Abstract
Familial pulmonary arterial hypertension (PAH) is associated with mutations in bone morphogenetic protein type II receptor (BMPR2). Many of these mutations occur in the BMPR2 tail domain, leaving the SMAD functions intact. To determine the in vivo consequences of BMPR2 tail domain mutation, we created a smooth muscle-specific doxycycline-inducible BMPR2 mutation with an arginine to termination mutation at amino acid 899. When these SM22-rtTA x TetO7-BMPR2R899X mice had transgene induced for 9 wk, starting at 4 wk of age, they universally developed pulmonary vascular pruning as assessed by fluorescent microangiography. Approximately one-third of the time, the induced animals developed elevated right ventricular systolic pressures (RVSP), associated with extensive pruning, muscularization of small pulmonary vessels, and development of large structural pulmonary vascular changes. These lesions included large numbers of macrophages and T cells in their adventitial compartment as well as CD133-positive cells in the lumen. Small vessels filled with CD45-positive and sometimes CD3-positive cells were a common feature in all SM22-rtTA x TetO7-BMPR2R899X mice. Gene array experiments show changes in stress response, muscle organization and function, proliferation, and apoptosis and developmental pathways before RVSP increases. Our results show that the primary phenotypic result of BMPR2 tail domain mutation in smooth muscle is pulmonary vascular pruning leading to elevated RVSP, associated with early dysregulation in multiple pathways with clear relevance to PAH. This model should be useful to the research community in examining early molecular and physical events in the development of PAH and as a platform to validate potential treatments.
Keywords: pulmonary circulation and disease, gene expression, genetically altered mice
idiopathic pulmonary arterial hypertension (PAH) is a disease characterized by increased pressure in the pulmonary circulation, the primary cause of which is not apparent. Although it is likely that the disease progresses over several years, in human patients, presentation usually occurs at a relatively late stage, leaving the initial events in the development of the disease obscure. PAH is associated with structural changes in the pulmonary vasculature, including hypertrophy and proliferation of both smooth muscle and endothelium, respectively, resulting in obliteration of small vessels (3). In addition, PAH is associated with perivascular inflammation (14).
The familial form is usually associated with mutations in the type II receptor for the bone morphogenetic protein pathway, BMPR2 (17). To determine the phenotype and in vivo molecular effect of BMPR2 mutation, we previously created a doxycycline-inducible transgenic mouse, which expressed an intracellular domain truncation of BMPR2, BMPR2delx4+, in smooth muscle on induction. These animals had increased right ventricular systolic pressures (RVSP) (36), increased cytokines (12, 33), loss of smooth muscle differentiation (33), and defects in vasoreactivity and vasoreactivity pathways (33, 42). However, pulmonary structural changes in this model were limited (36).
The mutation used in this original BMPR2delx4+ mouse was derived from family UK21 and consisted of a “T” base insertion at base 504, resulting in a premature stop 18 amino acids into the kinase domain (21). This resulted in loss of the entire intracellular domain and associated functions. The intracellular domain consists of a kinase domain at amino acids 200–500 responsible for phosphorylation of the type I BMP receptor leading to activation of SMAD transcription factors (4) and a long COOH-terminal tail from amino acids 500–1038. The kinase domain may also interact with other targets, including Rack1 (44). The BMPR2 tail has several poorly understood functions, including regulation of p38 and p42/44 MAPK (27, 40) and interaction with LIMK (10), c-Src (37), and Tctex (22). Some patient mutations are within this cytoplasmic tail and leave SMAD functions intact while causing disruptions to tail functions (21, 26). For example, an arginine to termination mutation at amino acid 899 (R899X) is found in family US33 (17).
We hypothesized that this mutation would result in a subset of the manifestations associated with the BMPR2delx4+ mutation, since SMAD activity remains intact with the R899X mutation. To test this hypothesis, we created transgenic mice that express the BMPR2 R899X mutation (BMPR2R899X) in smooth muscle when induced by doxycycline. After 9 wk of induction in adult mice, mice were hemodynamically phenotyped, fluorescent microangiography (FMA) was used to assess vascular pruning, and tissue sections were examined by immunohistochemistry. We found that expression of the BMPR2R899X mutation resulted in increased RVSP accompanied by substantial pulmonary vascular pruning and remodeling. Gene arrays were performed on transgenic mice with both high and low RVSP; we found that stress response, muscle organization and function, proliferation, apoptosis, and developmental pathways were all changed before RVSP increased. After elevation of RVSP, there were additional changes in these pathways as well as increased muscle structural genes and increased angiogenesis-related genes.
METHODS
Construction of transgenic mice.
TetO7-BMPR2R899X mice were generated at the University of Cincinnati Transgenic Mouse Core from plasmids we provided. A septad of the tetracycline response element and minimal cytomegalovirus promoter were used to drive expression of murine BMPR2 with an arginine to stop mutation at amino acid 899 with COOH-terminal FLAG tag, referred to as BMPR2R899X, followed by the β-globin intron and polyadenylation sequence. Complete sequence of the transgene has been deposited in GenBank as EU919427. Heterozygotes in TetO7-BMPR2R899X were crossed to homozygotes in our (36) previously published SM22-rtTA transgenic mouse with smooth muscle-specific expression of the reverse tetracycline transactivator. This resulted in mice that were universally heterozygous for the SM22-rtTA, and approximately half of which were heterozygous for TetO7-BMPR2R899X. We thus used transactivator-only littermate controls. Starting at 4 wk of age, all mice were fed doxycycline in chow (1 g/kg) for 9 wk when phenotyping was performed. All animal studies were preapproved by the University of Colorado Health Sciences Center Institutional Animal Care and Use Committee.
Heart catheterization and FMA.
