Abstract
A physiological cross talk operates between the tumor suppressor protein p53 and the bradykinin B2 receptor (BdkrB2) during renal organogenesis. Thus, although BdkrB2 is a target for p53-mediated transcriptional activation, BdkrB2 is required to restrict p53 proapoptotic activity. We previously demonstrated that BdkrB2−/− embryos exposed to gestational salt stress develop renal dysgenesis as a result of p53-mediated apoptosis of nephron progenitors and repression of the terminal differentiation program. Compared with wild-type kidneys, BdkrB2−/− express abnormally high levels of the Checkpoint kinase (Chk1), which activates p53 via Ser23 phosphorylation. To define the functional relevance of p53S23 phosphorylation, we generated a compound strain of BdkrB2−/− mice harboring a homozygous Ser23-to-Ala (S23A) mutation in the p53 gene by crossing BdkrB2−/− with p53S23A knockin mice. Unlike salt-stressed BdkrB2−/− pups, which exhibit renal dysgenesis, homozygous S23A;BdkrB2−/− littermates are protected and have normal renal development. Heterozygous S23A;BdkrB2−/− mice have an intermediate phenotype. The p53-S23A substitution was associated with amelioration of apoptosis and restored markers of nephrogenesis and tubulogenesis. Real-time quantitative RT-PCR of terminal differentiation genes demonstrated that the S23A substitution restored normal expression patterns of aquaporin-2, Na-Cl cotransporter, Na-K-2Cl cotransporter, Na-bicarbonate cotransporter, and Sglt1. We conclude that p53 phosphorylation on Ser23 is an essential step in the signaling pathway mediating the susceptibility of BdkrB2−/− mutants to renal dysgenesis.
Keywords: gene knockin, gene knockout, kidney development, nephrogenesis
a complex network of genes encoding transcription factors, soluble factors, receptors, and cell-cell and cell-matrix molecules regulate ureteric bud (UB) outgrowth from the Wolffian duct and subsequent branching, mesenchymal-to-epithelial transition, nephrogenesis, segmental patterning, and terminal differentiation (8, 28, 33, 45). Abnormal kidney development, the leading cause of chronic renal failure in children, results from disruption of one or more of these processes. Although monogenetic syndromes continue to be unraveled, a sizable proportion of cases are sporadic and considered the result of gene-environment interactions. However, how embryonic stressors combine with silent genetic mutations to induce congenital defects remains to be defined. A better understanding of these processes is necessary to advance preventive and therapeutic strategies for organ dysgenesis.
Our laboratory has developed and characterized a mouse model of renal dysgenesis that is produced by defined gene-environment interactions (21, 23, 24). This animal model established that the bradykinin B2 receptor, a G protein-coupled receptor, is required for renal development under conditions of fetal stress. The bradykinin B2 receptor (gene, BdkrB2; protein, B2R) is highly expressed in the developing kidney (15, 16, 19, 20). B2R signaling is linked to both mitogenic and differentiation signaling pathways, depending on the cellular context, developmental stage, and type of stress (3, 17). We reported that gestational salt loading (5% NaCl in maternal diet) induces abnormal renal development in homozygous BdkrB2−/− but not heterozygous or wild-type progeny. The aberrant renal phenotype is evident histologically on embryonic day 16 (E16), shortly after the onset of metanephric BdkrB2 gene expression, and consists of collecting duct dysgenesis, stromal expansion, and apoptosis (18, 21, 23, 24). All of these abnormalities are rescued by p53 gene deletion (24), implicating aberrant p53 activation in mediating the susceptibility to renal dysgenesis in BdkrB2−/− mice.
Previous studies that compared the proteomes of salt-stressed BdkrB2+/+ and BdkrB2−/− newborns revealed that the dysplastic kidney exhibits a dramatic induction of the checkpoint kinase (Chk1) and hyperphosphorylation of p53 on Ser23 (23, 24). Chk1 is activated in response to DNA damage and mediates p53 phosphorylation on the Ser23 residue (human p53 Ser20) (7, 43). Ser23 phosphorylation prevents the interaction of p53 with Mdm2, an E3 ubiquitin ligase that mediates p53 degradation (9, 12, 14). Taken together, our findings suggest two potential models: 1) embryonic salt stress cooperates with BdkrB2 inactivation to induce Chk1-induced p53S23 phosphorylation, metanephric growth arrest, and apoptosis, and hence renal dysplasia; or 2) p53S23 phosphorylation is a co phenomenon unrelated to the renal dysgenesis. To distinguish between these two possibilities, we employed an in vivo genetic targeting approach in which a homozygous Ser23-to-Ala knockin mutation (S23A) was introduced into the genome of BdkrB2−/− mice to specifically prevent p53S23 phosphorylation. The results demonstrate unequivocally that p53 phosphorylation on Ser23 is a critical step in the signaling pathway mediating the susceptibility of BdkrB2−/− offspring to renal dysgenesis.
MATERIALS AND METHODS
Animals and Breeding Strategy
BdkrB2−/− mice are bred on a C57BL/6 genetic background and have been described previously (10, 21). p53S23A/S23A knockin mice were generously provided by Dr. Tyler Jack's laboratory (MIT, Cambridge, MA) and are on a mixed 129/Sv × C57BL/6 background (35). In these mice, the DNA sequence of the p53 gene was the same as wild type, except for the single T-G base substitution targeted to exon 2, which results in S23A substitution (Fig. 1A). BdkrB2−/− and p53S23A/S23A mice were crossed to produce double heterozygous mice, which were intercrossed to generate double homozygous p53S23A/S23A;BdkrB2−/− mice (Fig. 1B). Pregnant mice were placed on high-salt (5% NaCl) isocaloric chow (Harlan Laboratories, Madison, WI) for the duration of gestation or as indicated in the experimental protocols. The animal study protocol was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee.
