Fig. 2.
Lung tissue endothelial nitric oxide synthase (eNOS) protein expression in sham-operated control and shunted lambs at 2, 4, and 8 wk of age. Top: representative Western blots are shown for eNOS protein extracts prepared from lung tissue separated on a 7.5% SDS-polyacrylamide gel, electrophoretically transferred to Hybond membranes, and analyzed using a specific antiserum raised against eNOS and reprobed with β-actin to demonstrate equal loading. Bottom: densitometric values for eNOS protein shown relative to control. *P < 0.05 vs. age-matched control; n = 5 for each group. Values are means ± SD.
Fig. 3.
In vivo expression of inducible NOS (iNOS) in the pulmonary vasculature of sham-operated control and shunted lambs at 2- (A), 4 (B), and 8 (C) wk of age. The expression in the smooth muscle cell layer was identified by colocalization with caldesmon and in the endothelial cell layer by colocalization with eNOS. iNOS expression is in green, and caldesmon and eNOS are in red. For each cell layer, controls are shown at the top and shunted are shown at the bottom. Images were taken as z-stack using a ×40 objective. The merged images are an orthogonal view showing a clear colocalization in x-, y-, and z-planes indicating that iNOS localized to both the smooth muscle cell and endothelial cell layers. The magnified view of the boxed area is also shown adjacent to merged image. Results are representative of 6 different sets of lambs. D represents the mean fluorescent intensity of iNOS for control and shunted lambs at each age group. iNOS quantification by fluorescent intensity did not demonstrate differences between control and shunted lambs at any age.
NOS activity.
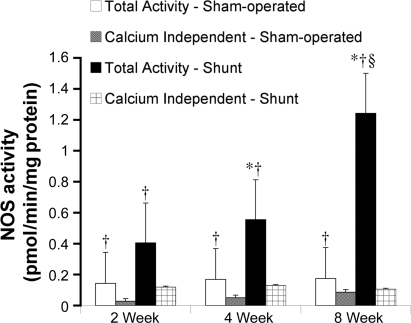
Total NOS activity and calcium-independent NOS activity in peripheral lung samples at 2, 4, and 8 wk of age in sham-operated control and shunted lambs are shown in Fig. 4. Total NOS activity in control lambs did not change from 2 to 8 wk of age. However, in shunted lambs, total NOS activity increased from 2 to 8 wk of age (ANOVA, P < 0.05) and was significantly greater at 4 and 8 wk of age than at 2 wk of age (P < 0.05). At 8 wk of age, NOS activity was greater in shunted lambs than controls (P < 0.05). Calcium-independent NOS activity did not change from 2 to 8 wk of age in either control or shunted lambs and was lower than total NOS activity at all ages for both groups (P < 0.05).
Fig. 4.
Total NOS activity and calcium-independent NOS activity in lung tissue from sham-operated control and shunted lambs at 2, 4, and 8 wk of age. In sham-operated control lambs, total NOS activity did not change from 2 to 8 wk of age (white bars). In shunted lambs, total NOS activity increased from 2 to 8 wk of age (ANOVA) and was greater at 4 and 8 wk of age than at 2 wk of age (black bars). Total NOS activity was greater than calcium-independent NOS activity in both sham-operated control lambs (gray bars) and shunted lambs (crossed bars) at all ages. n = 5 for each group. Values are means ± SD. *P < 0.05 compared with 2-wk shunt; †P < 0.05 compared with calcium-independent NOS activity within each age group; §P < 0.05 compared with 2-wk sham-operated control.
NOx levels.
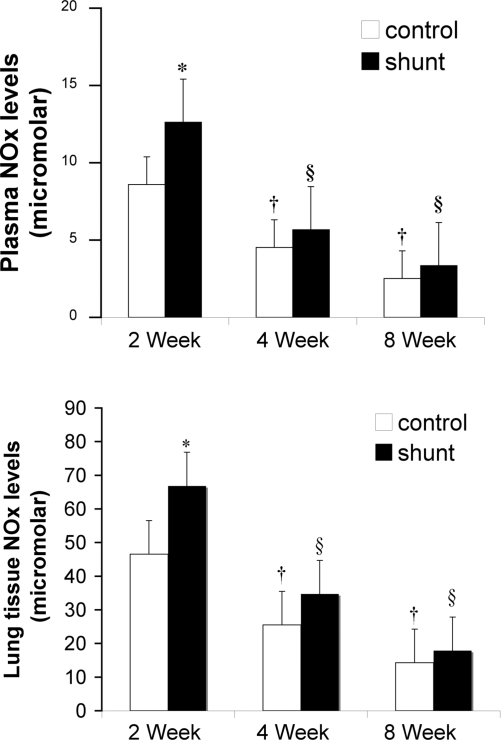
Plasma and tissue NOx levels from sham-operated control and shunted lambs at 2, 4, and 8 wk of age are shown in Fig. 5. At 2 wk, plasma and tissue NOx levels were higher in shunted lambs than control lambs (P < 0.05). Plasma and tissue NOx levels decreased below 2 wk levels at 4 and 8 wk of age in both control and shunted lambs (ANOVA, P < 0.05).
