Abstract
Inhibition of voltage-gated, L-type Ca2+ (CaL) channels by clinical calcium channel blockers provides symptomatic improvement to some pediatric patients with pulmonary arterial hypertension (PAH). The present study investigated whether abnormalities of vascular CaL channels contribute to the pathogenesis of neonatal PAH using a newborn piglet model of hypoxia-induced PAH. Neonatal piglets exposed to chronic hypoxia (CH) developed PAH by 21 days, which was evident as a 2.1-fold increase in pulmonary vascular resistance in vivo compared with piglets raised in normoxia (N). Transpulmonary pressures (ΔPtp) in the corresponding isolated perfused lungs were 20.5 ± 2.1 mmHg (CH) and 11.6 ± 0.8 mmHg (N). Nifedipine reduced the elevated ΔPtp in isolated lungs of CH piglets by 6.4 ± 1.3 mmHg but only reduced ΔPtp in lungs of N piglets by 1.9 ± 0.2 mmHg. Small pulmonary arteries from CH piglets also demonstrated accentuated Ca2+-dependent contraction, and Ca2+ channel current was 3.94-fold higher in the resident vascular muscle cells. Finally, although the level of mRNA encoding the pore-forming α1C-subunit of the CaL channel was similar between small pulmonary arteries from N and CH piglets, a profound and persistent upregulation of the vascular α1C protein was detected by 10 days in CH piglets at a time when pulmonary vascular resistance was only mildly elevated. Thus chronic hypoxia in the neonate is associated with the anomalous upregulation of CaL channels in small pulmonary arteries in vivo and the resulting abnormal Ca2+-dependent resistance may contribute to the pathogenesis of PAH.
Keywords: neonate, pulmonary circulation, vascular smooth muscle cells
chronic hypoxia during infancy may lead to severe pulmonary arterial hypertension (PAH) associated with high morbidity and mortality in infants and young children. Even if death does not occur, devastating neurological, respiratory, and cardiac complications often persist into childhood (1, 57). The pathogenesis of PAH appears to involve an initial, active vasoconstriction of pulmonary resistance vessels that may progress to fixed luminal narrowing, elevated pulmonary vascular resistance (PVR), thrombi formation, and, ultimately, right heart failure and death (1, 57). The contributing events may include a biochemical shift from the production of vasodilator to vasoconstrictor mediators, resulting in vasoconstriction and progressive activation of the pulmonary resistance vessels accompanied by cellular proliferation (1, 2, 5, 11, 16, 18–21, 28, 41, 53, 57). After the structural remodeling of pulmonary vessels has occurred, therapeutic interventions to restore PVR to normal levels are largely ineffective (1, 57). Therefore, drug therapies targeting cellular pathways that mediate the initial and reversible rise in PVR may be advantageous.
In this regard, calcium channel blockers (CCBs) have been used as long-term vasodilator therapies for PAH in the pediatric population that responds positively to an acute vasodilator test (4, 30, 32). The CCBs exert their vasodilator effect by binding with high affinity to long-lasting or “L-type” calcium (CaL) channels in vascular smooth muscle cells (VSMCs) to attenuate voltage-gated Ca2+ influx. In a comprehensive study by Barst et al. (4) of vasodilator therapies for PAH in 77 children, 42% of patients responded to oral nifedipine therapy with clinical improvement that included a fall in mean pulmonary arterial pressure. Patients under 2 yr of age had a ∼65% response rate to acute vasodilator therapy; the younger the child the greater the likelihood of eliciting vasodilation (4). In contrast to the therapeutic effect of CCBs in the pediatric population, only ∼15–20% of adult patients with PAH have a favorable response to the same drugs (46, 49). The higher therapeutic efficacy of CCBs in neonates and children may reflect less advanced pulmonary disease more amenable to vasodilator intervention or, alternatively, may indicate that CaL channels play a more central role in establishing the elevated PVR in pediatric than adult patients. In fact, CCBs including nifedipine and the longer acting amlodipine are used to treat chronic lung diseases of many causes in infants and children in whom the progression of PAH at least partially relies on the development of abnormal pulmonary vascular tone (4, 7, 30, 57).
The goal of the current study was to determine if CaL channel abnormalities contribute to the pathogenesis of PAH in an early pediatric population. We explored this possibility using an established model of hypoxia-induced PAH in neonatal piglets, an animal model that shares many biological features with human neonates exposed to chronic hypoxia, including pulmonary vasoconstriction, progressive elevation of PVR, and, ultimately, fixed vascular lesions and right heart failure (26–28). Importantly, in this model and similar to a subset of pediatric patients, administration of nifedipine blunts the development of PAH, suggesting a contribution of CaL channel abnormalities to the pathogenesis of the disease (14, 22). Taking advantage of this model, newborn piglets were exposed to 3, 10, or 21 days of either normoxia (N) or chronic hypoxia (CH). Subsequently, we compared resting pulmonary tone in vivo in N and CH piglets and assessed Ca2+-dependent vascular reactivity in isolated lungs and segments of small pulmonary arteries. These results were correlated with the levels of CaL channel mRNA, protein, and current in pulmonary VSMCs.
MATERIALS AND METHODS
Animal models.
Piglets were paired in environmental chambers at 3–4 days of age and exposed to N (FiO2 = 0.21) or CH (FiO2 = 0.10) for 3, 10, or 21 days. Ambient CO2 was maintained at <0.5% by a combination of high gas flow in the chamber and CO2 scrubbing with CaCO3 (24). Piglets were removed from the chamber for <1 h each day for cleaning and replacement of feed and were allowed to feed ad libitum. Those that did not feed well were bottle or gavage fed as needed. All animal protocols were approved by the Institutional Animal Care and Use Committees of the Zablocki Veterans Administration Medical Center and the Medical College of Wisconsin.
Pulmonary hypertension indexes.
Hematocrit, and the right ventricle to left ventricle plus septum (RV/LV + S) ratio, were measured in all piglets. Morphological correlates were performed using 1-cm cubes of tissue from a midsagittal slice of the left or right lower lobes of four piglets from each study group. The tissues were embedded in paraffin, sectioned, and stained with hematoxin and eosin. The percent muscle thickness (%MT = 2 × muscle thickness/external diameter) was measured for arteries ranging from <50 to 300 μm in outer diameter (40).
In vivo hemodynamics.
In vivo hemodynamics were measured in anesthetized N and CH piglets ventilated with room air (FiO2 = 0.21) to characterize the level of PAH, as described previously (25, 37). Briefly, the carotid artery was catheterized and a thermodilution catheter was introduced through the external jugular vein and floated into the pulmonary artery. Piglets were ventilated at the rate of 20 breaths/min using a tidal volume of 15 to 20 ml/kg. Baseline hemodynamic measurements were obtained during normoxia (21% O2) after which the gas mixture was made hypoxic (10% O2) to evaluate the acute pressor response to hypoxia. Finally, nitric oxide (NO; 10 ppm) was added to the hypoxic gas mixture and hemodynamic measurements were made a final time to assess the vasodilator responsiveness of the intact vasculature. Hemodynamic measurements included heart rate (HR), pulmonary artery pressure (PAP), pulmonary capillary wedge pressure (PCWP), and cardiac output measured by thermodilution in duplicate or triplicate. Cardiac output was divided by body weight to obtain cardiac index (CI). Pulmonary vascular resistance indexed to body weight was then calculated as pulmonary vascular resistance index (PVRI) = (PAP − PCWP)/CI and expressed in modified Wood units.