Mice are given tribromoethanol (500 mg/kg ip) to induce a surgical plane of anesthesia. The animals are then shaved to expose the surgical area. Mice are placed on a heated surgical table (872/1, 872/H; Harvard Apparatus, Holliston, MA) and secured with surgical tape. Systemic blood pressure and pulse is measured via a tail cuff and pulse transducer run through a PowerLab NIBP Controller (ADInstruments, Colorado Springs, CO). The surgical site is viewed using a Zeiss OPMI-1 surgical microscope. An incision of ∼1 in. in length is made extending from the animal's chin down to the right armpit. The thyroid gland is then blunt-dissected upward to expose the underlying tissue and the right jugular vein. The jugular vein is then separated from surrounding tissue using dissecting forceps until the body of the vessel is completely free from adherent tissues. The cranial end of the jugular is tied off completely, and a loose tie is then made at the caudal end of the exposed jugular using 4-0 braided silk suture. Four-inch microdissecting scissors are then used to make a small incision in the medial aspect of the right jugular vein. A Millar 1.4 French pressure-volume microtip catheter transducer (SPR-839; Millar Instruments, Houston, TX) connected to a PowerLab/8s (ADInstruments) is then inserted through the incision and gently threaded down into the right ventricle. Proper placement within the ventricle is determined through observation of the pressure-volume loop obtained from the catheter. The loose caudal suture is then tightened to secure the catheter in place. Once the catheter is properly placed, data are collected using Chart 5 (ADInstruments).
Once blood pressure and volume data are collected, the caudal suture is reloosened, and the catheter removed. One hundred units of heparin is then injected through the jugular incision to prevent clotting. The suture is then retightened to prevent bleeding. The animals are then removed from the surgical table to a dissecting area.
Plane of anesthesia is redetermined postsurgery, and an overdose of sodium pentobarbital is administered if the withdrawal reflex is returning. After complete loss of withdrawal reflex, an incision is made just below the xiphoid process, and the ventral portion of the rib cage is cut away being careful not to nick the lungs. A small cut is then made at the vertex of the left atrium. A needle is inserted into the right ventricle, and ∼10 ml of a flushing solution (10 mM sodium phosphate, 127 mM sodium chloride, 10 mM EDTA, 5 U/ml heparin) is flushed through the lungs and out of the left atrium. A 45°C solution of 10% (1:10 vol/vol) fluorescent microbeads (FluoSpheres F8811; Molecular Probes, Eugene, OR) in 1% low melting point (LMP) agarose in PBS is then slowly flushed via the right ventricle through the lungs until it runs out of the left atrium. Lungs are simultaneously inflated with a 0.8% solution of LMP agarose in PBS via the trachea. The chest is packed off with ice until the LMP agarose has congealed. The heart and lungs are removed en bloc and fixed 48 h in 4% paraformaldehyde. Sections for FMA are cut using a vibratome.
Luciferase reporter assay.
A7r5 [CRL-1444, American Type Culture Collection (ATCC)] cells were plated and grown in 12-well plates in DMEM with 10% FBS and 1% penicillin-streptomycin. When the cells were 60–70% confluent, wells were washed, and the media replaced with 0.5% FBS DMEM overnight. The following day, the cells were transfected with 3 μg of pBRE, 1.2 μg of TK Renilla (for internal control), and 3 μg of either control vector (pGEM), wild-type Bmpr2, or BMPR2 with R899X mutation, using FuGENE 6 (Roche, Indianapolis, IN) as transfecting medium at a 3:1 FuGENE-to-DNA ratio.
After 24 h, cells received 0, 5, 15, or 50 ng/ml recombinant BMP4. After 48 h from transfection, the cells were lysed directly in the plates using 200 μl of 1× lysis buffer from Dual-Luciferase Assay Kit (Promega). Cells were rocked for 15 min subjected to one round of freeze-thaw before analysis. Twenty microliters of the lysate and 100 μl each of Luciferase Assay Reagent II and Stop & Glo Reagent were used for each assay. The assays were carried out using a Turner BioSystems Luminometer with dual injectors.
Western blot and immunohistochemistry.
Western blots were performed as previously described (12) using primary antibodies at 1:200 dilution and secondary antibodies at 1:3,000 dilution. Primary antibodies included Id1 (sc-488; Santa Cruz Biotechnology, Santa Cruz, CA), phospho-Smad1 (cat. no. 9511; Cell Signaling Technology, Danvers, MA), and phospho-p38 (sc-7973, Santa Cruz Biotechnology).
Immunohistochemistry was performed as previously described (33) using the following primary antibodies and dilutions: Flag Ab-1 NeoMarkers (Fremont, CA) rabbit polyclonal, 1:250; actin A5441 Sigma (St. Louis, MO) mouse monoclonal, 1:1,000; actin Abcam (Cambridge, MA) ab5694 rabbit polyclonal, 1:1,000; von Willebrand factor (vWF) Dako A0082 rabbit polyclonal, 1:1,000; CD45 sc-25590 Santa Cruz Biotechnology rabbit polyclonal, 1:100; CD133 sc-30219 Santa Cruz Biotechnology rabbit polyclonal, 1:200; CD3 epsilon ab49943 Abcam rabbit polyclonal, 1:200; Mac-3 550292 BD Pharmingen (San Jose, CA) mouse monoclonal, 1:200; CD11b 550282 BD Pharmingen mouse monoclonal, 1:200; phospho-p42/44 9106 Cell Signaling Technology mouse monoclonal, 1:50.
Affymetrix arrays.
Total RNA was isolated using an RNeasy Mini Kit (QIAGEN, Valencia, CA) from 30 mg of right lung from six individual mice. Samples were prepared for Affymetrix arrays using 2.5 μg of total RNA. First and second strand complimentary DNA was synthesized using standard techniques. Biotin-labeled antisense complimentary RNA was produced by an in vitro transcription reaction. Mouse Genome 430 2.0 microarrays (Affymetrix, Foster City, CA) were hybridized with 20 μg of cRNA. Target hybridization, washing, staining, and scanning probe arrays were done following an Affymetrix GeneChip Expression Analysis Manual. All array results have been submitted to the NCBI gene expression and hybridization array data repository (GEO; http://www.ncbi.nlm.nih.gov/geo/) as series GSE11018.
Array analysis.
Affymetrix CEL files were loaded into dChip 2007 array analysis software. The dChip algorithm is capable of detecting significant differences at signal strengths lower than those usable in Microarray Suite (Affymetrix, Santa Clara, CA; Ref. 19). Overall signal strength from arrays was normalized to the median array, and expression levels were determined using the perfect match/mismatch (PM/MM) algorithm. Gene ontology was determined using the Classify Genes tool within dChip (45) with gene ontology files downloaded from the Gene Ontology Consortium (http://www.geneontology.org; Ref. 10a). Our definition for changed genes was a 1.2× change for each of four pairwise comparisons with a minimum absolute change of 100, which results in a false discovery rate of 27%.