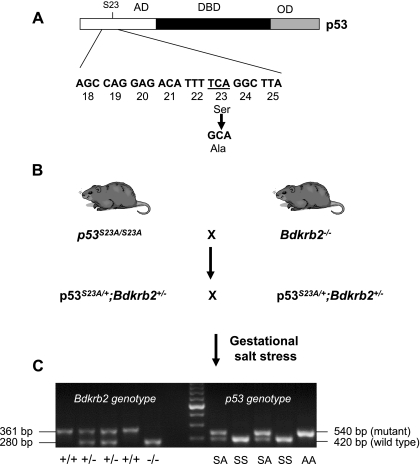
Fig. 1.
Animal breeding strategy and genotyping. A: the S23A mutation in p53 that is introduced by a single nucleotide substitution is represented. AD, transcriptional activation domain; DBD, DNA binding domain; OD, oligomerization domain. B: strategy of genetic crosses between p53S23A × BdkrB2−/− mice. BdkrB2, bradykinin B2 receptor gene. C: PCR genotyping of S23A mice (right) was based on the presence of a single loxP site remaining in intron 1 of the correctly targeted locus. PCR genotyping of BdkrB2−/− (left) shows the targeted allele (280 bp) and wild-type allele (361 bp).
p53 PCR genotyping was based on the presence of a single loxP site remaining in intron 1 of the correctly targeted locus. The following primers flanking the remaining loxP site were used: 5′-agcctgcctagcttcctcagg-3′ and 5′-cttggagacatagccacactg-3′. These primers amplify a 540-bp mutant band in the presence of a single loxP site and a 420-bp wild-type band. BdkrB2 genotyping was performed according to established protocols from the Jackson Laboratory (Bar Harbor, ME) (Fig. 1C).
Histological Analysis
Five-micrometer frontal corticomedullary sections taken through the hilus were stained with periodic acid-Schiff to delineate the proximal tubular brush border and glomerular mesangial matrix. On examination of the stained sections, particular attention was focused on the integrity of the nephrogenic zone, the organization of the medullary rays, the presence of tubular cysts/dysplasia and glomerular cysts, and the thickness and integrity of the renal microvessels. “Dysplasia” referred to abnormal spatial organization of nephron segments within the cortex or medulla, cystic dilatation or “ectasia” of the tubules, mesenchymal expansion, metaplasia, and primitive glomeruli or ducts. Histological evaluations of kidney tissue sections were performed in 7–10 mice per group.
In Situ Apoptosis Detection
Terminal deoxynucleotide transferase-mediated dUTP-biotin nick-end labeling (TUNEL) assays were performed as described previously (24) using an in situ cell death detection kit (Chemicon). After digestion with 20 μg/ml proteinase K for 15 min at room temperature, the sections were peroxidase quenched with 30% H2O2, and the TUNEL reaction mixture was added to cover each section. The slides were then incubated in a humidified chamber for 60 min at 37°C with a coverslip to prevent drying of sections. The reaction was stopped by a stop/wash buffer. Sections were incubated with anti-digoxigenin conjugate, and color was developed in a peroxidase substrate. The slides were counterstained with 0.5% methyl green and examined using light microscopy. The apoptosis index (percentage of TUNEL-positive cells) was determined in four microscopic fields (800 × 530 μm) of each kidney section.
Immunostaining
Kidneys were fixed in 10% buffered formalin, dehydrated in graded solutions of alcohol, and embedded in paraffin blocks, and 3- to 5-μm sections were cut and mounted on Vectabond-coated slides (Vector Laboratories, Burlingame, CA). Antigen retrieval was performed by immersing slides in 10 mM citric acid and boiling in a microwave for 20 min. Immunostaining was performed using the immunoperoxidase technique with a Vectastain Elite kit (Vector Laboratories). The primary antibody used was a polyclonal anti-phospho-Ser20 p53 (Ser23 in mouse) (1:1,000; Cell Signaling). Slides were photographed using an Olympus model SC35 camera mounted to an Olympus model BH-2 microscope, and digital images were captured using Adobe Photoshop software.
Whole Mount Immunofluorescence
Cultured metanephroi were fixed in 100% methanol for 10 min at room temperature and washed in PBST (phosphate-buffered saline with 1% Tween 20) twice for 20 min each. The primary antibodies diluted in PBST were placed on samples overnight and consisted of anti-cytokeratin (mouse monoclonal, 1:200; Sigma) to label the UB branches, WT1 (rabbit polyclonal, 1:400, C-19; Santa Cruz Biotechnology) to label the mesenchyme and glomeruli, and Lotus tetragonobulus agglutinin (LTA; 1:75; Vector Laboratories) to label the proximal tubules. The samples were then washed with PBST for 8 h, with the solution changed approximately every 30 min. Secondary antibody was diluted in PBST, FITC-conjugated goat anti-mouse (1:200), and goat anti-rabbit Alexa Fluor 594 (1:400) (Invitrogen) and placed on samples overnight. The samples were again washed in PBST for 8 h, with the solution changed every 30 min. They were mounted on glass slides with Dako fluorescent mounting medium (DAKO) and stored at 4°C until imaging.
Section Immunofluorescence
After the slides were deparaffinized in xylene followed by an ethanol series rehydration, they were washed in PBS twice before antigen retrieval was performed as described above. The sections were again washed twice for 10 min each before blocking with 10% goat serum in 0.1% BSA in PBS for 30 min in a humidity chamber. The primary antibody was diluted in 3% BSA in PBS and incubated overnight in a humidity chamber at 4°C. Primary antibodies included neural cell adhesion molecule (N-CAM; mouse monoclonal, 1:300; Sigma) and E-cadherin (mouse monoclonal, 1:300; BD Transduction). They were then washed in PBST (PBS with 0.1% Tween) three times for 10 min each before incubation with secondary antibody in PBS in a humidity chamber at room temperature in the dark for 1 h. Secondary antibodies included anti-rabbit Alexa Fluor 488 (1:2,000) and anti-rabbit Alexa Fluor 594 (1:2,000) (Molecular Probes) and anti-mouse FITC (1:200; Sigma). The sections were again washed three times for 10 min each in PBST followed by a final wash in PBS before being mounted with Dako anti-fade fluorescent mounting medium under glass coverslips.
