Abstract
Prostaglandin D2, the ligand for the G protein-coupled receptors DP1 and CRTH2, has been implicated in the pathogenesis of the allergic response in diseases such as asthma, rhinitis, and atopic dermatitis. This prostanoid also fulfills a number of physiological, anti-inflammatory roles through its receptor DP1. We investigated the role of PGD2 and CRTH2 in allergic pulmonary inflammation by using a highly potent and specific antagonist of CRTH2. Administration of this antagonist ameliorated inflammation caused by either acute or subchronic sensitization using the cockroach egg antigen. Gene expression and ELISA analysis revealed that there was reduced proinflammatory cytokine mRNA or protein produced, as well as a wide array of genes associated with the Th2-type proinflammatory response. Importantly, the CRTH2 antagonist reduced antigen-specific IgE, IgG1, and IgG2a antibody levels as well as decreased mucus deposition and leukocyte infiltration in the large airways. Collectively, these findings suggest that the PGD2-CRTH2 activation axis has a pivotal role in mediating the inflammation and the underlying immune response in a T cell-driven model of allergic airway inflammation.
Keywords: prostaglandin D2, asthma
prostaglandin d2 is the predominant prostanoid produced by activated mast cells and has been implicated in the pathogenesis of a number of allergic disorders including allergic asthma and atopic dermatitis (15). In a transgenic mouse model of mice overexpressing lipocalin-type PGD2 synthase, antigen challenge resulted in an increase of PGD2 in the lungs and an increase in eosinophil and lymphocyte numbers (6). In humans, elevated levels of PGD2 have been found in the alveolar lavage fluid from patients after antigen challenge (18, 20).
PGD2 is generated by cyclooxygenase (COX)-1 and COX-2 from arachidonic acid and exerts its effects through two G protein-coupled receptors, the PGD2 receptor (DP1; 3) and CRTH2 (chemoattractant receptor homologous molecule expressed on Th2 cells; DP2; 10, 21). The DP receptor provides both activation and inhibitory type signals to cells via its coupling to Gs-type G proteins (Gs) (11, 17). CRTH2 is coupled to Gi-type G proteins (Gi) and inhibits cAMP production and increases intracellular calcium (10), which mediates proinflammatory effects including migration or degranulation of eosinophils (7, 10), and migration of T lymphocytes (21) and bone marrow-derived mast cells (Boehme and Bacon, unpublished observations).
Many previous reports have demonstrated that the presence of CRTH2-positive cells and the CRTH2-specific ligand, PGD2, correlates with the severity of allergic responses; therefore, CRTH2 is considered to play important roles in the pathogenesis of allergic diseases. Despite these data, and perhaps because of the different expression pattern of CRTH2 that exists between human and rodents (1, 9, 27), the functional roles of this molecule in diseases are still unclear. Recently, it was shown that CRTH2 activation, but not DP1, can mediate the migration of inflammatory cells from the bone marrow to the peripheral blood in rats (27), or to inflamed tissues in a FITC-induced contact skin reaction in mice (31) by use of a nonselective CRTH2 small molecule antagonist, Ramatroban [Baynas, Bay u3405; (+)-(3R)-3-(4-fluorobenzenesulfonamide)-1,2,3,4-tetra-hydrocarbozole-9-propionic acid]. A clinical study also demonstrated the clear correlation between the expression levels of CRTH2 on circulating CD4+, CLA+ lymphocytes and disease severity in atopic dermatitis (12). Further insights into the role of CRTH2 in allergy have been provided from investigations in allergic rhinitis (22, 33). In this study, eosinophils and T cells recruited into nasal polyps from allergic rhinitis tissue were highly CRTH2 positive compared with healthy donors. Yoshimura-Uchiyama et al. (36) have subsequently revealed a functional correlation between the CRTH2 expression on eosinophils and basophils and their migration and activation in response to PGD2 derived from rhinitis tissue.
As a result of these findings, the role of CRTH2 in allergic inflammation and its potential for therapeutic intervention have been the focus of intense research over the last 5 years. With the use of a specific stimulatory ligand, 13,14-dihydro-15-keto-PGD2 (DK-PGD2), CRTH2 stimulation has been shown to promote the migration of Th2 cells, eosinophils, and basophils (10, 19, 27), stimulate the production of IL-4, IL-5, and IL-13 in Th2-type T cell lines (32, 35), and induce the in vivo migration of eosinophils to inflammatory sites in mouse models of atopic dermatitis and allergic asthma (29). Experiments utilizing mice with null mutations of the CRTH2 gene have supported the proinflammatory role for CRTH2. In a mouse model of asthma, the Th2 program of cytokine release, eosinophilia and T cell activation was completely switched off in CRTH2-deficient mice. Double knockout mice (DP1 and DP2) revealed an almost exclusive role for the CRTH2 receptor in inducing the inflammatory response (16, 8). In a separate study, CRTH2 gene-deficient mice bred to a BALB/c background showed a reduced inflammatory response in a number of mouse models of skin inflammation, along with lowered IgE levels (25). Surprisingly, however, an earlier study using DP1-deficient mice reported increased serum IgE levels and a decreased inflammatory infiltrate of lymphocytes and eosinophils in the lungs of knockout animals compared with wild type (17). The reason for the discrepancies noted in comparison of these studies is not fully understood either at the level of the technologies utilized or the role(s) DP1 and CRTH2 may be fulfilling. While gene compensation mechanisms are routinely invoked to explain differences, it is clear that the profound differences in signaling and cell specificity reflected in the activation of each receptor cannot easily be accounted for by compensatory mechanisms.
Here we use a highly specific and potent small molecule antagonist of CRTH2 to investigate the role of this PGD2 receptor in a chronic model of airway inflammation and the underlying immune response. Our results show that inhibition of CRTH2 leads to a decrease in the inflammatory infiltrate, locally produced proinflammatory cytokines and chemokines, as well as a reduction in antigen-specific antibodies. More strikingly, however, is the profound and widespread reduction and regulation of genes known to be involved in the proinflammatory response. Many of the regulated genes fit the paradigm of a profound Th2-type T cell-driven inflammation, whereas others are also found regulated in genome-wide screens of genetic risk factors for human atopy and asthma (5).
MATERIALS AND METHODS
Materials.
All reagents were purchased from Sigma (St. Louis, MO) unless otherwise stated. Compound A is a selective and proprietary CRTH2 antagonist developed at Actimis Pharmaceuticals, the intellectual property of which is represented under patent WO2005/073234A3. All receptor transfectants were generated according to protocols detailed in Refs. 27 and 30.
Radioligand binding assay.