After the hemodynamic studies were completed, piglets were given an additional dose of pentobarbital, heparinized (5,000 U iv), and exsanguinated. The N and CH piglets that were not used for in vivo hemodynamics were anesthetized, heparinized, and exsanguinated in a similar way. Subsequently, lungs or lung lobes were used for morphologic studies described above and/or for one or more of the protocols listed below.
Isolated lung preparation.
Isolated lungs from 21-day N and CH piglets were prepared as described previously (25, 37). Briefly, after an additional dose of anesthesia, the chest was opened by median sternotomy and the left lung was removed. The ductus arteriosus was ligated, and the left pulmonary artery and left atrium were cannulated. The left lung was perfused with a mixture of autologous blood and 3% Dextran-70 in Ringer lactate (hematocrit ∼12%) at a constant flow rate of 50 ml/kg body wt/min. PAP, left atrial pressure (Pla), and airway pressure (Paw) were constantly measured, and the transpulmonary pressure gradient (ΔPtp = PAP − Pla) was calculated. Since flow was constant, a change in ΔPtp reflected a change in vascular resistance. In all isolated lung experiments, pH, Pco2, and Po2 were measured at each oxygen tension and metabolic acidosis was corrected with 1 N NaHCO3 as needed. Isolated lungs from 21-day N and CH piglets were perfused under normoxic conditions to obtain baseline PAP. Subsequently, nifedipine (10 μmol/l) was added to the perfusate to assess the level of Ca2+-dependent tone. Preliminary studies demonstrated that 10 μmol/l nifedipine completely reversed a 1.4-fold rise in ΔPtp induced by perfusion of the CaL channel agonist Bay K 8644 (1 μmol/l), suggesting effective block of CaL channels even in the presence of a pharmacological opener (n = 3; data not shown). At the end of the experiment, papaverine (15-mg bolus) was additionally added to obtain maximal dilation.
Isolated vessel reactivity.
Small pulmonary arteries (3rd order, 2-mm length) were carefully dissected from lungs of 21-day N and CH piglets and mounted in a tension-recording system as described previously (44, 45). Optimal basal tension was established by evaluating the maximal contraction to successive challenges with 80 mmol/l KCl. After equilibration for 1 h, concentration-dependent responses to the CaL channel agonist Bay K 8644 (1 nmol/l to 1 μmol/l, half-log increments) were obtained to evaluate the availability of CaL channels to mediate contraction.
Whole cell Ca2+ currents.
Single VSMCs were enzymatically isolated from small pulmonary arteries (3rd- to 5th-order branches) of N and CH piglets, and whole cell CaL channel currents were recorded using a patch-clamp station and voltage protocols previously published (44). Briefly, recordings were obtained at 25°C using 10 mmol/l BaCl2 as a charge carrier to limit current rundown. With the use of −70 mV as the constant interpulse holding potential, whole cell currents were elicited in response to progressive 8-mV voltage steps from −70 to +50 mV. Peak amplitudes were measured and normalized to membrane capacitance to account for the different sizes of single cells. The pipette solution consisted of the following (in mmol/l): 145 cesium glutamate, 1 MgCl2, 10 HEPES, 10 EGTA, and 3 Na2ATP. The bath solution contained the following (in mmol/l): 10 BaCl2, 135 TEA, 1 MgCl2, 10 HEPES, and 10 glucose.
Western blot analysis.
Membrane proteins were isolated from small pulmonary arteries (3rd- to 5th-order branches) of N and CH piglets as described previously (44, 45). For comparison of protein expression between samples, equivalent amounts of membrane protein were added to adjacent lanes of a 4–15% gradient polyacrylamide gel, electrophoretically separated by size, and transferred to a nitrocellulose membrane. Membranes were washed in Tris-buffered saline containing 0.1% Tween 20 (TBST) and blocked with 10% nonfat dried milk in TBST overnight at 4°C. Membranes were subsequently incubated with a 1:200 dilution of polyclonal rabbit anti-α1C or anti-α1D for overnight and then subjected to four 10-min washes in TBST. Membranes were incubated with horseradish peroxidase conjugated goat anti-rabbit IgG in 5% milk/TBST for 1 h and washed at least several times. The bound antibody was detected by ECL on X-ray film, and immunoreactivity was calculated using National Institutes of Health Scion software. The smooth muscle-specific protein α-actin was used as an internal standard.
Real-time PCR.
Total RNA was isolated from small pulmonary arteries of 21-day N and CH piglets using an RNeasy minikit and QiaShredder columns (Qiagen Operon, Huntsville, AL). Genomic DNA contamination was removed using DNAse-free (Ambion, Austin, TX), and total RNA (1 μg) was reverse transcribed according to commercial instructions (iScript cDNA synthesis; Bio-Rad, Hercules, CA). The forward and reverse primers used to amplify cDNA corresponding to the CaL channel α1C-subunit were 5′-ccgcccactaccaagatcaac-3′ (forward) and 5′-gcatctcgggctcctcctc-3′ (reverse). The cDNA corresponding to the internal standard α-actin was amplified using 5′-cagggagtgatggttggaatgg-3′ (forward) and 5′-tggtgatgatgccgtgttctatc-3′ (reverse). The PCR mixture contained 50 μl of 1X iQ SYBR green Supermix (Bio-Rad), 200 nmol/l each of forward and reverse primers, and 10 ng of template. After initial denaturation at 95°C for 3 min, the following temperature-cycling profile was used for amplification (40 cycles): 95°C, 10 s for denaturing and 62°C, 1 min for annealing and extension. The amplification of the intended target was evaluated by melting curve analysis (iCycler, Bio-Rad) and size separation of cDNA products on a 1% agarose gel. The α1C-cycle threshold (CT) values were normalized using smooth muscle α-actin as an internal standard and analyzed by the ΔΔCT method for relative quantification of α1C-transcript (35).
Drugs and chemicals.
For the isolated lung studies, Dextran-70 (Sigma-Aldrich, St. Louis, MO) was dissolved in lactated Ringer to achieve a 3% solution. Nifedipine (Sigma-Aldrich) was dissolved in ethanol and an aliquot of <1 ml in volume was added to the blood/Dextran-lactated Ringer to achieve a 10 μmol/l solution. Papaverine was obtained from Bedford Laboratories (Bedford, OH) and added to the blood perfusate as supplied. For the isolated vessel studies, Bay K 8644 (Sigma-Aldrich) was dissolved in 70% ethanol as a 10 μmol/l stock solution and added in 50-μl aliquots to 15-ml tissue baths. The drug vehicle had no effect on basal vascular tone including values for ΔPtp in isolated perfused lungs. Salts and buffers were purchased from Sigma-Aldrich. Papain, dithiothreitol, collagenase, trypsin inhibitor, and PMSF also were purchased from Sigma-Aldrich. The CaL channel anti-α1C- and anti-α1D antibodies were purchased from Alomone Labs (Jerusalem, Israel).