Western Blotting
Immunoblotting was performed as described previously (27) using a polyclonal anti-phospho-Ser23 p53 (1:1,000; Cell Signaling). Membranes were reprobed with monoclonal α-actin antibody (1:4,000; Sigma) as a loading control. Signals were detected using enhanced chemiluminescence (Amersham), and protein expression was analyzed densitometrically using the Digital Imaging and Analysis Systems (Alpha Innotech).
Quantitative RT-PCR
Total kidney RNA was extracted using Trizol (Invitrogen) from three to five animals in each group. RNA was reverse transcribed, and real-time PCR was performed in quadruplicate using the Applied Biosystems 7500 Fast Real-time PCR system and TaqMan GenExpression Assays (Applied Biosystems) for aquaporin-2 (AQP2; Mm00437575_m1), Atp6v1v1/lysosomal V1-H-ATPase β1 (Mm00460309_m1), carbonic anhydrase II (Mm00501572_m1), nephrin (Mm00497828_m1), Slc12a1/Nkcc2 (Mm00441424_m1), Slc12a3/NCC (Mm00490213_m1), Slc4a4/NBC1 (Mm00489998_m1), Slc5a1/Sglt1 (Mm00451203_m1), Slc34a1/Npt2 (Mm00441450_m1), uromodulin (Mm00447649_m1), and GAPDH (endogenous control, FAM/MGB probe, non-primer limited; no. 4352932E). The setup of reaction consisted of 1 μl of cDNA (100 ng), 1 μl of TaqMan primer set, 10 μl Taq [TaqMan Fast Universal PCR master mix (2×), No AmpErase UNG; no. 4366072], and 8 μl of H2O under the following PCR conditions: step 1, 95°C for 20 s (enzyme activation); step 2, 95°C for 3 s (melting); and step 3, 60°C for 30 s (annealing and extension); steps 2 and 3 were repeated 40 times.
Statistical Analysis
Comparisons between the groups were performed using t-tests or one-way ANOVA. A P value <0.05 was considered significant.
RESULTS
Abnormal Kidney Development in Salt-Stressed BdkrB2−/− Mice: Role of p53-S23 Phosphorylation
We previously reported that if salt loading is initiated during embryogenesis, BdkrB2−/− mice acquire an aberrant renal phenotype, which consists of collecting duct dysgenesis, stromal expansion, and impaired nephrogenesis and terminal differentiation (21). These abnormalities are rescued by deletion of the p53 gene in vivo (23, 24). In support of a role for abnormal p53 activity are elevated p53 protein (not mRNA) levels in BdkrB2−/− kidneys accompanied by NH2-terminal hyperphosphorylation of p53 on Ser23 (24), a posttranslational modification that stabilizes the p53 protein by preventing mdm2-mediated degradation (12, 14, 35).
Generation of Double Mutant Mice
To determine the physiological significance of p53 Ser23 phosphorylation in mediating the renal phenotype, we generated compound BdkrB2−/− mutant mice harboring a S23A substitution in p53. p53S23A/+;BdkrB2+/− mice were intercrossed to generate offspring of various genotypes (Fig. 1). Pregnant mice were placed on either normal (0.3%) or high-salt (5% NaCl) isocaloric chow (Harlan Laboratories) for the duration of gestation or as indicated in the experimental protocols.
Homozygous p53 S23A Substitution Rescues the Susceptibility of BdkrB2−/− Mice to Renal Dysgenesis
We compared the individual and combined effects of gestational salt, p53-S23A knockin mutation, and BdkrB2 knockout on renal development.
Newborn mice.
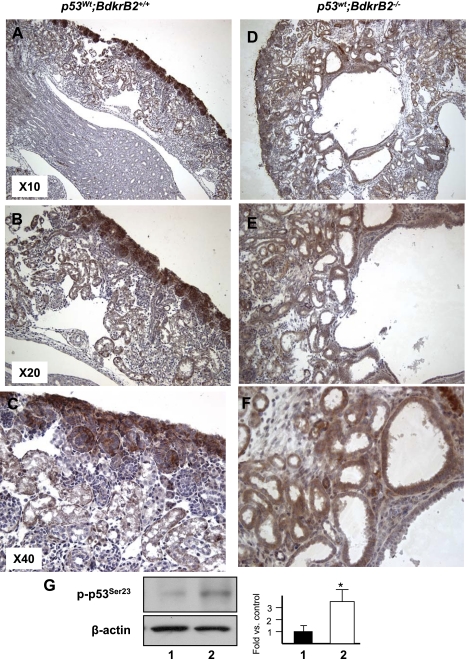
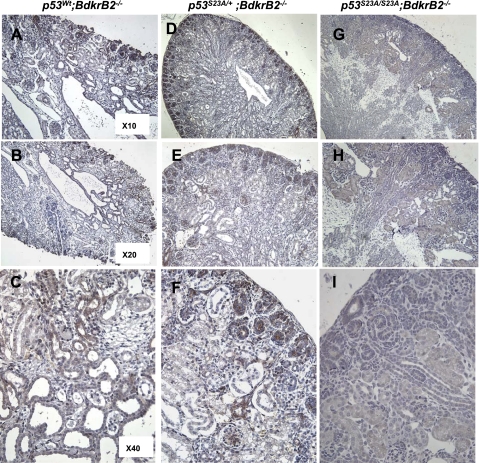
As previously reported (6, 21, 23, 24, 27), gestational high salt or BdkrB2 deletion individually had no significant effect on renal development (data not shown). On the other hand, the combination of gestational salt and BdkrB2−/− was detrimental to renal development. At postnatal day 1 (P1), salt-stressed p53wt;BdkrB2−/− pups exhibited tubular cysts in the medulla, expanded stroma, and thinning of the peripheral nephrogenic zone (Fig. 2, D–F). These changes were not observed in salt-stressed p53wt;BdkrB2+/+ littermates (Fig. 2, A–C). In p53wt;BdkrB2+/+ mice, immunoreactive phospho-p53S23 was expressed in the nephrogenic zone with lower levels in deeper, more mature nephrons (Fig. 2, A–C). In comparison, phospho-p53S23 was diffusely expressed in dysplastic kidneys (Fig. 2, D–F). Western blots of kidney nuclear extracts confirmed that dysplastic kidneys accumulated approximately threefold higher levels of phospho-p53S23 than normal kidneys (Fig. 2G). Remarkably, the dysplastic changes in the renal tubules of salt-stressed P1 p53wt;BdkrB2−/− mice (Fig. 3, A–C) were rescued partially by heterozygous p53S23A/+ (Fig. 3, D–F) and completely by homozygous p53S23A/S23A substitutions (Fig. 3, G–I). As predicted, p53S23A/S23A mice demonstrated lack of phospho-p53S23 immunoreactivity (Fig. 3C).