RESULTS
BMPR2R899X expression is correctly localized and results in increased p38MAPK and p42/44 phosphorylation but unchanged SMAD activity.
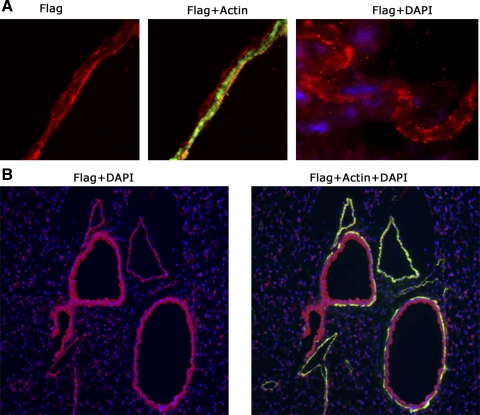
To verify that the transgene was expressed in the correct tissue type and was correctly trafficked to the cell surface, adult SM22-rtTA x TetO7-BMPR2R899X mice were fed doxycycline at 1 g/kg in chow for 3 days and then killed, and immunohistochemistry for the FLAG tag was performed. We found that the FLAG tag is correctly localized to the cell surface by confocal microscopy (Fig. 1A) and colocalizes with α-smooth muscle actin in both pulmonary artery and airway smooth muscle (Fig. 1B). Columnar epithelial cells and elastic lamina are false positive as shown by secondary antibody-only control (Supplemental Fig. 1A, available in the data supplement online at the AJP-Lung Cellular and Molecular Physiology web site). Supplemental Fig. 1B shows serial sections double-stained with either FLAG tag and actin or vWF (endothelial marker) and actin, showing that nonmuscularized vessels do not express FLAG tag, further supporting smooth muscle specificity. Although we cannot rule out expression below the threshold of detection in other tissue types, these data indicate that the transgene is substantially made in the correct cell type and is correctly expressed on the cell surface.
Fig. 1.
BMPR2R899X transgene is correctly expressed only in smooth muscle. A: 400× colocalization of α-smooth muscle actin and BMPR2R899X transgene FLAG tag. BMPR2R899X transgene is correctly trafficked to the cell surface. B: 100× colocalization of actin and FLAG tag in both vascular and bronchial smooth muscle. Columnar epithelial cells are false positive. DAPI, 4,6-diamidino-2-phenylindole.
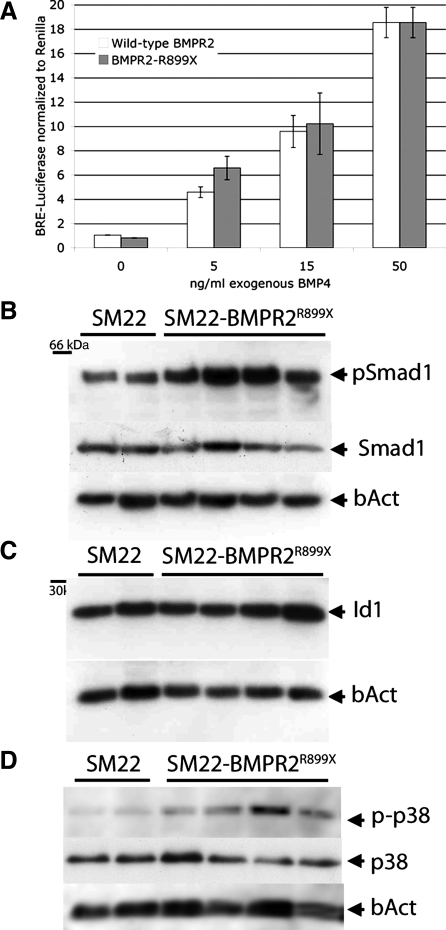
Although much of downstream signaling from the BMPR2 R899X mutation has previously been published (27), we wished to confirm the reported effects. We cotransfected vascular smooth muscle cells in culture with either wild-type or R899X mutant BMPR2 and the pBRE luciferase reporter construct for Smad activity (23). We found that there was no difference between wild-type and mutant receptor in either baseline activity or activity with 5, 15, or 50 ng/ml BMP4 added (Fig. 2A). We obtained similar results in COS-7 epithelial cells, suggesting that the effect of this mutation on Smad signaling is not cell type specific (Supplemental Fig. 2A). In whole animals, we saw a slight, but not statistically significant, increase in Smad1 phosphorylation (Fig. 2B). Id1 protein expression, a target of SMAD signaling, was not changed in the same animals, however (Fig. 2C), nor was expression of Id1, 2, or 3 (Supplemental Fig. 2B). Based on these data, we conclude that the R899X mutation does not have a significant effect on Smad activity in cell culture and in vivo may result in slightly increased, rather than decreased, Smad phosphorylation, likely via feedback not present in cell culture.
Fig. 2.
Effect of BMPR2R899X transgene on bone morphogenetic protein type II receptor (BMPR2) signaling pathways. A: A7r5 vascular smooth muscle cells transfected with pBRE luciferase reporter and either wild-type BMPR2 or BMPR2 with R899X mutation with increasing doses of recombinant BMP4. Two-way ANOVA shows a highly significant effect of added ligand (P < 0.0001) but no effect of vector or ligand by vector. B: SMAD1 phosphorylation (pSmad1) is not decreased (P = not significant by unpaired t-test). C: with the same samples and loading order as B, SMAD target Id1 protein remains unchanged. D: p38 phosphorylation increases in whole lung from mice expressing BMPR2R899X as normalized to total p38 (P < 0.05 by unpaired t-test). bAct, β-actin; BRE, BMP response element.
We also examined other downstream BMPR2 targets including p38, p42/44, and LIMK. We found a consistent increase in p38 phosphorylation (Fig. 2D) by densitometry almost 3× normalized to total p38 and ∼1.5× normalized to β-actin. We also found an increase in p42/44 phosphorylation by immunohistochemistry (Supplemental Fig. 2C), apparently in both endothelium and smooth muscle, implying a paracrine effect. Contrary to expectation, we did not see alteration in phosphorylation of cofilin, a LIMK target (Supplemental Fig. 2D) in whole lung homogenates. We cannot rule out a tissue-specific effect obscured by use of whole lung, as array data below suggest alterations in actin organization pathways.
Nine weeks of BMPR2R899X expression results in elevated RVSP in some mice and vascular pruning in all mice.
