Abstract
The goal of this study was to identify a mechanism regulating cholesterol accumulation in cystic fibrosis (CF) cells. Both CFTR activation and expression are regulated by the cAMP pathway, and it is hypothesized that a feedback response involving this pathway may be involved in the phenotype of cholesterol accumulation. To examine the role of the cAMP pathway in cholesterol accumulation, we treated two CF model cell lines with the Rp diastereomer of adenosine 3′,5′-cyclic monophosphorothioate (Rp-cAMPS) and visualized by filipin staining. Rp-cAMPS treatment eliminated cholesterol accumulation in CF cells, whereas 8-bromo-cAMP treatment led to cholesterol accumulation in wild-type cells. To confirm these findings in an independent model system, we also examined the role of cAMP in modulating cholesterol accumulation in Niemann-Pick type C (NPC) fibroblasts. Expression of the protein related to NPC, NPC1, is also directly regulated by cAMP; therefore, it is postulated that NPC cells exhibit the same cAMP-mediated control of cholesterol accumulation. Cholesterol accumulation in NPC cells also was reduced by the presence of Rp-cAMPS. Expression of β-arrestin-2 (βarr2), a marker of cellular response to cAMP signaling, was significantly elevated in CF model cells, Cftr−/− MNE, primary tissue obtained by nasal scrapes from CF subjects, and in NPC fibroblasts compared with respective controls.
Keywords: Rp diastereomer of adenosine 3′,5′-cyclic monophosphothioate
downstream consequences of lost cystic fibrosis transmembrane conductance regulator (CFTR) function have been examined in several studies with particular emphasis on epithelial inflammatory signaling pathways and in vivo inflammatory responses. Studies supporting the hypothesis of inherent inflammation in cystic fibrosis (CF) have shown increased inflammatory cytokine production in CF cell models (5, 6, 20, 28, 34, 39), various CF mouse models (13, 35, 36, 38), CF xenograph models (12, 37) and in samples taken from CF infants before detectable infection or significant obstruction (1, 2). Specific signaling alterations reported in primary CF tissues and cell models by our laboratory and others include reduced inducible nitric oxide synthase (NOS2) expression (7, 15, 24, 25, 26, 30), deficient signal transducer and activator of transcription-1 (STAT1) activation (16, 44, 45), and increased activation of the small GTPase RhoA (18, 28). Recently, we have demonstrated that abnormal perinuclear cholesterol accumulation in CF cells and tissues leads to elevated de novo cholesterol synthesis in response to lost CFTR expression or function (40, 41). Inhibition of cholesterol synthesis with mevastatin reverses the NOS2 expression and STAT1 activation in CF models (18). It is postulated from these results that interrupted cholesterol processing is a key intermediate step in propagating signaling changes that lead to an inherent elevation in inflammatory signaling in CF cells.
The goal of this study was to determine the mechanism responsible for cholesterol accumulation in CF cells. How the loss of CFTR function is related to cholesterol and related cell regulatory changes is unknown. Indeed, other studies have demonstrated that inflammatory signaling and cytokine production are not different in CF cells compared with wild-type controls (4, 14). These disparate findings regarding CFTR function and inflammatory signaling lead to the conclusion that CFTR function does not directly regulate inflammatory signaling. If lost CFTR function is not the direct cause of altered cellular regulation in CF cells, then a secondary event must be involved.
The hypothesis of this study is that feedback mechanisms involving the cAMP pathway triggered by the loss of CFTR function are responsible for secondary cell regulatory events in CF cells, specifically cholesterol accumulation in this study. An earlier study examined the cAMP pathway in CF, primarily focusing on cAMP production and whether the pathway was functional (42). This study by Widdicombe (42) concluded that total cAMP values are not significantly altered in CF epithelial cells compared with non-CF controls. More recent data on the cAMP pathway in CF tissues demonstrate that G protein-coupled receptor kinase-2 (grk2) expression and activation are increased in CF lung tissue, and this increased expression correlates with reduced β2-adrenergic receptor (β2-AR) expression on CF cells, a finding consistent with previous results (22, 33). The grk2 pathway is activated by an upregulation of the cAMP pathway and triggers β2-AR recycling from the plasma membrane as a mechanism to dampen further β2-AR-mediated cAMP production in a cell (9). Grk2 is activated by protein kinase A (PKA) and phosphorylates the β2-AR and other G protein-coupled receptors (GPCR). β2-AR phosphorylation by grk2 recruits the arrestins (β-arrestin-1 and β-arrestin-2), which facilitate receptor internalization into the endosomal/lysosomal pathway. Increased grk2 activation and subsequent β2-AR internalization reported in CF support the hypothesis of an intrinsic increase in cAMP-mediated signaling in CF cells and suggest a role for arrestins in CF cellular responses. Since both CFTR expression and activation are regulated by cAMP, increasing the cAMP pathway is a reasonable cellular feedback response to lost CFTR function (23, 31).
This postulated feedback mechanism is not limited to CF. On the basis of previously published similarities between CF and Niemann-Pick type C (NPC) cells regarding cholesterol accumulation and signaling (40, 41), the role of cAMP in NPC was also examined. Previous work has demonstrated that the expression of the NPC protein, NPC1, is regulated by cAMP (11). Although it is known that NPC1 is capable of binding cholesterol in late endosomal compartments, how the loss of its function leads to cholesterol accumulation is unclear (32). It is proposed that a similar cAMP-mediated response to lost NPC1 function may lead to the similarities between CF and NPC cells. Other than the regulation of NPC1 expression, no cAMP studies have been done in NPC cells. Data in this article demonstrate that cholesterol accumulation in both CF and NPC cells is reversible with Rp-adenosine-3′,5′-cyclic monophosphorothioate (Rp-cAMPS). Rp-cAMPS is a cell-permeable cAMP analog that competes for cAMP binding and is typically used as a PKA inhibitor. These data demonstrate that secondary cellular responses to lost CFTR function related to cAMP signaling are responsible for cholesterol processing patterns associated with CF cells. Similar cellular responses to lost NPC1 function also result in this phenotype in NPC cells. The shared cellular responses explain why such seemingly disparate diseases share so many characteristics at the cellular level.
MATERIALS AND METHODS
Cell culture.
