Abstract
Mechanical ventilation can overdistend the lungs or generate shear forces in them during repetitive opening/closing, contributing to lung injury and inflammation in patients with acute respiratory distress syndrome (ARDS). Repair of the injured lung epithelium is important for restoring normal barrier and lung function. In the current study, we investigated the effects of cyclic mechanical strain (CS), constant distention strain (CD), and simulated positive end-expiratory pressure (PEEP) on activation of Rac1 and wound closure of rat primary alveolar type 2 (AT2) cells. Cyclic stretch inhibited the migration of wounded AT2 cells in a dose-dependent manner with no inhibition occurring with 5% CS, but significant inhibition with 10% and 15% CS. PEEP conditions were investigated by stretching AT2 cells to 15% maximum strain (at a frequency of 10 cycles/min) with relaxation to 10% strain. AT2 cells were also exposed to 20% CD. All three types of mechanical strain inhibited wound closure of AT2 cells compared with static controls. Since lamellipodial extensions in migrating cells at the wound edge were significantly smaller in stretched cells, we measured Rac1 activity and found it to be decreased in stretched cells. We also demonstrate that Tiam1, a Rac1-specific guanine nucleotide exchange factor, was expressed mainly in the cytosol of AT2 cells exposed to mechanical strain compared with membrane localization in static cells. Downregulation of Tiam1 with 100 μM NSC-23766 inhibited activation of Rac1 and migration of AT2 cells, suggesting its involvement in repair mechanisms of AT2 cells subjected to mechanical strain.
Keywords: cyclic strain, mechanotransduction, guanine nucleotide exchange factor, GTP-Rac1
mechanical ventilation is the main supportive therapy for acute respiratory distress syndrome (ARDS) patients. Reduction in tidal volume from 12 ml/kg to 6 ml/kg reduced the mortality rate of ARDS patients (1) in a randomized clinical trial. Patients are now ventilated using a lung-protective strategy, low tidal volume (Vt), coupled with the use of positive end-expiratory pressure (PEEP). The use of lower tidal volumes is thought to reduce damage to the epithelial cells caused by overdistention of air-filled alveoli, whereas PEEP is thought to prevent damage caused by repeated collapse and reopening of alveolar units (21, 38, 39). Both high tidal volume mechanical ventilation and the absence of PEEP in injury models have been shown to induce epithelial injury in animals (13, 17, 18, 44, 59).
Epithelial damage is one of the hallmark features of acute lung injury, and recovery is dependent on repair of the epithelial barrier. We and others have examined factors involved in in vitro alveolar epithelial repair mechanisms including serum and transforming growth factor-α (32), soluble fibronectin (20), interleukin-1β (22), keratinocyte growth factor (2), vascular endothelial growth factor (51), β-agonists (47), and focal adhesion kinase and RhoA (13). However, few studies have examined how changes in the levels of mechanical forces may impact epithelial injury and repair. In vitro models using alveolar type 2 (AT2) epithelial cells have demonstrated the effects of mechanical stretch on changes in tight junctions (4), loss of epithelial barrier permeability (3), stretch-induced cell injury and death (3, 61), plasma membrane wounding and resealing (63, 64), the release of inflammatory cytokines (28), and reactive oxygen species production (6). Other studies have simulated epithelial injury caused by collapse and reopening using a bubble propagation model that leads to epithelial injury (27, 31). While many studies have focused on mechanical stress-induced injury, few studies have examined how increased mechanical stresses might alter epithelial repair mechanisms. We previously demonstrated that cyclic mechanical stretch inhibited repair of wounded bronchial epithelial cells (53), but no previous studies have examined the effect of mechanical stretch on wound healing of alveolar epithelial cells. Furthermore, the signaling pathways important in these repair processes that are affected by mechanical stretch have not been clearly defined.
Epithelial wound healing involves cell spreading, migration, and proliferation, but proliferation usually occurs in the latter stages of repair. Migration involves spreading of cells at the wound edge, where the cells extend into thin, sheet-like processes known as lamellipodia. These lamellipodia are regulated by the small GTPase Rac1 in response to a variety of stimuli, including growth factors and extracellular matrix (49, 55). Rho-GTPases function as molecular switches, cycling between an active GTP-bound and an inactive GDP-bound state. This cycling is regulated by guanine nucleotide exchange factors (GEFs), which facilitate exchange of GDP for GTP. The Rac-specific GEF, T-lymphoma invasion and metastasis 1 (Tiam1), has been demonstrated to regulate migration (25, 29, 50), cell-matrix adhesion (25, 41), and cell-cell adhesion (33, 37) by modulating the actin cytoskeleton (9, 37, 60) through Rac1. A previous study demonstrated that equibiaxial stretch caused decreased Rac1 activity in rat aortic smooth muscle cells, NIH/3T3 cells, and mouse embryonic fibroblasts (29). In another study, Kawamura et al. (30) found that activation of Rac1 localized in the caveolar compartment in neonatal rat cardiomyocytes was essential for sensing externally applied force and transducing this signal to the actin cytoskeleton. We hypothesized that mechanical stretch decreases alveolar epithelial repair through mechanisms involving downregulation of Tiam1/Rac1 signaling. In the current study, we used an in vitro model of cyclic mechanical stretch, constant distention, and simulated PEEP conditions to investigate AT2 repair processes and the roles of Tiam1 and Rac1.
MATERIALS AND METHODS
Reagents.