Statistics.
All data are shown as means ± SE. Single variables at different time points were compared using one-way ANOVA. Whole cell calcium channel current elicited at each voltage, and Western blots were compared between samples from N and CH piglets using unpaired t-tests. All differences were judged to be significant at the level of P < 0.05.
RESULTS
Development of PAH.
Three- to four-day-old newborn piglets were exposed to N (FiO2 = 0.21) or CH (FiO2 = 0.10) for 3, 10, or 21 days in environmental chambers. A total of 60 animals was studied. There was little evidence of hypoxia-induced PAH in the 3-day CH piglets (Table 1). Neither hematocrit, RV/LV + S ratio, nor normoxic PVRI differed significantly between 3-day N and CH piglets, respectively. Only a small but significant increase in %MT of pulmonary arteries measuring 50–300 μm in external diameter suggested initial vascular remodeling in CH piglets. Clinical signs of PAH were emerging in 10-day CH piglets. Hematocrit and PVRI did not differ between the 10-day N and CH groups, but an increased RV/LV + S ratio in the 10-day CH piglets was observed concurrent with an enhanced %MT. By 21 days of hypoxia, animals showed overt clinical signs of PAH. Hematocrit, RV/LV + S ratio, and PVRI values were significantly higher in 21-day CH compared with N piglets. In addition, there was a profound increase in %MT in the pulmonary arteries of CH piglets. Collectively, these findings indicated that PAH was well established in the neonatal piglets after 21 days of CH, and most of the subsequent studies focused on the 21-day animals.
Table 1.
Cardiovascular profile of neonatal piglets exposed to normoxia or chronic hypoxia for 3, 10 or 21 days
|
3 Days |
10 Days
|
21 Days
|
||||
|---|---|---|---|---|---|---|
| N | CH | N | CH | N | CH | |
| Hematocrit, % | 27.4±1.1 | 27.4±0.6 | 25.6±0.8 | 28.7±1.2 | 28.4±1.5 | 34.5±1.5* |
| RV/LV + S | 0.34±0.01 | 0.38±0.03 | 0.32±0.01 | 0.54±0.03* | 0.32±0.01 | 0.59±0.02* |
| Basal PVRI, modified Wood units | 31±3 | 42±4 | 33±5 | 42±1 | 39±4 | 83±8* |
| %MT | 13.1±0.6 | 15.6±0.4* | 9.7±0.2 | 15.2±0.4* | 10.4±0.4 | 22.8±0.6* |
Profiles of normoxic (N) and chronic hypoxic (CH) piglets after 3, 10, or 21 days of exposure to FIO2 = 0.21 (normoxia) or FIO2 = 0.10 (hypoxia), respectively. Values for hematocrit, right ventricle to left ventricle plus septal (RV/LV + S) ratio, basal pulmonary vascular resistance index (PVRI), and percent muscle thickness (% MT) of pulmonary arteries in the <50- to 300-μm external diameter range are shown. Values are means ± SE.
P < 0.05 between N and CH at the same time point. Sample sizes were as follows: 3 days (8 N, 8 CH), 10 days (7 N, 8 CH), and 21 days (15 N, 14 CH).
Hemodynamic evidence of a reactive pulmonary circulation in CH piglets at 21 days.
An elevated PVRI may reflect an abnormal but reversible vasoconstriction of the pulmonary circulation and/or the presence of a fixed vascular lesion characterized by a loss of vasoreactivity. In our study, although resting PVRI was 2.1-fold higher in lungs of CH compared with N piglets after 21 days of hypoxia (Table 1), the persistence of pulmonary vasomotor responses to constrictor and dilator stimuli indicated continued reactivity of the VSMCs. For example, vasoconstrictor responses to acute hypoxia were observed in lungs of both N and CH piglets in vivo (Fig. 1, A and B). Acute reductions in FiO2 from 0.21 to 0.10 increased PVRI in lungs of N piglets from 39 ± 3 to 69 ± 8 modified Wood units (n = 9) and increased PVRI in CH piglets from 83 ± 8 to 122 ± 4 modified Wood units (n = 10). The percent increases in PVRI induced by acute hypoxia were not significantly different between the two groups of animals (N, 84 ± 20%; CH, 57 ± 17%). Hypoxia-induced vasoconstriction was fully reversed by inhaled NO (10 ppm) in both groups, and, importantly, inhaled NO reduced PVRI in the lungs of CH piglets to below the basal level observed in normoxia (Fig. 1, B and C). This finding suggested that the elevated basal PVRI in the lungs of CH piglets was due, at least in part, to an active vasoconstriction of the pulmonary resistance vessels. We attributed the remaining elevated component of PVRI in CH lungs to vascular remodeling, although active tone insensitive to NO-induced dilation could not be ruled out.
Fig. 1.
Pulmonary circulation of 21-day chronic hypoxia (CH) piglets remained highly reactive to acute vasoconstrictor and dilator stimuli. A: resting PVRI was elevated in CH piglets compared with normoxia (N) piglets. B: acutely lowering FiO2 from 0.21 to 0.10 increased pulmonary vascular resistance index (PVRI) in N and CH piglets by 84 ± 20 and 57 ± 17%, respectively, indicating reactivity to pressor stimuli in both groups. C: inhaled NO reduced the PVRI below normoxic baseline in the CH piglets but only to baseline in the N piglets. Thus active vasoconstriction susceptible to dilator stimuli contributed significantly to the elevated resting PVRI in CH piglets. *Significant difference (P < 0.05) between PVRI of N and CH piglets under the same condition. +Significant difference (P < 0.05) between resting normoxic PVRI and other PVRI values in the same preparation. Sample sizes were 9 N and 10 CH.
Elevated Ca2+-dependent reactivity in isolated lungs and small arteries of CH piglets.
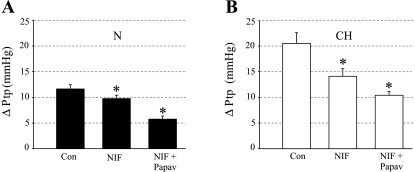
Subsequently, we compared the contribution of CaL channels to the resting transpulmonary pressure gradient (ΔPtp) between isolated, perfused lungs of 21-day N and CH piglets by evaluating the dilator response to nifedipine (NIF). The isolated lung model avoids the systemic hypotensive effect of CCBs that can confound the interpretation of hemodynamic measurements in vivo, thereby permitting the Ca2+-dependent tone of the pulmonary vasculature to be assessed independently. The nonselective vasodilator agent papaverine was further added to the lung perfusate to induce maximal vasodilation. The difference between control ΔPtp values and ΔPtp values after papaverine-induced dilation was regarded as the level of active pulmonary vascular tone. The isolated lungs of 21-day CH piglets showed an initial resting ΔPtp of 20.50 ± 2.09 mmHg (n = 5), which was 1.77-fold higher than the average value of 11.62 ± 0.82 mmHg (n = 4) in the N piglets (Fig. 2, A and B). Nifedipine reduced ΔPtp by 1.87 ± 0.24 mmHg, and papaverine further reduced ΔPtp by 4.00 ± 0.41 mmHg in lungs of N piglets, suggesting that 32 ± 4% of active tone depended on Ca2+ influx through CaL channels. In contrast, nifedipine reduced ΔPtp in the isolated lungs of CH piglets by 6.40 ± 1.35 mmHg, revealing a 3.42-fold higher CaL channel-dependent tone compared with the lungs of N piglets. Papaverine further reduced ΔPtp by 3.70 ± 0.81 mmHg in the affected lungs, suggesting that the majority (62 ± 7%) of the elevated active tone in the lungs of 21-day CH piglets was mediated by CaL channels.