Fig. 5.
Plasma (top) and lung tissue (bottom) nitrate and nitrite (NOx) levels at 2, 4, and 8 wk of age in sham-operated control and shunted lambs. Plasma and tissue NOx levels decreased from 2 to 8 wk of age in sham-operated control and shunted lambs (ANOVA). Plasma and tissue NOx levels were greater in shunted lambs than sham-operated controls at 2 wk. n = 5 for each group. Values are means ± SD. *P < 0.05 compared with age-matched sham-operated control; †P < 0.05 compared with 2-wk sham-operated control; §P < 0.05 compared with 2-wk shunt.
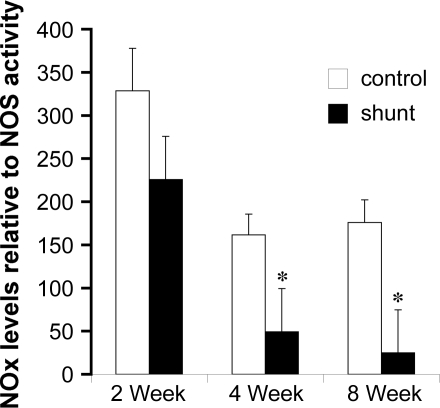
Lung tissue NOx levels relative to total NOS activity are shown for sham-operated control and shunted lambs at 2, 4, and 8 wk of age in Fig. 6. When evaluated relative to NOS activity, NOx levels were lower in shunted lambs than control lambs at 4 and 8 wk of age (Fig. 6; P < 0.05).
Fig. 6.
NOx levels expressed relative to total NOS activity at 2, 4, and 8 wk of age in sham-operated control and shunted lambs. Relative NOx levels were lower than controls at 4 and 8 wk of age. n = 5 for each group. *P < 0.05 compared with age-matched sham-operated control.
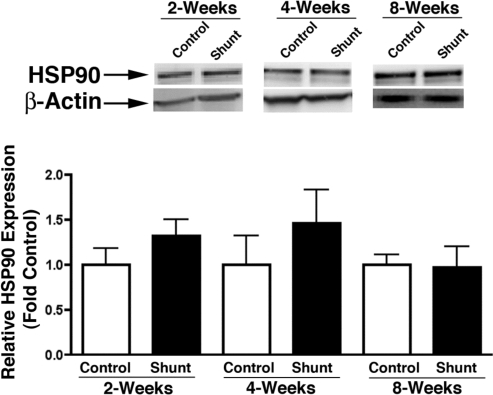
HSP90 protein levels.
Lung tissue HSP90 protein expression, as determined by Western blot analysis, was similar in sham-operated control and shunted lambs at 2, 4, and 8 wk of age (Fig. 7).
Fig. 7.
Lung tissue heat shock protein 90 (HSP90) expression in sham-operated control and shunted lambs at 2, 4, and 8 wk of age. Top: representative Western blots are shown for HSP90 protein extracts prepared from lung tissue separated on a 7.5% SDS-polyacrylamide gel, electrophoretically transferred to Hybond membranes, and analyzed using a specific antiserum raised against HSP90 and reprobed with β-actin to demonstrate equal loading. Bottom: densitometric values for HSP90 protein shown relative to control. n = 5 for each group. Values are means ± SD.
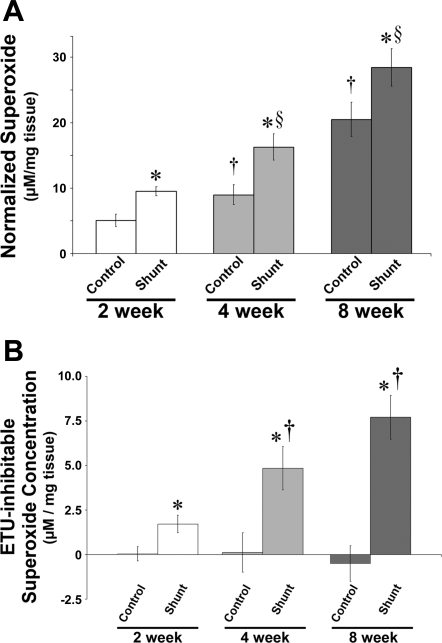
Superoxide quantitation.
