Abstract
IL-18 function is neutralized in IL-18 binding protein transgenic (IL-18BP Tg) mice. First, we determined whether IL-18BP Tg mice are protected against ischemic acute kidney injury (AKI). Ischemic AKI was induced by bilateral renal pedicle clamping. IL-18BP Tg mice were functionally and histologically protected against ischemic AKI as determined by blood urea nitrogen, serum creatinine, and acute tubular necrosis score. We have demonstrated that the injurious effect of IL-18 in the kidney is independent of neutrophils and lymphocytes. Thus the effect of IL-18 inhibition on renal macrophage infiltration was determined. The number of macrophages was significantly reduced in IL-18BP Tg compared with wild-type kidneys. To determine the cytokines and chemokines that are dependent on IL-18, we performed flow cytometry based assays. Multiple chemokines/cytokines, IL-3, IL-6, IL-15, IL-18, leukemia inhibitory factor, macrophage colony-stimulating factor, macrophage inflammatory protein-2, granulocyte-macrophage colony-stimulating factor, and monocyte chemotactic protein-1 were significantly increased in AKI vs. sham kidneys. Only CXCL1 (also known as KC or IL-8) was significantly increased in AKI vs. sham kidneys and significantly reduced in IL-18BP Tg AKI vs. wild-type AKI kidneys. To determine whether macrophages are the source of CXCL1 in the kidney, we depleted macrophages with liposomal encapsulated clodronate. CXCL1 was significantly decreased in macrophage-depleted vs. control AKI mice. In summary, in ischemic AKI in mice, 1) IL-18BP Tg mice are functionally and histologically protected, 2) macrophage infiltration in the kidney and CXCL1 are significantly reduced in IL-18BP Tg mice, and 3) macrophage depletion significantly reduces CXCL1 in the kidney. In conclusion, protection against ischemic AKI in IL-18BP Tg mice is associated with less macrophage infiltration and less production of CXCL1 in the kidney.
Keywords: renal chemokines/cytokines
wild-type mice injected with IL-18 antiserum before the induction of ischemia are protected against ischemic acute kidney injury (AKI), as we have previously demonstrated (25). The effect of other methods of IL-18 inhibition on ischemic AKI is not known. In the present study we determined whether mice that are transgenic for human IL-18-binding protein isoform a (hIL-18BPa) are protected against ischemic AKI. IL-18BP is a soluble decoy receptor for IL-18 that inhibits the biological functions of IL-18 (29). The function of IL-18 is neutralized in IL-18BP Tg mice (13). The IL-18BP Tg mouse has been described as a critical tool in the study of the mechanisms of IL-18-induced injury in experimental animal models of acute and chronic inflammatory diseases (13).
We have previously demonstrated that IL-18-mediated injury is independent of neutrophils (26) and CD4 T cells (14). Depletion of neutrophils in the kidney using the rat IgG2b monoclonal antibody RB6-8C5 or depletion of CD4 T cells with the antibody GK1.5 was not sufficient to prevent ischemic AKI in mice. Macrophage depletion using liposomal clodronate is protective against ischemic AKI, suggesting that macrophages play an injurious role in ischemic AKI (5, 20, 31). Macrophages are well-known sources and targets of IL-18 (2). The effect of IL-18 inhibition on macrophage infiltration in the kidney in ischemic AKI is not known. The mechanism of the protective effect of macrophage depletion in ischemic AKI is not known.
IL-18 is a proinflammatory cytokine and in turn results in production of other cytokines and chemokines including IL-1β, IL-6, macrophage inflammatory protein (MIP)-1, MIP-2, and monocyte chemotactic protein (MCP)-1 (2). Recently, the role of inflammation in AKI has been increasingly appreciated (1, 6, 17) with the involvement of leukocytes, adhesion molecules, chemokines, and cytokines. The proinflammatory cytokines/chemokines IFN-γ, IL-2, IL-10, granulocyte-macrophage colony-stimulating factor (GM-CSF), transforming growth factor (TGF)-β, CXCL1, IL-6, MIP-2, and MCP-1 are increased in the kidney in ischemic AKI (18, 22, 32, 33, 37). The effect of IL-18 inhibition on cytokines and chemokines in the kidney in AKI is not known.
With this background we developed the hypothesis that the protective effect of IL-18 inhibition in ischemic AKI may be mediated by inhibition of macrophage infiltration in the kidney with a resultant decrease in cytokines/chemokines produced by macrophages. The aims of the study were to determine the effect of IL-18 inhibition on kidney function and histology, macrophage infiltration, and cytokine/chemokine production by the kidney.
MATERIALS AND METHODS
Genotyping of IL-18 BP Tg mice by PCR.
The mice were genotyped as described previously (13). Briefly, a 592-base pair (bp) fragment (bases 51-643) of the hIL-18BPa gene was cloned into the EcoR1 sites of the pCAGGs expression vector. The pCAGGs/IL-18BPa vector was isolated and transiently transfected into RAW264.7 cells to confirm expression. The IL-18BPa genomic fragment was released from the vector backbone and separated from the pCAGGs vector. The purified fragment was then injected into oocytes from hepatitis B virus mice using standard protocols.
Mouse tail genomic DNA was obtained using the GenScript Tissue Direct Multiplex PCR system (GenScript) according to the manufacturer's instructions. PCR was performed with 1 μl of generated DNA. The PCR conditions included 30 cycles of 1.5 min of denaturizing at 94°C, 2 min of annealing at 62°C, and 2.5 min of extension at 72°C. The following primer was used: 5′ primer, ACA CCT GTC TCG CAG ACC AC, 3′ primer, TCA GCT GCT CCA GCA CCA A, size 328 bp. Human IL-18BPa was present in IL-18BP Tg mice but not in wild-type mice.
IL-18BPa in the kidney of IL-18BP Tg mice.