DMEM, penicillin-streptomycin, trypsin-EDTA solution, and PBS were purchased from GIBCO Life Technologies (Grand Island, NY). FBS obtained from Hyclone (Logan, UT) was heat-inactivated at 56°C for 30 min and used in the medium. HEPES, KCl, MgCl2, Triton X-100, SDS, sodium vanadate, PMSF, aprotinin, and leupeptin were purchased from Sigma (St. Louis, MO). Tween 20 was from Bio-Rad (Hercules, CA). NSC-23766, a specific inhibitor of Tiam1, was a gift from Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute. Rhodamine-phalloidin, DAPI, and SlowFade were obtained from Molecular Probes (Eugene, OR).
Cell culture.
Primary rat AT2 cells were isolated according to the methods described previously (13, 14). The animal use protocol was approved by the Institutional Animal Care and Use Committee. Briefly, AT2 cells were isolated from male Sprague-Dawley rats by elastase digestion and differential adherence on IgG-coated dishes. AT2 cells were identified using Nile Red (Sigma) staining of lamellar bodies, and >90% of the cells were Nile Red-positive on day 2. We used Bioflex plates coated with a rat lung fibroblast matrix deposited by RLF-6 cells (American Type Culture Collection) for stretching AT2 cells (13). Freshly isolated cells were seeded to confluence at 3.5 × 106 cells/well in AT2 culture medium (DMEM with 10% heat-inactivated FBS, 4 mM glutamine, 1% penicillin/streptomycin, and 0.25 μM amphotericin B), and experiments were performed on day 2 after isolation. A549 cells were cultured on plastic six-well plates in DMEM with 10% FBS.
Application of mechanical strain.
Strain was applied using a Flexercell FX-4000T tension unit (Flexcell International, Hillsborough, NC), a vacuum-driven device that applies biaxial strain to cells cultured on Silastic membranes. AT2 cells were cyclically stretched (CS) using strain levels of 5%, 10%, and 15% linear elongation at frequencies of 10–30 cycles/min (cpm). We investigated PEEP conditions by subjecting AT2 monolayers to a maximum strain of 15% with partial relaxation to 10% strain at a frequency of 10 cpm (10–15% CS). In addition, we exposed AT2 cells to 20% constant distention (CD; 0% relaxation). Preliminary studies demonstrated that AT2 cells did not remain adherent with cyclic stretch above 15% strain or with a frequency of 30 cpm with 15% strain, and these conditions were not subsequently used.
Lamellipodial width measurements.
AT2 cell monolayers were wounded and then treated with or without stretch for 16 h. After treatment, monolayers were fixed and stained for F-actin with 20% rhodamine-phalloidin (RP) solution for 30 min (12). Wells were rinsed with 2 ml of Dulbecco's phosphate-buffered saline (DPBS) and aspirated, and the membrane was cut out and placed cell-side down on a square microscope glass coverslip (Fisher Scientific, Pittsburgh, PA) with the wound perpendicular to one of the axes. Images of the RP staining (at least 12 images/well) at the wound edge of the membrane were acquired using Metamorph Imaging software (version 4.6; Universal Imaging, West Chester, PA). Lamellipodial width (μm) was calculated as lamellipodial area divided by wound edge length along the perimeter.
Cell migration.
To measure cell migration, confluent monolayers of AT2 or A549 cells were wounded by scraping a pipette tip across the monolayer to produce initial wounds of ∼1,100 μm. Cells were washed with DPBS, medium containing 10% serum with or without NSC-23766 was added, and cells were immediately exposed to static or stretch conditions. Images were collected with a Cool Snap cooled charge-coupled device camera (Roper Scientific, Trenton, NJ) mounted on an Eclipse TE300 inverted microscope with a ×4 phase contrast objective (Nikon, Melville, NY) using Metamorph imaging software. Images were obtained at the initial time of wounding and then at various times 24 h postwounding, and data were analyzed as described previously (12, 13). Metamorph software was used to record the coordinates for each wound location using a computer-controlled stage so that the same location was used for each measurement. AT2 cell proliferation is minimal in culture during this time (52). All results reported are from at least three independent wells from three separate experiments (n = 9).
Determination of activated Rac1.
Rac1 activity assays were performed according to the manufacturer's instructions (Upstate Cell Signaling Solutions, Lake Placid, NY). AT2 cells subjected to sham conditions or mechanical strain (3 wells pooled per measurement) were washed with ice-cold Tris-buffered saline (TBS) and lysed using 400 μl of lysis buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 10 mM MgCl2, 1 mM EDTA, 2% glycerol, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM sodium vanadate). Cell lysates were centrifuged at 14,000 g at 4°C for 10 min, and an equal volume of the lysates was incubated with glutathione-S-transferase-p21-activated kinase for Rac1 at 4°C for 45 min. The beads were pelleted by brief centrifugation (10 s, 14,000 g, 4°C) and washed three times with the lysis buffer. GTP-bound Rac1 was solubilized in Laemmli sample buffer and detected by Western blot analysis as described below, using anti-Rac1 (1:200; Santa Cruz Biotechnology). Blots were then incubated with IgG-horseradish peroxidase anti-mouse (MP Biomedicals, Aurora, OH) at a 1:1,000 dilution for 1 h at room temperature (RT).
Cytosolic and membrane fraction preparations.
AT2 monolayers subjected to sham conditions or mechanical strain were washed with cold DPBS. Cells (6 wells pooled/measurement) were lysed with buffer containing 10 mM Tris, pH 7.4, 1.5 mM MgCl2, 5 mM KCl, 0.2 mM sodium vanadate, 1 mM PMSF, 1 μg/ml aprotinin, and 1 μg/ml leupeptin and then centrifuged at 14,000 rpm at 4°C for 10 min; the cell pellet was discarded. Aliquots of the supernatant were collected as “total” protein fraction and were subjected to ultracentrifugation at 100,000 g at 4°C for 45 min. The supernatant was collected as the “cytosolic” fraction, and the pellet was redissolved in the lysis buffer, sonicated briefly on ice, and collected as the “membrane” fraction. Protein concentrations of the total, cytosolic, and membrane fractions were determined by the bicinchoninic acid (BCA) protein assay and were subjected to SDS-PAGE and immunoblot analysis as described below, using anti-Tiam1 (1:200; Santa Cruz Biotechnology). Blots were then incubated with IgG-horseradish peroxidase anti-rabbit (Amersham, Piscataway, NJ) at a 1:1,000 dilution for 1 h at RT.