Radioligand binding analyses were performed according to the methods of Sugimoto et al. (30). CRTH2-transfected cells were resuspended in binding buffer (50 mM Tris·HCl, pH 7.4, 40 mM MgCl2, 0.1% BSA) at a concentration of 4 × 106/ml at room temperature. Fifty microliters of cell suspension was incubated for 1 h at room temperature with gentle shaking in the presence of 10 μl of 3H-PGD2 (3 nM final), 10 μl of competitor (10−12 to 10−6 M dose range) or buffer alone, and the final volume adjusted to 100 μl with buffer. Incubations were performed in U-bottom polypropylene 96-well plates (Fisher Scientific, Pittsburgh, PA) for 60 min at room temperature, and the cell suspension transferred to filtration plates (MAFB; Millipore, Bedford, MA), pre-wet with polyethyleneimine (0.5%) buffer (Acros Organics, Morris Plains, NJ). The cell pellets were washed three times with buffer, and the plate was allowed to air dry. Scintillant (50 μl; Microscint 20, Perkin Elmer, Boston, MA) was added to each well, and the radioactivity counted on a TopCount (Packard Bioscience, Meriden, CT). Data analyses were performed using the Prizm graphics program using a one-site competition model (GraphPad Software, San Diego, CA). DK-PGD2 and BWA 868C were purchased from Cayman Chemical. Radiolabeled PGD2 {PGD2 [5,6,9,8,12,14,15–3H(N)]; specific activity 160 Ci/mmol} was purchased from Perkin Elmer (Waltham, MA). Prostanoid receptor binding analysis was performed as a part of routine PanLabs screening by MDS Pharma contract labs according to standard protocols.
Calcium mobilization assay.
CRTH2-L1.2 transfectants were labeled with 3 μM indo-1 AM dye (Invitrogen) for 60 min at 37°C. The cells were subsequently washed and resuspended in 1 ml of HBSS containing 1% BSA (fraction V, Sigma). Calcium mobilization in response to agonist, in the presence or absence of antagonist, was measured using a PTI fluorimeter (South Brunswick, NJ) recording the ratio of the dual emission spectra (405/482 λ) for calcium-bound and -free indo-1 AM.
Cockroach allergen model of disease.
These studies utilized Balb/c/J mice sensitized with soluble cockroach antigens (CRA; Cockroach Blatella germanica; Greer Laboratories, Lenoir, NC) in incomplete Freund's adjuvant intraperitoneally (10 μg at day 0). After 14 days, the mice were challenged with soluble CRA (2 μg) by an intranasal administration, followed 5 days later with an intratracheal (i.t.) injection of CRA (6 μg), followed by a second i.t. administration of allergen 48 h later. Before the final challenge, the allergic mice received the CRTH2 antagonist (Compound A) by oral gavage in a dose response from 10 mg/kg to 0.1 mg/kg, or a control treatment group. In a separate series of experiments designed to be a more chronic model, mice were sensitized for 14 days, challenged intranasally (4 challenges, 4 days apart each), then challenged i.t. 4 days later with a second i.t. challenge after a further 4 days. After 24-h postfinal allergen challenge, the mice were examined for airway hyperreactivity, and accumulation of leukocyte subsets was monitored in histology sections. All animal experiments were conducted according to protocols with full Institutional Animal Care and Use Committee approval.
Measurement of AHR.
Airways hyperreactivity (AHR) was measured as previously described using a direct ventilation mouse plethysmograph (Buxco, Troy, NY) specifically designed for low tidal volumes. Briefly, the mouse to be tested was anesthetized with pentobarbital sodium and intubated via cannulation of the trachea with an 18-gauge metal tube. The mouse was subsequently ventilated with a Harvard pump ventilator [tidal volume (VT) = 0.4 ml, frequency = 120 breaths/min, positive end-expiratory pressure 2.5–3.0 cmH2O], and the tail vein was cannulated with a 27-gauge needle for injection of the methacholine challenge. After determining a dose-response curve (0.001–0.5 mg/kg), an optimal dose was chosen, 0.1 mg/kg methacholine, which induced minimal AHR in naive control mice but a very significant induction of AHR in allergic animals. After the methacholine challenge, the response was monitored, and the peak airway resistance was recorded as a measure of AHR.
Statistics.
Statistical significance was determined using analysis of variance with P values less than 0.05 considered significant, followed by a Student-Newman-Keuls posttest.
Histological analysis.
Lungs were inflated and maintained in formalin for 24 h before being processed into paraffin using standard histological techniques. Lung tissue sections were stained with hematoxylin & eosin (H&E) for analysis of inflammatory cell accumulation and alcian blue/periodic acid-Schiff (PAS) for assessment of mucus production. To quantify the mucus production in the lung, PAS sections were randomized, examined, and scored on a scale from 1 to 4, with 1 representing no mucus cell content and 4 representing airways filled with mucus. All slides were imaged using a Zeiss Primostar microscope (Zeiss MicroImaging, Thornwood, NY), a Moticam 2000 camera, and Motic Images Plus 2.0 software (Motic, Richmond, BC, Canada).
Specific antibody ELISA.
Serum was isolated at the indicated times by cardiac puncture or tail bleeding and assayed for antigen-specific antibody levels. Quantitation of CRA-specific antibodies was as follows: 96-well EIA/RIA flat-bottom plates (Costar, Corning, NY) were coated with CRA antigens (diluted in PBS, 7.5 mg/ml, 100 μl/well) overnight, washed (PBS, Tween 20), and blocked for 1 h with 200 μl of 5% FCS (Lonza Biowhittaker, Portsmouth, NH) and 1% BSA (fraction V). The plates were washed, and the diluted serum (5% FCS, 1% BSA was the dilution buffer) was added (100 μl/well) and incubated for 2 h at room temperature. The serum was diluted as follows for analysis of the different isotypes: IgE-1/25, IgG1-1/1,500, and IgG2A-1/1,500. Following washing, the biotinylated detection antibodies were added [anti-mouse IgE (clone R35-118, BD-Pharmingen, San Diego, CA), anti-mouse IgG1 (clone A85-1, BD-Pharmingen), and anti-mouse IgG2A (clone R19-15, BD-Pharmingen)] together with the SA-HRP (BD-Pharmingen). After a second 2-h incubation, the plates were washed and developed with peroxidase substrate reagents (BD-Pharmingen). The absorption at 405 nm was read using an automated plate reader (Molecular Devices kinetic microplate reader, Sunnyvale, CA). CRA-specific antibody measurements were in the linear range of the standard curve, and final quantitation of antigen-specific antibody was expressed in arbitrary units.
Gene expression analysis.
The resected lungs were snap frozen in a dry ice/liquid nitrogen bath and stored at −80°C until RNA isolation. Total RNA was isolated using the Oligotex RNA isolation kit according to manufacturer's directions (Qiagen Sciences, Valencia, CA). Biotinylated cRNA was prepared using the Illumina RNA Amplification Kit according to the manufacturer's instructions (Ambion, Austin, TX). Messenger RNA was converted to cDNA and then amplified and labeled by T7 DNA polymerase. The Illumina Mouse 6 Sentrix Expression BeadChip was used (Illumina, San Diego, CA). Following hybridization and washing, the arrays were scanned on an Illumina BeadArray Reader. The signals were computed with weighted averages of pixel intensities, and local background was subtracted. Sequence-type signal was calculated by averaging corresponding bead signals from the three liver samples with outliers removed (median absolute deviation).