Human epithelium 9/HTEo- cells overexpressing the CFTR R domain (pCEPR) and mock-transfected 9/HTEo-cells (pCEP), the wild-type phenotype, were a generous gift from the laboratory of Dr. Pamela B. Davis (Case Western Reserve University). Cells were cared for as previously described (29). Niemann-Pick fibroblasts (NPC; GM03123A) containing two missense mutations in the NPC1 gene were obtained from Coriell Cell Repository (Camden, NJ). Control human fibroblasts (CRL-2076) were obtained from American Type Culture Collection (Manassas, VA). The cells were grown at 37°C in 95% O2-5% CO2 on Falcon 10-cm-diameter tissue culture dishes in MEM Eagle with 2 mM l-glutamine containing 15% fetal bovine serum (FBS) and 1 U/ml penicillin/streptomycin. IB3-1 cells (ΔF508/W1282X) and S9 cells (IB3-1 cell stably transfected with the full-length wild-type CFTR as controls) were developed by Pamela L. Zeitlin (Johns Hopkins University, Baltimore, MD). These cells were grown at 37°C in 95% O2-5% CO2 on Falcon 10-cm-diameter tissue culture dishes in LHC-8 basal medium (Biofluids) with 5% FBS. Cells treated with 50 μM Rp-cAMPS (BioLog, Hayward, CA) were exposed between 24 and 72 h.
Mice.
Mice lacking CFTR expression (CFTRtm1Unc) were obtained from Jackson Laboratories (Bar Harbor, MA). CFTR wild-type mice were siblings of Cftr−/− mice. All mice were used between 6 and 8 wk of age and were backcrossed over 10 generations onto a C57Bl/6 background. CF mice were fed a liquid diet as described by Eckman et al. (8). Mice were cared for by the CF Animal Core Facility following protocols approved by the Case Western Reserve University IACUC.
Human nasal scrapings.
Nasal epithelia from CF patients and control individuals were obtained as previously described (17). Four scrapes from each nasal passage were taken from four CF subjects (18–30 yr old) and four non-CF volunteers (26–53 yr old) were obtained and immediately placed in ice-cold lysis buffer (50 mM Tris, pH 7.5, 1% Triton X-100, 100 mM NaCl, 50 mM NaF, 200 μM Na3VO4, and 10 μg/ml gestating and leupeptin). After 30–60 min, tissue was homogenized and lysates were microcentrifuge at 4°C at 14, 000 rpm for 10 min. Supernatants were collected and stored at −20°C until Western blotting analysis was performed. The protocol was approved by the Institutional Review Board. Human patients and volunteers gave informed consent under protocol approved by the Institutional Review Board (University Hospitals, Cleveland, OH).
Filipin staining.
Cells were treated as previously described by Krutch et al. (19). Briefly, cells were grown to 75–90% confluency on Fisher brand coverslips. Cells were rinsed three times with PBS and then fixed with 2% paraformaldehyde for ∼30 min. Cells were rinsed once more with PBS and then incubated with 0.05 mg/ml filipin (Sigma-Aldrich, St. Louis, MO) in PBS for 1 h on a shaker in the dark. Filipin was dissolved freshly in dimethylsulfoxide before each experiment. Cells were rinsed in PBS before being mounted using SlowFade Light antifade (Molecular Probes) on slides. Cells were visualized in the ultraviolet range using a wide-field microscope on a Zeiss Axiovert 200 and Metamorph software. A ×63 objective was used for all images.
NBD-cholesterol staining.
Cells were seated at a density of 100,000 cells/well on Fisherbrand coverslips. Five micrograms per milliliter of NBD-cholesterol or 22-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-23,24-bisnor-5-cholen-3β-ol (Molecular Probes, Eugene, OR) was added for ∼24 h. Cells were then incubated in fresh medium for another 4 h. Cells were fixed in 2% paraformaldehyde for 30 min and then rinsed in PBS three times before being mounted using SlowFade Light antifade (Molecular Probes). Confocal images were taken using a Leica DMIRE2 confocal microscope (Leica Imaging Systems, Mannheim, Germany) with the HCX PL AP ×63 1.4 oil objective. Images are representatives of average pictures taken of the Z stacks.
Western immunoblotting.
Antibodies against βarr2, cAMP response element binding protein (CREB), and phosphorylated CREB (pCREB) were obtained from BD Transduction Laboratories (Billerica, MA). Antibody against actin (rabbit) was obtained from Sigma-Aldrich. Protein samples were prepared in 60-mm dishes of cultured cells in ice-cold lysis buffer (50 mM Tris, pH 7.5, 1% Triton X-100, 100 mM NaCl, 50 mM NaF, 200 μM Na3VO4, and 10 μg/ml pepstatin and leupeptin) for 30 min at 4°C while shaking. Cell lysates were microcentrifuged at 4°C at 14,000 rpm for 10 min. Proteins were separated using SDS-PAGE containing 20–40 μg of protein on a 6–12% acrylamide gel. The samples were transferred to an Immobilon-P membrane (Millipore, Bedford, MA) at 15 V for 30 min. The blots were blocked overnight in PBS (138 mM NaCl, 15 mM Na2HPO4, 1.5 mM KCl, and 2.5 mM KH2PO4) containing 5% nonfat dehydrated milk and 0.1% Tween 20 at 4°C. Blots were incubated overnight at 4°C for pCREB/CREB blots and for 3 h at room temperature for βarr2 (1:500 dilution) in PBS containing 5% nonfat dehydrated milk and 0.1% Tween 20. Blots were washed three times in PBS and incubated in secondary antibody conjugated to horseradish peroxidase for 1 h at room temperature (1: 4,000 dilution; Sigma). Blots were washed again in PBS before visualization using SuperSignal chemiluminescent substrate (Pierce, Rockford, IL) and the VersaDoc imaging system (Bio-Rad, Hercules, CA). Quantification of protein expression was accomplished using densitometry software on the VersaDoc (Quality One; Bio-Rad).
Immunostaining/filipin costaining.
Briefly, cells were grown to 55–60% confluency on Fisherbrand coverslips. Cells were rinsed three times with PBS and then fixed with 2% paraformaldehyde for ∼30 min. Cells were then filipin stained (as described in Filipin staining) and refixed for 5 min. Cells were rinsed once more with PBS and then permeablized in 0.1% Nonidet P-40 (Sigma-Aldrich) in PBS for 30 min at room temperature. Cells are washed three times in PBS and blocked with 10% goat serum in PBS for 30 min at room temperature. Cells were then incubated with anti-β2-AR or anti-βarr2 (1:100 dilution) from Santa Cruz Biotechnology (Santa Cruz, CA) in 10% goat serum in PBS for 1 h at room temperature. After slides were washed three times in PBS, cells are incubated with Alexa Fluor 488 goat anti-mouse IgG at 10 μg/ml in 10% goat serum in PBS for 1 h at room temperature in the dark. Results were visualized in the ultraviolet range using wide-field microscope on a Zeiss Axiovert 200 and Metamorph software. A ×40 oil objective with a green fluorescent protein filter was used for all images.