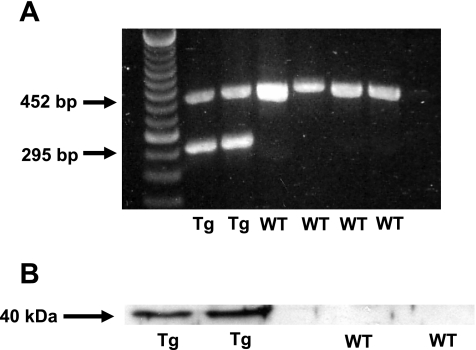
Total RNA was extracted from IL-18BP Tg and wild-type mouse kidney using the Trizol method (Life Technologies, GIBCO BRL). Total RNA (1 μg) was reverse transcribed using the SuperScript Synthesis system (catalog no. 11904-018; Invitrogen). One-tenth of the reaction mixture was used as a template for PCR amplification with the following primers: hIL-18BPa, sense CCTCTACTGGCTGGGCAATGG, antisense TTAACCCTGCTGTGGAC; and GAPDH, sense ACCACAGTCCATGCCATCAC, antisense TCCACCACCCTGTTGCTGTA. The amplification profile was 94°C for 1.5 min, followed by 35 cycles at 94°C for 30 s, 58°C for 30 s, and 72°C for 60 s, with a final extension at 72°C for 7 min. Products were then separated in a 1.5% agarose gel in the presence of ethidium bromide, and bands were analyzed in a UV box. GAPDH was included as an internal control. RT-PCR products were 295 bp for hIL-18BPa and 452 bp for GAPDH. Human IL-18BPa mRNA was present in kidneys from IL-18BP Tg mice but not in kidneys from wild-type mice (Fig. 1).
Fig. 1.
Demonstration of human IL-18 binding protein isoform a (hIL-18BPa) in kidney of IL-18BP transgenic (Tg) mice. A: on RT-PCR analysis, human IL-18BPa mRNA (295 bp) was present in kidneys from IL-18BP Tg mice but not from wild-type (WT) mice. GAPDH (452 bp) was included as an internal control. B: on immunoblot analysis, hIL-18BPa protein (40 kDa) was present in kidneys from IL-18BP Tg mice but not in kidneys from WT mice.
Immunoblotting.
Whole kidney was homogenized in radioimmunoprecipitation assay buffer plus proteinase inhibitors and immunoblotted using a rabbit polyclonal anti-IL-18 BP antibody that recognizes amino acid residues 44-55 of human IL-18BP (STKDPCPSQPPVC; catalog no. NB200-201; Novus Biologicals, Littleton, CO). Human IL-18BPa protein was present in kidneys from IL-18BP Tg mice but not in kidneys from wild-type mice (Fig. 1B).
Ischemia protocol.
The IL-18BP Tg mice, backcrossed into C57BL/6 mice, were obtained from Dr. Charles Dinarello (13). Littermates were used as normal wild-type controls. Male mice (C57BL/6) ages 8–10 wk were used. Mice weighing 20–25 g were anesthetized with an intraperitoneal injection of Avertin (2,2,2-tribromoethanol; Sigma-Aldrich, Milwaukee, WI). A midline incision was made, and both the renal pedicles were clamped for 22 min with microaneurysm clamps as previously described (25, 26). The time of ischemia was chosen to obtain a reversible model of ischemic AKI. Sham surgery consisted of the same surgical procedure, except that clamps were not applied. Studies in ischemic AKI were performed at 24 h of postischemic reperfusion, unless otherwise stated. Blood urea nitrogen (BUN) and serum creatinine were measured using quantitative colorimetric urea determination (QuantiChrom urea assay kit DIUR-500; Bioassay Systems, Hayward, CA) and quantitative colorimetric creatinine determination (QuantiChrom creatinine assay kit DICT-500; Bioassay Systems).
Histological examination.
Paraformaldehyde-fixed (4%) and paraffin-embedded kidneys were sectioned at 4 μm and stained with hematoxylin-eosin and periodic acid-Schiff (PAS) using standard methods. Histological examinations were performed by the renal pathologist in a blinded fashion. Histological changes due to tubular necrosis in the outer stripe of the outer medulla were quantitated by counting the percentage of tubules that displayed cell necrosis, loss of brush border, cast formation, and tubule dilatation as follows: 0, none; 1, <10%; 2, 11–25%; 3, 26–45%; 4, 46–75%; and 5, >76%. At least 5–10 fields (×200) were reviewed for each slide.
The number of neutrophils per high-power field (HPF; ×400) was quantitatively assessed on PAS-stained tissue by the renal pathologist. At least 10 fields were counted in the outer stripe of the outer medulla for each slide.
Morphological criteria were used to count apoptotic tubular cells on PAS-stained tissue. Morphological characteristics included cellular rounding and shrinkage, nuclear chromatin condensation, and formation of apoptotic bodies. Apoptotic tubular cells were quantitatively assessed per 10 HPF in the outer stripe of the outer medulla by the renal pathologist in a blinded fashion. At least 10 fields were counted for each slide.
Immunofluorescence studies.
Kidney tissues were embedded in OCT, snap frozen in liquid nitrogen, and stored at −80°C until sectioning. Cryostat sections (5 μm) were fixed in 70% acetone-30% methanol and prepared for immunofluorescence studies as previously described (9). The primary antibodies used were 1) a rat anti-mouse CD11b monoclonal antibody (catalog no. MCA74; Serotec, Oxford, UK) and 2) a goat polyclonal antibody against KC/GROα (catalog no. sc-16961; Santa Cruz Biotechnology, Santa Cruz, CA). CD11b-positive cells were counted in five HPF of the outer stripe of the outer medulla, and the mean number of cells per HPF was determined by a blinded observer.
Renal cytokines.
Frozen tissue samples (one-half kidney) were homogenized in 500 μl of cell lysis buffer (Bio-Rad, Hercules, CA) containing 0.4% 500 mM PMSF and 1% protease inhibitor cocktail (Sigma, St. Louis, MO). Samples were sonicated (model VC500; Sonics & Materials, Danbury, CT) on ice using five 5-s pulses with time allowed for samples to cool between pulses. Samples were then centrifuged at 4,500 g for 15 min at 4°C. Supernatants were collected, analyzed for protein content using a Bio-Rad DC protein assay kit with bovine serum albumin as standard, and stored at −20°C until use.