Relative superoxide levels, as determined by EPR on peripheral lung taken from sham-operated control and shunted lambs at 2, 4, and 8 wk of age, are shown in Fig. 8A. Superoxide levels (plotted as micromolar per milligram of tissue) increased from 2 to 8 wk of age (ANOVA, P < 0.05) in both groups. In addition, superoxide levels were elevated in shunted lambs vs. controls at each age (P < 0.05). Specificity of the EPR assay for superoxide was confirmed by a significant reduction in the waveform amplitude with the addition of PEG-SOD to the samples (data not shown; Ref. 45). To determine to what extent the contribution of superoxide production arose from uncoupled NOS, samples were incubated with 200 μM ETU for 30 min on ice before the addition of CMH. ETU-mediated inhibition of NOS resulted in almost no change in EPR amplitude of control animals, whereas shunt animals showed up to ∼30% reduction in signal (Fig. 8B).
Fig. 8.
A: superoxide levels in lung tissue in sham-operated control and shunted lambs at 2, 4, and 8 wk of age estimated by electron paramagnetic resonance (EPR) assay using 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine·HCl (CMH), which forms a stable chemical product with superoxide. Superoxide concentrations increased from 2 to 8 wk of age in both sham-operated control and shunted lambs (ANOVA) and were greater in shunted lambs than sham-operated control lambs at each age. B: S-ethylisothiourea hydrobromide (ETU)-mediated inhibition of NOS resulted in almost no change in EPR amplitude of control lambs, whereas shunted lambs showed up to ∼30% reduction in signal, suggesting that uncoupled eNOS contributed to superoxide levels in shunted but not control lambs. n = 5 for each group. Values are means ± SD. *P < 0.05 compared with age-matched sham-operated control; †P < 0.05 compared with previous sham-operated control; §P < 0.05 compared with previous shunt.
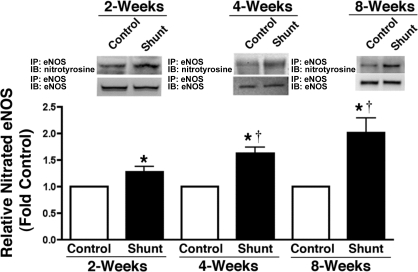
eNOS nitration.
Lung tissue-nitrated eNOS protein levels were determined by immunoprecipitation and Western blot analysis in sham-operated control and shunted lambs at 2, 4, and 8 wk of age. Nitrated eNOS protein levels were greater in shunted lambs than control lambs at each age (P < 0.05) and increased from 2 to 8 wk in shunted lambs (ANOVA, P < 0.05; Fig. 9).
Fig. 9.
Developmental increases in eNOS nitration in shunted lambs. Nitrated eNOS protein levels were determined by immunoprecipitation (IP) using specific antiserum raised against eNOS in tissue extracts prepared from 2- (n = 4), 4- (n = 5), and 8-wk-old (n = 4) shunted and age-matched control lambs. Immunoprecipitated extracts were separated on 4–20% SDS-polyacrylamide gradient gels, electrophoretically transferred to Hybond membranes, and analyzed using antisera against either eNOS or 3-nitrotyrosine residues. Representative Western Blots are shown. Densitometric analysis indicates that the levels of nitrated eNOS protein are increased in shunted lambs and that this nitration increases over time. Values are means ± SE. *P < 0.05 vs. age matched control; †P < 0.05 vs. previous age. IB, immunoblot.
DISCUSSION
This study used a large aorta to pulmonary artery vascular graft placed in late gestation fetal lambs to model a congenital cardiac defect with significantly increased pulmonary blood flow. In agreement with human studies, over time, shunted lambs developed impaired endothelium-dependent pulmonary vascular relaxation, whereas endothelium-independent pulmonary vascular relaxation remained intact (11, 31). Thus this model mimics the clinical manifestations of pulmonary vascular disease that are most responsible for morbidity in the current era of early surgical repair (31). In addition, our investigational timeframe captured the development of this selective endothelial dysfunction, which occurred between 2 and 4 wk of age. In association with this development, we found progressive decreases in plasma and lung tissue NOx levels, which were not due to decreased lung tissue NOS protein levels or activity, and progressive increases in lung tissue superoxide and nitrated eNOS protein levels, an indirect measure of peroxynitrite formation. Together, these data suggest that decreased bioavailable NO, in part due to scavenging by superoxide, may contribute to impaired endothelium-dependent pulmonary vascular relaxation and increased constriction that develops in association with abnormally increased pulmonary blood flow.