Nineteen 4-wk-old SM22-rtTA x TetO7-BMPR2R899X mice and 14 SM22-rtTA-only littermate controls were fed doxycycline for 9 wk, systemic pressures were determined by tail cuff, right ventricular pressures were assessed by closed-chest Millar catheter, FMA were performed on some, and the frozen and fixed tissue were collected. We found that 5 of 14 SM22-rtTA x TetO7-BMPR2R899X mice from which RVSP could be obtained had elevated RVSP (Fig. 3A); right ventricular muscularization was consistent with this (P < 0.02 by correlation z-test, data not shown). Counts of <100-μm muscularized vessels per field by immunohistochemistry showed an almost perfect correlation with RVSP (Fig. 3B). Thus there was strong support in both heart and pulmonary vessel muscularization for the pressures measured. Systemic pressures were not different between transgenic mice and controls and did not correlate with RVSP (data not shown).
Fig. 3.
A: right ventricular systolic pressure (RVSP) is increased above controls (Ctl) in 5/14 SM22-rtTA x TetO7-BMPR2R899X mice in which readings could be obtained. B: muscularized vessels per field is increased and correlates with RVSP in transgenic mice (P < 0.001 by z-correlation test).
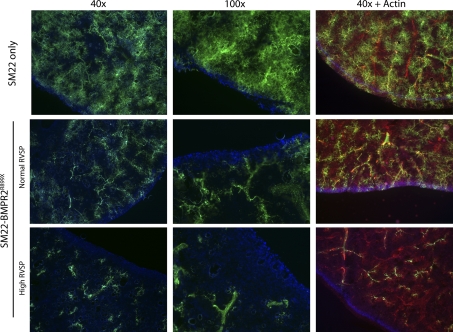
FMA, in which 0.2-μm fluorescent beads are perfused through the pulmonary vasculature, was used to determine the number of vessels that could be perfused. This indicated that SM22-rtTA x TetO7-BMPR2R899X mice with normal RVSP had substantial vascular pruning in all mice tested (n = 4, with 5 SM22-rtTA-only controls) with greater loss of vessels in mice with elevated RVSP (n = 2; Fig. 4). Staining FMA sections with actin demonstrated that the vessels were still present but could not be perfused. These results suggest that activation of the BMPR2R899X transgene is causing blockage but not loss of vessels in all mice and that RVSP only increases when this pruning is far advanced.
Fig. 4.
SM22-rtTA x TetO7-BMPR2R899X mice show decreased perfusion even when RVSP is normal by fluorescent microangiography; perfusion is reduced still further in mice with high RVSP. All mice continue to show extensive actin staining (red, right panels).
BMPR2R899X mice with elevated RVSP have vessels obliterated by muscle or endothelium.
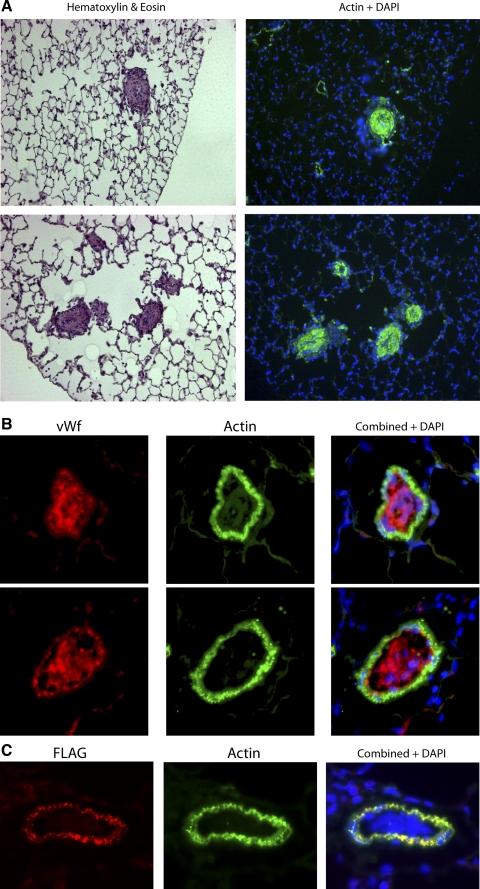
Examination of tissue sections from mice with elevated RVSP revealed some vessels with substantial structural changes. Some vessels were filled with well-organized layers of smooth muscle (Fig. 5A), whereas some were filled with endothelial cells (Fig. 5B). This means that in some lesions, the cells filling the vessels do not express transgene (Fig. 5C), demonstrating that, in this case, the effect of the mutation is necessarily through paracrine signaling. In fact, some nonmuscularized vessels also appear to be filled with endothelial cells (Supplemental Fig. 3A), and increased proliferating cells (by PCNA staining) do not appear to be limited to muscularized vessels (Supplemental Fig. 3B), suggesting that the mutation in this context may lead to a paracrine proliferative effect. These sorts of structural changes are never seen in hypoxic mice, indicating that they are unlikely to be the result of increased pressure alone.
Fig. 5.
SM22-rtTA x TetO7-BMPR2R899X mice with high RVSP develop extensive pulmonary vascular structural changes, including well-organized muscular lesions; actin is stained green in the right panels (A), and filling with endothelial cells; von Willebrand factor (vWF) is red, and actin is green (B). Two examples of endothelial lesions are shown on different lines. C: the cells filling the lumen in some lesions do not express R899X transgene (blue nuclei seen in combined panel do not also express FLAG tag).
The transgene is expressed in all smooth muscle in the body (Supplemental Fig. 3C shows expression in smooth muscle in kidney), however, other organs do not appear to have the gross abnormalities seen in lungs (Supplemental Fig. 3D shows liver and kidney). The only systemic abnormality found was a slight (6%) but statistically significant (P = 0.03 by 2-way ANOVA with sex and genotype as variables) increase in body weight in SM22-rtTA x TetO7-BMPR2R899X compared with age-matched littermate SM22-rtTA-only mice. Although one might expect loss of proper BMPR2 function to have a phenotype in any organ, limitation of obvious abnormality to lung matches the phenotype seen in human BMPR2-related PAH.
Muscular lesions are surrounded by macrophages and T cells and include some CD133+ cells.