Renal cytokines were determined using two bead-based multiplex cytokine kits (Bio-Rad) in conjunction with flow-based protein detection and the Luminex LabMAP multiplex system (Luminex, Austin, TX) according to the manufacturer's directions. This technique allows the detection of these cytokines with 20 μl of a single sample. The detection limit for each cytokine was 1.95 pg/ml. Value are reported as picograms per milligram of tissue protein. Two separate multiplex kits were utilized, a 23-plex kit and a 9-plex kit. The 23-plex kit measured IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-17, Eotaxin, granulocyte colony-stimulating factor (G-CSF), GM-CSF, IFN-γ, CXCL1 (KC), MCP-1, MIP-1α, MIP-1β, RANTES (regulated on activation normal T cell expressed and secreted), and tumor necrosis factor (TNF)-α. The 9-plex kit measured IL-15, IL-18, basic fibroblast growth factor, leukemia inhibitory factor (LIF), macrophage colony-stimulating factor (M-CSF), monokine induced by IFN-γ (MIG), MIP-2, PDGF-BB, and VEGF.
Preparation of mouse proximal tubules.
Proximal tubules were isolated from the kidney cortex using collagenase digestion and Percoll centrifugation as we have previously described in detail (10).
IFN-γ ELISA.
A mouse IFN-γ ELISA kit II (catalog no. 558258; BD Biosciences, San Diego, CA) was used. The performance characteristics of the kit (per manufacturer's instructions) are as follows: 1) no cross-reactivity with other cytokines (e.g., IL-1α, IL-1β, IL-2, IL-3, IL-5, IL-6, IL-7, IL-10, IL-12, TNF, and TNF receptor II) was identified; 2) the intra-assay and interassay variability is between 3 and 6%; 3) the minimum detectable dose of IFN-γ is 0.762 pg/ml.
IL-6 ELISA.
A mouse IL-6 ELISA kit (catalog no. M6000B; R&D Systems, Minneapolis, MN) was used. The intra-assay and interassay variability is between 3 and 9%. The detection limit of the kit is 1.3 pg/ml.
CXCL1 ELISA.
A mouse CXCL1 ELISA kit (catalog no. MKC00B; R&D Systems) was used. The intra-assay and interassay variability is between 3 and 10%. The detection limit of the kit is 2.0 pg/ml.
Macrophage depletion studies.
Empty liposomes (vehicle) and liposome-encapsulated clodronate (LEC) were prepared as previously described in detail (39–41). Clodronate was obtained from Roche Diagnostics (Mannheim, Germany). Briefly, macrophages phagocytose the liposomes, resulting in the release of clodronate into the cytoplasm and death of the macrophage. Empty liposomes, not containing clodronate and prepared under exactly the same conditions as the LEC, were used as a control. Mice received a tail vein injection of 100 μl/10 g body wt of empty liposomes or LEC at 6 and 2 days before induction of ischemia by bilateral renal pedicle clamp.
Statistical analysis.
Non-normally distributed data were analyzed using the nonparametric unpaired Mann-Whitney test. Multiple group comparisons were performed using ANOVA with posttest according to Newman-Keuls. A P value <0.05 was considered statistically significant. Values are expressed as means ± SE.
RESULTS
Demonstration of IL-18BPa in kidney of IL-18BP Tg mice.
On RT-PCR analysis, human IL-18BPa mRNA (295 bp) was present in kidneys from IL-18BP Tg mice but not in kidneys from wild-type mice (Fig. 1A). On immunoblot analysis, human IL-18BPa protein was present in kidneys from IL-18BP Tg mice but not in kidneys from wild-type mice (Fig. 1B).
Renal function.
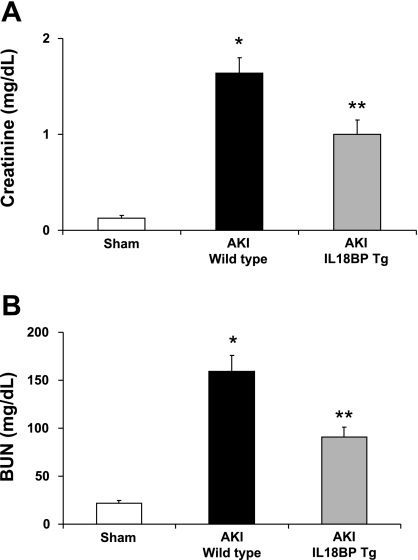
IL-18BP Tg mice were functionally protected against ischemic AKI. Serum creatinine was 0.1 ± 0.02 mg/dl in sham-operated mice, 1.6 ± 0.2 mg/dl in wild-type mice with ischemic AKI (P < 0.001 vs. sham), and 1.0 ± 0.15 mg/dl in IL-18BP Tg mice with ischemic AKI (P < 0.01 vs. wild type). BUN was 22 ± 2.6 mg/dl in sham-operated mice, 159 ± 17 mg/dl in wild-type mice with ischemic AKI (P < 0.001 vs. sham), and 91 ± 10 mg/dl in IL-18BP Tg mice with ischemic AKI (P < 0.01 vs. wild type; n = 18–34 per group) (Fig. 2, A and B).
Fig. 2.
Renal function. IL-18BP Tg mice were functionally protected against ischemic acute kidney injury (AKI). IL-18 BP Tg mice with AKI had a significantly lower serum creatinine (A) and blood urea nitrogen (BUN; B) than WT with AKI. *P < 0.001 vs. sham. **P < 0.01 vs. WT AKI.
Histology.