Simultaneous normalization of multiple microarrays was done using the “mloess” method (24). Genes were ranked according to interest. The interest statistic was devised following modification of the methods of Cole et al. (4) and their software package Focus. The interest statistic reflects a biologist's view that a gene with a greater fold change (in absolute value) than other genes is potentially the more interesting one. Also, given two genes with the same fold changes, it is the gene with a higher expression level (and therefore higher absolute change) that is the more interesting one.
Array data have been deposited in the EBI Array Express Database (acc. no. is pending).
Quantitative real-time PCR analysis.
Relative mRNA transcript levels were measured by real-time quantitative RT-PCR in a LightCycler 480. Total RNA was extracted from lungs from mice treated with PBS or CRA ± Compound A as described above and reverse-transcribed using the Roche Transcriptor kit, and 50 ng cDNA was quantified using LightCycler 480 Probe Master kit. Duplicate biological samples were used. Each sample was run as a technical duplicate, and mean values were reported. Normalized gene expression values were obtained using LightCycler Relative Quantification software. Relative gene copy numbers were derived by efficiency-corrected relative quantification using the formula 2ΔCT where ΔCT is the difference in amplification cycles required to detect amplification product from equal starting concentrations of RNA. The sequences of the oligonucleotide primers and the corresponding Universal Probe Library probe (Roche) are provided as a Supplemental Table (available online at the AJP-Lung web site). Results were expressed as fold change compared with untreated cells.
Analysis of culture supernatants for cytokine production.
Cytokine or chemokine levels in the protein lysates were assessed by luminex technology using the Bioplex system (Bio-Rad). All assays were used according to the manufacturer's protocol as provided with standards that demonstrated sensitivity down to 1 pg/ml cytokine. Statistical analysis was done using one-way ANOVA followed by Student-Newman-Keuls posttest analysis between control and treatment groups.
RESULTS
Compound A is a potent and selective CRTH2 antagonist.
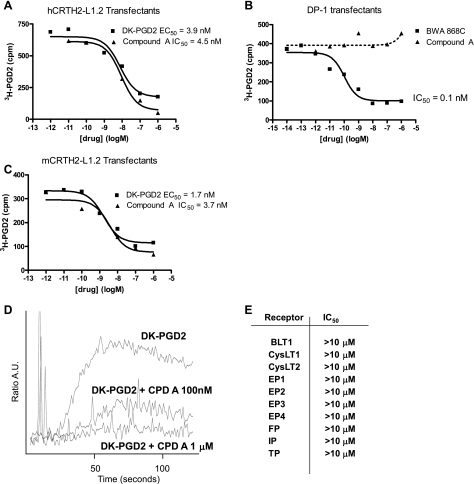
Pharmacological characterization of the CRTH2 antagonist (Compound A) used in these studies is shown in Fig. 1. Tritiated PGD2 binding was saturable, and, while Compound A exhibited low nM potency as an antagonist of human or mouse CRTH2 in transfected cells (Fig. 1, A and C), there was no activity on DP1 (Fig. 1B), other prostanoid, thromboxane, and cysteinyl leukotriene receptors (Fig. 1E), or full panel of G protein-coupled receptors as measured in standard PanLabs screens (MDS Pharma; data not shown). Compound A was also a functional antagonist as shown by standard calcium fluorimetry assays (Fig. 1D).
Fig. 1.
Pharmacological characterization of Compound A. A–C: equilibrium binding studies and heterologous competition studies of Compound A on CRTH2 and DP1 transfectants. Radioligand binding analysis was performed as detailed in materials and methods, with triplicate wells per concentration of ligand in a minimum of n = 4 separate experiments. D: effect of Compound A on DK-PGD2-induced calcium mobilization in CRTH2-L1.2 transfectants (representative of n = 3 separate experiments). DK-PGD2 was used at concentrations ranging from 1 to 100 nM, the trace represented in Fig. 1 being from an experiment using 10 nM. E: lack of competitive inhibition by Compound A on the binding of cognate ligands to prostaglandin, leukotriene, and thromboxane receptors (see materials and methods; triplicate assessments in n = 3 separate experiments).
Blockade of CRTH2 alters allergen-induced pathophysiology.
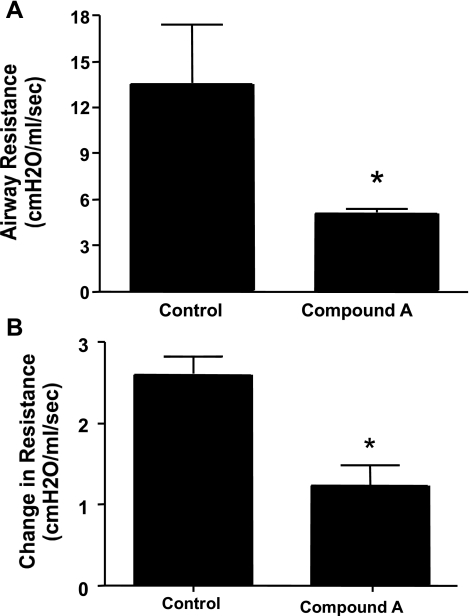
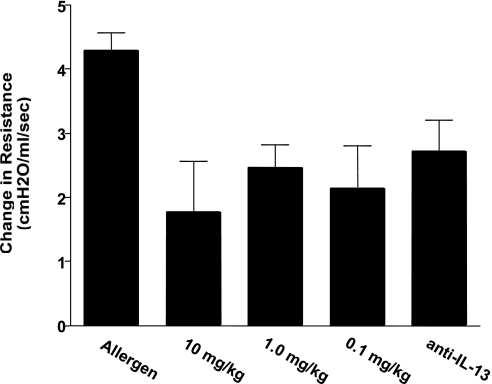
Based on previous data (not shown) for in vivo efficacy, we began studies using three doses of CRTH2 specific Compound A, 10, 1.0, and 0.1 mg/kg. The animals were sensitized systemically with cockroach allergen and challenged into the airway. The animals were treated with Compound A or vehicle according to the denoted protocols and 24 h later assessed for changes in airway function (AHR) upon a methacholine challenge. The data in Fig. 2 illustrate significant inhibition of AHR in mice treated with 10 mg/kg in either an acute (Fig. 2A) or more chronic (Fig. 2B) sensitization and challenge regimen. Figure 3 shows that even at the lower dose of 0.1 mg/kg, a reduction in AHR was evident. In all cases, the reduction in AHR was comparable to the positive control, a neutralizing antibody to IL-13. In separate studies using lower doses of compound, 0.01 mg/kg, the attenuating effect was lost with the compound (data not shown).
Fig. 2.