NOS function was assessed in several ways. Using whole animal hemodynamic responses, we determined agonist-induced pulmonary vascular relaxation after the administration of ACh, an agonist that requires eNOS to produce NO, and iNO that does not require NOS function but rather directly stimulates pulmonary vascular smooth muscle cells. Impaired agonist-induced NOS function was demonstrated to occur between 2 and 4 wk, since by 4 wk of age the NOS-dependent response was lost, but the NOS-independent response remained intact. NOS function in response to stimulation may or may not relate to its function under basal conditions. Therefore, we evaluated basal NOS function by determining NOS activity and NOx levels. Interestingly, we found that whereas NOS activity increased, the product NO decreased, as determined by NOx levels. Furthermore, NOx levels relative to NOS activity were markedly lower in shunted lambs than age-matched sham-operated controls. These data suggest that, in addition to agonist-induced NOS dysfunction, basal NOS function is also disrupted by chronic exposure to abnormally increased pulmonary blood flow. Moreover, these data indicate a central role for altered NO bioavailability in pulmonary vascular dysfunction under these conditions.
To investigate a potential role for oxidative stress in this pathology, we measured superoxide levels in lung tissue by EPR and found that they were greater in shunted lambs than age-matched controls at each age group and increased over time in both groups. In addition, our analysis indicated that eNOS uncoupling contributed to superoxide levels to an increasing degree from 2 to 8 wk in shunted lambs. Thus, although there is a normal developmental increase in superoxide generation, this response appears to be exaggerated by the stimulus of increased pulmonary blood flow. Previously, we demonstrated increased lung tissue superoxide levels, as measured by dihydroethidium oxidation, in shunted lambs at 4 wk of age that resulted, in part, from an increase in NADPH oxidase activity and eNOS uncoupling (15). In addition, in a previous study, we (20) found that superoxide scavenging normalized the responses of isolated 5th generation pulmonary arteries, harvested from 4-wk shunted lambs. The present study confirms these previous in vivo and ex vivo findings using a different, more sensitive technique to capture lung tissue superoxide levels and, more importantly, expands the investigation to include 2-wk shunted lambs that have not yet developed impaired pulmonary vascular responses and 8-wk-old lambs that have been exposed to twice the duration of abnormal pulmonary blood flow.
Given our previous studies, we anticipated finding increased superoxide levels in shunted lambs, which we hypothesized would result in increased peroxynitrite production due to a rapid reaction with NO. The determination of peroxynitrite levels in vivo is challenging. Peroxynitrite readily nitrates protein tyrosine residues, and thus Western blot analysis of nitrotyrosine provides an indirect measure of peroxynitrite levels. Thus to focus this type of analysis on a relevant protein in our study, we used a combined immunoprecipitation-Western blot technique to determine levels of nitrated eNOS protein in shunted and control lambs. We found that nitrated eNOS protein levels mirrored superoxide levels in shunted lambs over time. These findings suggest that peroxynitrite production may be an important mechanism that decreases bioavailable NO in shunted lambs, in part reconciling the discrepancy between NOx levels and NOS protein expression and activity.
The potential effects of nitration on eNOS are unclear. In a previous study of normal lambs chronically exposed to exogenous NO, we (26) demonstrated increased superoxide and nitrated eNOS protein levels. Furthermore, in an earlier in vitro study using cultured pulmonary artery smooth muscle cells, we (44) demonstrated that endothelin-1 exposure induced increases in superoxide, peroxynitrite, and eNOS nitration. In both these studies, eNOS nitration was associated with a decrease in eNOS activity, contrary to the findings in the present study. Importantly, the conditions resulting in increased superoxide generation differed between these studies and the present study of shunted lambs. It is possible that differences in the amount of peroxynitrite generated in response to exogenous NO compared with that produced by the uncoupling of eNOS explains this difference. Differences in the amount of peroxynitrite in the pulmonary vasculature might alter the number and location of the tyrosine residues nitrated, which could, in turn, determine whether eNOS is partially uncoupled or completely inhibited. For example, our recent study demonstrated that 19 of the 30 tyrosine residues found in eNOS could be nitrated (50). Interestingly, our data identified 4 such tyrosine residues in the region of eNOS responsible for the binding to HSP90 (13). We (40) and others (29) have shown previously that HSP90 is responsible for ensuring efficient coupling of eNOS. Furthermore, we (36, 40) have previously found that eNOS-HSP90 interactions are decreased in 2- and 4-wk-old shunted lambs. Therefore, in the present study, we measured lung tissue HSP90 protein levels in shunted and control lambs at each time point but found no differences. Further studies to assess developmental alterations in eNOS-HSP90 interactions are ongoing in our laboratory and will be required to determine whether nitration of these residues actually alters eNOS-HSP90 interactions.