The adventitial component of the vascular lesions contained many CD45+ cells, indicating that they are derived from circulating cells (data not shown). Further staining for T cells and macrophages showed that the vessels were surrounded closely by macrophages (Fig. 6A) with a large number of T cells making up the extended lesion (Fig. 6B). Some of the adventitial cells are also positive for progenitor marker CD34 (Supplemental Fig. 4). Lungs from SM22-rtTA-only mice have only a few alveolar macrophages and no apparent T cells (Fig. 6, A and B, right). Although the muscle making up the central lesion did not stain positive for CD45 (data not shown), there appeared to be a substantial number of cells that were positive for both actin and CD133 (Fig. 6C), suggesting that at least some of the muscle cells differentiated from circulating cells. Vessels that were filled primarily with CD133-positive cells were also CD45 positive (Fig. 6D); from our current study design, we cannot determine whether this is an earlier stage in the development of lesions, such as those in Fig. 6C, or a different type of lesion. In lungs from control mice, only a few isolated alveolar macrophages can be found, and both T cells and CD133 staining were essentially never found.
Fig. 6.
Adventitial lesions are substantially made up of macrophages, as determined by Mac-3 antibody (A), and T cells, as determined by CD3 antibody (B). C: some muscle cells filling the lumen are CD133 positive, suggesting circulating origin (D). CD133-positive cells are also CD45 positive. Control lungs (top, right box) have very few macrophages and T cells (columnar epithelial cells are false positive). Arrows indicate alveolar macrophages.
Nine weeks of BMPR2R899X expression results in small vessels filling with CD45+ cells in all mice.
The structural changes referred to in the previous two sections were only visible in mice with high RVSP and had a frequency of only a few instances per tissue section, too few to allow reliable statistical representation of frequency. Much more common was the finding of small vessels filled with multiple large mononuclear cells (Fig. 7, A and B). These were found with approximately the frequency indicated in Fig. 7B in every SM22-rtTA x TetO7-BMPR2R899X mouse with both normal and high RVSP. These cells were often weakly CD45 positive, suggesting origin as circulating cells (Fig. 7C); they were never positive for macrophage or neutrophil markers (data not shown) and were sometimes positive for T-cell markers (Fig. 7D).
Fig. 7.
A: the most common lesion in SM22-rtTA x TetO7-BMPR2R899X mice is obliteration of small vessels with large nucleated cells. B: these are extremely common; red arrows indicate a large number in this representative field. These cells are CD45+ as seen in these serial sections (C) and can be CD3+ (D).
BMPR2R899X mice with normal RVSP show altered gene expression in muscle structure and function, stress response, cell cycle, and developmental pathways.
Affymetrix gene expression arrays were used to examine changes in gene expression in whole lung that might underlie the morphological and hemodynamic results. Three groups with two arrays each were examined: control SM22-rtTA-only mice, SM22-rtTA x TetO7-BMPR2R899X mice with normal RVSP, and SM22-rtTA x TetO7-BMPR2R899X mice with elevated RVSP, all with 9 wk of treatment with doxycycline.
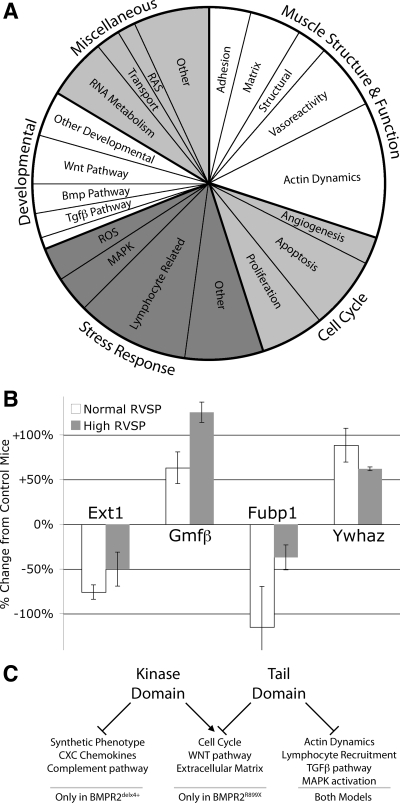
Comparison of controls to BMPR2R899X mice with normal RVSP allows examination of pathways dysregulated by mutation and compensation for the mutation without the complicating factor of additional changes caused by elevated pulmonary pressure. In this comparison, we found 181 unique, named genes with altered expression using criteria with a false discovery rate of 27%; our goal was sensitivity over specificity. When sorted into gene ontology groups, we found that the majority fell into four broad categories: developmental genes, stress response, muscle structure and function, and cell cycle (Fig. 8A and Supplemental Table 1).
Fig. 8.
A: functional distribution of all genes differentially regulated in SM22-rtTA x TetO7-BMPR2R899X mice with normal RVSP compared with controls. B: changes in gene expression of examples of developmental (Ext1), stress response (Gmfb), cell cycle (Fubp1), and actin organization (Ywhaz) genes, as normalized to SM22-rtTA-only mice. C: comparison to previously published BMPR2delx4+ mice suggests pathways downstream of BMPR2 kinase and tail domains. Tgfβ, transforming growth factor-β; ROS, reactive oxygen species.
Examples of these genes are shown in Fig. 8B. These include the developmental gene exostoses 1 (Ext1), involved in establishing morphogen gradients in BMP, hedgehog, and Wnt signaling (13); the stress-related gene glia maturation factor (Gmfb), an inducer of cytokines implicated in some autoimmune disorders (43); actin organization gene 14-3-3 zeta (Ywhaz), which binds BMPR2-interactor LIMK (1); and cell cycle gene Fubp1, a Tgfβ target that regulates c-myc (16). Of course, we cannot determine from these data whether the change in each of these specific genes is part of pathogenesis or compensatory. In either case, however, the ontology group to which they belong is implicated in the early etiology of BMPR2-mediated PAH.
Statistically overrepresented gene ontology groups are likely to have a lower number of falsely discovered genes. For instance, ∼4% of genes on the array are involved with actin dynamics; with 181 genes altered, one would expect about (0.04 × 181) seven to be actin dynamics-related if they were randomly distributed, with two of these spurious. Since we actually have 22 genes in this category, the category is highly overrepresented and thus likely to be a real change; in addition, we still only expect two of the genes in this category to be falsely discovered.