A: Six- to eight-week-old age- and sex-matched BALB/c mice were sensitized with CRA for 14 days, challenged intranasally (4 times every day), and then challenged intratracheally after 5 days. Forty-eight hours later, a second intratracheal challenge was given, and 24 h later, AHR was measured according to the protocol listed in materials and methods. B: BALB/c mice were sensitized as above, challenged intranasally (4 separate times with 4 days separating the challenges), and challenged intratracheally after 4 days. A second intratracheal challenge followed after a further 4 days, and 24 h after the last challenge, AHR was analyzed as above. Drug was administered by oral gavage 24 h before the final challenge. Five mice per group were used. *P < 0.01.
Fig. 3.
BALB/c mice were sensitized and challenged according to the subchronic protocol in Fig. 2A. A dose response to drug (0.1–10 mg/kg by oral gavage 24 h before final challenge) was assessed. Anti-IL-13 antibody (0.5 ml of serum; Ref. 26) was administered intraperitoneally at 30 min before challenge and used as a known control inhibitor of the allergic response.
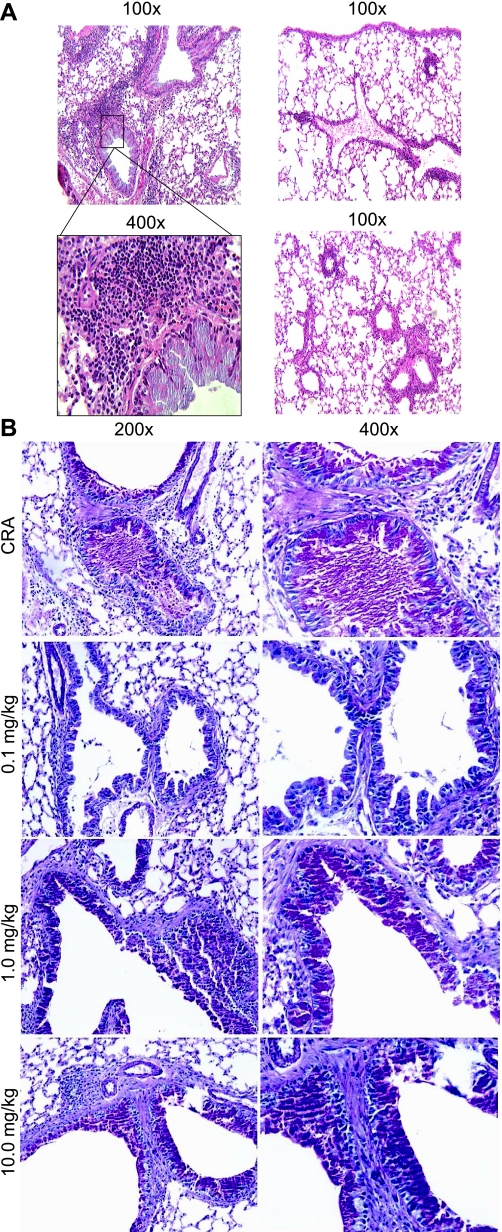
A second parameter that reflects the intensity of the response is the induction of goblet cell hyperplasia and mucus production in the airways. Figure 4A (left) shows a section of large airways upon H&E staining, revealing profound leukocyte infiltrates into the airway parenchyma and significant mucus cell hyperplasia. This inflammation and hyperplasia is dramatically reduced in mice treated with Compound A (right). Upon histological examination of PAS-stained slides, the vehicle control-treated animals displayed airways that were clogged with mucus and intense goblet cell hyperplasia (Fig. 4B). In contrast, while the goblet cells were still present, there appeared to be no secretion into the airways, and they were relatively clear of mucus and debris upon treatment with Compound A. These latter data likely demonstrate an important change in the development of the pathophysiology and may reflect an alteration of specific beneficial aspects of the response.
Fig. 4.
Formalin-fixed lung sections from challenged mice ± drug treatment were stained with hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS) as described in materials and methods. A: H&E sections under ×100 power showing perivascular and parenchymal inflammation (bottom left represents a × 400 magnification of the delineated area above). Right panels represent 2 independent sections from lungs treated with Compound A, as described in materials and methods. B: alcian blue/PAS staining for assessment of mucus production. The illustrations are representative of multiple experiments and depict the overall changes observed with treatment of allergic mice with compound A.
Alteration of cytokine production by CRTH2 blockade.
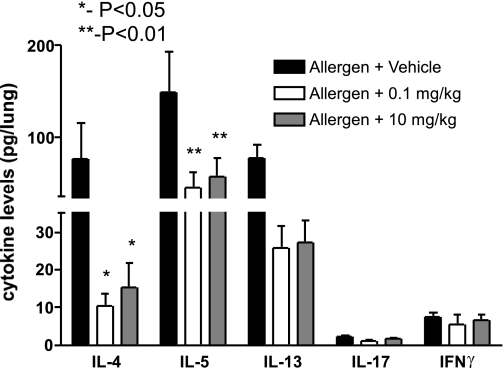
One of the clearest associations with allergic asthmatic disease is the overproduction of Th2 cytokines that leads to the above changes in airway pathophysiology. To assess changes in cytokine responses in the lung, we set up separate studies using two doses of Compound A based on our above results with 10 and 0.1 mg/kg in an acute sensitization and challenge protocol. After the final airway allergen challenge (24 h), the lungs were harvested and processed for cytokine analysis in 1 ml of protease inhibitor buffer (see materials and methods). The debris-free supernatant was then assayed for cytokine levels using luminex beads (Bioplex, Bio-Rad). The data in Fig. 5 indicate that there was a significant reduction in all of the Th2 cytokines examined, IL-4, IL-5, and IL-13, in the Compound A-treated animals. In addition, when IL-17 and IFNγ were examined, although lower levels were detected, a significant reduction of these cytokines was also observed in the treated group. Together, these data demonstrate that through the blockade of CRTH2, one of the likely mechanisms in the observed changes in the pathophysiology was by reduction of Th2 and proinflammatory cytokines.
Fig. 5.
Cytokine analysis by Bioplex. Lung samples were prepared by homogenizing lungs in PBS buffer containing a protease inhibitor cocktail (Complete) and 0.05% Triton X-100 nonionic detergent. The debris-free supernatant was prepared using high-speed centrifugation. Samples were assayed using the multiplex proteomics analysis as prescribed by the Bio-Rad protocols. Data represent means ± SE from 8–10 mice/group.
Reduction in serum antibody levels during allergic responses by CRTH2 blockade.