The present study used lung tissue obtained from distal lung biopsies for determinations of superoxide and nitrated eNOS levels. In addition to uncoupled eNOS, the lung contains multiple other potential sources for superoxide including lipoxygenase, cyclooxygenase, xanthine oxidase, NADPH oxidase, and the mitochondrion. Indeed, we (15, 36) have previously shown that both NADPH oxidase and mitochondria may be involved in the oxidative stress associated with increased pulmonary blood flow in shunted lambs. However, further studies will be required to determine the potential role of other sources of superoxide in the shunted lambs and whether their role is altered over time (9, 15).
Measurements of NOS activity in the setting of uncoupled eNOS warrant discussion. We determined NOS activity from lung tissue using an in vitro assay in which activity was determined under maximal velocity (Vmax) conditions with excess substrate and cofactors. Thus our measurements represented the maximal potential of NOS but not necessarily actual in vivo activity. Previously, we (15, 33) demonstrated that eNOS uncoupling in 4-wk-old shunted lambs occurred, in part, secondary to an increase in the oxidation of tetrahydrobiopterin (BH4) to dihydrobiopterin (BH2) and a decrease in plasma l-arginine levels. Thus decreases in NOS activity occurring for these reasons would be masked by the assay conditions used in the present study.
Importantly, the changes we observed in NOS activity in shunted lambs did not relate to decreased NOS protein expression. There are three isoforms of NOS, eNOS, nNOS, and iNOS, which could contribute to total NOS activity. The two constitutive forms, eNOS and nNOS, are calcium- and calmodulin-dependent, whereas iNOS activity is calcium independent (21, 35, 47). Various diseases that cause inflammation have been shown to increase iNOS, greatly increasing NO production (22). Since chronically increased pulmonary blood flow is a potential stimulus for inflammation, we performed NOS activity assays both with and without calcium to determine the fraction of total NOS activity attributable to iNOS. We found that calcium-independent NOS activity did not change from 2 to 8 wk of age in either sham-operated control or shunted lambs. In addition, since in shunted lambs total NOS activity increased from 2 to 8 wk of age, the proportion of calcium-independent activity actually decreased over time. Furthermore, Western blot analysis and immunohistochemistry revealed similar levels of iNOS in lung tissue prepared from control and shunted lambs at each age that localized to pulmonary vascular endothelial and smooth muscle cells. Together, these data suggest that inflammatory alterations in iNOS do not play a significant role in the pulmonary vascular alterations induced by chronically increased pulmonary blood flow in this model. The precise contributions of eNOS and nNOS and the specific cell types contributing to NO production in this model were not determined. NOS protein levels were determined from peripheral lung samples, which likely increased sampling of the distal vasculature compared with other potential sites such as the large airways. This sampling bias may have accounted, in part, for the increased detection of eNOS relative to nNOS. However, the associated finding of impaired endothelium-dependent pulmonary vascular relaxation in 4- and 8-wk shunted lambs suggests that pulmonary vascular endothelial cells contributed to these changes.
The current study confirmed our previous studies that found an increase in eNOS protein expression at 4 wk of age in shunted lambs above normal age-matched control lambs (4). Interestingly, this increased eNOS protein expression in shunted lambs above controls did not persist at 8 wk of age. The regulation of eNOS occurs at the transcriptional, posttranscriptional, and posttranslational levels (14, 28). For example, laminar shear stress, which is increased under conditions of increased pulmonary blood flow, increases eNOS transcription (51). Since the relative size of the shunt decreases with body growth, shear stress may decrease over time. In the present study, the decrease in left pulmonary artery blood flow per kilogram of body weight from 2 to 8 wk did not reach significance; however, the transduction of mechanical stimuli into molecular events such as protein expression may not be captured by gross whole animal physiological measurements. In addition, several lines of evidence suggest that ROS can regulate eNOS gene expression and activity (42–44). Finally, the disparity between eNOS protein levels and NOS activity in shunted and control lambs over time may relate, in part, to other potential posttranslational factors such as intracellular location, protein-protein interactions (e.g., calmodulin and caveolin), phosphorylation, and substrate and cofactor availability (14, 28, 30, 37). Further studies are necessary to clarify these potential mechanisms.