SDS-PAGE.
Protein concentration was determined by the BCA protein assay method, resolved by 4–12% SDS-PAGE, and electrophoretically transferred onto nitrocellulose membranes. Membranes were blocked 1 h in 5% TBS containing 0.01% Tween 20 (TBST) and incubated overnight at 4°C in 1% TBST with the appropriate primary antibody. Membranes were washed with TBST and then incubated with the secondary antibody for 1 h at RT. Immunoreactive proteins were detected with Supersignal West Dura Extended chemiluminescent substrate (Pierce Biotechnology, Rockford, IL). Images of blots were acquired using the MultiImager chemiluminescence imaging system (Bio-Rad Fluor-S; Bio-Rad, Hercules, CA) and quantitated using Bio-Rad Quantity One imaging software.
Statistical analysis.
All values are presented as means ± SE. Statistical analysis was performed with the SigmaStat statistical package (version 3.5; Jandel Scientific, San Rafael, CA). A t-test was used when only two groups were compared, and one-way ANOVA with the Holm-Sidak method was performed for comparisons of multiple treatments to determine significant differences between individual conditions. Significant differences were determined based on a threshold of P < 0.05.
RESULTS
Mechanical strain inhibited migration of alveolar epithelial cells.
We investigated migration of alveolar epithelial cells by wounding confluent monolayers of rat AT2 cells cultured on an RLF-6 matrix and exposing them to varying levels of CS. As shown in Fig. 1A, cells under static conditions migrated to 52.9% of the original wound after 24 h. Cells exposed to 5% CS at a frequency of 30 cpm (1 s of stretch and 1 s of relaxation) were unaffected by mechanical stretch, whereas cells stretched at 10% CS (30 cpm) migrated significantly slower than static cells (73.2% of original wound width remaining after 24 h). When cells were stretched with 15% CS and 10 cpm (3 s of stretch and 3 s of relaxation), 82.7% of the original wound width remained after 24 h. Increased levels of CS and higher frequencies with 15% CS caused significant cell detachment from the membranes, and these conditions were not examined further.
Fig. 1.
Mechanical strain inhibited migration of alveolar type 2 (AT2) cells. AT2 cells were plated (3.5 × 106 cells/well) on 6-well Flexercell plates coated with RLF-6 matrix. Confluent monolayers were wounded with a pipette tip, cells were exposed to either static or stretch conditions immediately, and wound closure was monitored for 24 h. A: cyclic strain (CS) at 0–5% (30 cpm), 0–10% (30 cpm), 0–15% (10 cpm) compared with static cells. B: static cells compared with cells stretched with 20% constant distention (CD) or conditions simulating PEEP (10–15% CS, 10 cpm). #P < 0.05 vs. static control; measurements were made in at least 3 wells from 3 different isolations (n = 9).
To mimic PEEP conditions, we used a model in which we stretched AT2 cells at 15% CS at a frequency of 10 cpm (3 s of stretch and 3 s of relaxation) and allowed relaxation to only 10% strain (10–15% CS). In addition, we investigated the effect of 20% CD on migration of AT2 cells to simulate conditions in which no relaxation occurs. After 24 h, the wound widths were 54.4%, 82.7%, 68.5%, and 66.7% of the original wound width for static, 0–15% CS, 10–15% CS, and CD, respectively. Cell migration was significantly inhibited by 0–15% CS, 20% CD, and by conditions simulating PEEP (10–15% CS, Fig. 1B). In addition, the inhibition due to 0–15% CS was significantly greater than the inhibition due to CD or 10–15% CS (PEEP).
Mechanical strain inhibited lamellipodial extensions.
Since the early stages of wound healing involve cell spreading and migration of cells, we investigated the effect of mechanical strain on lamellipodial formation at the wound edge. Figure 2A shows F-actin staining in cells migrating at the wound edge after 16 h and indicates the approach for measurement of lamellipodial width. As shown in Fig. 2B, CS (0–15%) for 16 h caused a significant reduction in lamellipodial width compared with unstretched cells.
Fig. 2.
Mechanical strain inhibited lamellipodial extensions in migrating AT2 cells. Confluent monolayers were wounded, subjected to 0–15% CS (10 cpm) for 16 h, and fixed and stained with rhodamine-phalloidin for F-actin. Images were acquired using Z series, and lamellipodial extensions at the wound edge were traced and quantitated using Metamorph software. A: low-magnification images of cells at the wound edge as well as higher-magnification views of the indicated regions. Lamellipodial width (μm) was calculated as lamellipodial area/wound edge length as indicated (A, bottom). B: the measured lamellipodial width for static and 0–15% CS cells. Data are expressed as means ± SE of 3 independent experiments. #P < 0.05 vs. static control.
Rac1 activity is inhibited by mechanical strain.
Since lamellipodial extensions are regulated by Rac1, we investigated whether the stretch-induced inhibition of lamellipodial extensions was due to decreased Rac1 activity. Figure 3A shows that Rac1 activity was significantly decreased in AT2 cells subjected to 2 h of 0–15% CS, and the inhibition remained after 12 h of CS. Figure 3, B and C, indicate that 10–15% CS (PEEP) and CD conditions also significantly decreased Rac1 activity after 2 and 12 h.
Fig. 3.