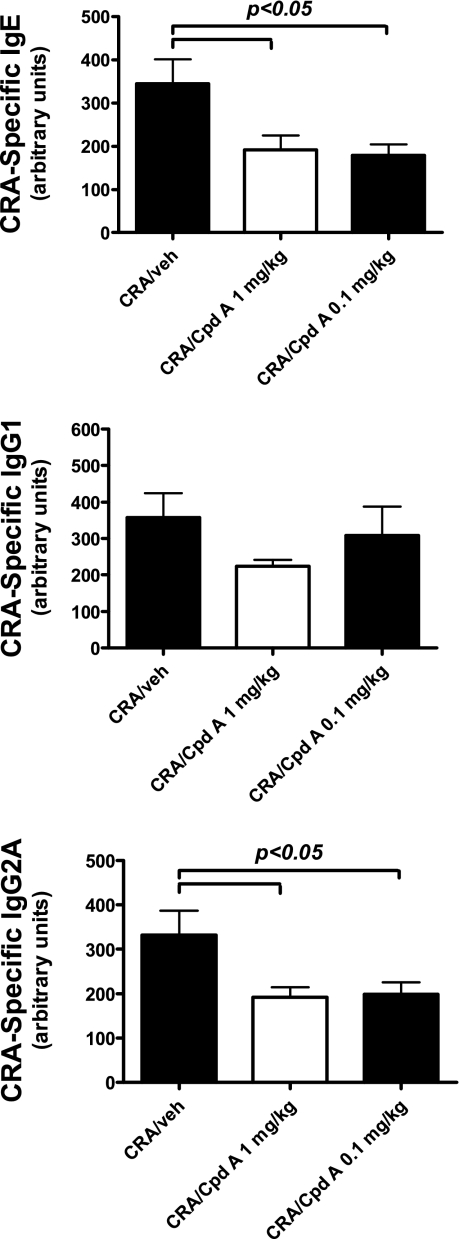
The data above indicate an overall reduction in cytokine levels in the lungs of the allergic mice treated with the CRTH2 antagonist Compound A. An important consequence of T cell-derived cytokines is the production of antibodies that can directly alter immune responses. In particular, IgE levels often correlate with severity of allergic disease, as it is responsible for early induction of mast cell and basophil activation upon allergen introduction. To assess the impact of CRTH2 blockade on antibody levels, serum from the previous experiment (acute challenge) was collected at the same time as lung harvest for cytokine analysis. The data in Fig. 6 illustrate that while all serum antibody isotypes were reduced in the 10 and 0.1 mg/kg treatment groups, only IgE levels were significantly reduced. These data reflect the reduction in pulmonary Th2 cytokine levels above, as both IL-4 and IL-13 have the ability to induce an isotype switch to IgE in B cells and are also known to further promote the production of antibody in antigen-specific B cells. Thus, a further benefit in the responses is the reduction in IgE, which may have a benefit on responses in future allergen exposures in chronic disease.
Fig. 6.
Levels of CRA-specific IgE, IgG1, and IgG2a from challenged mice with and without drug treatment. CRA-specific IgE levels were quantitated by comparison to a standard curve for absolute units; antigen-specific antibody levels are in arbitrary units. Mice were treated with 2 different concentrations of Compound A, 10 and 0.1 mg/kg. Each column and error bars display the means ± SE. A minimum of 5 mice/treatment group was analyzed, and representative results from 3 independent experiments are shown.
Gene array analysis of inflammatory responses in lungs of allergic animals treated with compound A.
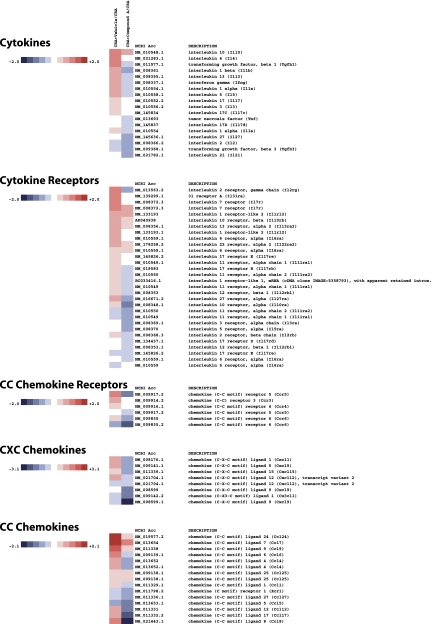
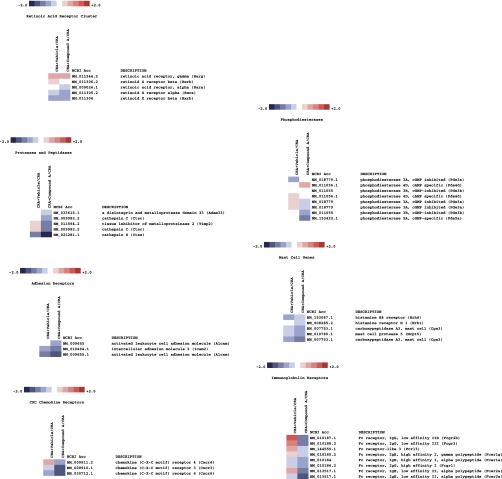
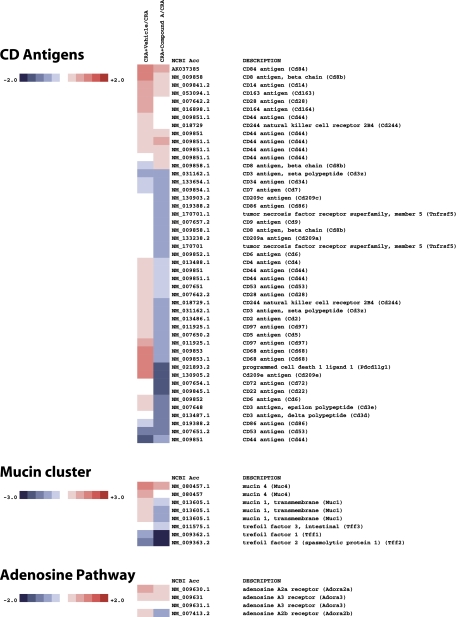
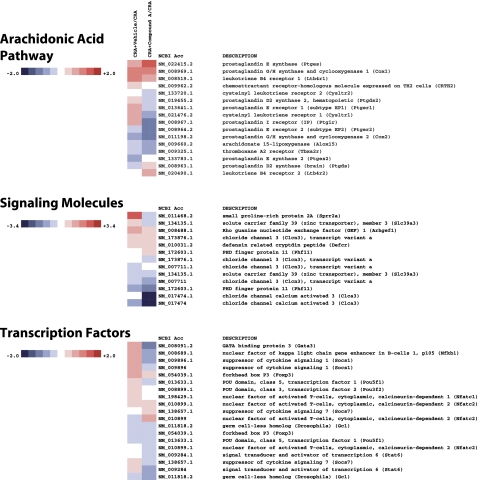
Genome-wide analysis of the effect of Compound A in CRA-challenged mice revealed a surprisingly profound and wide-ranging effect of CRTH2 blockade. As shown in Figs. 7–10 on a selection of genes clustered according to loose family associations, a clear inhibition of the Th2 profile could be observed as well as numerous genes that have been implicated either as a result of direct functional evidence for bioactivity or through biomarker strategies, for human atopic disease including asthma.
Fig. 7.