RESULTS
Functional impact of the cAMP pathway on cholesterol processing in CF model cells.
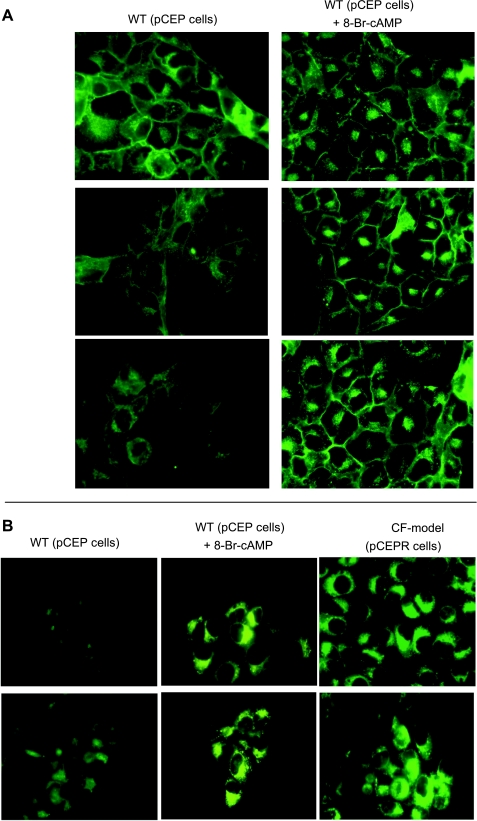
A primary goal of this study was to determine the mechanism of CF-related cholesterol accumulation, since the mechanism of cholesterol accumulation associated with CF has been debated in the literature (10). We have proposed previously that cholesterol accumulation is an important intermediate step in a variety of CF-related cell signaling changes that have been verified in primary tissues (7, 15, 16, 18, 24, 25, 27, 30, 40, 44, 45). It is postulated that feedback mechanisms related to lost CFTR function could impact the cAMP pathway and, subsequently, cholesterol processing. The first question to be addressed was whether direct upregulation of the cAMP pathway could lead to CF-like perinuclear cholesterol accumulation. 9/HTEo- control cells were treated for 72 h with 8-bromo-cAMP (8-Br-cAMP; 100 μM) and examined for free cholesterol accumulation by filipin staining. Cells treated with 8-Br-cAMP exhibited clear perinuclear filipin staining, indicative of free cholesterol accumulation (Fig. 1A). No toxicity from 8-Br-cAMP treatment was observed using trypan blue exclusion assay (not shown). Since imaging data can be subjective, a second technique was utilized to verify filipin staining results. Cholesterol accumulation was visualized using the fluorescent cholesterol analog NBD-cholesterol. One caveat to using NBD-cholesterol is that the NBD group is a large hydrophobic molecule and can influence trafficking. In this study, NBD-cholesterol was only used to verify filipin staining results. 9/HTEo- cells treated with 8-Br-cAMP showed perinuclear accumulation of NBD-cholesterol in a manner identical to CF model cells (Fig. 1B). These data demonstrate that a direct increase in the cAMP pathway is sufficient to mimic CF-like cholesterol accumulation.
Fig. 1.
Cholesterol accumulation in 9/HTEo- cells initiated by 8-bromo-cAMP (8-Br-cAMP). A: 3 representative images of endogenous free cholesterol content visualized by filipin staining in wild-type (WT) 9/HTEo- (pCEP) cells in the absence or presence of the cell-permeable cAMP analog 8-Br-cAMP (100 μM for 72 h). Representative images taken from at least 27 images for each condition over 4 separate experiments are shown. B: representative images of the trafficking of the fluorescent cholesterol analog NBD-cholesterol in WT 9/HTEo- cells in the absence or presence of 8-Br-cAMP (100 μM for 72 h) and in untreated cystic fibrosis (CF) model 9/HTEo- (pCEPR) cells for comparison. Images are representative at least 18 images from triplicate experiments.
CF-related changes in cAMP pathway regulatory proteins.
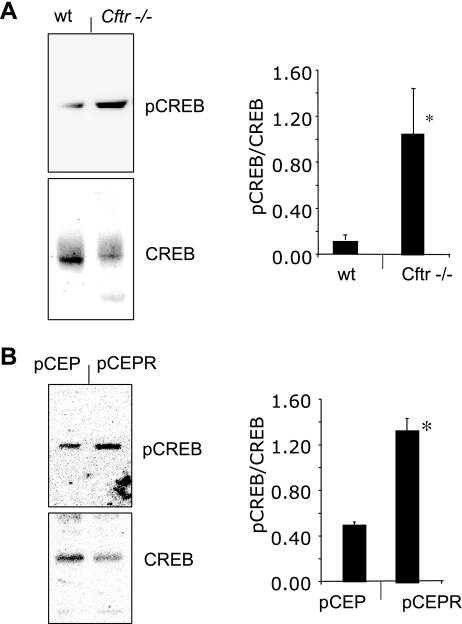
Since elevated cAMP content can mimic the phenotype of cholesterol accumulation in epithelial cells, aspects of cAMP signaling were examined in CF model cells and tissues. pCREB levels were determined in a cultured cell model of CF, 9/HTEo- cells stably expressing the regulatory domain, resulting in a functional inhibition of CFTR (9/HTEo- pCEPR cells), and in control (9/HTEO- pCEP) cells (29). These cells were essential for this study because they accurately reflect cholesterol processing and signaling changes observed in CF mouse models and human tissue (15, 18, 40, 41). CF model pCEPR cells exhibited significant increases in pCREB content compared with control pCEP cells, suggesting an inherent increase in CREB activation (Fig. 2B). Despite their utility, pCEP and pCEPR cells are transformed clonal lines and may exhibit non-CF-related cell regulatory changes. To verify results in an in vivo model, we excised nasal epithelium from S489X Cftr−/− mice and assayed for pCREB content compared with nasal epithelium from age-matched wild-type mice. Consistent with cell data, pCREB content was significantly increased in Cftr−/− tissue compared with controls (Fig. 2A). These data indicate a functional increase in the cAMP pathway in two separate CF models despite similar total cAMP levels.
Fig. 2.
cAMP response element binding protein (CREB) phosphorylation in CF mouse nasal epithelium (MNE) and cell models. A: representative gel and densitometry data of phosphorylated CREB (pCREB)/total CREB ratio in MNE from WT and Cftr−/− mice (n = 6 for each condition). Error bars represent SE, and significance was determined by t-test (*P < 0.01). B: representative gel and densitometry data of pCREB/total CREB ratio in 9/HTEo- WT (pCEP) and CF model (pCEPR) cells (n = 3 for each condition). Error bars represent SE, and significance was determined by t-test (*P < 0.001).