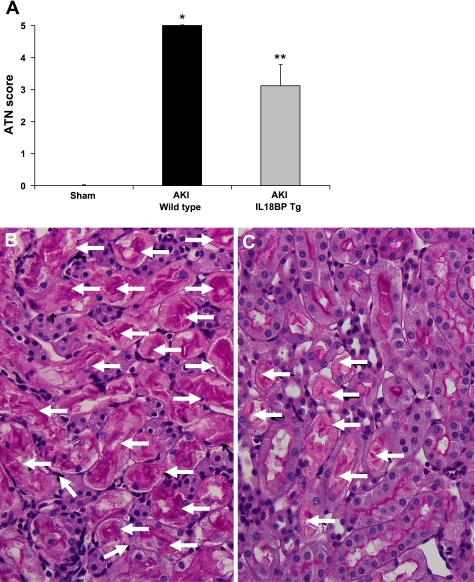
IL-18BP Tg mice were histologically protected against ischemic AKI. Acute tubular necrosis (ATN) score was 5.0 ± 0.01 in wild type mice with ischemic AKI and 3.1 ± 0.6 in IL-18 BP Tg mice with ischemic AKI (P < 0.05 vs. wild type; n = 10 per group) (Fig. 3A). Representative images of the renal histology of wild-type AKI mice and IL-18 BP Tg AKI mice are demonstrated in Fig. 3, B and C.
Fig. 3.
Acute tubular necrosis (ATN) score. IL-18BP Tg mice were histologically protected against ischemic AKI. IL-18 BP Tg mice with AKI had a significantly lower ATN score than WT mice with AKI (A). *P < 0.001 vs. sham. **P < 0.05 vs. WT AKI. Representative images of the renal histology of WT AKI mice (B) and IL-18 BP Tg AKI mice (C) are shown. No tubular necrosis was seen in sham-operated mice. In WT AKI mice most of the renal tubules were necrotic (B; arrows) compared with less tubular necrosis in IL-18 BP Tg AKI mice (C; arrows).
The number of neutrophils in AKI kidneys was significantly different between wild-type and IL-18 BP Tg mice. The number of neutrophils per 10 HPF was 154 ± 35 in wild-type mice with ischemic AKI and 64 ± 35 in IL-18 BP Tg mice with ischemic AKI (P < 0.05 vs. wild type; n = 10 per group).
The number of apoptotic tubular cells in AKI kidneys was not significantly different between wild-type and IL-18 BP Tg mice. The number of apoptotic tubular cells was 22.5 ± 4 in wild-type mice with ischemic AKI and 17 ± 5 in IL-18 BP Tg mice with ischemic AKI (P = not significant vs. wild type; n = 10 per group). The protection against acute tubular necrosis without a significant effect on apoptosis of tubular cells may suggest that apoptosis plays a role in the residual injury in the IL-18BP Tg mice.
Cd11b-positive cells.
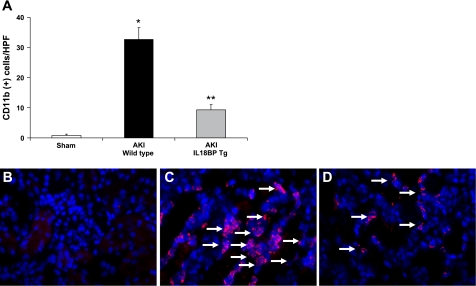
The number of CD11b+ macrophages in ischemic AKI was significantly reduced in IL-18BP Tg kidneys compared with wild-type kidneys. The number of CD 11b+ cells per HPF (×400) in the outer stripe of the outer medulla was 0.8 ± 0.5 in sham-operated mice, 33 ± 4 in wild-type mice with ischemic AKI (P < 0.001 vs. sham), and 9 ± 1.6 in IL-18 BP Tg mice with ischemic AKI (P < 0.01 vs. wild type) (Fig. 4A; n = 5 per group). Representative images of CD 11b-positive staining in macrophages is demonstrated in Fig. 4, B–D.
Fig. 4.
Macrophage infiltration. The number of CD11b+ cells in the outer stripe of the outer medulla in ischemic AKI was significantly reduced in IL-18BP Tg kidneys compared with WT kidneys (A). *P < 0.001 vs. sham. **P < 0.01 vs. WT AKI. Representative images of CD11b-positive staining in macrophages (arrows) in sham-operated mice (B), WT mice with AKI (C), and IL-18BP Tg mice with AKI (D) are shown.
Cytokines/chemokines in the kidney.
The effect of ischemic AKI on cytokines/chemokines is demonstrated in Table 1. The following cytokines/chemokines were significantly increased in ischemic AKI vs. sham- operated controls: IL-6, IL-3, GM-CSF, MCP-1, IL-15, IL-18, LIF, M-CSF, MIP-2, and CXCL1. Of the cytokines that were increased in ischemic AKI, CXCL1 was the only cytokine that was significantly decreased in ischemic AKI in IL-18BP Tg mice (Table 1).
Table 1.