Several limitations of the current study are notable. First, the in vivo measurement of NO is difficult, as NO has a short half-life and reacts rapidly with ROS, oxygen, metals, sulfhydryls, disulfides, and hemoglobin (38). In this study, we used a validated chemiluminescence method of detecting NO and its metabolic products nitrite and nitrate, which together are termed NOx (12, 18, 48, 49). Plasma NOx levels are representative of whole body NO generation and thus can be influenced by various factors including diet, renal function, and total body water composition. Therefore, we determined NOx levels in plasma and lung tissue and found that the levels were correlated. NOx levels decreased similarly in control and shunted animals from 2 to 8 wk, indicating a normal developmental decrease in total body NO. However, the decrease in normal lambs was associated with stable NOS activity throughout the 2-mo study period, whereas in shunted lambs the decrease in NOx levels occurred despite an increase in NOS activity, suggesting NOS dysfunction in addition to normal developmental changes in these animals.
Second, we used lung tissue to assess alterations in NOS and HSP90 protein levels, NOS activity, and ROS quantification. Therefore, these analyses do not identify the specific cell types within the lung that are responsible for these changes. Ongoing studies are investigating potential differential contributions between large and small pulmonary arteries and veins to these processes. However, the demonstration of a selective impairment in agonist induced pulmonary vascular endothelial-dependent relaxation suggests that the alterations in NOS activity and NOx levels represent or at least influence pulmonary vascular derangements.
Third, it is well-accepted that a number of pathways, especially the prostacyclin and endothelin-1 systems, are disrupted in patients with abnormally increased pulmonary blood flow. Undoubtedly, cross talk between NO signaling with these and other pathways influences the development of pulmonary vascular dysfunction in our model. These interactions are the focus of past and present investigations.
In summary, we conclude that increased pulmonary blood flow results in progressive impairment of basal and agonist-induced NOS function. We speculate that that scavenging of NO by superoxide to produce peroxynitrite is one mechanism that decreases bioavailable NO. Several therapies for infants and children with congenital cardiac defects resulting in pulmonary vascular disease are aimed at augmenting NO-cGMP signaling such as iNO and phosphodiesterase type 5 inhibition (sildenafil). These data suggest that antioxidant therapies may also have potential efficacy for these patients. Future studies to evaluate further the mechanisms of NOS dysfunction under conditions of increased pulmonary blood flow may result in novel therapies and treatment strategies.
GRANTS
This research was supported, in part, by National Heart, Lung, and Blood Institute Grants K08 HL-086513 (to P. E. Oishi), HL-60190 (to S. M. Black), HL-67841 (to S. M. Black), HL-72123 (to S. M. Black), HL-70061 (to S. M. Black), and HL-61284 (to J. R. Fineman) and by a grant from the Fondation Leducq (to S. M. Black, S. Fratz, and J. R. Fineman).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Black S, Fineman J, Johengen M, Bristow J, Soifer S. Increased pulmonary blood flow alters the molecular regulation of vascular reactivity in the lamb (Abstract). Chest 114: 39S, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Black SM, Bekker JM, Johengen MJ, Parry AJ, Soifer SJ, Fineman JR. Altered regulation of the ET-1 cascade in lambs with increased pulmonary blood flow and pulmonary hypertension. Pediatr Res 47: 97–106, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Black SM, Bekker JM, McMullan DM, Parry AJ, Ovadia B, Reinhartz O, Lakshminrushimha S, Mata-Greenwood E, Steinhorn RH, Fineman JR. Alterations in nitric oxide production in 8-week-old lambs with increased pulmonary blood flow. Pediatr Res 52: 233–244, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Black SM, Fineman JR, Steinhorn RH, Bristow J, Soifer SJ. Increased endothelial NOS in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol Heart Circ Physiol 275: H1643–H1651, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Black SM, Heidersbach RS, McMullan DM, Bekker JM, Johengen MJ, Fineman JR. Inhaled nitric oxide inhibits NOS activity in lambs: potential mechanism for rebound pulmonary hypertension. Am J Physiol Heart Circ Physiol 277: H1849–H1856, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Black SM, Sanchez LS, Mata-Greenwood E, Bekker JM, Steinhorn RH, Fineman JR. sGC and PDE5 are elevated in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 281: L1051–L1057, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Burrows FA, Klinck JR, Rabinovitch M, Bohn DJ. Pulmonary hypertension in children: perioperative management. Can Anaesth Soc J 33: 606–628, 1986. [DOI] [PubMed] [Google Scholar]