Fig. 2.
Calcium-dependent resting tone is elevated in isolated lungs of 21-day CH compared with N piglets. A: resting transpulmonary pressures (ΔPtp) of 11.62 ± 0.82 mmHg in lungs of N piglets (n = 4) was reduced by 1.87 ± 0.24 mmHg by nifedipine (NIF; 10 μmol/l) and an additional 4.00 ± 0.41 mmHg by papaverine (Papav; 15-mg bolus). Thus L-type Ca2+ (CaL) channels accounted for 32% of active tone sensitive to papaverine. B: nifedipine had a more pronounced attenuating effect on the elevated resting ΔPtp of 20.50 ± 2.09 mmHg in lungs of CH piglets (n = 5). In these lungs, nifedipine reduced ΔPtp by 6.40 ± 1.35 mmHg, and papaverine lowered ΔPtp an additional 3.70 ± 0.81 mmHg. Thus 62% of the elevated ΔPtp sensitive to papaverine was dependent on Ca2+ influx through CaL channels. *Significantly different (P < 0.05) from control (Con) ΔPtp in the same preparation.
To verify that the enhanced Ca2+-dependent tone in the isolated lungs from CH piglets reflected a CaL channel abnormality localized to the VSMCs, we isolated third-order small pulmonary arteries from lungs of 21-day N and CH piglets for use in isometric tension recording. In these studies, depolarization-induced Ca2+ influx in response to KCl (80 mmol/l) caused more pronounced contractions in arterial segments from lungs of CH compared with N piglets (Fig. 3, A and B). Additionally, the pharmacological activation of CaL channels by the dihydropyridine agonist Bay K 8644 only contracted arteries from lungs of CH but not N piglets. The maximal contractile responses to Bay K 8644 averaged 62 ± 14% of the contraction to 80 mmol/l KCl in arteries from CH piglets but were absent in arteries from N piglets that instead passively relaxed below initial basal tension (n = 5; Fig. 3, C and D). Collectively, these findings suggested an increased number of CaL channels available to mediate Ca2+ influx and vascular contraction in the pulmonary arteries of the CH piglets.
Fig. 3.
Small pulmonary arteries of 21-day CH piglets have more functional CaL channels. A: representative traces of isometric tension recordings. Compared with arteries of 21-day N piglets, arteries of 21-day CH piglets showed enhanced amplitudes of Ca2+-dependent contractions elicited by a depolarizing concentration (80 mmol/l) of KCl. B: average amplitude of KCl-induced contractions in arteries of 21-day N and CH piglets (n = 5 each). *Significant difference (P < 0.05) between values subjected to an unpaired t-test. C: CaL channel opener, Bay K 8644, dose dependently contracted arteries of 21-day CH piglets, whereas arteries of 21-day N piglets did not respond. D: averaged dose-response curve to Bay K 8644. Only arteries of 21-day CH piglets contracted in response to the CaL channel opener, whereas arteries of 21-day N piglets slowly relaxed during the experiment (n = 5 each). Contraction is plotted as percentage of the maximal contraction to 80 mmol/l KCl in the same vessel. *Significant difference (P < 0.05) between values at the same drug concentration.
Increased Ca2+ channel current density in VSMCs of CH piglets.
Patch-clamp studies also detected an abnormally high level of Ca2+ influx in freshly isolated VSMCs from small pulmonary arteries (3rd- to 5th-order branches) of 21-day CH piglets with PAH. Membrane capacitance, an indicator of cell membrane area, was similar between VSMCs from N and CH lungs averaging 9.59 ± 0.73 pF (n = 10) and 8.46 ± 0.73 pF (n = 5), respectively. The tiny neonatal VSMCs were extremely fragile, which permitted the acquisition of high quality recordings in only a small number of cells. In a sample of 10 VSMCs from N piglets, families of whole cell inward currents elicited by 8-mV depolarizing steps from a constant holding potential of −70 mV to a peak voltage of +50 mV showed a uniformly low density of inward current. In contrast, CaL channel current densities in five pulmonary VSMCs from CH piglets were highly increased. Nifedipine (5 μmol/l) blocked the inward currents, confirming CaL channels as the conducting pathway (Fig. 4A). The average current densities elicited at each voltage were plotted in current-voltage (I-V) relationships to determine if the CaL channel current was elevated in VSMCs from CH compared with N animals (Fig. 4B). The I-V curve generated from the VSMCs of CH animals was significantly enhanced at most voltages. Peak CaL channel current was increased between 1.93- and 10.23-fold in VSMCs from CH piglets (n = 5) compared with the average peak current in VSMCs from N piglets (n = 10).
Fig. 4.
Isolated pulmonary vascular smooth muscle cells (VSMCs) of CH piglets show increased Ca2+ current density. A: patch-clamp recordings of Ca2+ current in freshly isolated pulmonary VSMCs from 21-day N and CH piglets. Constant holding potential was −70 mV. Currents were elicited by 8-mV depolarizing steps from −70 mV to +50 mV (top traces). Nifedipine (5 μmol/l) reduced current amplitude in both preparations (bottom traces). B: current-voltage relationships show an increased CaL channel current density in VSMCs of CH compared with N piglets (n = 5, 10). *Significant difference (P < 0.05) between N and CH values obtained at the same voltage.
Increased α1C-subunit protein but not mRNA in arteries of CH piglets.
Subsequently, we investigated if the mechanism of enhanced CaL channel function in pulmonary arteries of 21-day CH piglets reflected an increased expression of these channels in the pulmonary vasculature of the hypoxic animals. In these studies, small pulmonary arteries (3rd- to 5th-order branches) were microdissected from the lungs of four N and four CH piglets. Equal amounts of vascular proteins from a single animal were loaded into adjacent lanes. The transferred proteins were probed using an anti-α1C polyclonal antibody directed against the pore-forming α1C-subunit of the CaL channel. In Western blots, the α1C-subunit is recognized as a 200- to 240-kDa doublet band representing its short and long forms, respectively (44, 45). A biochemical truncation of the carboxyl terminus results in the shorter form of α1C that still retains function (29, 36). In the present study, the anti-α1C immunoblots confirmed a profound upregulation of the α1C-subunit in the small pulmonary arteries of the CH piglets. The expression of an internal standard, smooth muscle α-actin, was unchanged in the same vessels (Fig. 5A).
Fig. 5.