Chemokines/cytokines in ischemic AKI kidneys
| Chemokine/cytokine, pg/mg | Sham Wild-Type (n = 5) | AKI Wild-Type (n = 8–10) | AKI IL-18BP Tg (n = 8–10) |
|---|---|---|---|
| IL-1α | 1.0±0.3 | 1.2±0.1 | 1.5±0.2 |
| IL-1β | 2±0.5 | 2.8±0.1 | 3.9±0.4† |
| IL-2 | 2.5±1.1 | 3.5±0.3 | 3.9±0.7 |
| IL-3 | 0.03±0.01 | 0.06±0.02 | 0.1±0.03* |
| IL-4 | 0.02±0.01 | 0.03±0.002 | 0.03±0.01 |
| IL-5 | 0.07±0.03 | 0.03±0.003 | 0.09±0.03 |
| IL-6 | 1.5±0.2 | 11.9±1.6* | 25.4±9.5* |
| IL-9 | 20.6±7.7 | 26.1±1.9 | 32±4.7 |
| 1L-10 | 1.6±0.2 | 1.7±0.1 | 2.2±0.4 |
| IL-12 (p40) | 0.9±0.1 | 1.1±0.1 | 1.3±0.1 |
| IL-13 | 7.3±1.6 | 7.2±0.7 | 9.1±1.7 |
| IL-15 | 3.8±1.8 | 20.2±2.2† | 20.3±2.2† |
| IL-17 | 0.4±0.06 | 0.4±0.04 | 0.5±0.09 |
| IL-18 | 86.4±42.1 | 129.4±15.9 | 192.3±23.2* |
| GM-CSF | 0.8±0.2 | 1.3±0.1 | 1.8±0.3† |
| G-CSF | 1.5±0.6 | 5.7±1.7 | 4.1±0.9 |
| M-CSF | 22.1±4.8 | 48±4.5* | 49.6±7.4* |
| IFN-γ | 1.2±0.2 | 1.3±0.1 | 1.8±0.3 |
| MIG | 22.5±10.5 | 26.9±2.7 | 35.6±3.9 |
| TNF-α | 4.3±1 | 4.3±0.3 | 5.9±0.9 |
| MIP-1α | 6.1±1 | 8.9±0.9 | 12.8±1.6† |
| MCP-1 | 4±0.3 | 15.6±2† | 15.3±2.5† |
| MIP-1α | 1.4±0.2 | 1.5±0.1 | 2.1±0.3 |
| MIP-2 | 0.5±0.4 | 40.6±6.5* | 31.7±6.1* |
| RANTES | 6.1±1.1 | 11.8±1 | 14.1±2 |
| Eotaxin | 14.2±5.2 | 18.5±1.5 | 23.7±3.0 |
| FGF-basic | 34.6±17.8 | 50.2±3.9 | 54.5±5 |
| LIF | 7.3±7.3 | 87.9±14.8* | 84.5±9.4* |
| CXCL1 (KC; IL-8) | 2.4±0.5 | 158.8±24.6‡ | 94.2±8.3§ |
| PDGF-BB | 24.3±12.4 | 52.6±6.3 | 55.6±9.2 |
| VEGF | 18.8±10.4 | 4.8±1.2 | 4±0.5* |
Values are means ± SE in sham-operated wild-type mouse kidneys (n = 5), acute kidney injury (AKI) wild-type mouse kidneys (n = 8–10), and AKI IL-18 binding protein transgenic (IL-18BP Tg) mouse kidneys (n = 8–10). GM-CSF, granulocyte-macrophage colony-stimulating factor; G-CSF, granulocyte colony-stimulating factor; M-CSF, macrophage colony-stimulating factor; IFN-γ, interferon-γ; MIG, monokine induced by IFN-γ; TNF-α, tumor necrosis factor-α; MIP-1, macrophage inflammatory protein-1; MCP-1, monocyte chemotactic protein-1; RANTES, regulated upon activation, normal T-cell expressed and secreted; FGF, fibroblast growth factor; LIF, leukemia inhibitory factor; CXCL1, chemokine (CXC) motif ligand 1 (also known as KC or IL-8); PDGF, platelet-derived growth factor; VEGF, vascular endothelial growth factor.
P < 0.05;
P < 0.01;
P < 0.001 vs. sham.
P < 0.01 vs. AKI wild-type.
In addition to the multiplex cytokine kit (Table 1), both IL-6 and IFN-γ were measured by ELISA assay. The ELISA assay for IL-6 confirmed the increase in IL-6 between sham and wild-type AKI kidneys and confirmed that IL-6 was not decreased in IL-18BP Tg AKI kidneys. IL-6 was 1.9 ± 0.5 pg/mg in sham-kidneys, 14 ± 2.4 pg/mg in wild-type AKI kidneys (P < 0.01 vs. sham), and 18 ± 5 pg/mg in IL-18BP Tg kidneys (P < 0.01 vs. sham; n = 9–10). The ELISA assay for IFN-γ confirmed no significant differences in IFN-γ among sham, wild-type AKI, and IL-18 BP Tg AKI kidneys. IFN-γ was 1.5 ± 0.06 pg/ml in sham-kidneys, 1.0 ± 0.16 pg/ml in wild-type AKI kidneys, and 1.25 ± 0.25 pg/ml in IL-18BP Tg kidneys (n = 6).
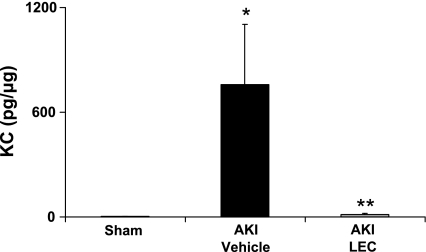
To determine whether macrophages are the source of CXCL1 in ischemic AKI, we measured CXCL1 in the kidney in macrophage-depleted mice. We have previously demonstrated that administration of LEC resulted in a 70% reduction in macrophages in the spleen, attenuated the increase in macrophage infiltration in the kidney, and protected against AKI as determined by renal function and ATN score (31). The increase in CXCL1 in ischemic AKI was virtually completely inhibited by macrophage depletion. CXCL1 was 3.1 ± 2 pg/mg in sham-operated mice, 760 ± 343 pg/mg in vehicle-treated mice with AKI (P < 0.01 vs. sham), and 13 ± 8 pg/mg in LEC-treated mice with AKI (P < 0.05 vs. vehicle-treated AKI; n = 5 per group) (Fig. 5).
Fig. 5.
Effect of macrophage depletion on CXCL1 (KC). To determine whether macrophages are the source of CXCL1 in ischemic AKI, we measured CXCL1 in the kidney in macrophage-depleted mice using 2 bead-based multiplex cytokine kits. The increase in CXCL1 in ischemic AKI was virtually completely inhibited by macrophage depletion with liposomal encapsulated clodronate (LEC). *P < 0.01 vs. sham. **P < 0.05 vs. AKI + vehicle (empty liposomes).
Localization of CXCL1 in the kidney.