Mechanical strain inhibited Rac1 activation. Confluent monolayers of AT2 cells on RLF matrix were exposed to mechanical strain for 2 and 12 h. GTP-Rac1 levels in AT2 cells exposed to static conditions, cyclic strain (0–15% CS) (A) and 10–15% CS (PEEP) conditions (B), and constant distention strain (CD) (C) were determined via a pull-down assay; a representative blot is shown at the top of each panel. The bar graphs summarize the data expressed as means ± SE, from 4 independent experiments, the values of which are expressed as the ratio of active GTP-bound Rac1 to total Rac1. #P < 0.05 vs. static controls at 2 and 12 h.
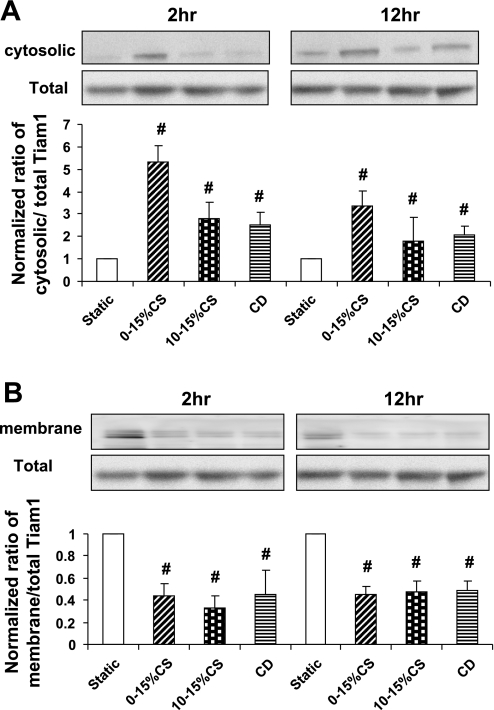
Mechanical strain caused translocation of Tiam1 to the cytosol.
Tiam1, a GDP-GTP exchange factor specific for Rac-GTPase, contributes to the cytoskeletal reorganization required during cell migration. To determine whether the stretch- induced decrease in Rac1 activity was due to changes in Tiam1 localization, we quantified levels of Tiam1 expression in total, cytosolic, and membrane fractions of AT2 cells subjected to mechanical strain. Tiam1 localization to the membrane has previously been demonstrated to increase Rac activity (43). Figure 4 demonstrates that total Tiam1 levels remained unaltered following exposure to each type of mechanical strain, but mechanical strain induced cytosolic localization (Fig. 4A) of Tiam1 compared with membrane localization (Fig. 4B) seen in static controls. The increased cytosolic localization was significant after both 2 and 12 h of mechanical strain.
Fig. 4.
Mechanical strain caused translocation of Tiam1 from membrane to the cytosol. Confluent monolayers of AT2 cells on RLF-6 matrix were exposed to mechanical strain for 2 and 12 h. Cells were lysed, aliquots of total fraction withdrawn, and cell lysates further subjected to high-speed centrifugation to prepare the cytosolic and membrane fractions. The total, cytosolic (A), and membrane (B) levels of Tiam1 were assessed by Western blotting. The blots are representative of 3 independent experiments from separate isolations, and the bar graphs summarize the densitometry data expressed as means ± SE; n = 3 independent experiments. #P < 0.05 vs. static controls at 2 and 12 h.
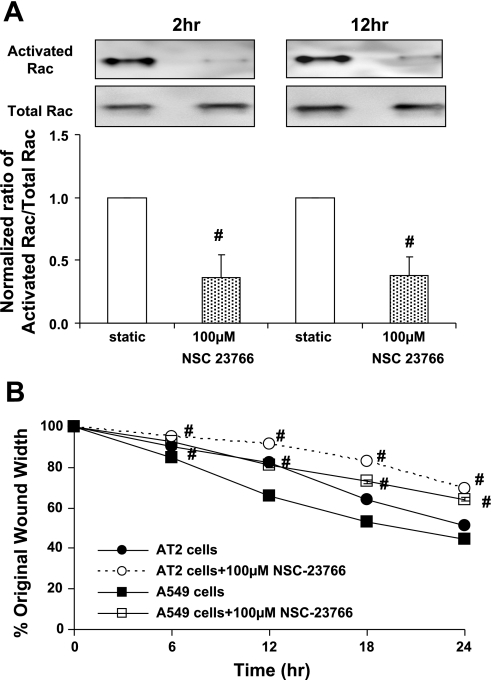
Inhibition of Tiam1 downregulated Rac1 activity and inhibited migration of A549 and AT2 cells.
To determine whether Tiam1 activity was necessary for Rac1-mediated cell migration, we used a specific inhibitor of Tiam1, NSC-23766. We observed a concentration-dependent inhibition of GTP-Rac1 in AT2 cells treated for 12 h with NSC-23766 with maximal inhibition with 100 μM NSC-23766 (data not shown). AT2 cells treated with 100 μM NSC-23766 showed significant inhibition of activated Rac1 after 2 and 12 h (Fig. 5A). In addition, we observed that inhibition with 100 μM NSC-23766 caused delayed wound closure in both A549 and AT2 cells (Fig. 5B). These results are consistent with our data demonstrating that downregulation of activated Rac1 leads to inhibition of migration of AT2 cells exposed to mechanical strain.
Fig. 5.
Inhibition of Tiam1 downregulated both Rac1 activity and migration of AT2 and A549 cells. Confluent monolayers of AT2 cells were treated with the Tiam1 inhibitor NSC-23766. A: GTP-Rac1 levels in AT2 cells treated with 100 μM NSC-23766 for 2 and 12 h. Representative Western blot was probed for activated Rac1 and total Rac1 levels. B: wound closure of AT2 and A549 cells treated with 100 μM NSC-23766. Data are expressed as means ± SE of 4 independent experiments. #P < 0.05 vs. untreated corresponding condition.