Western blot comparisons of the expression of α1C-subunits in small and large pulmonary arteries between 21-day N and CH piglets. A: proteins isolated from small pulmonary arteries of N piglets were loaded in the four left lanes; proteins from CH piglets were loaded in the four right lanes. The α1C-subunit, which was recognized as a 200- to 240-kDa doublet band, was profoundly increased in the small pulmonary arteries of CH piglets. α-Actin was the internal standard. B: Western blot analysis of the α1C-subunit of the CaL channel in large pulmonary arteries of N and CH piglets. No significant difference in α1C expression was observed. α-Actin was the internal standard. C: graphical representation of normalized α1C immunoreactive signal from small and large pulmonary arteries of 21-day N and CH piglets. *Significant difference (P < 0.05) in immunodensity of α1C between N and CH values.
Micropuncture studies (17) in lungs of CH piglets have attributed the increased vascular resistance in PAH to a compartment containing small arteries as the site of greatest reactivity. Thus we reasoned that if CaL channels contributed to the increased vascular resistance of PAH, their upregulation should be localized to small arteries and not a feature of larger diameter vessels. Indeed, Western blots comparing α1C expression between large pulmonary arteries (1st and 2nd branches) from four N and CH piglets showed variable α1C expression but no pattern of CaL channel upregulation in the large arteries of the CH animals (Fig. 5B). Collectively, the immunodensity of the doublet band corresponding to the short and long forms of the CaL channel α1C-subunit was 7.2-fold higher in the small pulmonary arteries of CH compared with N piglets (n = 8 each), whereas α1C immunoreactivity was nearly identical between large pulmonary arteries from the same animals (n = 4 each; Fig. 5C).
We further reasoned that if CaL channels contributed to the development of PAH, their anomalous expression should be evident in small pulmonary arteries of CH piglets at an early stage of the disease. With the use of pooled proteins obtained from small pulmonary arteries of 3-, 10-, and 21-day N and CH piglets (n = 3–4 each) used in the hemodynamic studies (Table 1), Western blot analyses indicated that the expression of the CaL channel α1C-subunit was similar between small pulmonary arteries obtained from 3-day N and CH piglets animals (Fig. 6). However, by 10 days of hypoxia, the immunoreactivity associated with α1C increased 1.9-fold in the small pulmonary arteries of CH compared with N piglets. PVRI was mildly but insignificantly elevated at this early stage of PAH, but an increased RV/LV + S ratio and %MT signaled the onset of hypertension (Table 1). The upregulation of α1C in the small pulmonary arteries of CH piglets persisted at 21 days when PAH was overt, showing a 3.2-fold increase in expression in this subset of CH animals.
Fig. 6.
Western blot analysis of the CaL channel α1C-subunit in small pulmonary arteries of 3-, 10-, and 21- day N and CH piglets. Vascular proteins were pooled from 3 or 4 piglets for each preparation. The α1C-subunit was upregulated in small pulmonary arteries of 10-day and 21-day CH piglets compared with respective arteries from N piglets obtained at the same time point. In contrast, there was no difference between α1C expression between arteries of 3-day N and CH piglets. α-Actin was used as an internal standard.
Recently, a functional role for another dihydropyridine-sensitive, voltage-gated CaL channel composed of the pore-forming α1D (Cav1.3)-subunit has been identified in several tissues, including in cardiac atria in which it drives pacemaker activity (29, 43, 59). Although the physiological role of α1D in VSMCs is unknown, we investigated whether an upregulation of this CaL channel type also occurred in small pulmonary arteries of CH piglets. As an initial step, we investigated if α1D was expressed in small pulmonary arteries using piglet brain as a positive expression control. In lanes equally loaded with brain or vascular protein lysates (50 μg each), the α1D-subunit was resolved as a faint immunoreactive band at 190 kDa that was sparsely expressed in pulmonary arteries compared with brain tissue (Fig. 7A). Subsequently, the expression level of α1D was compared between samples of small pulmonary arteries from four 21-day N and CH piglets. The sparse expression of α1D noted in the small arteries of N piglets also was a feature of CH piglets (Fig. 7B). In the same preparations, α1C was profoundly upregulated in the small arteries of the CH animals (Fig. 7C). Densitometric analysis showed that the expression of α1D was similar (0.02 ± 0.26-fold difference) between small pulmonary arteries of 21-day N and CH piglets. In contrast, the α1C signal was elevated 5.88 ± 0.77-fold in the small arteries of the same diseased animals (Fig. 7D). These findings suggest that chronic hypoxia preferentially upregulates the α1C protein and CaL channels composed of this particular pore-forming protein likely contribute to the anomalous pulmonary vascular tone in the CH animals.
Fig. 7.
Western blot analysis of the CaL channel α1D-subunit in small pulmonary arteries of 21-day N and CH piglets. A: piglet brain lysate (Br; left lane) was used as a positive expression control to screen for α1D in piglet small pulmonary arteries (PA; right lane). Immunoreactive band corresponding to α1D was resolved at 190 kDa. The α1D protein was only sparsely expressed in small pulmonary arteries compared with brain. B: expression of the α1D-subunit was not different between small pulmonary arteries of 21-day N and CH piglets. C: in the same preparations, the α1C-subunit was profoundly upregulated in small pulmonary arteries of CH animals. D: normalized α1D and α1C immunoreactive signals calculated from the Western blots in B and C. *Significant difference (P < 0.05) in immunodensity of α1C between N and CH values.
Since hypoxia has been shown to transcriptionally regulate the expression of other ion channels in VSMCs (42, 58), we used real-time PCR to evaluate if an increased expression of α1C mRNA was associated with the overexpression of α1C protein in small pulmonary arteries of 21-day CH piglets. The average cycle dependency of α1C amplification was similar between the vascular samples from N and CH piglets (n = 5; Fig. 8A). In five different N and CH animals, the average threshold values of 29.98 ± 0.21 (N) and 29.87 ± 0.45 (CH) were obtained. Threshold values were variable between piglets from the same group, a finding confirmed by displaying the PCR products at 30 cycles corresponding to α1C mRNA on an agarose gel (Fig. 8B). The α1C CT values normalized to α-actin and analyzed by the ΔΔCT method for relative quantification confirmed no difference between α1C mRNA levels between small pulmonary arteries of N and CH animals.
Fig. 8.
Small pulmonary arteries of 21-day N and CH piglets show similar expression levels of α1C transcript. A: real-time PCR showed no difference in amplified product corresponding to α1C mRNA between small pulmonary arteries of 21-day N and CH piglets (n = 5 each). B: amplified products obtained at cycle 30 (α1C) and cycle 24 (α-actin) displayed on an agarose gel indicate no significant difference between arteries of 21-day N and CH piglets. RFU, relative fluorescent units.
DISCUSSION
The findings of our study show for the first time that the response of the neonatal lung to hypoxia involves an increased Ca2+-dependent PVRI that is associated with a profound upregulation of the CaL channel α1C-subunit in small pulmonary arteries. This anomalous CaL channel expression occurs by 10 days after exposure to hypoxia at an early time point when the initial signs of PAH including an increased RV/LV + S ratio and enhanced arterial muscle thickness are detected but PVRI is not significantly elevated. By 21 days after hypoxia when PAH is established in CH piglets, the upregulation of CaL channels is a striking feature in small pulmonary arteries, which also exhibit enhanced Ca2+-dependent contractions after isolation from the lung parenchyma. Further compelling evidence of a CaL channel abnormality was provided by patch-clamp studies that revealed an increased density of voltage-gated CaL channel current in pulmonary VSMCs of CH piglets, suggesting an overabundance of functional CaL channels in the plasma membrane. Collectively, all of these findings raise the possibility that anomalous CaL channel expression may be involved in the initiation or maintenance of hypoxia-induced PAH in the piglet model of this disease.