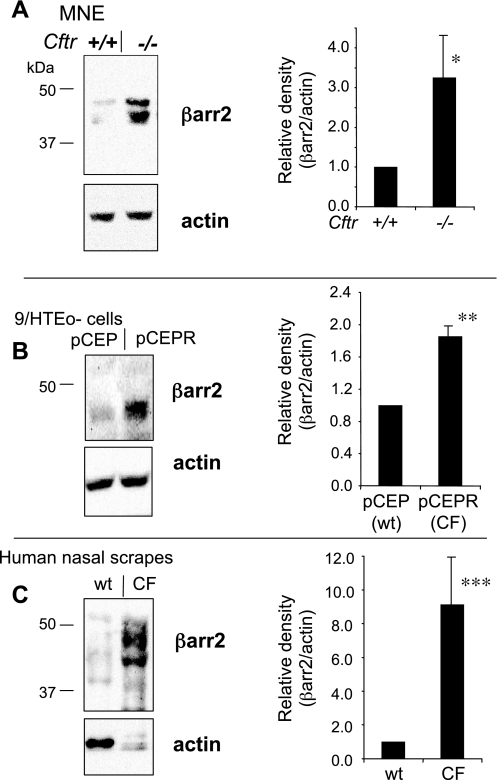
A good candidate to link the cAMP pathway to cholesterol processing regulation is the protein β-arrestin-2 (βarr2). βarr2 participates in the internalization of GPCRs such as the β2-AR, which are sorted into the endosomal/lysosomal pathway where cholesterol accumulation occurs (26). A chronic activation of the cAMP pathway as indicated by basal CREB activation would be expected to desensitize the β2-AR system, which is regulated in part by receptor internalization mediated by grk2 and βarr2. Previous studies have noted that grk2 exhibits increased expression in CF tissues and a corresponding decrease in β2-AR surface expression (22, 33). To further examine the β2-AR desensitization pathway, we examined the protein expression of βarr2. Figure 3B demonstrates that βarr2 exhibited increased protein expression in 9/HTEo- pCEPR cells compared with control pCEP cells. Protein expression of βarr2 was examined in excised nasal epithelium from Cftr−/− mice and wild-type controls to verify cell model results. Consistent with cell model results, βarr2 expression was significantly elevated in nasal tissue from Cftr−/− mice compared with controls (Fig. 3A). Finally, since increased βarr2 expression appears to be a distinct feature of these CF models, its expression was examined in tissue obtained by nasal scraping from four separate CF subjects and four non-CF controls. As shown in Fig. 3C, increased βarr2 expression in CF was conserved in primary human tissue. These data demonstrate in primary human and mouse tissue and in a cultured cell model of CF that there is an intrinsic alteration in the cAMP pathway in CF cells and tissues. Attempts were made to measure the content of pCREB/total CREB in nasal scraping samples to correlate to cell model and mouse tissue results. However, no immunoreactive CREB, either pCREB or total CREB, was detectable.
Fig. 3.
β-Arrestin-2 (βarr2) protein content in CF epithelium. A: representative gel showing βarr2 and actin expression in MNE from WT (+/+) and Cftr−/− (−/−) mice and densitometry analysis of βarr2 protein expression normalized to actin content (βarr2/actin). Significance was determined by t-test (n = 7; *P = 0.03). B: representative gel showing βarr2 and actin expression in 9/HTEo- pCEP and pCEPR cells and densitometry analysis of βarr2 protein expression normalized to actin content. Significance was determined by t-test (n = 4; **P = 0.004). C: representative gel showing βarr2 and actin expression in human nasal scrape tissue obtained from non-CF (WT) and CF subjects and densitometry analysis of βarr2 protein expression normalized to actin content. Significance was determined by t-test (n = 4; ***P = 0.02).
Colocalization of β2-AR and cholesterol in CF model cells.
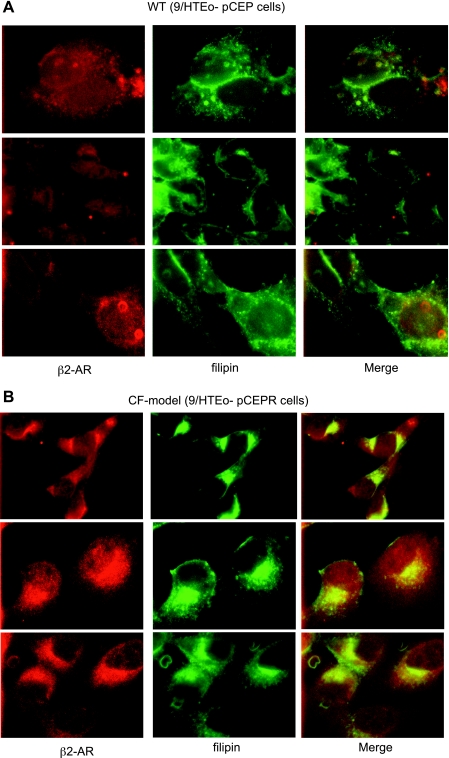
The above-described data demonstrate that elevating the cAMP pathway is sufficient to initiate cholesterol accumulation. Moore et al. (26) have demonstrated that internalized β2-AR is trafficked to endosomal/lysomal pathway for degradation. To assess whether the βarr2 system is implicated in cholesterol processing, it was hypothesized that a known ligand for βarr2-mediated internalization and recycling would colocalize with cholesterol if the βarr2 pathway were involved. The localization of the β2-AR was examined in CF model cells by immunostaining, and its localization relative to cholesterol was determined. This study demonstrated that cholesterol and the β2-AR colocalized in CF model cells, suggesting that the βarr2 pathway is involved in cholesterol processing (Fig. 4). This finding is consistent with a report demonstrating that βarr2 is responsible for LDL receptor (LDLR) internalization (43). It is important to note that these data do not demonstrate a direct role for βarr2 in CF-related cholesterol accumulation, only a correlation with the βarr2-mediated internalization pathway.
Fig. 4.
β2-Adrenergic receptor (β2-AR) and cholesterol colocalize in CF model 9/HTEo- pCEPR cells. Immunostaining for β2-AR and cholesterol visualization with filipin in WT 9/HTEo- pCEP (A) and CF model pCEPR cells (B) is shown. In merged images, yellow color denotes colocalization. Three representative images taken from at least 25 images for each cell line over 4 separate experiments are shown. Control studies using secondary antibody only were performed to ensure images represent primary antibody binding. No staining was observed in control images (not shown).