Comparing genes dysregulated in the BMPR2R899X model to those we have previously reported in our SM22-rtTA x TetO7-BMPR2delx4+ model, we found both substantial overlap as well as major differences, which allows us to make guesses as to domain function (Fig. 8C). The most striking differences are genes dysregulated in BMPR2delx4+ mice but not BMPR2R899X mice. BMPR2R899X mice do not show the broad loss of smooth muscle markers seen in the earlier model (33), suggesting that smooth muscle differentiation state is driven by SMAD activity. More surprisingly, given the apparently inflammatory phenotype seen in BMPR2R899X mice, they do not have the broad increase in cytokines, CXC chemokines, or complement activation seen in the BMPR2delx4+ mice (33), at least at this time point. Conversely, BMPR2R899X mice had several categories of genes changed not seen in BMPR2delx4+ mice, including cell cycle and Wnt pathway genes. Other pathways were dysregulated in both models and had substantial overlap in specific genes altered (marked in Supplemental Table 1).
BMPR2R899X mice with elevated RVSP show additional changes in angiogenesis, vasoreactivity, and injury response.
The BMPR2R899X mice with elevated RVSP had substantial additional changes to gene expression; using the same criteria as above, an additional 269 changed (Supplemental Table 2). These include many genes that are clearly suggestive of response to injury. In Fig. 9A, these include heme oxygenase-1 (Hmox1), an oxidative stress response (28), CD44, a cell adhesion marker characteristic of response to endothelial barrier injury (31), and myotrophin (Mtpn), a pressure and stretch sensor that triggers hypertrophy (30). Angiogenesis factors also increase with elevated RVSP (Fig. 9B), including canonical angiogenesis genes VegfA and angiopoietin-1 but also PDGF receptor-α (PdgfRA), required for fibroblast recruitment in angiogenesis (7). Finally, the advent of high right ventricular pressure results in increases in vasoreactivity-related genes (Fig. 9C) such as endothelin receptor-α (EdnRA), nitric oxide signal transducer guanylate cyclase (Gucy1a3), and thioredoxin interacting protein (Txnip), a peroxisome proliferator-activated receptor-γ (PPARγ)-interacting protein that regulates oxidative stress (38) and contractile energy homeostasis (41). For all of these genes, expression in the normal RVSP group is also trending upward, although not yet significantly; we assume that this is because these also are starting to have difficulty regulating pressure. Broadly, additional genes differentially regulated in BMPR2R899X mice with elevated RVSP but not normal RVSP likely indicate genes that are regulated in response to pressure or stretch, not in response to BMPR2 dysfunction.
Fig. 9.
Genes dysregulated with elevated RVSP in SM22-rtTA x TetO7-BMPR2R899X mice include genes directly related to oxidative stress and damage (A), angiogenesis-related genes (B), and vasoreactivity (C). Values are normalized to expression in control SM22-rtTA-only animals; *P < 0.05 for difference compared with control by Fisher post hoc after ANOVA. Mtpn, myotrophin; Ho1, heme oxygenase-1; VegfA, VEGF canonical angiogenesis gene; PdgfRA, PDGF receptor-α; EdnRA, endothelin receptor-α; Txnip, thioredoxin interacting protein; Gucy1a3, nitric oxide signal transducer guanylate cyclase.
DISCUSSION
SM22-rtTA x TetO7-BMPR2R899X mice produce a very close approximation of human PAH using induction in adult animals of murine Bmpr2 with a mutation derived from a human patient family. These mice produce a distinct hemodynamic and histological phenotype with a number of potentially separable elements. These elements include vascular pruning (Fig. 4), recruitment of circulating cells to the lumen of very small vessels (Fig. 7), recruitment of CD133-positive cells (Fig. 6), production of adventitial lesions largely consisting of macrophages and T cells (Fig. 6), elevation of RVSP (Fig. 3), conventional muscularization of the pulmonary vasculature (Fig. 3), and development of large pulmonary lesions similar to those characteristic of human PAH consisting of either rings of smooth muscle or endothelial cells (Fig. 5).
Gene arrays for SM22-rtTA x TetO7-BMPR2R899X mice assayed before RVSP increases show alterations in expression of genes involved in development, stress response, cell cycle, and muscle structure and function (Fig. 8). Although it is impossible to determine whether the change in any individual gene is etiologic or compensatory, changes in entire classes of genes implicate those pathways in disease development, thereby narrowing the focus for downstream effector pathways for mutant BMPR2. Of equal importance, they also exclude pathways; angiogenesis and conventional vasoreactivity pathways do not show substantial altered expression until after RVSP increases (Fig. 9), suggesting that they are a response to increased pulmonary vascular pressure, not the cause of it.
Integrating the data from this study with our (33, 36) past studies and recent data from the literature (15, 32), we hypothesize that a decrease in BMP signal results in activation of injury repair mechanisms with each downstream BMPR2 effector pathway, SMAD, MAPK, and actin organization, playing a different role. Decreased SMAD signaling through BMPR2 results in both a switch in smooth muscle from a contractile to a synthetic state and increased cytokine expression (12, 33). Increased p38MAPK phosphorylation (Fig. 2D) drives downstream stress responses, possibly including proliferation (Ref. 39; Fig. 8, A and B, and Supplemental). Direct BMPR2-mediated alterations in actin organization through LIMK1 and Tctex may result in multiple effects seen in PAH in patients, including muscle migration (18), matrix deposition (2), and inflammatory cell adhesion (8, 29). Different BMPR2 mutations may cause dysfunction in any or all of these elements, but one can easily imagine how overactive injury response through any of these pathways would predispose to PAH.
These studies were done at a single time point and so cause and effect relationships between these elements are essentially a matter for speculation. However, the differences between those animals that had normal RVSP and those that had high RVSP leads us to the following hypothesis for pathogenesis.
Loss of proper tail domain function leads to alterations in actin organization pathways through LIMK and Tctex. These either directly lead to recruitment of circulating cells through alteration in adhesion factors or they lead to pulmonary vascular stiffening or constriction. In the latter case, this is insufficient to directly increase RVSP, but since shear stress is exquisitely sensitive to vessel diameter (25), even subtle changes in vessel diameter can radically alter shear stress and thus endothelial injury. Some combination of direct effects of BMPR2 mutation, signaling from the circulating cells, and direct endothelial injury result in increased proliferation. This proliferation results in blocked small vessels in the pulmonary vasculature (Fig. 7). When pruning is sufficiently advanced, RVSP starts to increase, leading to conventional muscularization of all pulmonary vessels. The combination of increased pressure and either the Bmpr2 mutation or the presence of inflammatory cells (Fig. 6) leads to the larger lesions; although these happen in secondary forms of human PAH, they are never seen purely as the result of increased pressure in mice. The fact that some of the concentric muscular lesions are CD133-positive suggests that they are least partially populated by recruitment of circulating progenitors.