Heatmap of select genes downregulated in CRA-challenged mice treated with Compound A. Total RNA was extracted and analyzed using Illumina bead arrays. Levels of gene expression were investigated in 3 experimental groups, CRA treated, CRA + vehicle, and CRA + compound A, as outlined in the text. Data for the vehicle and drug treatments are presented as a fold increase or decrease relative to mice treated solely with CRA. The data array is representative of 3 independent experiments (biological replicates). The fold changes are noted on the respective color scales. In this clustergram, genes are grouped into cytokines, cytokine receptors, CC chemokines, CC chemokine receptors, and CXC chemokines.
Fig. 10.
Heatmap of select genes downregulated in CRA-challenged mice treated with Compound A. In this clustergram, genes are grouped into miscellaneous families.
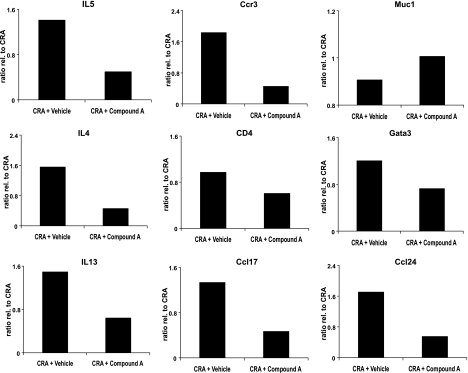
Of the “classic” Th2 phenotype, IL-5, IL-4, and IL-13 were all significantly reduced on the arrays, and these data were supported by direct Q-PCR analysis of these genes (Fig. 11). Other genes often associated with the asthma phenotype that were downregulated included the receptors CysLT1 and IL-5R, the chemokine receptors CCR3 and CCR5, the chemokine CCL8, and the adenosine A2b receptor, to name a few. Genes, whose products may be of significance to the remodeling process and mucin deposition included TGF-β1, the calcium-activated chloride channel 3, the solute carrier family 39 members, and Tff (Trefoil factor) members. Interestingly, the transcription factor GATA-3 was also downregulated, as was NF-κB.
Fig. 11.
Levels of chemokine receptor 3 (Ccr3), GATA binding protein 3 (Gata3), interleukin-4 (IL4), interleukin-13 (IL13), interleukin-5 (IL5), chemokine (CC motif) ligand 24 (Ccl24), chemokine (CC motif) ligand 17 (Ccl17), mucin 1 transmembrane (Muc1), and CD4 antigen (CD4) gene expression as determined by RT-qPCR. The data are presented as a fold increase or decrease relative to mice treated solely with CRA. The 28S rRNA is shown as a control. The RT-qPCR is representative of 2 independent experiments (biological replicates) performed in duplicate.
Of interest and importance to note, the genes for CRTH2 and the hematopoietic cell-derived PGD synthase were both significantly reduced by Compound A treatment. Other important inflammation-related genes of the cyclooxygenase and lipoxygenase pathways that were downregulated were Alox-15, COX-2, and the receptor EP1.
While by no means an exhaustive catalog of relevant genes to the multifactorial “atopic march,” this series of experiments certainly reveal a profound ability of the CRTH2 antagonist, Compound A, to ameliorate the proinflammatory process.
DISCUSSION
The induction and maintenance of chronic airway inflammation has been closely associated with alteration of specific Th2-driven immune responses, including IL-4, IL-5, and IL-13 production as well as eosinophil accumulation and mucus hypersecretion. Using the cockroach allergen model of allergic airway inflammation that is dependent on the development of Th2 responses, these studies have demonstrated that antagonism of CRTH2 is a relevant therapeutic modality.
Targeting CRTH2 has been shown to be a potentially useful strategy in the therapeutic intervention of allergic disease. We have further validated this approach using a highly selective and potent CRTH2 small-molecular-weight receptor antagonist, Compound A. A significant body of data now exists showing the proinflammatory role of PGD2-induced CRTH2 activation. This evidence, in part supported through the use of a variety of receptor antagonist and gene knockout approaches, begins to clarify the role of PGD2 in physiology and inflammation, which up to the present has been somewhat contradictory. Initial evidence through the use of both nonselective and selective antagonists has suggested that the antagonism of CRTH2 would, at least, serve as a useful anti-inflammatory strategy in the inhibition of eosinophilia into the airways. Shiraishi et al. (28) showed that PGD2-induced lung eosinophilia was almost completely inhibited by the nonselective antagonist Ramatroban, which was shown to be an inhibitor of the thromboxane A2 receptor as well as CRTH2 and DP1. The highly selective CRTH2 antagonist TM30089 (34) was shown to inhibit airway eosinophilia in a murine asthma model, as well as reduce goblet cell hyperplasia. Evidence from gene knockout studies has revealed that a total lack of the CRTH2 gene would serve to prevent the development of a Th2-type allergic response (8, 16). In all cases, it would appear that strategies for the antagonism solely of DP1 as an anti-inflammation therapeutic modality are less supported in favor of CRTH2.
Using compounds from our proprietary small molecule CRTH2 antagonist portfolio, we have undertaken a significant number of studies using a variety of experimental paradigms to further support the utility of this strategy in the development of anti-allergy medicines. Compound A is a selective CRTH2 antagonist having no discernable activity towards any other prostanoid receptor, or a full panel of GPCRs and enzymes. Compound A is derived from a chemical series of competitive, reversible CRTH2 antagonists displaying Ki values of less than 10 nM and between 60–80% bioavailability in rodents, suggesting that it would be a useful tool to analyze the biology of CRTH2 in animal models of allergic inflammation. In this specific example of a T cell-driven model of pulmonary inflammation, we have shown that CRTH2 antagonism has a profound ability to ameliorate the inflammatory phenotype. Analysis of lung function in challenged mice reveals a significant reduction in airway hyperreactivity that is mirrored in the clear improvement in pathology when mucus deposition and inflammation are analyzed by histopathology. Given the nature of the response and the role of proinflammatory cytokines in disease progression, we also see a clear and robust downregulation of the genes encoding these cytokines, as assessed by array and quantitative PCR technologies. In addition to reductions in the cytokines themselves, the production of allergen-specific immunoglobulins, specifically IgE, known for their role in atopy and the maintenance of the allergic response, is also significantly inhibited. It is also highly interesting that our ability to modulate the pathophysiology is comparable with that of the anti-IL-13 control. It will be very interesting to analyze the compound in a more chronic setting, and with chronic drug administration, to determine its efficacy in disease modification.