Fig. 2.
Renal phenotype of newborn (P1) BdkrB2−/− and BdkrB2+/+ mice subjected to gestational high salt. Brown staining represents phospho-p53S23 (p-p53S23) distribution. A–C: salt-stressed BdkrB2+/+ pups have normal overall kidney morphology, medullary development, and corticomedullar differentiation. P-p53S23 is predominantly expressed in metanephric mesenchyme and nephron progenitors with lower levels in mature tubules. wt, Wild type. D–F: salt-stressed BdkrB2−/− kidneys are cystic-dysplastic. P-p53S23 is expressed diffusely and ectopically. G: Western blot analysis of p-p53S23 and β-actin (loading control). Phospho-p53S23 levels are approximately 3-fold higher in dysplastic than normal kidneys. Lane 1, p53wt;BdkrB2+/+; lane 2, p53wt;BdkrB2−/−.
Fig. 3.
p53-S23A substitution rescues renal dysgenesis in BdkrB2 null mice. A–C: tubular dysgenesis in a salt-stressed BdkrB2−/− P1 mouse. P-p53S23 is expressed diffusely. Compound heterozygous p53S23A/+;BdkrB2−/− (D–F) and nullizygous p53S23A/S23A;BdkrB2−/− mice (G–I) exhibit a partial or complete rescue of kidney development, respectively. As expected, homozygous S23A mice show background nonspecific staining for p-p53S23 (G–I).
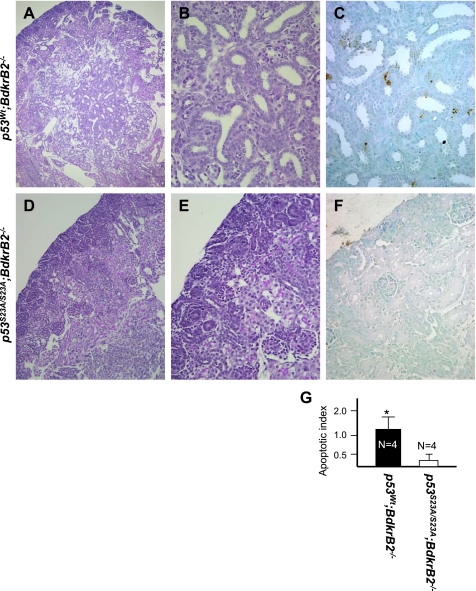
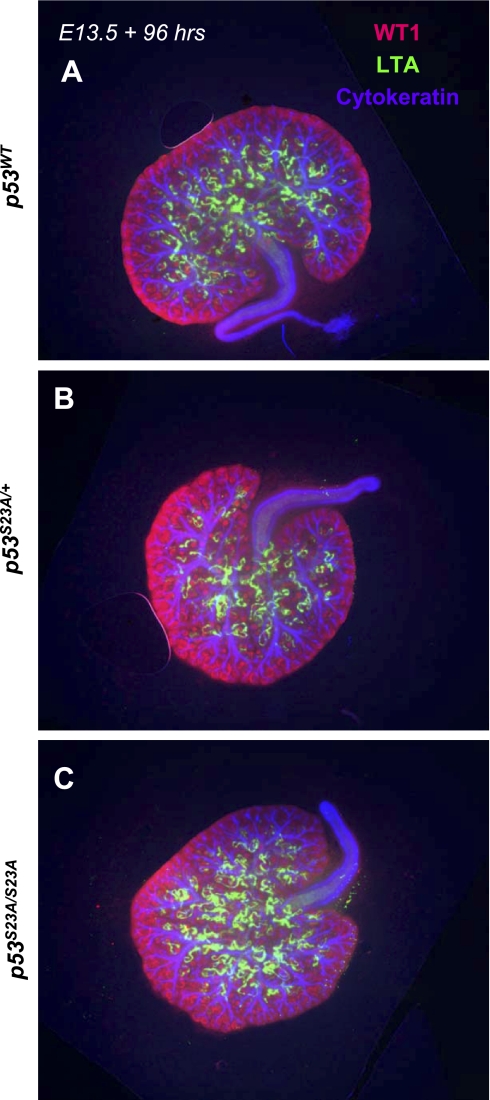
In our previous studies, we observed up to a 10-fold higher apoptosis index in the tubules and collecting ducts of salt-stressed fetal and newborn p53wt;BdkrB2−/− than control littermates (22). This was rescued by deletion of p53 (22). In this study, we examined whether loss of p53 phosphorylation on Ser23 mimics the salutary effects of p53 deletion. Figure 4, A–C, depicts a kidney section from a representative salt-stressed P1 p53wt;BdkrB2−/− mouse revealing focal tubular dysplasia accompanied by excessive cellular apoptosis. In comparison, a p53S23A/S23A;BdkrB2−/− littermate exhibited no evidence of tubular dysplasia or abnormal cell death (Fig. 4, D–G). Thus our data indicate that loss of p53 phosphorylation on Ser23 mimics p53 deletion, protecting BdkrB2 null mice from renal dysgenesis. It is important to note that the p53S23A/S23A substitution per se had no apparent effect on glomerular, proximal tubular, or collecting duct development ex vivo (Fig. 5, A–C) or in vivo (not shown).