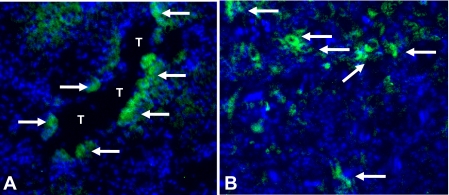
Immunofluorescence staining for CXCL1 in the kidney demonstrates CXCL1 staining in macrophages in the outer stripe of the outer medulla (Fig. 6A) as well as staining in proximal tubules (Fig. 6B). To confirm the presence of CXCL1 in proximal tubules, we performed an ELISA assay for CXCL1 on freshly isolated mouse proximal tubules. Proximal tubules from 10 different proximal tubule preparations were prepared for the CXCL1 ELISA assay according to the ELISA kit instructions. There was 4.5 ± 1.3 pg/mg CXCL1 in the tubular extracts.
Fig. 6.
Immunofluorescence staining for CXCL1 in mouse kidneys. CXCL1 staining (green) is demonstrated in macrophages in the outer stripe of the outer medulla (A; arrows) as well as in a proximal tubule (T) (B; arrows). No glomerular staining was seen. Nuclei are stained with 4′,6-diamidino-2-phenylindole (blue).
DISCUSSION
IL-18BP was first purified from urine and then sequenced, cloned, and expressed in COS7 cells (30). IL-18BP is a soluble decoy receptor for IL-18 that inhibits the biological functions of IL-18 both in vitro and in vivo (29) (7). IL-18BP binds IL-18 with a high affinity and at equimolar ratios inhibits 50–70% of IL-18. IL-18BP Tg mice demonstrate global high expression of human IL-18BP, and the IFN-γ-inducing activity of exogenously administered IL-18 is completely neutralized in these mice. Neutralization of IL-18 has potential therapeutic effects. Blockade of IL-18 with the use of neutralizing antibodies (12) or exogenous IL-18BP (11) protects mice from liver necrosis. IL-18BP reduces ischemic dysfunction in a suprafused human atrial myocardium model (7). Strategies to block IL-18 using IL-18BP are underway in clinical trials of rheumatoid arthritis (8). In the present study we have demonstrated functional and histological protection against ischemic AKI in IL-18BP Tg mice. In view of the protection against ischemic AKI in IL-18 BP Tg mice, strategies to inhibit IL-18 merit further study as a potential therapy for ischemic AKI.
We have demonstrated that wild-type mice injected with IL-18 antiserum before ischemia are protected against ischemic AKI (25). This is in contrast to our other studies, where IL-18 inhibition was not protective against endotoxemia-induced AKI (42) and cisplatin-induced AKI (15). The differences in the protective effect of IL-18 inhibition in the different AKI models may be related to differences in tubular injury and inflammation in the models. In our ischemic AKI model, >75% of the tubules demonstrate acute tubular necrosis and there is a macrophage infiltrate in the interstitium. Macrophage depletion with LEC is protective in the ischemic AKI model. In our cisplatin-induced AKI model, there is a macrophage infiltrate in the interstitium, but the acute tubular necrosis scores are lower than in ischemic AKI and there is prominent tubular injury due to apoptosis. Macrophage depletion with LEC is not protective in the cisplatin-induced AKI model (24). In our endotoxemia model, there is minimal inflammation and minimal acute tubular necrosis. Thus IL-18 inhibition is protective in the ischemic AKI model, where macrophages play an injurious role and in which there is severe acute tubular necrosis rather than tubular apoptosis. The precise mechanism of IL-18-mediated injury in ischemic AKI is unclear but may be related to inhibition of CXCL1 in macrophages, as demonstrated in the current study.
We have previously demonstrated that IL-18-mediated injury is independent of neutrophils (26). In our previous study, neutrophil-depleted mice had a significant increase in caspase-1 and IL-18 in the kidney but had no reduction in the ATN score despite a lack of neutrophil infiltration in the kidney. In addition, IL-18-antiserum-treated neutrophil- depleted mice were protected against ischemic AKI. These results suggested a novel neutrophil-independent mechanism of IL-18-mediated ischemic AKI.
Our data demonstrate that IL-18-mediated ischemic AKI is independent of CD4+ T cells (14). CD4+ T-cell depletion by injection of the monoclonal antibody GK1.5 was not protective against ischemic AKI and did not prevent the increase in IL-18 in the kidney. However, CD4+ T cells (3), natural killer (NK) T cells (23), and resident dendritic cells (43) have been demonstrated to be a source of IFN-γ in ischemic AKI. In view of our data that IL-18-mediated injury is independent of neutrophils and T cells, macrophages were investigated as a target of IL-18 in ischemic AKI. Monocyte/macrophages express the IL-18 receptor (IL-18R), and IL-18 can result in activation of macrophages (2). In the present study, IL-18 inhibition significantly reduced macrophage infiltration in the kidney in ischemic AKI.
IL-18 is a proinflammatory cytokine and in turn results in production of other cytokines and chemokines. IL-18 is a potent activator of Th1 cells and induces IFN-γ production and lymphocyte proliferation. In synergy with IL-23, IL-18 activates Th17 cells to produce IL-17. IL-18 inhibition reduces IL-1β, IL-6, MIP-1 and MIP-2 in streptococcal arthritis (38) and reduces MCP-1 production in macrophages (44). Of the multiple cytokines/chemokines increased in the kidney in AKI, CXCL1 was the only cytokine that was reduced in the kidney in the IL-18 BP Tg mice. These data suggest that CXCL1 production in the ischemic kidney is dependent on IL-18.
CXCL1, the prototypic CXC chemokine, is a neutrophil chemoattractant and attracts both neutrophils and T lymphocytes to the site of inflammation. (16). It has been previously demonstrated that CXCL1 is increased in the kidney in ischemic AKI (28, 33, 37). Studies suggest that CXCL1 is a mediator of ischemic AKI. Injection of a neutralizing antibody to CXCL1 in mice results in decreased neutrophil infiltration in the kidney and protection against ischemic AKI (27). CXCL1 binds to the chemokines receptors CXCR1 and CXCR2. In a rat model of renal transplantation, repertaxin, a CXCR2 inhibitor, prevents granulocyte infiltration in the kidney and prevents deterioration of kidney function (4). Thus part of the protection against ischemic AKI in IL-18 BP Tg mice may be related to CXCL1 inhibition.