What is also of interest in the assessment of this wide array of modulated genes is the distinction between those that are potentially involved in more acute initiation of the inflammatory and hyperreactivity responses such as the prostanoids and cysteinyl-leukotrienes, and the genes that are regulated on a later time scale and involved in chronic maintenance, remodeling, and or resolution of the pulmonary inflammatory response. All lung tissue-derived RNA for the array studies has been isolated at the end of the sensitization and challenge periods. This interestingly suggests a profound effect of the compound in inhibiting the acute phase of the response as a result of chronic drug treatment, as well as the later phases from which prolonged cytokine and immunoglobulin production is seen. While extensive and comprehensive time-course experiments, as well as full molecular and genetic studies have yet to be undertaken, the current evidence clearly reveals a prominent role for CRTH2 in the inflammatory responses seen in this model. As an example, the modulation of IL-4 production (as well as other cytokines and chemokines) clearly points to important effects of the compound on T lymphocytes as well as basophils (as producers of IL-4). However, it is not yet clear from these studies at what point in the inflammatory cascade the CRTH2 receptor is functional, as a direct effect on the lymphocytes, or indirectly as a result of secondary effects mediated through eosinophil or basophil action. Similarly, other cytokines that are produced by lymphocytes and modified by the antagonist suggest an important role for this receptor at the level of dendritic cell-T lymphocyte interactions. Such mechanistic and pathway impact may have significant bearing on the performance of this or related compounds in a clinical setting.
While the functionality of many of these regulated genes has yet to be confirmed, or even analyzed in the current model, it is interesting to note that a fair number of those listed have emerged in recent years as significant biomarkers in the clinic for human asthma, or even as genetic risk factors for atopy and asthma from whole population studies (5). Specifically, the Th2-type cytokines IL-4, IL-5, and IL-13, as well as the monocyte marker CD14, the protease ADAM 33, and the chemokines eotaxin and RANTES, have all been noted in numerous population studies and are seen in this series of experiments to be significantly downregulated. Other genes, for which the associations are not as clear, have potentially relevant roles in the remodeling process as well as the control of mucus deposition, including the Muc1 gene, the calcium-activated chloride channel (subtypes 1 and 3), the solute carrier family members, and Treffoil factors (14, 23).
In summary, our data reveal a profound role for the PGD2/CRTH2 stimulatory axis in this murine model of airway inflammation, which suggests a capacity to significantly impact more than just the airway eosinophilia shown by previous studies (28, 34) as this compound class moves to clinical applications. Since we did not compare the effects of selective CRTH2 antagonism vs. dual DP1/CRTH2 antagonism, the utility of selective antagonism of this target in the clinic is still open to debate. It will be highly interesting, therefore, to determine and compare the effect of selective DP1 antagonists, for example, on the array of biological effects seen affected in this study. To date, the only published clinical evidence showing that CRTH2 may play a role in human allergic disease comes from studies using the nonselective CRTH2 antagonist Ramatroban/Baynas (2, 13). In a small cohort of asthmatics, the hyperresponsiveness to methacholine was significantly reduced following a 2-wk administration of the drug. Separately, PGD2-induced, but not histamine-induced, bronchoconstriction (13) was shown to be inhibited. While those authors conclude that the effects were due to the inhibition of TXA2R activation, these studies were performed long before Ramatroban was shown to be a CRTH2 antagonist (30). No further studies with Ramatroban have progressed clinically for asthma, although it was approved as an anti-rhinitic in Japan and has been shown to affect both human eosinophils and basophils in facilitating this clinical effect (36). Given the knowledge that this compound can significantly impact not only these leukocyte types but also T lymphocytes and the robust proinflammatory cytokine program they regulate, it will now be highly interesting to monitor their therapeutic efficacy in allergic disease.
DISCLOSURES
S. A. Boehme, T. W. Ly, and K. B. Bacon are Actimis Pharmaceuticals shareholders and employed by Actimis. G. Hardiman, R. Šášik, L. J. Sprague, N. W. Lukacs, and A. A. Berlin were paid-for-service consultants. Actimis is the patent holder on the compound described in the article.
GRANTS
G. Hardiman was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 1-P30-DK-063491-03.
Fig. 8.
Heatmap of select genes downregulated in CRA-challenged mice treated with Compound A. In this clustergram, genes are grouped into CD antigens, mucin cluster, and adenosine pathway.
Fig. 9.
Heatmap of select genes downregulated in CRA-challenged mice treated with Compound A. In this clustergram, genes are grouped into arachidonic acid pathway, signaling molecules, and transcription factors.
Acknowledgments
We thank J. Lapira at the UCSD BIOGEM Laboratory for Illumina Beadarray processing and quantitative RT-PCR analysis, and I. Wick for help with the preparation of figures.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abe H, Takeshita T, Nagata K, Arita T, Endo Y, Fujita T, Takayama H, Kubo M, Sugamura K. Molecular cloning, chromosome mapping and characterization of the mouse CRTH2 gene, a putative member of the leukocyte chemoattractant receptor family. Gene 227: 71–77, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Aizawa H, Shigyo M, Nogami H, Hirose T, Hara N. BAY u3405, a thromboxane A2 antagonist, reduces bronchial hyperresponsiveness in asthmatics. Chest 109: 338–342, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Boie Y, Sawyer N, Slipetz DM, Metters KM, Abramovitz M. Molecular cloning and characterization of the human prostanoid DP receptor. J Biol Chem 270: 18910–18916, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Cole SW, Galic Z, Zack JA. Controlling false-negative errors in microarray differential expression analysis: a PRIM approach. Bioinformatics 19: 1808–1816, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Cookson W The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat Rev Immunol 4: 978–988, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Fujitani Y, Kanaoka Y, Aritake K, Uodome N, Okazaki-Hatake K, Urade Y. Pronounced eosinophilic lung inflammation and Th2 cytokine release in human lipocalin-type prostaglandin D synthase transgenic mice. J Immunol 168: 443–449, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Gervais FG, Cruz RP, Chateauneuf A, Gale S, Sawyer N, Nantel F, Metters KM, O'Neill GP. Selective modulation of chemokinesis, degranulation, and apoptosis in eosinophils through the PGD2 receptors CRTH2 and DP. J Allergy Clin Immunol 108: 982–988, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalo J, Qiu Y, Coyle AJ, Hodge MR. CRTH2 (DP2) and not the DP1 receptor mediate allergen induced mucus production and airway hyperresponsiveness. The 2005 International Conference Abstracts of the American Thoracic Society. http://www.thoracic.org/publications/abstracts.asp. Poster: L41, 2005.