Chronic hypoxia in infants during the postnatal period may result from congenital cardiac defects, obstructive pulmonary disease, sleep-disordered breathing, or broncho-pulmonary dysplasia (1, 57). The relevance of the developing pulmonary circulation of neonatal piglets to lungs of human infants has been documented (26–28), and the neonatal piglet model of hypoxia-induced PAH has been characterized by many laboratories (18–24, 37, 53). It demonstrates early changes in hemodynamic parameters and pulmonary vascular morphology consistent with the development of PAH in human infants exposed to hypoxia at birth (23, 26–28, 53). In earlier studies (23, 24, 37), elevated PVRI was detected by 10–14 days of hypoxia. In our study, the first sign of PAH was an increased medial thickening in 3-day CH piglets, which suggested vascular wall hypertrophy instead of the normal thinning of the pulmonary arterial musculature that occurs after birth (28). After 10 days of hypoxia, an increased RV/LV + S ratio also was evident coincident with medial arterial thickening. Thus critical events in the pathogenesis of hypoxia-induced PH that portend hemodynamic disruption appear to occur by 10 days of hypoxia. At this time point, we also initially detected an upregulation of CaL channels in the small pulmonary arteries of the CH animals. By 21 days of hypoxia when PVRI was markedly elevated in CH piglets, the upregulation of CaL channels in small pulmonary arteries was profound but the pulmonary vasculature was still highly reactive, suggesting an early stage of PAH. We chose this time point of therapeutic relevance to evaluate Ca2+-dependent tone and vascular CaL channel abnormalities.
Sixty-two percent of the elevated ΔPtp in the lungs of 21-day CH piglets was nifedipine sensitive, which suggested a strong contribution of CaL channels to abnormal vasoconstriction. The residual dihydropyridine-insensitive ΔPtp abolished by papaverine was attributed to vasoconstrictor mechanisms other than CaL channels, since papaverine primarily inhibits phosphodiesterase to induce relaxation (10, 52). However, since papaverine also weakly inhibits CaL channels (31), the pharmacological block by papaverine of residual Ca2+ influx may have accounted for a minor component of its dilator effect. The presence of active vascular tone insensitive to nifedipine infers that other sources of activator Ca2+ in addition to CaL channels contribute to the increased pulmonary vascular tone in PAH. Receptor and store-operated Ca2+-permeable channels and a Na+/Ca2+ exchanger are expressed in pulmonary VSMCs, and intracellular Ca2+ can be mobilized by the opening of ryanodine receptors or inositol 3-phosphate receptors (33, 38, 48). Endothelin-1 and reactive oxygen species reportedly mobilize these stores in pulmonary VSMCs during hypoxia to increase cytosolic free calcium (48, 56). Additionally, lower threshold T-type Ca2+ channels that are voltage gated but insensitive to nifedipine have been described in cultured pulmonary VSMCs and observed to promote cell proliferation (47). In neuroendocrine cells, chronic hypoxia is reported to upregulate T-type Ca2+ channels, although this event has not been reported in VSMCs to our knowledge (8, 13). We did not search for these channels in our preparation by using alternative antibody probes or patch-clamp protocols designed to detect low-threshold currents. However, the resistance of T-type Ca2+ channels to nifedipine-induced block suggests that they are not responsible for the anomalous nifedipine-sensitive vascular tone that we observed in 21-day CH piglets. A role for these channels in pulmonary vascular remodeling is not ruled out by our findings, however.
Earlier studies (50, 51, 55) primarily using rodent models of PAH have shown that hypoxia acutely inactivates voltage-gated K+ channels in small pulmonary arteries and subsequently suppresses their expression in the plasma membrane by 24–60 h. The resulting depolarization of the affected VSMCs mediates CaL channel opening and voltage-gated Ca2+ influx (3, 51). Initially, this Ca2+-dependent vasoconstrictor response to hypoxia may represent a physiological event that permits ventilation-perfusion matching in alveolar networks. However, our new findings suggest that if hypoxia persists for 10 days, it may trigger a more profound and pathogenic mechanism to promote Ca2+-dependent constriction in the pulmonary circulation. In the neonatal lung, this response appears to involve a strong upregulation of CaL channel α1C-subunits in small pulmonary arteries that coincides with the emergence of PAH. The anomalous expression of α1C appears to intensify as PAH worsens between 10 and 21 days. Notably, the CaL channel α1D-subunit that is encoded by a different gene (Cav1.3) than the Cav1.2 gene that encodes α1C is also sensitive to dihydropyridine drugs (34, 59). However, our findings suggest that the α1D-subunit is only sparsely expressed in small pulmonary arteries and its expression levels do not change significantly in response to chronic hypoxia. Thus the abnormal nifedipine-sensitive tone of neonatal PAH appears to be fueled specifically by a high density of CaL channels composed of α1C-subunits in the pulmonary VSMCs. Notably, the concept that CaL channels are involved in the pathogenesis of neonatal PAH also is supported by the findings of Fike and Kaplowitz (22). These authors reported that sublingual nifedipine blunted the rise in PVRI in newborn piglets exposed to hypoxia for 10–12 days. Thus Ca2+ influx mediated by CaL channels appears to be requisite for establishing at least the initial phase of hypoxia-induced PAH in neonatal piglets.
It is important to acknowledge that many disease-related alterations may fuel abnormal vascular tone in PAH independently of increases of CaL channel expression. For example, we considered the possibility that the increased Ca2+-dependent tone in isolated lungs of 21-day CH piglets reflected an exaggerated release of vasoconstrictor substances from the lung parenchyma rather than a CaL channel abnormality of the pulmonary vasculature (25). The production of thromboxane A2 is reportedly enhanced in neonatal piglets exposed to hypoxia (20, 21, 41), and elevated urine levels of thromboxane A2 metabolites and plasma endothelin-1 have been measured in adult and pediatric patients with PAH, respectively (2, 11, 41). However, our in vitro studies confirmed that the enhanced CaL channel function in small pulmonary arteries of CH piglets was intrinsic to the VSMCs. First, the activation of voltage-gated CaL channels by a depolarizing KCl stimulus or Bay K 8644 elicited more robust contractions in isolated arteries of 21-day CH than N piglets. Second, patch-clamped VSMCs isolated from small pulmonary arteries of 21-day CH piglets exhibited elevated CaL channel currents compared with VSMCs from N piglets. Notably, this increase in functional CaL channels appeared less pronounced than the profound increase in immunoreactivity corresponding to the α1C protein on Western blot. The reason for this discrepancy is not readily apparent, but it is possible that some of the overexpressed α1C protein was not trafficked from its site of assembly in the endoplasmic reticulum to the plasma membrane for insertion as a functional CaL channel. The latter process involves ancillary channel proteins and other cytoplasmic factors that were not analyzed in our study (6, 9, 15).