- 8.Bush PA, Gonzalez NE, Ignarro LJ. Biosynthesis of nitric oxide and citrulline from l-arginine by constitutive nitric oxide synthase present in rabbit corpus cavernosum. Biochem Biophys Res Commun 186: 308–314, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci 24: 471–478, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Celermajer DS, Cullen S, Deanfield JE. Impairment of endothelium-dependent pulmonary artery relaxation in children with congenital heart disease and abnormal pulmonary hemodynamics. Circulation 87: 440–446, 1993. [DOI] [PubMed] [Google Scholar]
- 12.Ewing JF, Janero DR. Specific S-nitrosothiol (thionitrite) quantification as solution nitrite after vanadium(III) reduction and ozone-chemiluminescent detection. Free Radic Biol Med 25: 621–628, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, Tsuruo T, Sessa WC. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ Res 90: 866–873, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Govers R, Rabelink TJ. Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol 280: F193–F206, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol 290: L1069–L1077, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Handy RL, Wallace P, Moore PK. Inhibition of nitric oxide synthase by isothioureas: cardiovascular and antinociceptive effects. Pharmacol Biochem Behav 55: 179–184, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Hanley FL, Heinemann MK, Jonas RA, Mayer JE Jr, Cook NR, Wessel DL, Castaneda AR. Repair of truncus arteriosus in the neonate. J Thorac Cardiovasc Surg 105: 1047–1056, 1993. [PubMed] [Google Scholar]
- 18.Ignarro LJ, Fukuto JM, Griscavage JM, Rogers NE, Byrns RE. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from l-arginine. Proc Natl Acad Sci USA 90: 8103–8107, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ignarro LJ, Harbison RG, Wood KS, Kadowitz PJ. Activation of purified soluble guanylate cyclase by endothelium-derived relaxing factor from intrapulmonary artery and vein: stimulation by acetylcholine, bradykinin and arachidonic acid. J Pharmacol Exp Ther 237: 893–900, 1986. [PubMed] [Google Scholar]
- 20.Lakshminrusimha S, Wiseman D, Black SM, Russell JA, Gugino SF, Oishi P, Steinhorn RH, Fineman JR. The role of nitric oxide synthase-derived reactive oxygen species in the altered relaxation of pulmonary arteries from lambs with increased pulmonary blood flow. Am J Physiol Heart Circ Physiol 293: H1491–H1497, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamas S, Marsden PA, Li GK, Tempst P, Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci USA 89: 6348–6352, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Forstermann U. Nitric oxide in the pathogenesis of vascular disease. J Pathol 190: 244–254, 2000. [DOI] [PubMed] [Google Scholar]
- 23.McMullan DM, Bekker JM, Johengen MJ, Hendricks-Munoz K, Gerrets R, Black SM, Fineman JR. Inhaled nitric oxide-induced rebound pulmonary hypertension: role for endothelin-1. Am J Physiol Heart Circ Physiol 280: H777–H785, 2001. [DOI] [PubMed] [Google Scholar]
- 24.McMullan DM, Bekker JM, Parry AJ, Johengen MJ, Kon A, Heidersbach RS, Black SM, Fineman JR. Alterations in endogenous nitric oxide production after cardiopulmonary bypass in lambs with normal and increased pulmonary blood flow. Circulation 102: III172–III178, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Murad F Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest 78: 1–5, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oishi P, Grobe A, Benavidez E, Ovadia B, Harmon C, Ross GA, Hendricks-Munoz K, Xu J, Black SM, Fineman JR. Inhaled nitric oxide induced NOS inhibition and rebound pulmonary hypertension: a role for superoxide and peroxynitrite in the intact lamb. Am J Physiol Lung Cell Mol Physiol 290: L359–L366, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Ovadia B, Reinhartz O, Fitzgerald R, Bekker JM, Johengen MJ, Azakie A, Thelitz S, Black SM, Fineman JR. Alterations in ET-1, not nitric oxide, in 1-week-old lambs with increased pulmonary blood flow. Am J Physiol Heart Circ Physiol 284: H480–H490, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Papapetropoulos A, Rudic RD, Sessa WC. Molecular control of nitric oxide synthases in the cardiovascular system. Cardiovasc Res 43: 509–520, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Pritchard K, Stepp D, Konduri G. Inhibition of heatshock protein 90 activity impairs vasorelaxation by increasing superoxide anion generation by endothelial nitric oxide synthase (eNOS) (Abstract). Circulation 104: 500, 2001.