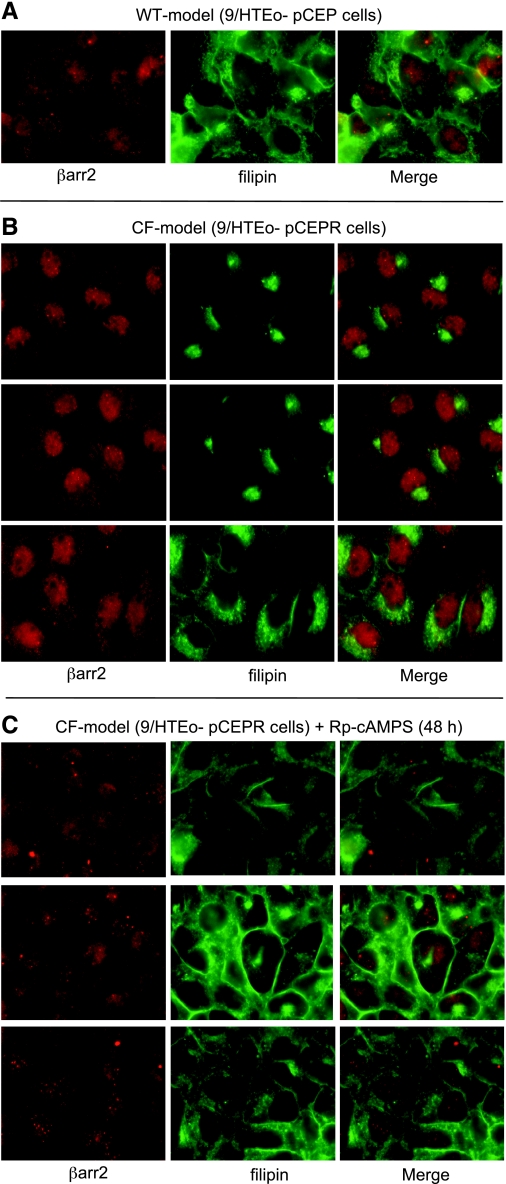
Correction of cholesterol accumulation in CF model cells with Rp-cAMPS and association with βarr2.
If direct upregulation of the cAMP pathway can lead to CF-like cholesterol accumulation, as shown in Fig. 1, then cholesterol accumulation in CF model cells should be reversible with Rp-cAMPS. CF model pCEPR cells were exposed to Rp-cAMPS (50 μM) for 48 h and examined by filipin staining and costained for βarr2 detection to determine whether there was any colocalization with cholesterol and βarr2. Rp-cAMPS treated CF model cells exhibited a wild-type staining pattern compared with untreated CF model cells, suggesting effective reversal of the cholesterol phenotype (Fig. 5). However, βarr2 showed no colocalization with cholesterol by immunostaining, which could be a factor of staining intensity. This finding does not rule out a role for βarr2 in the cholesterol processing, but this interaction needs to be further explored.
Fig. 5.
Correction of cholesterol transport in CF model cells with Rp-cAMPS. A: representative image of WT 9/HTEo- cells costained for βarr2 and endogenous free cholesterol (filipin). B: 3 representative images of CF model 9/HTEo- pCEPR cells costained for βarr2 and cholesterol (filipin). C: 3 representative images of CF model 9/HTEo- pCEPR cells costained for βarr2 and cholesterol (filipin) in the presence of the cAMP binding competitor Rp-cAMPS (50 μM for 48 h). Images are representative of at least 17 images for each condition taken over 5 separate experiments. Control studies using secondary antibody only were performed to ensure images represent primary antibody binding. No staining was observed in control images (not shown).
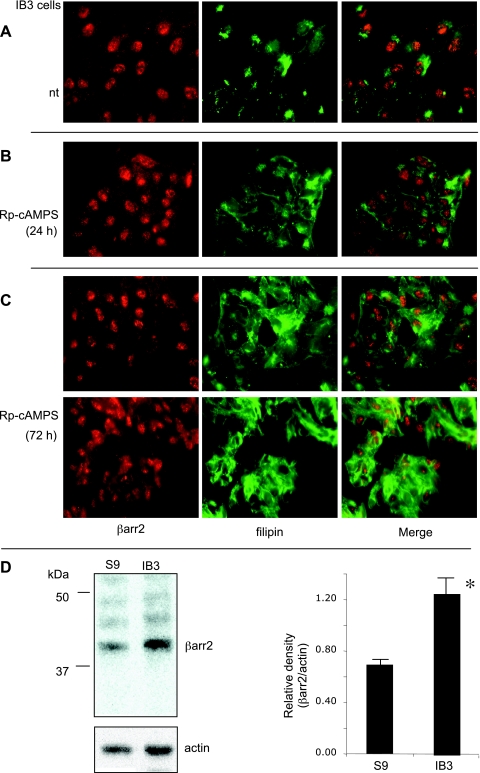
The overexpression of the regulatory domain in pCEPR cells could potentially impact the cAMP pathway. To test whether Rp-cAMPS could reverse cholesterol accumulation in another CF cell line, we performed the same study in IB3 cells. As shown in Fig. 6A, Rp-cAMPS treatment diminished cholesterol accumulation in IB3 cells as well. Minimal correction was observed after 24-h treatment, but 72-h exposure to Rp-cAMPS resulted in cholesterol redistribution. To determine whether IB3 cells also would exhibit increased βarr2 expression as shown in other CF cells and tissues, we performed Western blot analysis of βarr2 in IB3 cells compared with S9 cells (IB3 cells stably transfected with full-length CFTR). Consistent with other CF models and human nasal tissue, IB3 cells exhibited a twofold increase in βarr2 protein expression compared with S9 cells. These data demonstrate that the effect of Rp-cAMPS is not limited to one CF cell model and that increased βarr2 protein expression in CF cells is a consistent marker and is reversible with normalized CFTR function.
Fig. 6.
Correction of cholesterol transport in CF model IB3 cells with Rp-cAMPS. A: representative image of IB3 cells costained for βarr2 and endogenous free cholesterol (filipin). B: representative images of CF model IB3 cells costained for βarr2 and cholesterol (filipin) treated with Rp-cAMPS (50 μM) for 24 h. C: 2 representative images of CF model IB3 cells costained for βarr2 and cholesterol (filipin) in the presence of Rp-cAMPS (50 μM) for 72 h. Images are representative of at least 17 images for each condition taken over duplicate experiments. Control studies using secondary antibody only were performed to ensure images represent primary antibody binding. No staining was observed in control images (not shown). D: representative gel showing βarr2 and actin expression in S9 (WT) and IB3 (CF model) cells and densitometry analysis of βarr2 protein expression normalized to actin content. Significance was determined by t-test (n = 3; *P = 0.005).
cAMP control of cholesterol processing in NPC fibroblasts.