Interestingly, we did not detect a difference in the expression levels of α1C mRNA between small pulmonary arteries of 21-day N and CH piglets, although the α1C-subunit protein was highly upregulated in the arteries of CH piglets. Thus the mechanisms by which chronic hypoxia increases CaL channel expression apparently do not involve steady-state increases in the corresponding mRNA. Alternative processes that may be functional in PAH include enhanced translational efficiency, an increased rate of α1C trafficking to the cell surface, or reduced α1C turnover in the plasma membrane. Similar mechanisms have been invoked to explain the overabundance of CaL channels in peripheral arteries of genetic and renal rat models of systemic hypertension. In these animals, the α1C transcript is mildly elevated in several vascular beds during the established phase of hypertension, but this event apparently does not fully account for the marked upregulation of α1C protein (44, 45).
In conclusion, our study has linked chronic hypoxia in newborn piglets to the upregulation of CaL channels in small pulmonary arteries and the development of PAH. These findings provide initial insight into an apparently key mechanism by which abnormal Ca2+-dependent tone develops during the pathogenesis of neonatal PAH. An abnormality of CaL channels also appears to contribute to some forms of pediatric PAH, since CCBs partially reversed elevated pulmonary arterial pressures in a subset of infants and young children (1, 4, 7, 30). Unfortunately, many infants with hypoxia-induced PAH are not identified until they have advanced disease that fails to respond to either CCBs or other vasodilator therapies. In this regard, our study suggests that prophylactic drug therapies designed to suppress CaL channel expression in the pulmonary vasculature during chronic hypoxia may mitigate abnormal Ca2+-dependent tone and the development of PAH.
GRANTS
Funding was provided by National Heart, Lung, and Blood Institute Grants R01-HL-083013 (to N. J. Rusch and J. B. Gordon) and R01-HL-19298 (to J. B. Gordon).
Acknowledgments
We thank the staff of the Veterinary Medical Unit at the Zablocki Veterans Administration Medical Center in Milwaukee for capable assistance. In addition, we thank Aleksandra Pesic and Miodrag Pesic for assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abman SH, Steinhorn RH. Persistent pulmonary hypertension of the newborn: Pathophysiology and treatment. In: Hypoxic Pulmonary Vasoconstriction: Cellular and Molecular Mechanisms, edited by Jason JX-J. Boston, MA: Kluwer Academic, chapt. 27, p. 471–495, 2004.
- 2.Allen SW, Chatfield BA, Koppenhafer SA, Schaffer MS, Wolfe RR, Abman SH. Circulating immunoreactive endothelin-1 in children with pulmonary hypertension. Am Rev Respir Dis 148: 519–522, 1993. [DOI] [PubMed] [Google Scholar]
- 3.Archer SL, Michelakis E. The mechanism of hypoxic pulmonary vasoconstriction: Potassium channels, redox O2 sensors, and controversies. News Physiol Sci 17: 131–137, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Barst RJ, Maislin G, Fishman AP. Vasodilator therapy for primary pulmonary hypertension in children. Circulation 99: 1197–1208, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Berkenbosch JW, Baribeau J, Perreault T. Decreased synthesis and vasodilation to nitric oxide in piglets with hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 278: L276–L283, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Birnbaumer L, Qin N, Olcese R, Tareilus E, Platano D, Costantin J, Stefani E. Structures and functions of calcium channel β subunits. J Bioenerg Biomembr 30: 357–375, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Brownlee JR, Beekman RH, Rosenthal A. Acute hemodynamic effects of nifedipine in infants with bronchopulmonary dysplasia and pulmonary hypertension. Pediatr Res 24: 186–190, 1988. [DOI] [PubMed] [Google Scholar]
- 8.Carabelli V, Marcantoni A, Comunanza V, de Luca A, Díaz J, Borges R, Carbone E. Chronic hypoxia up-regulates alpha1H T-type channels and low-threshold catecholamine secretion in rat chromaffin cells. J Physiol 584: 149–165, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catterall WA Structure and regulation of voltage-gated Ca2+ channels. Ann Rev Cell Develop Biol 16: 521–555, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Chang KC, Chong WS, Lee IJ. Different pharmacological characterstics of structurally similar bezylisoquinoline analogs, papaverine, higenamine, and GS 389, on isolated rat aorta and heart. Can J Physiol Pharmacol 72: 327–334, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Christman BW Lipid mediator dysregulation in primary pulmonary hypertension. Chest 114: 205–207, 1998. [DOI] [PubMed] [Google Scholar]
- 12.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky E, Brundage BH, Detre KM, Fishman AP, Goldring RM, Kernis JT, Levy PS, Pietra GG, Reid LM, Reeves JT, Rich S, Vreim CE, Williams GW, Wu M. Survival in patients with primary pulmonary hypertension. Ann Intern Med 115: 343–349, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Del Toro R, Levitsky KL, López-Barneo J, Chiara MD. Induction of T-type calcium channel gene expression by chronic hypoxia. J Biol Chem 278: 22316–22324, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Dickstein PJ, Trindade O, Goldberg RN, Bancalari E. The effect of calcium antagonists on hypoxic pulmonary hypertension in piglets. Pediatr Res 18: 1262–1265, 1984. [DOI] [PubMed] [Google Scholar]
- 15.Dolphin AC Beta subunits of voltage-gated calcium channels. J Bioenerg Biomembr 35: 599–620, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Durmowicz AG, Orton EC, Stenmark KR. Progressive loss of vasodilator responsive component of pulmonary hypertension in neonatal calves exposed to 4,570 m. Am J Physiol Heart Circ Physiol 265: H2175–H2183, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Fike CD, Gordon JB, Kaplowitz MR. Micropipette and vascular occlusion pressures in isolated lungs of newborn lambs. J Appl Physiol 75: 1854–1860, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Fike CD, Kaplowitz MR, Thomas CJ, Nelin LD. Chronic hypoxia decreases nitric oxide production and endothelial nitric oxide synthase in newborn pig lungs. Am J Physiol Lung Cell Mol Physiol 274: L517–L526, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Fike CD, Aschner JL, Zhang Y, Kaplowitz MR. Impaired NO signaling in small pulmonary arteries of chronically hypoxic newborn piglets. Am J Physiol Lung Cell Mol Physiol 286: L1244–L1254, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Fike CD, Pfister SL, Kaplowitz MR, Madden JA. Cyclooxygenase contracting factors and altered pulmonary vascular responses in chronically hypoxic newborn pigs. J Appl Physiol 92: 67–74, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Fike CD, Kaplowitz MR, Pfister SL. Arachidonic acid metabolites and an early stage of pulmonary hypertension in chronically hypoxic newborn pigs. Am J Physiol Lung Cell Mol Physiol 284: L316–L323, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Fike CD, Kaplowitz MR. Nifedipine inhibits pulmonary hypertension but does not prevent decreased lung eNOS in hypoxic newborn pigs. Am J Physiol Lung Cell Mol Physiol 277: L449–L456, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Fike CD, Kaplowitz MR. Effect of chronic hypoxia on pulmonary vascular pressures in isolated lungs of newborn pigs. J Appl Physiol 77: 2853–2862, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Gordon JB, Halla TR, Fike CD, Madden JA. Mediators of alkalosis-induced relaxation in pulmonary arteries from normoxic and chronically hypoxic piglets. Am J Physiol Lung Cell Mol Physiol 276: L155–L163, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Gordon JB, VanderHeyden MA, Halla TR, Cortez EP, Hernandez G, Haworth Dawson CA ST, Madden JA. What leads to different mediators of alkalosis-induced vasodilation in isolated and in situ pulmonary vessels? Am J Physiol Lung Cell Mol Physiol 284: L799–L807, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Haworth SG, Hislop AA. Adaptation of the pulmonary circulation to extra-uterine life in the pig and its relevance to the human infant. Cardiovasc Res 15: 108–119, 1981. [DOI] [PubMed] [Google Scholar]