- 30.Pritchard KA Jr, Ackerman AW, Gross ER, Stepp DW, Shi Y, Fontana JT, Baker JE, Sessa WC. Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric-oxide synthase. J Biol Chem 276: 17621–17624, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Rabinovitch M, Keane JF, Norwood WI, Castaneda AR, Reid L. Vascular structure in lung tissue obtained at biopsy correlated with pulmonary hemodynamic findings after repair of congenital heart defects. Circulation 69: 655–667, 1984. [DOI] [PubMed] [Google Scholar]
- 32.Reddy VM, Meyrick B, Wong J, Khoor A, Liddicoat JR, Hanley FL, Fineman JR. In utero placement of aortopulmonary shunts. A model of postnatal pulmonary hypertension with increased pulmonary blood flow in lambs. Circulation 92: 606–613, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Reddy VM, Wong J, Liddicoat JR, Johengen M, Chang R, Fineman JR. Altered endothelium-dependent responses in lambs with pulmonary hypertension and increased pulmonary blood flow. Am J Physiol Heart Circ Physiol 271: H562–H570, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Rudolph AM, Kurland MD, Auld PA, Paul MH. Effects of vasodilator drugs on normal and serotonin-constricted pulmonary vessels of the dog. Am J Physiol 197: 617–623, 1959. [DOI] [PubMed] [Google Scholar]
- 35.Sessa WC, Harrison JK, Luthin DR, Pollock JS, Lynch KR. Genomic analysis and expression patterns reveal distinct genes for endothelial and brain nitric oxide synthase. Hypertension 21: 934–938, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Sharma S, Sud N, Wiseman DA, Carter AL, Kumar S, Hou Y, Rau T, Wilham J, Harmon C, Oishi P, Fineman JR, Black SM. Altered carnitine homeostasis is associated with decreased mitochondrial function and altered nitric oxide signaling in lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 294: L46–L56, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaul PW Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol 64: 749–774, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science 258: 1898–1902, 1992. [DOI] [PubMed] [Google Scholar]
- 39.Steinhorn RH, Russell JA, Lakshminrusimha S, Gugino SF, Black SM, Fineman JR. Altered endothelium-dependent relaxations in lambs with high pulmonary blood flow and pulmonary hypertension. Am J Physiol Heart Circ Physiol 280: H311–H317, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Sud N, Sharma S, Wiseman DA, Harmon C, Kumar S, Venema RC, Fineman JR, Black SM. Nitric oxide and superoxide generation from endothelial NOS: modulation by HSP90. Am J Physiol Lung Cell Mol Physiol 293: L1444–L1453, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Tworetzky W, Moore P, Bekker JM, Bristow J, Black SM, Fineman JR. Pulmonary blood flow alters nitric oxide production in patients undergoing device closure of atrial septal defects. J Am Coll Cardiol 35: 463–467, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Villamor E, Kessels CG, Fischer MA, Bast A, de Mey JG, Blanco CE. Role of superoxide anion on basal and stimulated nitric oxide activity in neonatal piglet pulmonary vessels. Pediatr Res 54: 372–381, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Wedgwood S, Black SM. Endothelin-1 decreases endothelial NOS expression and activity through ETA receptor-mediated generation of hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol 288: L480–L487, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Wedgwood S, McMullan DM, Bekker JM, Fineman JR, Black SM. Role for endothelin-1-induced superoxide and peroxynitrite production in rebound pulmonary hypertension associated with inhaled nitric oxide therapy. Circ Res 89: 357–364, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Wiseman DA, Wells SM, Hubbard M, Welker JE, Black SM. Alterations in zinc homeostasis underlie endothelial cell death induced by oxidative stress from acute exposure to hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol 292: L165–L177, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Wiseman DA, Wells SM, Wilham J, Hubbard M, Welker JE, Black SM. Endothelial response to stress from exogenous Zn2+ resembles that of NO-mediated nitrosative stress, and is protected by MT-1 overexpression. Am J Physiol Cell Physiol 291: C555–C568, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Xie QW, Cho HJ, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Ding A, Troso T, Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 256: 225–228, 1992. [DOI] [PubMed] [Google Scholar]
- 48.Yang F, Troncy E, Francoeur M, Vinet B, Vinay P, Czaika G, Blaise G. Effects of reducing reagents and temperature on conversion of nitrite and nitrate to nitric oxide and detection of NO by chemiluminescence. Clin Chem 43: 657–662, 1997. [PubMed] [Google Scholar]
- 49.Zeballos GA, Bernstein RD, Thompson CI, Forfia PR, Seyedi N, Shen W, Kaminiski PM, Wolin MS, Hintze TH. Pharmacodynamics of plasma nitrate/nitrite as an indication of nitric oxide formation in conscious dogs. Circulation 91: 2982–2988, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Zickus M, Fonseca FV, Tummala M, Black SM, Ryzhov V. Identification of the tyrosine nitration sites in human endothelial nitric oxide synthase by liquid chromatography-mass spectrometry. Eur J Mass Spectrom (Chichester, Eng) 14: 239–247, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ziegler T, Silacci P, Harrison VJ, Hayoz D. Nitric oxide synthase expression in endothelial cells exposed to mechanical forces. Hypertension 32: 351–355, 1998. [DOI] [PubMed] [Google Scholar]