CXCL1 is made by monocytes/macrophages, fibroblasts, keratinocytes, and endothelial cells (16, 35). In the present study CXCL1 staining was detected in macrophages and tubular epithelial cells. Macrophage depletion is known to be protective against ischemic acute renal failure in mice (5, 20, 31). A key question is the mechanism of this protective effect. A possible mechanism of this protective effect is inhibition of production of CXCL1 by macrophages in ischemic AKI. Thus we determined whether macrophages were the source of CXCL1 in the kidney in ischemic AKI. Inhibition of macrophage infiltration in the kidney using LEC prevented the increase in CXCL1 in the kidney, suggesting that macrophages are the main source of CXCL1 in ischemic AKI. In view of the inhibition of CXCL1 production in the kidney by macrophage depletion and the documented protective effect of CXCL1 inhibition in ischemic AKI (4, 27), it is possible that part of the protective effect of macrophage depletion in ischemic AKI is mediated by inhibition of CXCL1. CXCL1 is a predominantly a neutrophil chemoattractant but also attracts T lymphocytes to the site of inflammation (16). It is thought that the protective effect of CXCL1 inhibition in ischemic AKI is mediated by prevention of neutrophil infiltration in the ischemic kidney (27). However, expression of CXCR1, the receptor for CXCL1, is also found on other inflammatory cells, including NK cells and monocytes (19, 34). In early atherosclerotic lesions CXCL1 triggers monocyte recruitment (19). Thus, in view of our data that IL-18-mediated injury is independent of neutrophils and T cells, it is possible that the decrease in CXCL1 production in the ischemic kidney leads to a further decrease in monocyte/macrophage infiltration in the kidney. These studies have important clinical relevance, because macrophage-derived cytokine targeting is used in patients with Crohn's disease (21).
Ischemic AKI resulted in an increase in multiple cytokines (IL-6, IL-3, GM-CSF, MCP-1, IL-15, IL-18, LIF, M-CSF, MIP-2, and CXCL1) in the kidney. Of these cytokines, IL-6, GM-CSF, MCP-1, IL-18, M-CSF, MIP-2, and CXCL1 have been described to increase in the kidney in ischemic AKI (18, 22, 25, 32, 33, 37). We describe, for the first time, an increase in IL-3, IL-15, and LIF in the ischemic kidney. IL-3 is produced by CD4+ T cells and is involved in neutrophil and monocyte differentiation in the setting of GM-CSF (35). IL-3 prevents apoptosis in target cells (36). IL-15 is made by macrophages and endothelial cells and plays a role in maintenance of NK cells and T cells and plays a crucial role in the innate immune system (35). LIF is involved in tubular regeneration after experimental AKI in rats (45). The role of IL-3, IL-15, and LIF in ischemic AKI merits further study.
In summary, IL-18BP Tg mice are functionally and histologically protected against ischemic AKI. Macrophage infiltration and CXCL1 in the kidney in ischemic AKI are significantly reduced in IL-18BP Tg mice. Macrophage depletion significantly reduces CXCL1 in the kidney. In conclusion, the protective effect of IL-18 inhibition and macrophage depletion in the kidney may, in part, be mediated by inhibition of CXCL1 production.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK-56851 (to C. L. Edelstein), K08 DK-65022 (to S. Faubel), K08 DK-0695121 (to A. Jani), and F32 DK-079547-01 (to L. Lu).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int 66: 480–485, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Boraschi D, Dinarello CA. IL18 in autoimmunity: review. Eur Cytokine Netw 17: 224–252, 2006. [PubMed] [Google Scholar]
- 3.Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O'Donnell MP, Rabb H. Identification of the CD4+ T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest 108: 1283–1290, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cugini D, Azzollini N, Gagliardini E, Cassis P, Bertini R, Colotta F, Noris M, Remuzzi G, Benigni A. Inhibition of the chemokine receptor CXCR2 prevents kidney graft function deterioration due to ischemia/reperfusion. Kidney Int 67: 1753–1761, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Day YJ, Huang L, Ye H, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of macrophages. Am J Physiol Renal Physiol 288: F722–F731, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Devarajan P Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Dinarello CA Novel targets for interleukin 18 binding protein. Ann Rheum Dis 60, Suppl 3: iii18–iii24, 2001. [DOI] [PMC free article] [PubMed]
- 8.Dinarello CA Interleukin-18 and the treatment of rheumatoid arthritis. Rheum Dis Clin North Am 30: 417–434, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Dursun B, He Z, Somerset H, Oh DJ, Faubel S, Edelstein CL. Caspases and calpain are independent mediators of cisplatin-induced endothelial cell necrosis. Am J Physiol Renal Physiol 291: F578–F587, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Edelstein CL Rat renal proximal tubules, hypoxia, ionomycin and calpain. In: Calpain Methods and Protocols, edited by Elce JS. Totowa, NJ: Humana, 2000, p. 225–238. [DOI] [PubMed]
- 11.Faggioni R, Cattley RC, Guo J, Flores S, Brown H, Qi M, Yin S, Hill D, Scully S, Chen C, Brankow D, Lewis J, Baikalov C, Yamane H, Meng T, Martin F, Hu S, Boone T, Senaldi G. IL-18-binding protein protects against lipopolysaccharide-induced lethality and prevents the development of Fas/Fas ligand-mediated models of liver disease in mice. J Immunol 167: 5913–5920, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Faggioni R, Jones-Carson J, Reed DA, Dinarello CA, Feingold KR, Grunfeld C, Fantuzzi G. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor alpha and IL-18. Proc Natl Acad Sci USA 97: 2367–2372, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fantuzzi G, Banda NK, Guthridge C, Vondracek A, Kim SH, Siegmund B, Azam T, Sennello JA, Dinarello CA, Arend WP. Generation and characterization of mice transgenic for human IL-18-binding protein isoform a. J Leukoc Biol 74: 889–896, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Faubel SG, Ljubanovic D, Poole B, Dursun B, Cushing S, He Z, Gill RG, Edelstein CL. Peripheral CD4 T cell depletion is not sufficient to prevent ischemic acute renal failure. Transplantation 80: 643–649, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Faubel SG, Ljubanovic D, Reznikov LL, Somerset H, Dinarello CA, Edelstein CL. Caspase-1-deficient mice are protected against cisplatin-induced apoptosis and acute tubular necrosis. Kidney Int 66: 2202–2213, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Frangogiannis NG Chemokines in ischemia and reperfusion. Thromb Haemost 97: 738–747, 2007. [PubMed] [Google Scholar]