Fig. 4.
p53-S23A prevents metanephric apoptosis in salt-stressed BdkrB2−/− mice. Periodic acid-Schiff (PAS) staining is shown in A, B, D, and E and in situ terminal deoxynucleotide transferase-mediated dUTP-biotin nick-end labeling (TUNEL) staining in C and F. A–C: medullary dysgenesis in a P1 p53wt;BdkrB2−/− mouse. Note immature ureteric bud-like collecting ducts. TUNEL-positive cells are observed in and around the dysplastic tubules. D–F: a p53S23A/S23A;BdkrB2−/− littermate mouse showing normal tubulogenesis and lack of apoptosis. G: apoptotic index in P1 salt-stressed p53wt;BdkrB2−/− and p53S23A/S23A;BdkrB2−/− mice. *P < 0.05.
Fig. 5.
The p53-S23A mutation by itself has no effect on renal development. Embryonic day 13.5 (E13.5) kidneys were cultured in vitro for 96 h and then fixed and processed for triple immunofluorescence for WT1, Lotus tetragonobulus agglutinin (LTA), and cytokeratin. No apparent abnormalities were observed in heterozygous (B) or homozygous p53S23A mice (C) compared with wild-type control littermates (A).
E14.5–E17.5 mice.
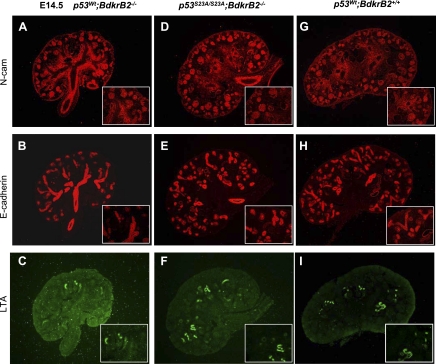
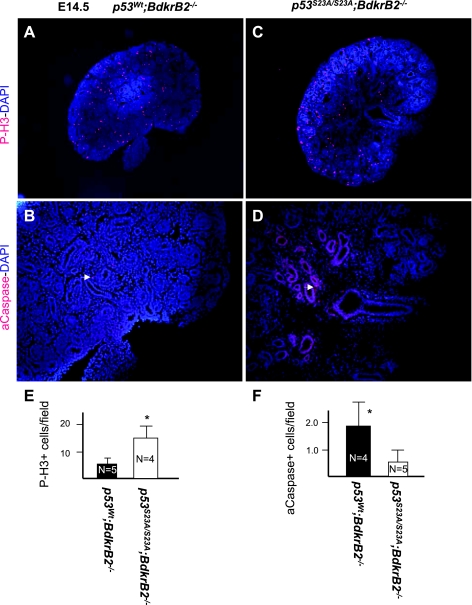
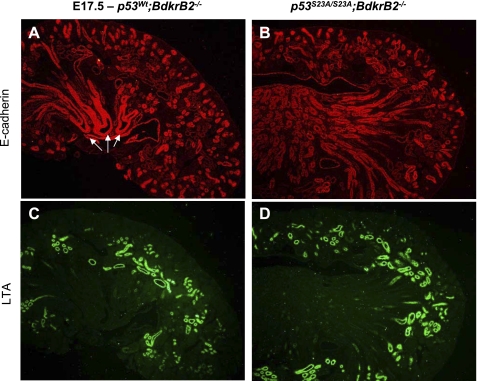
We previously reported that the onset of tubular dysgenesis in salt-stressed BdkrB2−/− mice at E16.5 is preceded by apoptosis (24). In this study, we confirmed that at E14.5, the integrity of nephron progenitors (N-CAM positive), proximal (LTA positive), and distal (E-cadherin positive) nephron segments was more preserved in salt-stressed p53S23A/S23A; BdkrB2−/− than p53wt;BdkrB2−/− littermates (Fig. 6, A–I). This was accompanied by lower rates of apoptosis and higher rates of proliferation (Fig. 7, A–F). On E17.5, salt-stressed p53wt; BdkrB2−/− mice exhibited short, dilated collecting ducts (Fig. 8A, arrows). These abnormalities were not observed in p53S23A/S23A;BdkrB2−/− mice (Fig. 8B). No structural abnormalities were observed in salt-stressed p53wt;BdkrB2+/− or p53S23A/+; BdkrB2−/− mice (not shown), indicating that the induction of renal dysgenesis requires wild-type p53 alleles and homozygous BdkrB2 gene disruption.
Fig. 6.
Effect of p53-S23A substitution on nephrogenesis and tubulogenesis in salt-stressed BdkrB2−/− mice. Metanephroi were harvested at E14.5 and processed for section immunofluorescence. LTA, E-cadherin, and neural cell adhesion molecule (N-CAM) abundance was restored in p53S23A/S23A;BdkrB2−/− mice (D–F) to a level similar to that in wild-type p53wt;BdkrB2+/+ (G–I) compared with p53wt;BdkrB2−/− mice (A–C).