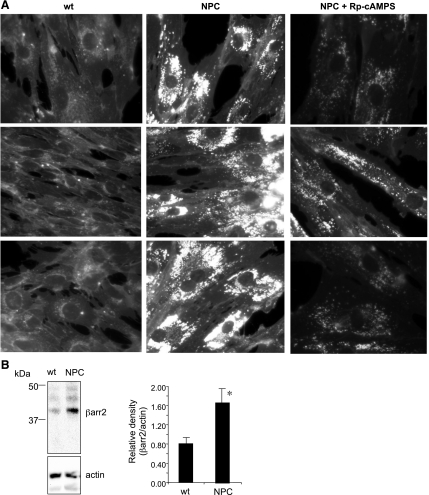
Combined with data demonstrating increased βarr2 expression in multiple models, including primary tissue, correction of cholesterol accumulation with Rp-cAMPS in two separate CF cell models suggests that the relationship between the cAMP pathway and cholesterol processing is not limited to a single model system. However, it is not clear whether this relationship holds in other disease models. Based on the previously published similarities between CF and NPC cells regarding cholesterol accumulation and signaling, the impact of the cAMP pathway on cholesterol accumulation in NPC fibroblasts was examined. NPC fibroblasts were treated with Rp-cAMPS and examined for cholesterol accumulation by filipin staining. Treated cells exhibited significantly reduced perinuclear cholesterol accumulation compared with untreated cells (Fig. 7A).
Fig. 7.
Correction of cholesterol transport in Niemann-Pick type C (NPC) fibroblasts. A: 3 representative image of untreated WT fibroblasts, 3 representative images of untreated NPC fibroblasts, and 3 representative images of NPC fibroblasts treated with Rp-cAMPS (50 μM) for 48 h. Fibroblasts are stained for free cholesterol (filipin). Images are representative of at least 25 images for each condition taken over 5 separate experiments. B: representative gel showing βarr2 and actin expression in WT and NPC fibroblasts and densitometry analysis of βarr2 protein expression normalized to actin content. Significance was determined by t-test (n = 3; *P = 0.04).
To determine whether NPC fibroblasts exhibit evidence of altered cAMP regulation, βarr2 expression was examined, since this is a consistent marker in CF cells and tissues. NPC fibroblasts also exhibit elevated βarr2 expression compared with wild-type controls (Fig. 7B). These data confirm that cAMP regulation of cholesterol accumulation is not due to the characteristics of a particular CF model system. These NPC studies also demonstrate that cholesterol accumulation is not likely due to any direct effect of lost CFTR function but is more likely due to secondary cellular responses that are shared by NPC cells.
DISCUSSION
Understanding the relationship between CFTR function and cell regulatory changes associated with CF has been a long-term goal. The hypothesis of this study is that CF-related perinuclear accumulation of cholesterol identified by this laboratory is not directly regulated by CFTR function, but rather occurs due to a cellular feedback response to the loss of CFTR function. Because both CFTR expression and activation are regulated by the cAMP pathway, a reasonable feedback response to the loss of CFTR function is the upregulation of the cAMP pathway. Other studies have reported decreased β-adrenergic receptor numbers in CF tissue (33), a finding consistent with this study and the identification of increased grk2/5 activity and receptor internalization (22).
An apparent anomaly is the lack of an increase in total cAMP content in CF cells previously reported (42) and supported in this study. This finding can be explained by two mechanisms. First, localized cAMP accumulation can have profound signaling implications without significantly raising total cellular cAMP values (3). Second, cell signaling regulation is essentially an achieved cellular balance between positive and negative regulatory factors. Localized production of cAMP may be skewed in CF toward nonadrenergic mechanisms such as soluble adenylate cyclase, or consumption may be reduced due to reduced phosphodiesterase function or expression. CF cells appear to have achieved a new regulatory balance leading to increased basal activation of CREB and increased βarr2 expression in CF cells and tissues compared with non-CF controls despite a lack of a measurable increase in total cAMP content. This new balance is likely the consequence of cell survival mechanisms adapting to the chronic presence of a functionally intended acute feedback response. Essentially, the feedback response meant to increase CFTR function never achieves this goal and thus never resolves.
The consequences of a chronic shift in the cAMP pathway would be profound, because several pathways are influenced. This study does not attempt an exhaustive characterization of cAMP signaling; instead, we chose to focus on the role of cAMP in cholesterol processing. Previously, we have demonstrated that cholesterol accumulation in CF cells is an important intermediate step in propagating a number of CF-related cell signaling changes, including increased RhoA activation, poor STAT1 activation, and reduced NOS2 expression (40). Understanding the mechanisms leading to cholesterol accumulation is important to unraveling the cell regulatory mechanisms associated with CF.
A recent report (10) has postulated that misprocessing of ΔF508 CFTR is responsible for lipid trafficking abnormalities in CF. Gentzsch et al. (10) demonstrated that the expression of ΔF508 CFTR in cell lines that do not normally express CFTR [baby hamster kidney (BHK) and Chinese hamster ovary (CHO) cells] results in perinuclear cholesterol accumulation. The expression of the inactive but properly trafficked G551D CFTR mutant has no effect on cholesterol transport. The authors concluded that the cholesterol defect in CF is due to aberrant ΔF508 CFTR trafficking. These data confirm that CFTR function is likely not involved directly in cholesterol transport and that misfolded protein accumulation can impact cholesterol processing. However, these results do not fully describe the CF situation. We have shown that Cftr−/− mice exhibit cholesterol processing defects (41), and we have demonstrated that cholesterol processing in IB3 (W1282X/ΔF508) is restored in CFTR-corrected IB3 cells (S9 cells) (40). S9 cells still express ΔF508 CFTR but have had function restored, indicating cholesterol transport is at least indirectly related to CFTR function. The feedback mechanism reported in this study satisfies both of these apparently contradictory works. Expression of G551D CFTR in CHO cells is unlikely to stimulate a feedback response. This model system excels in examining direct effects of CFTR on various outcomes but is ill suited for examining CF-related signaling alterations. The feedback mechanism also satisfies the observations that CFTR function is related to, but not directly involved in, cholesterol processing.