At the start of this study, our goal was to find out which subset of the phenotype found in the earlier SM22-rtTA x TetO7-BMPR2delx4+ mouse model was due to loss of the tail domain and, by deduction, the loss of the kinase domain. The earlier model had increased RVSP likely due to defects in vasoreactivity and loss of complete smooth muscle differentiation (33, 36, 42). Although it also had defects in cytokine signaling (12, 33), these had no obvious impact on phenotype. The BMPR2delx4+ mouse looked like it was best considered a model of asymptomatic mutation carriers, who may have some baseline defects in vasoreactivity (11) but are in need of a second hit to develop disease.
The much more penetrant phenotype seen in the SM22-rtTA x TetO7-BMPR2R899X mice, which at least nominally have a less severe mutation, was thus somewhat surprising. There are two potential explanations for this. First, it may just be a dose effect; the BMPR2delx4+ mice downregulated their own promoter and had approximately threefold lower expression of transgene on average than BMPR2R899X mice (data not shown), which may have limited their phenotype. The more interesting hypothesis is that loss of proper tail domain function has a stronger phenotype when kinase domain function is preserved; this is suggested by the recent data from the model in which expression of a small interfering RNA to BMPR2 causes defects in pulmonary smooth muscle but not PAH (20).
This model system has several limitations. Although inducible overexpression of a dominantly acting mutant overcomes limitations of BMPR2 knockouts or constitutive mutations, it is not representative of the stoichiometry found in any human patient, which may alter the relative importance of different BMPR2 downstream pathways. It is also possible that the native BMPR2 R899X mutation found in patients is subject to exon skipping or nonsense mediated decay. Furthermore, the doxycycline that we use to induce the transgene itself inhibits matrix metalloproteinases and through these angiogenesis (9); inhibition of angiogenesis exacerbates phenotype in the rat models of PAH (34, 35). On the other hand, doxycycline has been proposed as a treatment for pulmonary diseases such as lymphangioleiomyomatosis (24) and Marfan syndrome (6). We have attempted to control for potential effects by administering doxycycline to control mice as well, but the effect of matrix may be over- or underrepresented in this model. Furthermore, limitation of the transgene to smooth muscle removes potentially important effects in endothelium, circulating cells, or other tissues. This model is thus best understood as one mechanism by which BMPR2 mutation can directly lead to PAH rather than the only mechanism.
In summary, this is the first report of an animal model demonstrating extensive pulmonary vascular structural changes resulting from Bmpr2 mutation in vivo. The power of this model is that it will now be possible to dissect the molecular etiology of the disease in detail with correlation of small molecule inhibitors to alleviation of specific subsets of the molecular, hemodynamic, and histological phenotype. This model should be extremely useful to the research community both in examining early molecular and physical events in the development of PAH and as a platform to validate potential treatments.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-072058, HL-071596, HL-079315, and HL-066328.
Supplementary Material
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Birkenfeld J, Betz H, Roth D. Identification of cofilin and LIM-domain-containing protein kinase 1 as novel interaction partners of 14-3-3 zeta. Biochem J 369: 45–54, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapados R, Abe K, Ihida-Stansbury K, McKean D, Gates AT, Kern M, Merklinger S, Elliott J, Plant A, Shimokawa H, Jones PL. ROCK controls matrix synthesis in vascular smooth muscle cells: coupling vasoconstriction to vascular remodeling. Circ Res 99: 837–844, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Chazova I, Loyd JE, Zhdanov VS, Newman JH, Belenkov Y, Meyrick B. Pulmonary artery adventitial changes and venous involvement in primary pulmonary hypertension. Am J Pathol 146: 389–397, 1995. [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors 22: 233–241, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Chung AW, Yang HH, Radomski MW, van Breemen C. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in Marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circ Res 102: e73–e85, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Dong J, Grunstein J, Tejada M, Peale F, Frantz G, Liang WC, Bai W, Yu L, Kowalski J, Liang X, Fuh G, Gerber HP, Ferrara N. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J 23: 2800–2810, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J 22: 358–363, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Fainaru O, Adini I, Benny O, Bazinet L, Pravda E, D'Amato R, Folkman J. Doxycycline induces membrane expression of VE-cadherin on endothelial cells and prevents vascular hyperpermeability. FASEB J. In press. [DOI] [PubMed]
- 10.Foletta VC, Lim MA, Soosairajah J, Kelly AP, Stanley EG, Shannon M, He W, Das S, Massague J, Bernard O, Soosairaiah J. Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1. J Cell Biol 162: 1089–1098, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Gene Ontology Consortium. The Gene Ontology (GO) project in 2006. Nucleic Acids Res 34: D322–D326, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grunig E, Janssen B, Mereles D, Barth U, Borst MM, Vogt IR, Fischer C, Olschewski H, Kuecherer HF, Kubler W. Abnormal pulmonary artery pressure response in asymptomatic carriers of primary pulmonary hypertension gene. Circulation 102: 1145–1150, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Hagen M, Fagan K, Steudel W, Carr M, Lane K, Rodman DM, West J. Interaction of interleukin-6 and the BMP pathway in pulmonary smooth muscle. Am J Physiol Lung Cell Mol Physiol 292: L1473–L1479, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Han C, Belenkaya TY, Khodoun M, Tauchi M, Lin X, Lin X. Distinct and collaborative roles of Drosophila EXT family proteins in morphogen signalling and gradient formation. Development 131: 1563–1575, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Herve P, Humbert M, Sitbon O, Parent F, Nunes H, Legal C, Garcia G, Simonneau G. Pathobiology of pulmonary hypertension. The role of platelets and thrombosis. Clin Chest Med 22: 451–458, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Ichikawa T, Suenaga Y, Koda T, Ozaki T, Nakagawara A. DeltaNp63/BMP-7-dependent expression of matrilin-2 is involved in keratinocyte migration in response to wounding. Biochem Biophys Res Commun 369: 994–1000, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Kim MJ, Park BJ, Kang YS, Kim HJ, Park JH, Kang JW, Lee SW, Han JM, Lee HW, Kim S. Downregulation of FUSE-binding