Fig. 7.
p53-S23A substitution restores renal cell proliferation and apoptosis in E14.5 salt-stressed BdkrB2−/− mice. A and C: phospho-histone H3 (P-H3). B and D: activated caspase-3 (aCaspase; arrows). Nuclei were costained with 4,6-diamidino-2-phenylindole (DAPI; blue). E and F: quantitative analysis of proliferating and apoptotic cells. *P < 0.05.
Fig. 8.
p53-S23A substitution corrects collecting duct dysgenesis and renal development in E17.5 salt-stressed wild-type (A and C) and homozygous p53S23A (B and D) BdkrB2−/− mice. Arrows in A depict the dysplastic collecting ducts.
Reversal of Gene Expression Changes in p53S23A-BdkrB2−/− Mice
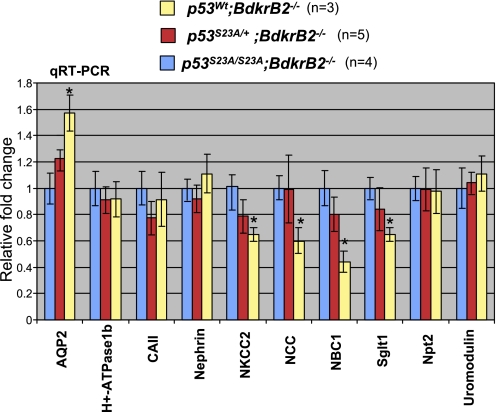
In view of the importance of p53S23 phosphorylation in mediating the susceptibility to renal dysgenesis in BdkrB2−/− mice, we examined the impact of the p53S23A mutation on expression of terminal differentiation markers by quantitative real-time PCR. Rescue of renal development in p53S23A/S23A;BdkrB2−/− P1 mice was associated with a significant increase in expression of several markers. For example, NKCC2 (Na-K-2Cl cotransporter), NCC (Na-Cl cotransporter), NBC1 (Na-bicarbonate cotransporter), and Sglt1 mRNA levels were 25–50% higher in p53S23A/S23A;BdkrB2−/− than p53wt;BdkrB2−/− mice (Fig. 9). Others, such as Npt2, nephrin, and uromodulin, were not affected. Rescue of collecting duct development in p53S23A/S23A;BdkrB2−/− was also accompanied by a 50% decrease in AQP2 mRNA levels. Levels of H+-ATPase β1 and carbonic anhydrase II mRNA, both markers of intercalated cells, did not change appreciably. Compound heterozygous mice exhibited intermediate changes in gene expression (Fig. 9). There were no differences in gene expression between p53S23A/S23A;BdkrB2−/− and wild-type mice (data not shown).
Fig. 9.
Quantitative real-time RT-PCR of renal function genes. Renal dysplasia in salt-stressed P1 p53wt;BdkrB2−/− mice is associated with upregulation of aquaporin-2 (AQP2) and repression of Na-Cl cotransporter (NCC), Na-bicarbonate cotransporter (NBC1), Na-K-2Cl cotransporter (NKCC2), and Sglt1. These changes in gene expression were corrected by p53-S23A. CAII, carbonic anhydrase II. *P < 0.05.
DISCUSSION
The present study demonstrates that phosphorylation of p53 on Ser23 is an essential requirement for the susceptibility to renal dysgenesis in BdkrB2 null mice. Aberrant p53 activity induces metanephric apoptosis and alters the terminal differentiation program, leading to renal dysgenesis.
The bradykinin B2 receptor (BdkrB2, B2R) is a developmentally regulated G protein-coupled receptor that is highly expressed in differentiating collecting ducts (20). Previous work from our laboratory has shown that the tumor suppressor protein p53 is a direct transcriptional activator of the BdkrB2 gene (37, 42). During collecting duct differentiation, p53 recruits the histone acetylase cAMP response element binding protein (CREB)-binding protein (CBP) to the promoter region of the BdkrB2 gene (41). Genetic deletion of p53 in mice impairs CBP recruitment and BdkrB2 expression, supporting the notion that BdkrB2 is a physiological target of p53 (41). Other studies have shown that BdkrB2-deficient mice are prone to salt-sensitive hypertension (1, 2, 11, 13, 36). Interestingly, salt sensitivity in BdkrB2−/− mice is established during embryogenesis; salt-stressed BdkrB2−/− embryos exhibit repression of terminal differentiation genes, e.g., E-cadherin, Dlg, HNF-1β, and renin (23), hyperphosphorylation of p53 on Ser23, and metanephric apoptosis (24). All of these abnormalities are rescued by deletion of p53 (23, 24). Accordingly, existing data strongly support the presence of genetic epistasis between p53 and BdkrB2.