- 27.Haworth SG Development of the normal and hypertensive pulmonary vasculature. Exp Physiol 80: 843–853, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Haworth SG, Hislop AA. Effect of hypoxia on adaptation of the pulmonary circulation to extra-uterine life in the pig. Cardiovasc Res 16: 293–303, 1982. [DOI] [PubMed] [Google Scholar]
- 29.Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel α1 subunits. J Cell Biol 123: 949–962, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houde C, Bohn DJ, Freedom RM, Rabinovitch M. Profile of paediatric patients with pulmonary hypertension judged by responsiveness to vasodilators. Br Heart J 70: 580–587, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iguchi M, Nakajima T, Hisada T, Sugimoto T, Kurachi Y. On the mechanism of papaverine inhibition of the voltage-dependent Ca++ current in isolated smooth muscle cells from the guinea pig trachea. J Pharmacol Exp Therap 263: 194–200, 1992. [PubMed] [Google Scholar]
- 32.Johnson CE, Beekman RH, Kostyshak DA, Nguyen T, Oh DM, Amidon GL. Pharmacokinetics and pharmacodynamics of nifedipine in children with bronchopulmonary dysplasia and pulmonary hypertension. Pediatr Res 29: 500–503, 1991. [DOI] [PubMed] [Google Scholar]
- 33.Landsberg JW, Yuan JXJ. Calcium and TRP channels in pulmonary vascular smooth muscle cell proliferation. News Physiol Sci 19: 44–50, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Lipscombe D, Helton TD, Xu W. L-type calcium channels: The low down. J Neurophysiol 92: 2633–2641, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Rusch NJ, Striessnig J, Sarna SK. Down-regulation of L-type calcium channels in inflamed circular smooth muscle cells of the canine colon. Gastroenterology 120: 480–489, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Lee KJ, Hernandez G, Gordon JB. Hypercapnic acidosis and compensated hypercapnia in control and pulmonary hypertensive piglets. Pediatr Pulmonol 36: 94–101, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Mauban JR, Remillard CV, Yuan JXJ. Hypoxic pulmonary vasoconstriction: Role of ion channels. J Appl Physiol 98: 415–420, 2005. [DOI] [PubMed] [Google Scholar]
- 39.McMurtry IF, Petrun MD, Reeves JT. Lungs from chronically hypoxic rats have decreased pressor response to acute hypoxia. Am J Physiol Heart Circ Physiol 235: H104–H109, 1978. [DOI] [PubMed] [Google Scholar]
- 40.Michel RP, Gordon JB, Chu K. Development of the pulmonary vasculature in newborn lambs: structure-function relationships. J Appl Physiol 70: 1255–1264, 1991. [DOI] [PubMed] [Google Scholar]
- 41.Olschewski H, Rose F, Grünig E, Ghofrani AH, Walmrath D, Schulz R, Schermuly R, Grimminger F, Seeger W. Review article: Cellular pathophysiology and therapy of pulmonary hypertension. J Lab Clin Med 138: 367–377, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, Wang JY, Rubin LJ, Yuan JXJ. Chronic hypoxia decreases KV channel expression and function in pulmonary artery myocytes. Am J Physiol Lung Cell Mol Physiol 280: L801–L812, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sionatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 102: 89–97, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Pesic A, Madden JA, Pesic M, Rusch NJ. High blood pressure upregulates L-type Ca2+ channels. Is membrane depolarization the signal? Circ Res 94: e97–e104, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Pratt PF, Bonnet S, Ludwig LM, Bonnet P, Rusch NJ. Upregulation of L-type Ca2+ channels in mesenteric and skeletal arteries of SHR. Hypertension 40: 214–219, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium channel blockers on survival in primary pulmonary hypertension. N Engl J Med 327: 76–81, 1992. [DOI] [PubMed] [Google Scholar]
- 47.Rodman DM, Reese K, Harral J, Fouty B, Wu S, West J, Hoedt-Miller M, Tada Y, Li KX, Cool C, Fagan K, Cribbs L. Low-voltage-activated (T-type) calcium channels control proliferation of human pulmonary artery myocytes. Circ Res 96: 864–872, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Shimoda LA, Wang J, Sylvester JT. Ca2+ channels and chronic hypoxia. Microcirculation 13: 657–670, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Sitbon O, Humbert M, Simonneau G. Primary pulmonary hypertension: current therapy. Prog Cardiovasc Dis 45: 115–128, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Smirnov SV, Robertson TP, Ward JP, Aaronson PI. Chronic hypoxia is associated with reduced delayed rectifier K+ current in rat pulmonary artery muscle cells. Am J Physiol Heart Circ Physiol 266: H365–H370, 1994. [DOI] [PubMed] [Google Scholar]
- 51.Sweeney M, Yuan JXJ. Hypoxic pulmonary vasoconstriction: Role of voltage-gated potassium channels. Respir Res 1: 40–48, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Truss MC, Uckert S, Stief CG, Schulz-Knappe P, Hess R, Forssmann WG, Jonas U. Porcine detrusor cyclic nucleotide phosphodiesterase isoenzymes: Charcterization and functional effects of various phosphodiesterase inhibitor in vitro. Urology 45: 893–901, 1995. [DOI] [PubMed] [Google Scholar]
- 53.Tulloh RM, Hislop AA, Boels PJ, Deutsch J, Haworth SG. Chronic hypoxia inhibits postnatal maturation of porcine intrapulmonary artery relaxation. Am J Physiol Heart Circ Physiol 272: H2436–H2445, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Turley JE, Nelin LD, Kaplowitz MR, Zhang Y, Fike CD. Exhaled NO is reduced at an early stage of hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 284: L489–L500, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Juhaszova M, Rubin LJ, Yuan JXJ. Hypoxia inhibits gene expression of voltage-gated K+ channel alpha subunits in pulmonary artery smooth muscle cells. J Clin Invest 100: 2347–2353, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res 91: 719–726, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Widlitz A, Barst RJ. Pulmonary arterial hypertension in children. Eur Respir J 21: 155–176, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JXJ. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA 101: 13861–13866. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Z, He Y, Tuteja D, Xu D, Timofeyev V, Zhang Q, Glatter KA, Xu Y, Shin HS, Low R, Chiamvimonvat N. Functional roles of Cav1.3 (α1D) calcium channels in atria insights gained from gene targeted null mutant mice. Circulation 112: 1936–1944, 2005. [DOI] [PubMed] [Google Scholar]