protein and c-myc by tRNA synthetase cofactor p38 is required for lung cell differentiation. Nat Genet 34: 330–336, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA 3rd, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet 26: 81–84, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Lawrie A, Waterman E, Southwood M, Evans D, Suntharalingam J, Francis S, Crossman D, Croucher P, Morrell N, Newman C. Evidence of a role for osteoprotegerin in the pathogenesis of pulmonary arterial hypertension. Am J Pathol 172: 256–264, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci USA 98: 31–36, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu D, Wang J, Kinzel B, Mueller M, Mao X, Valdez R, Liu Y, Li E. Dosage-dependent requirement of BMP type II receptor for maintenance of vascular integrity. Blood 110: 1502–1510, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Machado RD, Pauciulo MW, Thomson JR, Lane KB, Morgan NV, Wheeler L, Phillips JA 3rd, Newman J, Williams D, Galiè N, Manes A, McNeil K, Yacoub M, Mikhail G, Rogers P, Corris P, Humbert M, Donnai D, Martensson G, Tranebjaerg L, Loyd JE, Trembath RC, Nichols WC. BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am J Hum Genet 68: 92–102, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machado RD, Rudarakanchana N, Atkinson C, Flanagan JA, Harrison R, Morrell NW, Trembath RC. Functional interaction between BMPR-II and Tctex-1, a light chain of Dynein, is isoform-specific and disrupted by mutations underlying primary pulmonary hypertension. Hum Mol Genet 12: 3277–3286, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Monteiro RM, de Sousa Lopes SM, Korchynskyi O, ten Dijke P, Mummery CL. Spatio-temporal activation of Smad1 and Smad5 in vivo: monitoring transcriptional activity of Smad proteins. J Cell Sci 117: 4653–4663, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Moses MA, Harper J, Folkman J. Doxycycline treatment for lymphangioleiomyomatosis with urinary monitoring for MMPs. N Engl J Med 354: 2621–2622, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Murray CD The physiological principle of ninimum work: I. The vascular system and the cost of blood volume. Proc Natl Acad Sci USA 12: 207–214, 1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishihara A, Watabe T, Imamura T, Miyazono K. Functional heterogeneity of bone morphogenetic protein receptor-II mutants found in patients with primary pulmonary hypertension. Mol Biol Cell 13: 3055–3063, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudarakanchana N, Flanagan JA, Chen H, Upton PD, Machado R, Patel D, Trembath RC, Morrell NW. Functional analysis of bone morphogenetic protein type II receptor mutations underlying primary pulmonary hypertension. Hum Mol Genet 11: 1517–1525, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Ryter SW, Choi AM. Heme oxygenase-1: redox regulation of a stress protein in lung and cell culture models. Antioxid Redox Signal 7: 80–91, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol 25: 1767–1775, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Sil P, Gupta S, Young D, Sen S. Regulation of myotrophin gene by pressure overload and stretch. Mol Cell Biochem 262: 79–89, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Singleton PA, Salgia R, Moreno-Vinasco L, Moitra J, Sammani S, Mirzapoiazova T, Garcia JG. CD44 regulates hepatocyte growth factor-mediated vascular integrity. Role of c-Met, Tiam1/Rac1, dynamin 2, and cortactin. J Biol Chem 282: 30643–30657, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Song Y, Coleman L, Shi J, Beppu H, Sato K, Walsh K, Loscalzo J, Zhang YY. Inflammation, endothelial injury, and persistent pulmonary hypertension in heterozygous BMPR2-mutant mice. Am J Physiol Heart Circ Physiol 295: H677–H690, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tada Y, Majka S, Carr M, Harral J, Crona D, Kuriyama T, West J. Molecular effects of loss of BMPR2 signaling in smooth muscle in a transgenic mouse model of PAH. Am J Physiol Lung Cell Mol Physiol 292: L1556–L1563, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 15: 427–438, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Vieillard-Baron A, Frisdal E, Eddahibi S, Deprez I, Baker AH, Newby AC, Berger P, Levame M, Raffestin B, Adnot S, d'Ortho MP. Inhibition of matrix metalloproteinases by lung TIMP-1 gene transfer or doxycycline aggravates pulmonary hypertension in rats. Circ Res 87: 418–425, 2000. [DOI] [PubMed] [Google Scholar]
- 36.West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, Hoedt-Miller M, Tada Y, Ozimek J, Tuder R, Rodman DM. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res 94: 1109–1114, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Wong WK, Knowles JA, Morse JH. Bone morphogenetic protein receptor type II C-terminus interacts with c-Src: implication for a role in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 33: 438–446, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World CJ, Yamawaki H, Berk BC. Thioredoxin in the cardiovascular system. J Mol Med 84: 997–1003, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Yang X, Lee PJ, Long L, Trembath RC, Morrell NW. BMP4 induces HO-1 via a Smad-independent, p38MAPK-dependent pathway in pulmonary artery myocytes. Am J Respir Cell Mol Biol 37: 598–605, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Yang X, Long L, Southwood M, Rudarakanchana N, Upton PD, Jeffery TK, Atkinson C, Chen H, Trembath RC, Morrell NW. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res 96: 1053–1063, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Yoshioka J, Imahashi K, Gabel SA, Chutkow WA, Burds AA, Gannon J, Schulze PC, MacGillivray C, London RE, Murphy E, Lee RT. Targeted deletion of thioredoxin-interacting protein regulates cardiac dysfunction in response to pressure overload. Circ Res 101: 1328–1338, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Young KA, Ivester C, West J, Carr M, Rodman DM. BMP signaling controls PASMC KV channel expression in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol 290: L841–L848, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Zaheer A, Zaheer S, Sahu SK, Yang B, Lim R. Reduced severity of experimental autoimmune encephalomyelitis in GMF-deficient mice. Neurochem Res 32: 39–47, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Zakrzewicz A, Hecker M, Marsh LM, Kwapiszewska G, Nejman B, Long L, Seeger W, Schermuly RT, Morrell NW, Morty RE, Eickelberg O. Receptor for activated C-kinase 1, a novel interaction partner of type II bone morphogenetic protein receptor, regulates smooth muscle cell proliferation in pulmonary arterial hypertension. Circulation 115: 2957–2968, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Zhong S, Li C, Wong WH. ChipInfo: software for extracting gene annotation and gene ontology information for microarray analysis. Nucleic Acids Res 31: 3483–3486, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.