- 9.Hata AN, Zent R, Breyer MD, Breyer RM. Expression and molecular pharmacology of the mouse CRTH2 receptor. J Pharmacol Exp Ther 306: 463–470, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, Ichimasa M, Sugamura K, Nakamura M, Takano S, Nagata K. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med 193: 255–261, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirata M, Kakizuka A, Aizawa M, Ushikubi F, Narumiya S. Molecular characterization of a mouse prostaglandin D receptor and functional expression of the cloned gene. Proc Natl Acad Sci USA 91: 11192–11196, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwasaki M, Nagata K, Takano S, Takahashi K, Ishii N, Ikezawa Z. Association of a new-type prostaglandin D2 receptor CRTH2 with circulating T helper 2 cells in patients with atopic dermatitis. J Invest Dermatol 119: 609–616, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Johnston SL, Bardin PG, Harrison J, Ritter W, Joubert JR, Holgate ST. The effects of an oral thromboxane TP receptor antagonist BAY u3405, on prostaglandin D2- and histamine-induced bronchoconstriction in asthma, and relationship to plasma drug concentrations. Br J Clin Pharmacol 34: 402–408, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang C, Murgia C, Leong M, Tan LW, Perozzi G, Knight D, Ruffin R, Zalewski P. Anti-inflammatory effects of zinc and alterations in zinc transporter mRNA in mouse models of allergic inflammation. Am J Physiol Lung Cell Mol Physiol 292: L577–L584, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Lewis RA, Soter NA, Diamond PT, Austen KF, Oates JA, Roberts LJ. Prostaglandin D2 generation after activation of rat and human mast cells with anti-IgE. J Immunol 129: 1627–1631, 1982. [PubMed] [Google Scholar]
- 16.Lora JM, Al-Gawari A, Zhang DM, Gutierrez Ramos JC, Coyle AJ, Hodge MH. CRTH2 mediates in vivo leukocyte migration and regulates IL-4 expression in allergic inflammation models. [D21] [Poster: 909], Novel advances in pulmonary immunology. The 2004 International Conference Abstracts of the American Thoracic Society. 100th Meeting, May 21–26, Orlando, Florida. http://www.thoracic.org/publications/abstracts.asp.
- 17.Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, Sugimoto Y, Kobayashi T, Ushikubi F, Aze Y, Eguchi N, Urade Y, Yoshida N, Kimura K, Mizoguchi A, Honda Y, Nagai H, Narumiya S. Prostaglandin D2 as a mediator of allergic asthma. Science 287: 2013–2017, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Miadonna A, Tedeschi A, Brasca C, Folco G, Sala A, Murphy RC. Mediator release after endobronchial antigen challenge in patients with respiratory allergy. J Allergy Clin Immunol 85: 906–913, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Monneret G, Gravel S, Diamond M, Rokach J, Powell WS. Prostaglandin D2 is a potent chemoattractant for human eosinophils that acts via a novel DP receptor. Blood 98: 1942–1948, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Murray JJ, Tonnel AB, Brash AR, Roberts 2nd LJ, Gosset P, Workman R, Capron A, Oates JA. Release of prostaglandin D2 into human airways during acute antigen challenge. N Engl J Med 315: 800–804, 1986. [DOI] [PubMed] [Google Scholar]
- 21.Nagata K, Tanaka K, Ogawa K, Kemmotsu K, Imai T, Yoshie O, Abe H, Tada K, Nakamura M, Sugamura K, Takano S. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol 162: 1278–1286, 1999. [PubMed] [Google Scholar]
- 22.Nantel F, Fong C, Lamontagne S, Wright DH, Giaid A, Desrosiers M, Metters KM, O'Neill GP, Gervais FG. Expression of prostaglandin D synthase and the prostaglandin D2 receptors DP and CRTH2 in human nasal mucosa. Prostaglandins Other Lipid Mediat 73: 87–101, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Nikolaidis NM, Zimmermann N, King NE, Mishra A, Pope SM, Finkelman FD, Rothenberg ME. Trefoil factor-2 is an allergen-induced gene regulated by Th2 cytokines and STAT6 in the lung. Am J Respir Cell Mol Biol 29: 458–464, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Sásik R, Woelk CH, Corbeil J. Microarray truths and consequences. J Mol Endocrinol 33: 1–9, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Satoh T, Moroi R, Aritake K, Urade Y, Kanai Y, Sumi K, Yokozeki H, Hirai H, Nagata K, Hara T, Utsuyama M, Hirokawa K, Sugamura K, Nishioka K, Nakamura M. Prostaglandin D2 plays an essential role in chronic allergic inflammation of the skin via CRTH2 receptor. J Immunol 177: 2621–2629, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Schaller MA, Kallal LE, Lukacs NW. A key role for CC chemokine receptor 1 in T-cell-mediated respiratory inflammation. Am J Pathol 172: 386–394, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shichijo M, Sugimoto H, Nagao K, Inbe H, Encinas JA, Takeshita K, Bacon KB, Gantner F. Chemoattractant receptor-homologous molecule expressed on Th2 cells activation in vivo increases blood leukocyte counts and its blockade abrogates 13,14-dihydro-15-keto-prostaglandin D2-induced eosinophilia in rats. J Pharmacol Exp Ther 307: 518–525, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Shiraishi Y, Asano K, Nakajima T, Oguma T, Suzuki Y, Shiomi T, Sayama K, Niimi K, Wakaki M, Kagyo J, Ikeda E, Hirai H, Yamaguchi K, Ishizaka A. Prostaglandin D2-induced eosinophilic airway inflammation is mediated by CRTH2 receptor. J Pharmacol Exp Ther 312: 954–960, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Spik I, Brénuchon C, Angéli V, Staumont D, Fleury S, Capron M, Trottein F, Dombrowicz D. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J Immunol 174: 3703–3708, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Sugimoto H, Shichijo M, Iino T, Manabe Y, Watanabe A, Shimazaki M, Gantner F, Bacon KB. An orally bioavailable small molecule antagonist of CRTH2, ramatroban (BAY u3405), inhibits prostaglandin D2-induced eosinophil migration in vitro. J Pharmacol Exp Ther 305: 347–352, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Takeshita K, Yamasaki T, Nagao K, Sugimoto H, Shichijo M, Gantner F, Bacon KB. CRTH2 is a prominent effector in contact hypersensitivity-induced neutrophil inflammation. Int Immunol 16: 947–959, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka K, Hirai H, Takano S, Nakamura M, Nagata K. Effects of prostaglandin D2 on helper T cell functions. Biochem Biophys Res Commun 316: 1009–1014, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Terada N, Yamakoshi T, Hasegawa M, Tanikawa H, Nagata H, Maesako K, Konno A. Effect of a thromboxane A2 receptor antagonist, ramatroban (BAY u3405), on inflammatory cells, chemical mediators, and non-specific nasal hyperreactivity after allergen challenge in patients with perennial allergic rhinitis. Allergology Int 47: 59–67, 1998. [Google Scholar]
- 34.Uller L, Mosolff Mathiesen J, Alenmyr L, Korsgren M, Ulven T, Högberg T, Andersson G, Persson CGA, Kostenis E. Antagonism of the prostaglandin D2 receptor CRTH2 attenuates asthma pathology in mouse eosinophilic airway inflammation. Respir Res 8: 16–26, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue L, Gyles SL, Wettey FR, Gazi L, Townsend E, Hunter MG, Pettipher R. Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol 175: 6531–6536, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimura-Uchiyama C, Iikura M, Yamaguchi M, Nagase H, Ishii A, Matsushima K, Yamamoto K, Shichijo M, Bacon KB, Hirai K. Differential modulation of human basophil functions through prostaglandin D2 receptors DP and chemoattractant receptor-homologous molecule expressed on Th2 cells/DP2. Clin Exp Allergy 34: 1283–1290, 2004. [DOI] [PubMed] [Google Scholar]