Based on previous findings demonstrating a number of similarities between CF and NPC cells regarding cholesterol accumulation and cell signaling regulation (11), the impact of the cAMP pathway on cholesterol accumulation in NPC fibroblasts was also examined. NPC1 expression is also regulated by cAMP, and it was postulated that similar feedback responses might be responsible for the cellular similarities with CF. As shown, NPC fibroblasts have increased protein expression of βarr2 compared with wild-type cells, and cholesterol accumulation is reversible with Rp-cAMPS. These data demonstrate that findings are not due to an anomaly of a CF model and that the cAMP regulation of cholesterol processing is not limited to CF cells. CFTR and NPC1 are structurally unrelated and share no known function. The only commonality between CF and NPC1 that can explain the cellular similarities is the shared dependence between CFTR and NPC1 on the cAMP pathway. Recently, a study by Lemberger et al. (21) demonstrated that tissue-specific deletion of CREB from mouse forebrain results in neuronal cholesterol accumulation. Put into context with the present study, loss of function of three unrelated proteins (CFTR, NPC1, CREB) results in filipin-positive cellular cholesterol accumulation. The only commonality shared by these proteins is that the cAMP pathway predominantly regulates each. Any perturbation where the cell could restore lost function of a target protein by elevating cAMP signaling results in perinuclear cholesterol accumulation. These findings suggest that CFTR function has no direct role in cholesterol accumulation; rather, it is the cellular response to lost CFTR function that leads to this phenotype.
This study details the role of cAMP in regulating cholesterol accumulation in CF and NPC cells, a likely consequence of a cellular response to the loss of function to CFTR and NPC1, both of which are regulated by the cAMP pathway (11). This finding is significant, because it is the first report of a CF-related phenotype not being the direct result of lost CFTR function, but rather the cellular feedback response to lost CFTR function. What aspect of lost CFTR function is triggering the feedback response is unknown. Studies performed in chloride-free Ringer or bicarbonate-free media had no influence on cholesterol processing, although these studies need to be expanded (not shown). Also, specific alterations within the cAMP pathway need to be further characterized, as does the specific role of βarr2 in cholesterol accumulation in each of these systems.
GRANTS
This work is supported by a grant from the Cystic Fibrosis Foundation and by National Heart, Lung, and Blood Institute Grant HL080319. Technical support for this project was provided by core facilities of the cystic fibrosis center (P30 DK 27651).
Acknowledgments
We thank Dr. Pam Davis, Case Western Reserve University, for providing cell lines necessary for completion of this study and P. Bead for technical assistance. Technical assistance also was provided by M. Malloy, S. Troy, D. Davis, K. Ploetze, C. Miranda, and M. Stein from the University of Notre Dame.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Armstrong DS, Grimwood K, Carlin JB, Carzino R, Gutièrrez JP, Hull J, Olinsky A, Phelan EM, Robertson CF, Phelan PD. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Respir Crit Care Med 156: 1197–1204, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DS, Hook SM, Jamsen KM, Nixon GM, Carzino R, Carlin JB, Robertson CF, Grimwood K. Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatr Pulmonol 40: 500–510, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Barnes AP, Livera G, Huang P, Sun C, O'Neal WK, Conti M, Stutts MJ, Milgram SL. Phosphodiesterase 4D forms a cAMP diffusion barrier at the apical membrane of the airway epithelium. J Biol Chem 280: 7997–8003, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Becker MN, Sauer MS, Muhlebach MS, Hirsh AJ, Wu Q, Verghese MW, Randell SH. Cytokine secretion by cystic fibrosis airway epithelial cells. Am J Respir Crit Care Med 169: 645–653, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Boncoeur E, Criq VS, Bonvin E, Roque T, Henrion-Caude A, Gruenert DC, Clement A, Jacquot J, Tabary O. Oxidative stress induces extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase in cystic fibrosis lung epithelial cells: potential mechanism for excessive IL-8 expression. Int J Biochem Cell Biol 40: 432–446, 2008. [DOI] [PubMed] [Google Scholar]
- 6.DiMango E, Ratner AJ, Bryan R, Tabibi S, Prince A. Activation of NF-kappaB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J Clin Invest 101: 2598–2605, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dötsch J, Puls J, Klimek T, Rascher W. Reduction of neuronal and inducible nitric oxide synthase gene expression in patients with cystic fibrosis. Eur Arch Otorhinolaryngol 259: 222–226, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Eckman EA, Cotton CU, Kube DM, Davis PB. Dietary changes improve survival of CFTR S489X homozygous mutant mouse. Am J Physiol Lung Cell Mol Physiol 269: L625–L630, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson SS, Zhang J, Barak LS, Caron MG. Molecular mechanisms of G protein-coupled receptor desensitization and resensitization. Life Sci 62: 1561–1565, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Gentzsch M, Choudhury A, Chang XB, Pagano RE, Riordan JR. Misassembled mutant ΔF508 CFTR in the distal secretory pathway alters cellular lipid trafficking. J Cell Sci 120: 447–455, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Gévry NY, Lalli E, Sassone-Corsi P, Murphy BD. Regulation of Niemann-Pick C1 gene expression by the 3′,5′-cyclic adenosine monophosphate pathway in steroidogenic cells. Mol Endocrinol 17: 704–715, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Hajj R, Lesimple P, Nawrocki-Raby B, Birembaut P, Puchelle E, Coraux C. Human airway surface epithelial regeneration is delayed and abnormal in cystic fibrosis. J Pathol 211: 340–350, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Heeckeren A, Walenga R, Konstan MW, Bonfield T, Davis PB, Ferkol T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest 100: 2810–2815, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hybiske K, Fu Z, Schwarzer C, Tseng J, Do J, Huang N, Machen TE. Effects of cystic fibrosis transmembrane conductance regulator and ΔF508CFTR on inflammatory response, ER stress, and Ca2+ of airway epithelia. Am J Physiol Lung Cell Mol Physiol 293: L1250–L1260, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Kelley TJ, Drumm ML. Inducible nitric oxide synthase expression is reduced in cystic fibrosis murine and human airway epithelial cells. J Clin Invest 102: 1200–1207, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley TJ, Elmer HL. In vivo alterations of IFN regulatory factor-1 and PIAS1 protein levels in cystic fibrosis epithelium. J Clin Invest 106: 403–410, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konstan MW, Davis PB, Wagener JS, Hilliard KA, Stern RC, Milgram LJ, Kowalczyk TH, Hyatt SL, Fink TL, Gedeon CR, Oette SM, Payne JM, Muhammad O, Ziady AG, Moen RC, Cooper MJ. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Hum Gene Ther 15: 1255–1269, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Kreiselmeier NE, Kraynack NC, Corey DA, Kelley TJ. Statin-mediated correction of STAT1 signaling and inducible nitric oxide synthase expression in cystic fibrosis epithelial cells. Am J Physiol Lung Cell Mol Physiol 285: L1286–L1295, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Kruth HS, Comly ME, Butler JD, Vanier MT, Fink JK, Wenger DA, Patel S, Pentchev PG. Type C Niemann-Pick disease. Abnormal metabolism of low density lipoprotein in homozygous and heterozygous fibroblasts. J Biol Chem 261: 16769–16774, 1986. [PubMed] [Google Scholar]