DISCUSSION
Ventilator-induced lung injury (VILI) is known to significantly contribute to the high mortality associated with ARDS (1, 65). Mechanical ventilation with lung-protective strategies involving low tidal volumes and the use of PEEP is the recommended approach for patients with ARDS (36). ARDS results in heterogeneous lung injury, with areas of acutely injured stiff and noncompliant lung regions adjacent to areas of normal lung compliance. Consequently, the pressures required to inflate the injured lung areas are much higher than those needed to inflate the healthy or more compliant regions. This results in shunting of excessive tidal volume into the healthy lung, causing injury by either alveolar overdistention (10, 15, 16) or recruitment/derecruitment (8, 58, 59). In a recent study using an intact rat model, Sinclair et al. (56) measured deformation of small airways in vivo during mechanical ventilation in the presence of PEEP and found that mechanical ventilation results in significant airway mechanical strain that is heterogeneously distributed in the uninjured lung. Animals ventilated with PEEP exhibited higher overall mechanical strain, but the changes in strain during a breath were smaller compared with animals ventilated without PEEP.
Although numerous studies have demonstrated the role of mechanical forces in causing injury to the lung epithelium, few studies have examined the role of mechanical forces in the repair processes. The rapid regeneration of a continuous alveolar epithelium in VILI is critical for restoring normal lung function and occurs on a substrate undergoing cyclic deformation. Since the effect of mechanical strain on the repair mechanisms of alveolar epithelial wounds was not previously demonstrated, we investigated wound healing of AT2 cells subjected to different types of mechanical strain. We found that low levels of cyclic stretch, 5%, had no effect on cell migration, but levels of 10% and above caused significant inhibition (Fig. 1). In preliminary studies, we found that AT2 cells grown on collagen- or fibronectin-coated Silastic membranes (collagen I plates as provided by Flexcell and coated with human plasma fibronectin) rapidly dissociated from the membrane when stretched cyclically at levels above 10%. To overcome this limitation, we first cultured rat lung fibroblasts on the membranes and then removed the fibroblasts and cultured the AT2 cells. Using this approach, we were able to stretch cells up to 15% without cell detachment. Cavanaugh and Margulies (3) previously reported that AT2 cells grown on matrix deposited by day 5 cultures of AT2 cells tolerated higher levels of mechanical stretch. A limitation of these approaches, however, is that the matrix deposited by the RLF-6 or AT2 cells is undefined. We attempted to minimize potential variation in matrix composition by controlling passage number, seeding densities, and days in culture. Another variable in our experiments was the frequency of stretch. Increasing the amplitude of stretch from 5% to 10% caused a significant inhibition of cell migration when the frequency was kept constant at 30 cycles/min (Fig. 1). When we increased the amplitude to 15% stretch with 30 cycles/min, the morphology of the AT2 cells changed, and they detached from the membrane. Therefore, we could not accurately measure wound healing with the higher levels of stretch and the higher frequency (0–15% and 30 cycles/min). In previous studies with bronchial epithelial cells, we showed that higher frequencies of stretch (or more accurately, shorter intervals of relaxation in a cycle) inhibited cell migration to a greater extent than lower frequencies (53). We also found in the current study that higher levels of stretch (20%) with 10 cycles/min caused cell detachment, and wound closure could not be measured. Thus, we restricted the reported results to those conditions that did not cause cell detachment. While others have reported that AT2 cell proliferation does not occur until after 3 days in culture (52), we did not measure proliferation in our studies, and thus we cannot rule out the possibility that stretch also inhibited proliferation.
Few studies have directly measured the deformation of alveolar epithelial cells in an intact alveolus to define the levels of mechanical stain. Perlman and Bhattacharya (48) recently measured the deformation of alveolar segments in surface alveoli of isolated rat lungs. They found that inflation from an alveolar pressure of 5 cmH2O to 20 cmH2O (considered to be hyperinflation) resulted in changes in alveolar segment lengths averaging between 10 and 20%, although there was substantial heterogeneity. Steinberg et al. (57) also demonstrated significant alveolar deformation in surfactant-deactivated lungs. Our findings suggest that low levels of cyclic stretch (≤5%) would have little effect on epithelial cell migration, but that higher levels of inflation (≥10%) would lead to inhibition of cell migration. We simulated PEEP using an in vitro model of 15% maximum strain at a frequency of 10 cpm with relaxation to 10% (10–15% CS) and compared it to 15% maximum strain at a frequency of 10 cpm (0–15% CS) and CD (20%). We also found that 15% CD caused inhibition of cell migration (data not shown). Our data demonstrate that PEEP and CD had less inhibitory effects than CS on the migration of AT2 cells (Fig. 1B). However, inhibition of Rac activity was similar under all strain conditions. We speculate that this may be due to differential effects on other factors important in wound closure such as RhoA activation and remodeling of focal adhesions. Ventilation strategies involving PEEP (10–15% CS) and CD, compared with 0–15% CS, may be beneficial in terms of accelerated migration of AT2 cells. These results are consistent with our previous study demonstrating that CS at 20% maximum strain with a frequency of 30 cpm inhibited migration of human and cat airway epithelial cells (53).