p53 is a tumor suppressor and a short-lived protein due to its rapid proteosomal degradation by the E3 ubiquitin ligase Mdm2 (5, 25, 31, 34, 39). Genotoxic stress prolongs p53 half-life and activates its transcriptional activity as a result of posttranslational modifications, e.g., phosphorylation and acetylation. Phosphorylation of the NH2 terminus of p53 on one or more serine residues is believed to relieve p53 from mdm2-mediated inhibition and degradation (4, 9, 32). Phosphorylation of p53 on Ser23 (corresponding to Ser20 in humans) by the checkpoint kinase Chk1 is accompanied by stabilization of p53 (7, 43). Mice with a knockin S23A mutation in the p53 gene have defective capacity to induce cell death and cell cycle arrest in response to DNA damage (35). Previously, we showed that newborn BdkrB2−/− mice exhibit overexpression of renal Chk1 and phospho-p53S23 (24). Although p53 deletion rescues the susceptibility to renal dysgenesis in BdkrB2−/− mice, the exact role of p53S23 phosphorylation was unknown. The present study provides a genetic proof that salt-stressed BdkrB2−/− mice lacking the ability to phosphorylate p53 on Ser23 do not exhibit metanephric apoptosis and have normal tubular differentiation. Moreover, quantitative real-time PCR confirmed that salt-stressed BdkrB2−/− mice manifest repression of several terminal differentiation genes, including NCC, NKCC2, Sglt1, and NBC1, compared with wild-type littermates. These aberrations in gene expression were corrected in BdkrB2−/− mice harboring the p53-S23A gene. In previous studies, we reported that c-ret expression is maintained in the tips of UB branches despite impaired branching in salt-stressed BdkrB2−/− embryonic kidneys (24). Similarly, Eya1, Pax2, and Wnt11 are unaltered (Stefkova J and El-Dahr SS, unpublished observations). Thus the precise developmental pathway affected in BdkrB2−/− mice remains to be defined. Interestingly, unlike other differentiation markers, AQP2 expression was upregulated in the dysplastic kidneys and is reminiscent of enhanced AQP2 in polycystic kidney disease (26). The cAMP-PKA pathway regulates AQP2 gene expression, and cAMP-mediated signaling is altered in PKD epithelia (40, 44). Whether a similar abnormality exists in kidneys of BdkrB2−/− mice remains to be determined.
In summary, the present study demonstrates that Chk1-induced p53S23 phosphorylation mediates the susceptibility of BdkrB2−/− mice to renal dysgenesis. The mechanism(s) by which Chk1 is induced by the combination of gestational salt stress and BdkrB2 disruption and how phospho-p53S23 represses terminal differentiation genes and induces apoptosis remain to be investigated. Previous studies have shown that phosphorylation of p53 on Ser46 targets the MDM2 promoter in preference to that for PTEN (38), suggesting that p53 phosphorylation alters target gene selection. Along this line, it is conceivable that p53 phosphorylation on Ser23 alters p53 function in salt-stressed BdkrB2−/− mice, leading to selective activation of apoptosis genes and repression of terminal differentiation. The precise mechanism(s) is not clear but may involve interaction with transcriptional coregulators and other interacting proteins (9, 29, 30).
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-56264 and DK-62250.
Acknowledgments
We thank the laboratory of Dr. Tyler Jacks for providing p53S23A mutant mice. We also thank Drs. Oliver Wessely and Zubaida Saifudeen and their laboratory personnel for insightful discussions and technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alfie ME, Sigmon DH, Pomposiello SI, Carretero OA. Effect of high salt intake in mutant mice lacking bradykinin-B2 receptors. Hypertension 29: 483–487, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Alfie ME, Yang XP, Hess F, Carretero OA. Salt-sensitive hypertension in bradykinin B2 receptor knockout mice. Biochem Biophys Res Commun 224: 625–630, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Alric C, Pecher C, Schanstra JP, Bascands JL, Girolami JP. Bradykinin-induced inhibition of cell proliferation and tyrosine kinase activity in rat mesangial cells. Int J Mol Med 5: 85–93, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Ashcroft M, Taya Y, Vousden KH. Stress signals utilize multiple pathways to stabilize p53. Mol Cell Biol 20: 3224–3233, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashcroft M, Vousden KH. Regulation of p53 stability. Oncogene 18: 7637–7643, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Baricos WH, Cortez SL, El-Dahr SS, Schnaper HW. ECM degradation by cultured human mesangial cells is mediated by a PA/plasmin/MMP-2 cascade. Kidney Int 47: 1039–1047, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3: 421–429, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Bates CM Kidney development: regulatory molecules crucial to both mice and men. Mol Genet Metab 71: 391–396, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer 4: 793–805, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Borkowski JA, Ransom RW, Seabrook GR, Trumbauer M, Chen H, Hill RG, Strader CD, Hess JF. Targeted disruption of a B2 bradykinin receptor gene in mice eliminates bradykinin action in smooth muscle and neurons. J Biol Chem 270: 13706–13710, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Cervenka L, Harrison-Bernard LM, Dipp S, Primrose G, Imig JD, El-Dahr SS. Early onset salt-sensitive hypertension in bradykinin B2 receptor null mice. Hypertension 34: 176–180, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Chao C, Herr D, Chun J, Xu Y. Ser18 and 23 phosphorylation is required for p53-dependent apoptosis and tumor suppression. EMBO J 25: 2615–2622, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao C, Madeddu P, Wang C, Liang Y, Chao L, Chao J. Differential regulation of kallikrein, kininogen, and kallikrein-binding protein in arterial hypertensive rats. Am J Physiol Renal Fluid Electrolyte Physiol 271: F78–F86, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Chehab NH, Malikzay A, Stavridi ES, Halazonetis TD. Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc Natl Acad Sci USA 96: 13777–13782, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Dahr SS Spatial expression of the kallikrein-kinin system during nephrogenesis. Histol Histopathol 19: 1301–1310, 2004. [DOI] [PubMed] [Google Scholar]