- 20.Kube D, Sontich U, Fletcher D, Davis PB. Proinflammatory cytokine responses to P. aeruginosa infection in human airway epithelial cell lines. Am J Physiol Lung Cell Mol Physiol 280: L493–L502, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Lemberger T, Parkitna JR, Chai M, Schütz G, Engblom D. CREB has a context-dependent role in activity-regulated transcription and maintains neuronal cholesterol homeostasis. FASEB J 22: 2872–2879, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Mak JC, Chuang TT, Harris CA, Barnes PJ. Increased expression of G protein-coupled receptor kinases in cystic fibrosis lung. Eur J Pharmacol 436: 165–172, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Matthews RP, McKnight GS. Characterization of the cAMP response element of the cystic fibrosis transmembrane conductance regulator gene promoter. J Biol Chem 271: 31869–31877, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Meng QH, Polak JM, Edgar AJ, Chacon MR, Evans TJ, Gruenert DC, Bishop AE. Neutrophils enhance expression of inducible nitric oxide synthase in human normal but not cystic fibrosis bronchial epithelial cells. J Pathol 190: 126–132, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Meng QH, Springall DR, Bishop AE, Morgan K, Evans TJ, Habib S, Gruenert DC, Gyi KM, Hodson ME, Yacoub MH, Polak JM. Lack of inducible nitric oxide synthase in bronchial epithelium: a possible mechanism of susceptibility to infection in cystic fibrosis. J Pathol 184: 323–331, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Moore RH, Tuffaha A, Millman EE, Dai W, Hall HS, Dickey BF, Knoll BJ. Agonist-induced sorting of human beta2-adrenergic receptors to lysosomes during downregulation. J Cell Sci 112: 329–338, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Morrissey BM, Schilling K, Weil JV, Silkoff PE, Rodman DM. Nitric oxide and protein nitration in the cystic fibrosis airway. Arch Biochem Biophys 406: 33–39, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Perez A, Issler AC, Cotton CU, Kelley TJ, Verkman AS, Davis PB. CFTR inhibition mimics the cystic fibrosis inflammatory profile. Am J Physiol Lung Cell Mol Physiol 292: L383–L395, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Perez A, Risma KA, Eckman EA, Davis PB. Overexpression of R domain eliminates cAMP-stimulated Cl− secretion in 9/HTEo- cells in culture. Am J Physiol Lung Cell Mol Physiol 271: L85–L92, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Poschet JF, Fazio JA, Timmins GS, Ornatowski W, Perkett E, Delgado M, Deretic V. Endosomal hyperacidification in cystic fibrosis is due to defective nitric oxide-cyclic GMP signalling cascade. EMBO Rep 7: 553–559, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rommens JM, Dho S, Bear CE, Kartner N, Kennedy D, Riordan JR, Tsui LC, Foskett JK. cAMP-inducible chloride conductance in mouse fibroblast lines stably expressing the human cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA 88: 7500–7504, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott C, Higgins ME, Davies JP, Ioannou YA. Targeting of NPC1 to late endosomes involves multiple signals, including one residing within the putative sterol-sensing domain. J Biol Chem 279: 48214–48223, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Sharma RK, Jeffery PK. Airway beta-adrenoceptor number in cystic fibrosis and asthma. Clin Sci (Lond) 78: 409–417, 1990. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava M, Eidelman O, Zhang J, Paweletz C, Caohuy H, Yang Q, Jacobson KA, Heldman E, Huang W, Jozwik C, Pollard BS, Pollard HB. Digitoxin mimics gene therapy with CFTR and suppresses hypersecretion of IL-8 from cystic fibrosis lung epithelial cells. Proc Natl Acad Sci USA 101: 7693–7698, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teichgräber V, Ulrich M, Endlich N, Riethmüller J, Wilker B, De Oliveira-Munding CC, van Heeckeren AM, Barr ML, von Kürthy G, Schmid KW, Weller M, Tumbler B, Lang F, Grasse H, During G, Goblins E. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med 14: 382–391, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Thomas GR, Costelloe EA, Lunn DP, Stacey KJ, Delaney SJ, Passey R, McGlinn EC, McMorran BJ, Ahadizadeh A, Geczy CL, Wainwright BJ, Hume DA. G551D cystic fibrosis mice exhibit abnormal regulation of inflammation in lungs and macrophages. J Immunol 164: 3870–3877, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Tirouvanziam R, de Bentzmann S, Hubeau C, Hinnrasky J, Jacquot J, Péault, B Puchelle E. Inflammation and infection in naive human cystic fibrosis airway grafts. Am J Respir Cell Mol Biol 23: 121–127, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Van Heeckeren AM, Schluchter MD, Drumm ML, Davis PB. Role of Cftr genotype in the response to chronic Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol 287: L944–L952, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Weber AJ, Soong G, Bryan R, Saba S, Prince A. Activation of NF-κB in airway epithelial cells is dependent on CFTR trafficking and Cl− channel function. Am J Physiol Lung Cell Mol Physiol 281: L71–L78, 2001. [DOI] [PubMed] [Google Scholar]
- 40.White NM, Corey DA, Kelley TJ. Mechanistic similarities between cultured cell models of cystic fibrosis and Niemann-Pick type C. Am J Respir Cell Mol Biol 31: 538–543, 2004. [DOI] [PubMed] [Google Scholar]
- 41.White NM, Jiang D, Burgess JD, Bederman IR, Previs SF, Kelley TJ. Altered cholesterol homeostasis in cultured and in vivo models of cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 292: L476–L486, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Widdicombe JH Cystic fibrosis and β-adrenergic response of airway epithelial cell cultures. Am J Physiol Regul Integr Comp Physiol 251: R818–R822, 1986. [DOI] [PubMed] [Google Scholar]
- 43.Wu JH, Peppel K, Nelson CD, Lin FT, Kohout TA, Miller WE, Exum ST, Freedman NJ. The adaptor protein beta-arrestin2 enhances endocytosis of the low density lipoprotein receptor. J Biol Chem 278: 44238–44245, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Xu W, Zheng S, Goggans TM, Kiser P, Quinones-Mateu ME, Janocha AJ, Comhair SA, Slee R, Williams BR, Erzurum SC. Cystic fibrosis and normal human airway epithelial cell response to influenza a viral infection. J Interferon Cytokine Res 26: 609–627, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Zheng S, Xu W, Bose S, Banerjee AK, Haque SJ, Erzurum SC. Impaired nitric oxide synthase-2 signaling pathway in cystic fibrosis airway epithelium. Am J Physiol Lung Cell Mol Physiol 287: L374–L381, 2004. [DOI] [PubMed] [Google Scholar]