Migration involves reorganization of the actin cytoskeleton and requires Rho-GTPases. RhoA activation induces cell contractility, whereas Rac1 promotes cell spreading. The reciprocal balance between these GTPases determines both cell morphology and migration. In our previous study, we demonstrated that both RhoA and Rac are required for efficient migration of human bronchial epithelial cells (12). We also recently demonstrated that RhoA activity was significantly increased in AT2 cells isolated from rats ventilated with a high tidal volume (25 ml/kg) and that cell migration was significantly decreased in AT2 cells expressing a constitutively active from of RhoA (13). We have not yet examined Rac1 activity in vivo. In support of our current findings involving lamellipodial extensions in the current study (Fig. 2), it has been reported that NIH/3T3 cells plated on flexible substrates exert less tension and also show increased rates of lamellipodial activity and motility compared with cells on rigid substrates (11, 46). In another study, adhesion of fibroblasts to surfaces coated with high concentrations of fibronectin inhibited Rac1 activation and decreased migration, compared with moderate coating densities (11). Mechanical stretch has previously been shown to increase Rac1 activity in a mouse myoblastic cell line (C2C12) (34) and in the intestinal epithelial cell line CaCo-2 (7), whereas others have demonstrated a stretch-induced decrease in Rac1 activity in rat aortic smooth muscle cells (29) and in rat gastric mucosal cells RGM1 (45). However, stretch-induced effects on Rac1 signaling have not previously been demonstrated in AT2 cells. Because we measured a significant decrease in AT2 cell migration in cells exposed to cyclic stretch (Fig. 1), we hypothesized that mechanical strain caused decreased Rac1 activation that led to inhibition of lamellipodial spreading (Fig. 2). We observed decreased Rac1 activity in cells exposed to CS, PEEP, and CD conditions after both 2 and 12 h (Fig. 3), consistent with our measurements of inhibition of wound healing. A limitation of our approach was that our measurements of Rac1 activity were made in unwounded cells exposed to cyclic stretch, whereas our measurements of wound healing were made in monolayers with a single wound exposed to stretch. It would be impractical to measure Rac1 activity in a monolayer with a single wound because there would not be a sufficient percentage of the cells near the wound edge to detect a change in activity. In addition, when we applied multiple wounds to the monolayer and subjected the cells to cyclic stretch, there was a loss of the epithelial sheet integrity, cell-cell contacts were lost, and cell rounding occurred. This did not occur when stretch was applied to monolayers with a single wound. Therefore, we do not believe that monolayers stretched with multiple wounds can be reliably compared.
Rho-GTPases cycle between the active GTP-bound state and the inactive GDP-bound state (24, 62). The Rho proteins become activated through interaction with a class of positive regulators, the Dbl family guanine nucleotide exchange factors, which facilitate GTP binding to Rho GTPases (5, 54, 67). It has been demonstrated that the Rac1-specific GEF Tiam1 (T-lymphoma invasion and metastasis 1) regulates migration, cell-matrix adhesion, and cell-cell adhesion by modulating the actin cytoskeleton through the Rac-GTPase (23, 26, 40, 60). Therefore, in the present study, we examined the specific role of Tiam1 in the migration of mechanically stretched AT2 cells. Our results demonstrated that Tiam1 is translocated from the membrane to the cytosolic fraction of mechanically stretched cells (Fig. 4). This translocation of Tiam1 correlated with reduced Rac1 activity, decreased lamellipodial extensions, and inhibition of cell migration. Since Tiam1 localization to the cytosol is known to decrease Rac1 activity, our results suggest, but do not definitively prove, that stretch-induced inhibition of cell migration was in part caused by Tiam1 translocation. To support our hypothesis, we treated cells with the Tiam1 inhibitor NSC-23766. NSC-23766 is a small-molecule inhibitor of Rac-GTPase targeting Rac1 activation by GEF. It was identified by applying a structure-based virtual screening approach to search for Rac-GEF interaction-specific small-molecule inhibitors (19). When we blocked Rac1 activation using the Tiam1 inhibitor, migration of both AT2 cells and A549 cells was significantly inhibited. Recent studies have demonstrated that increased Tiam1 expression correlates with increased cell migration in keratinocytes, endothelial cells, breast carcinoma cells, and Schwann cells (25, 35, 42, 66). Moissoglu et al. (43) investigated in vivo dynamics of Rac1 utilizing a photobleaching method and found that overexpression of Tiam1 decreased the dissociation rate constant k (off) of membrane-bound Rac, most likely by converting membrane-bound GDP-Rac to GTP-Rac. In the current study, we demonstrated the novel findings that 1) Tiam1 regulated Rac1 activity in alveolar epithelial cell migration, and 2) mechanical stretch induced translocation of Tiam1 from membrane to cytosol.
Our study demonstrates a previously unrecognized role of Tiam1 in the migration of AT2 cells exposed to mechanical strain. We observed that cytosolic localization of Tiam1 leads to downregulation of Rac1 activity, decreased lamellipodial extensions, and inhibition of migration of alveolar epithelial cells exposed to mechanical strain. Identification of the molecular mechanisms regulating wound closure of AT2 cells will help in the development of ventilatory and pharmacological therapeutic strategies aimed at preventing the deleterious effects of mechanical ventilation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-064981 (C. M. Waters).