- 16.El-Dahr SS, Chao J. Spatial and temporal expression of kallikrein and its mRNA during nephron maturation. Am J Physiol Renal Fluid Electrolyte Physiol 262: F705–F711, 1992. [DOI] [PubMed] [Google Scholar]
- 17.El-Dahr SS, Dipp S, Baricos WH. Bradykinin stimulates the ERK→Elk-1→Fos/AP-1 pathway in mesangial cells. Am J Physiol Renal Physiol 275: F343–F352, 1998. [DOI] [PubMed] [Google Scholar]
- 18.El-Dahr SS, Dipp S, Meleg-Smith S, Pinna-Parpaglia P, Madeddu P. Fetal ontogeny and role of metanephric bradykinin B2 receptors. Pediatr Nephrol 14: 288–296, 2000. [DOI] [PubMed] [Google Scholar]
- 19.El-Dahr SS, Dipp S, Yosipiv IV, Carbini LA. Activation of kininogen expression during distal nephron differentiation. Am J Physiol Renal Physiol 275: F173–F182, 1998. [DOI] [PubMed] [Google Scholar]
- 20.El-Dahr SS, Figueroa CD, Gonzalez CB, Muller-Esterl W. Ontogeny of bradykinin B2 receptors in the rat kidney: implications for segmental nephron maturation. Kidney Int 51: 739–749, 1997. [DOI] [PubMed] [Google Scholar]
- 21.El-Dahr SS, Harrison-Bernard LM, Dipp S, Yosipiv IV, Meleg-Smith S. Bradykinin B2 null mice are prone to renal dysplasia: gene-environment interactions in kidney development. Physiol Genomics 3: 121–131, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Fan H, Harrell JR, Dipp S, Saifudeen Z, El-Dahr SS. A novel pathological role of p53 in kidney development revealed by gene-environment interactions. Am J Physiol Renal Physiol 288: F98–F107, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Fan H, Harrell JR, Dipp S, Saifudeen Z, El-Dahr SS. A novel pathological role of p53 in kidney development revealed by gene-environment interactions. Am J Physiol Renal Physiol 288: F98–F107, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Fan H, Stefkova J, El-Dahr SS. Susceptibility to metanephric apoptosis in bradykinin B2 receptor null mice via the p53-Bax pathway. Am J Physiol Renal Physiol 291: F670–F682, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs SY, Adler V, Buschmann T, Wu X, Ronai Z. Mdm2 association with p53 targets its ubiquitination. Oncogene 17: 2543–2547, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Gattone VH 2nd, Maser RL, Tina C, Rosenberg JM, Brandon MG. Developmental expression of urine concentration-associated genes and their altered expression in murine infantile-type polycystic kidney disease. Dev Genet 24: 309–318, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Harrison-Bernard LM, Dipp S, El-Dahr SS. Renal and blood pressure phenotype in 18-mo-old bradykinin B2R(−/−)CRD mice. Am J Physiol Regul Integr Comp Physiol 285: R782–R790, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Hu MC, Resemble ND. Genetic regulation of branching morphogenesis: lessons learned from loss-of-function phenotypes. Pediatr Res 54: 433–438, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Kari S, Sakaguchi K, Shimohigashi Y, Samaddar S, Banerjee R, Basu G, Swaminathan V, Kundu TK, Roy S. Effect of phosphorylation on the structure and fold of transactivation domain of p53. J Biol Chem 277: 15579–15585, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Knights CD, Catania J, Giovanni SD, Muratoglu S, Perez R, Swartzbeck A, Quong AA, Zhang X, Beerman T, Pestell RG, Avantaggiati ML. Distinct p53 acetylation cassettes differentially influence gene-expression patterns and cell fate. J Cell Biol 173: 533–544, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature 387: 299–303, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Lakin ND, Jackson SP. Regulation of p53 in response to DNA damage. Oncogene 18: 7644–7655, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Lechner MS, Dressler GR. The molecular basis of embryonic kidney development. Mech Dev 62: 105–120, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Lozano G, Montes de Oca Luna R. MDM2 function. Biochim Biophys Acta 1377: M55–M59, 1998. [DOI] [PubMed] [Google Scholar]
- 35.MacPherson D, Kim J, Kim T, Rhee BK, Van Oostrom CT, DiTullio RA, Venere M, Halazonetis TD, Bronson R, De Vries A, Fleming M, Jacks T. Defective apoptosis and B-cell lymphomas in mice with p53 point mutation at Ser 23. EMBO J 23: 3689–3699, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madeddu P, Varoni MV, Palomba D, Emanueli C, Demontis MP, Glorioso N, Dessi-Fulgheri P, Sarzani R, Anania V. Cardiovascular phenotype of a mouse strain with disruption of bradykinin B2-receptor gene. Circulation 96: 3570–3578, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Marks J, Saifudeen Z, Dipp S, El-Dahr SS. Two functionally divergent p53-responsive elements in the rat bradykinin B2 receptor promoter. J Biol Chem 278: 34158–34166, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Mayo LD, Seo YR, Jackson MW, Smith ML, Rivera Guzman J, Korgaonkar CK, Donner DB. Phosphorylation of human p53 at serine 46 determines promoter selection and whether apoptosis is attenuated or amplified. J Biol Chem 280: 25953–25959, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Moll UM, Petrenko O. The MDM2-p53 interaction. Mol Cancer Res 1: 1001–1008, 2003. [PubMed] [Google Scholar]
- 40.Putnam WC, Swenson SM, Reif GA, Wallace DP, Helmkamp GM Jr, Grantham JJ. Identification of a forskolin-like molecule in human renal cysts. J Am Soc Nephrol 18: 934–943, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Saifudeen Z, Dipp S, Fan H, El-Dahr SS. Combinatorial control of the bradykinin B2 receptor promoter by p53, CREB, KLF-4, and CBP: implications for terminal nephron differentiation. Am J Physiol Renal Physiol 288: F899–F909, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Saifudeen Z, Du H, Dipp S, El-Dahr SS. The bradykinin type 2 receptor is a target for p53-mediated transcriptional activation. J Biol Chem 275: 15557–15562, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev 14: 289–300, 2000. [PMC free article] [PubMed] [Google Scholar]
- 44.Torres VE, Harris PC. Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2: 40–55; quiz 55, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Vainio S, Lin Y. Coordinating early kidney development: lessons from gene targeting. Nat Rev Genet 3: 533–543, 2002. [DOI] [PubMed] [Google Scholar]