Acknowledgments
We thank Scott E. Sinclair for helpful discussions and Charlean Luellen for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Atabai K, Ishigaki M, Geiser T, Ueki I, Matthay MA, Ware LB. Keratinocyte growth factor can enhance alveolar epithelial repair by nonmitogenic mechanisms. Am J Physiol Lung Cell Mol Physiol 283: L163–L169, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Cavanaugh KJ, Margulies SS. Measurement of stretch-induced loss of alveolar epithelial barrier integrity with a novel in vitro method. Am J Physiol Cell Physiol 283: C1801–C1808, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Cavanaugh KJ, Oswari J, Margulies SS. Role of stretch on tight junction structure in alveolar epithelial cells. Am J Respir Cell Mol Biol 25: 584–591, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Cerione RA, Zheng Y. The Dbl family of oncogenes. Curr Opin Cell Biol 8: 216–222, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Chapman KE, Sinclair SE, Zhuang D, Hassid A, Desai LP, Waters CM. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol 289: L834–L841, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Chaturvedi LS, Marsh HM, Shang X, Zheng Y, Basson MD. Repetitive deformation activates focal adhesion kinase and ERK mitogenic signals in human Caco-2 intestinal epithelial cells through Src and Rac1. J Biol Chem 282: 14–28, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Cheng KC, Zhang H, Lin CY, Slutsky AS. Ventilation with negative airway pressure induces a cytokine response in isolated mouse lung. Anesth Analg 94: 1577–1582, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Connolly BA, Rice J, Feig LA, Buchsbaum RJ. Tiam1-IRSp53 complex formation directs specificity of rac-mediated actin cytoskeleton regulation. Mol Cell Biol 25: 4602–4614, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbridge TC, Wood LD, Crawford GP, Chudoba MJ, Yanos J, Sznajder JI. Adverse effects of large tidal volume and low PEEP in canine acid aspiration. Am Rev Respir Dis 142: 311–315, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Cox EA, Sastry SK, Huttenlocher A. Integrin-mediated adhesion regulates cell polarity and membrane protrusion through the Rho family of GTPases. Mol Biol Cell 12: 265–277, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai LP, Aryal AM, Ceacareanu B, Hassid A, Waters CM. RhoA and Rac1 are both required for efficient wound closure of airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 287: L1134–L1144, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Desai LP, Sinclair SE, Chapman KE, Hassid A, Waters CM. High tidal volume mechanical ventilation with hyperoxia alters alveolar type II cell adhesion. Am J Physiol Lung Cell Mol Physiol 293: L769–L778, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Dobbs LG Isolation and culture of alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 258: L134–L147, 1990. [DOI] [PubMed] [Google Scholar]
- 15.Dreyfuss D, Saumon G. Role of tidal volume, FRC, and end-inspiratory volume in the development of pulmonary edema following mechanical ventilation. Am Rev Respir Dis 148: 1194–1203, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 157: 294–323, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Frank JA, Gutierrez JA, Jones KD, Allen L, Dobbs L, Matthay MA. Low tidal volume reduces epithelial and endothelial injury in acid-injured rat lungs. Am J Respir Crit Care Med 165: 242–249, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Gajic O, Lee J, Doerr CH, Berrios JC, Myers JL, Hubmayr RD. Ventilator-induced cell wounding and repair in the intact Lung. Am J Respir Crit Care Med 167: 1057–1063, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA 101: 7618–7623, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garat C, Kheradmand F, Albertine KH, Folkesson HG, Matthay MA. Soluble and insoluble fibronectin increases alveolar epithelial wound healing in vitro. Am J Physiol Lung Cell Mol Physiol 271: L844–L853, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Gattinoni L, Caironi P, Carlesso E. How to ventilate patients with acute lung injury and acute respiratory distress syndrome. Curr Opin Crit Care 11: 69–76, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Geiser T, Jarreau PH, Atabai K, Matthay MA. Interleukin-1β augments in vitro alveolar epithelial repair. Am J Physiol Lung Cell Mol Physiol 279: L1184–L1190, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Habets GG, Scholtes EH, Zuydgeest D, van der Kammen RA, Stam JC, Berns A, Collard JG. Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell 77: 537–549, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Hall A Rho GTPases and the actin cytoskeleton. Science 279: 509–514, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Hamelers IH, Olivo C, Mertens AE, Pegtel DM, van der Kammen RA, Sonnenberg A, Collard JG. The Rac activator Tiam1 is required for (alpha)3(beta)1-mediated laminin-5 deposition, cell spreading, and cell migration. J Cell Biol 171: 871–881, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hordijk PL, ten Klooster JP, van der Kammen RA, Michiels F, Oomen LC, Collard JG. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science 278: 1464–1466, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Huh D, Fujioka H, Tung YC, Futai N, Paine R 3rd, Grotberg JB, Takayama S. Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proc Natl Acad Sci USA 104: 18886–18891, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jafari B, Ouyang B, Li LF, Hales CA, Quinn DA. Intracellular glutathione in stretch-induced cytokine release from alveolar type-2 like cells. Respirology 9: 43–53, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Katsumi A, Milanini J, Kiosses WB, del Pozo MA, Kaunas R, Chien S, Hahn KM, Schwartz MA. Effects of cell tension on the small GTPase Rac. J Cell Biol 158: 153–164, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawamura S, Miyamoto S, Brown JH. Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J Biol Chem 278: 31111–31117, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Kay SS, Bilek AM, Dee KC, Gaver DP 3rd. Pressure gradient, not exposure duration, determines the extent of epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol 97: 269–276, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Kheradmand F, Folkesson HG, Shum L, Derynk R, Pytela R, Matthay MA. Transforming growth factor-α enhances alveolar epithelial cell repair in a new in vitro model. Am J Physiol Lung Cell Mol Physiol 267: L728–L738, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Kraemer A, Goodwin M, Verma S, Yap AS, Ali RG. Rac is a dominant regulator of cadherin-directed actin assembly that is activated by adhesive ligation independently of Tiam1. Am J Physiol Cell Physiol 292: C1061–C1069, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A, Murphy R, Robinson P, Wei L, Boriek AM. Cyclic mechanical strain inhibits skeletal myogenesis through activation of focal adhesion kinase, Rac-1 GTPase, and NF-kappaB transcription factor. FASEB J 18: 1524–1535, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Lee SH, Kunz J, Lin SH, Yu-Lee LY. 16-kDa prolactin inhibits endothelial cell migration by down-regulating the Ras-Tiam1-Rac1-Pak1 signaling pathway. Cancer Res 67: 11045–11053, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Malhotra A Low-tidal-volume ventilation in the acute respiratory distress syndrome. N Engl J Med 357: 1113–1120, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malliri A, van Es S, Huveneers S, Collard JG. The Rac exchange factor Tiam1 is required for the establishment and maintenance of cadherin-based adhesions. J Biol Chem 279: 30092–30098, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol 33: 319–327, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendez JL, Hubmayr RD. New insights into the pathology of acute respiratory failure. Curr Opin Crit Care 11: 29–36, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Michiels F, Habets GG, Stam JC, van der Kammen RA, Collard JG. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature 375: 338–340, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Minard ME, Ellis LM, Gallick GE. Tiam1 regulates cell adhesion, migration and apoptosis in colon tumor cells. Clin Exp Metastasis 23: 301–313, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Minard ME, Kim LS, Price JE, Gallick GE. The role of the guanine nucleotide exchange factor Tiam1 in cellular migration, invasion, adhesion and tumor progression. Breast Cancer Res 84: 21–32, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Moissoglu K, Slepchenko BM, Meller N, Horwitz AF, Schwartz MA. In vivo dynamics of Rac-membrane interactions. Mol Biol Cell 17: 2770–2779, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 149: 1327–1334, 1994. [DOI] [PubMed] [Google Scholar]
- 45.Osada T, Watanabe S, Tanaka H, Hirose M, Miyazaki A, Sato N. Effect of mechanical strain on gastric cellular migration and proliferation during mucosal healing: role of Rho dependent and Rac dependent cytoskeletal reorganisation. Gut 45: 508–515, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pelham RJ, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA 94: 13661–13665, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perkins GD, Nathani N, McAuley DF, Gao F, Thickett DR. In vitro and in vivo effects of salbutamol on neutrophil function in acute lung injury. Thorax 62: 36–42, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perlman CE, Bhattacharya J. Alveolar expansion imaged by optical sectioning microscopy. J Appl Physiol 103: 1037–1044, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Price LS, Leng J, Schwartz MA, Bokoch GM. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell 9: 1863–1871, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ray RM, Vaidya RJ, Johnson LR. MEK/ERK regulates adherens junctions and migration through Rac1. Cell Motil Cytoskeleton 64: 143–156, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Roberts JR, Perkins GD, Fujisawa T, Pettigrew KA, Gao F, Ahmed A, Thickett DR. Vascular endothelial growth factor promotes physical wound repair and is anti-apoptotic in primary distal lung epithelial and A549 cells. Crit Care Med 35: 2164–2170, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Samuelsen JT, Schwarze PE, Huitfeldt HS, Thrane EV, Lag M, Refsnes M, Skarpen E, Becher R. Regulation of rat alveolar type 2 cell proliferation in vitro involves type II cAMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol 292: L232–L239, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Savla U, Waters CM. Mechanical strain inhibits repair of airway epithelium in vitro. Am J Physiol Lung Cell Mol Physiol 274: L883–L892, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt A, Hall A. The Rho exchange factor Net1 is regulated by nuclear sequestration. J Biol Chem 277: 14581–14588, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz MA, Shattil SJ. Signaling networks linking integrins and rho family GTPases. Trends Biochem Sci 25: 388–391, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Sinclair SE, Molthen RC, Haworth ST, Dawson CA, Waters CM. Airway strain during mechanical ventilation in an intact animal model. Am J Respir Crit Care Med 176: 786–794, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinberg J, Schiller HJ, Halter JM, Gatto LA, Dasilva M, Amato M, McCann UG, Nieman GF. Tidal volume increases do not affect alveolar mechanics in normal lung but cause alveolar overdistension and exacerbate alveolar instability after surfactant deactivation. Crit Care Med 30: 2675–2683, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Suh GY, Koh Y, Chung MP, An CH, Kim H, Jang WY, Han J, Kwon OJ. Repeated derecruitments accentuate lung injury during mechanical ventilation. Crit Care Med 30: 1848–1853, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Taskar V, John J, Evander E, Robertson B, Jonson B. Surfactant dysfunction makes lungs vulnerable to repetitive collapse and reexpansion. Am J Respir Crit Care Med 155: 313–320, 1997. [DOI] [PubMed] [Google Scholar]
- 60.Ten Klooster JP, Evers EE, Janssen L, Machesky LM, Michiels F, Hordijk P, Collard JG. Interaction between Tiam1 and the Arp2/3 complex links activation of Rac to actin polymerization. Biochem J 397: 39–45, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tschumperlin DJ, Oswari J, Margulies AS. Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med 162: 357–362, 2000. [DOI] [PubMed] [Google Scholar]
- 62.Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev 11: 2295–2322, 1997. [DOI] [PubMed] [Google Scholar]
- 63.Vlahakis NE, Schroeder MA, Pagano RE, Hubmayr RD. Deformation-induced lipid trafficking in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 280: L938–L946, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Vlahakis NE, Schroeder MA, Pagano RE, Hubmayr RD. Role of deformation-induced lipid trafficking in the prevention of plasma membrane stress failure. Am J Respir Crit Care Med 166: 1282–1289, 2002. [DOI] [PubMed] [Google Scholar]
- 65.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. [DOI] [PubMed] [Google Scholar]
- 66.Yamauchi J, Miyamoto Y, Tanoue A, Shooter EM, Chan JR. Ras activation of a Rac1 exchange factor, Tiam1, mediates neurotrophin-3-induced Schwann cell migration. Proc Natl Acad Sci USA 102: 14889–14894, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng Y Dbl family guanine nucleotide exchange factors. Trends Biochem Sci 26: 724–732, 2001. [DOI] [PubMed] [Google Scholar]