- 17.Friedwald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int 66: 486–491, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Goes N, Urmson J, Ramassar V, Halloran PF. Ischemic acute tubular necrosis induces an extensive local cytokine response. Evidence for induction of interferon-gamma, transforming growth factor-beta 1, granulocyte-macrophage colony-stimulating factor, interleukin-2, and interleukin-10. Transplantation 59: 565–572, 1995. [PubMed] [Google Scholar]
- 19.Huo Y, Weber C, Forlow SB, Sperandio M, Thatte J, Mack M, Jung S, Littman DR, Ley K. The chemokine KC, but not monocyte chemoattractant protein-1, triggers monocyte arrest on early atherosclerotic endothelium. J Clin Invest 108: 1307–1314, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jo SK, Sung SA, Cho WY, Go KJ, Kim HK. Macrophages contribute to the initiation of ischemic acute renal failure in rats. Nephrol Dial Transplant 21: 1231–1239, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Kanai T, Uraushihara K, Totsuka T, Okazawa A, Hibi T, Oshima S, Miyata T, Nakamura T, Watanabe M. Macrophage-derived IL-18 targeting for the treatment of Crohn's disease. Curr Drug Targets Inflamm Allergy 2: 131–136, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Kielar ML, John R, Bennet M, Richardson JA, Shelton JM, Chen L, Jeyarajah DR, Zhou XJ, Zhou H, Chiquett B, Nagami GT, Lu CY. Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol 16: 3315–3325, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 178: 5899–5911, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Lu L, Oh DJ, Dursun B, He Z, Hoke TS, Faubel S, Edelstein CL. Increased macrophage infiltration and fractalkine expression in cisplatin-induced acute renal failure in mice. J Pharmacol Exp Ther 324: 111–117, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, Schrier RW, Edelstein CL. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest 107: 1145–1152, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melnikov VY, Faubel SG, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL. Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest 110: 1083–1091, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miura M, Fu X, Zhang QW, Remick DG, Fairchild RL. Neutralization of Gro alpha and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am J Pathol 159: 2137–2145, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizutani A, Okajima K, Uchiba M, Noguchi T. Activated protein C reduces ischemia/reperfusion-induced renal injury in rats by inhibiting leukocyte activation. Blood 95: 3781–3787, 2000. [PubMed] [Google Scholar]
- 29.Muhl H, Kampfer H, Bosmann M, Frank S, Radeke H, Pfeilschifter J. Interferon-gamma mediates gene expression of IL-18 binding protein in nonleukocytic cells. Biochem Biophys Res Commun 267: 960–963, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity 10: 127–136, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Oh DJ, Dursun B, He Z, Lu L, Hoke TS, Ljubanovic D, Faubel S, Edelstein CL. Fractalkine receptor (CX3CR1) inhibition is protective against ischemic acute renal failure in mice. Am J Physiol Renal Physiol 294: F264–F271, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Rice JC, Spence JS, Yetman DL, Safirstein RL. Monocyte chemoattractant protein-1 expression correlates with monocyte infiltration in the post-ischemic kidney. Ren Fail 24: 703–723, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Safirstein R, Megyesi J, Saggi SJ, Price PM, Poon M, Rollins BJ, Taubman MB. Expression of cytokine-like genes JE and KC is increased during renal ischemia. Am J Physiol Renal Fluid Electrolyte Physiol 261: F1095–F1101, 1991. [DOI] [PubMed] [Google Scholar]
- 34.Segerer S, Henger A, Schmid H, Kretzler M, Draganovici D, Brandt U, Noessner E, Nelson PJ, Kerjaschki D, Schlondorff D, Regele H. Expression of the chemokine receptor CXCR1 in human glomerular diseases. Kidney Int 69: 1765–1773, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Sigal LH Basic science for the clinician 33: interleukins of current clinical relevance (part 1). J Clin Rheumatol 10: 353–359, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Thatte U, Dahanukar S. Apoptosis: clinical relevance and pharmacological manipulation. Drugs 54: 511–532, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Thurman JM, Lenderink AM, Royer PA, Coleman KE, Zhou J, Lambris JD, Nemenoff RA, Quigg RJ, Holers VM. C3a is required for the production of CXC chemokines by tubular epithelial cells after renal ischemia/reperfusion. J Immunol 178: 1819–1828, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Tissi L, McRae B, Ghayur T, von Hunolstein C, Orefici G, Bistoni F, Puliti M. Role of interleukin-18 in experimental group B streptococcal arthritis. Arthritis Rheum 50: 2005–2013, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 174: 83–93, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Van Rooijen N, Sanders A. Kupffer cell depletion by liposome-delivered drugs: comparative activity of intracellular clodronate, propamidine, and ethylenediaminetetraacetic acid. Hepatology 23: 1239–1243, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Van Rooijen N, Sanders A. Elimination, blocking, and activation of macrophages: three of a kind? J Leukoc Biol 62: 702–709, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Wang W, Faubel SG, Ljubanovic D, Mitra A, Kim J, Tao Y, Soloviev A, Reznikov L, Dinarello CA, Schrier RW, Edelstein CL. Endotoxemic acute renal failure is attenuated in caspase-1 deficient mice. Am J Physiol Renal Physiol 288: F997–F1004, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Wu CJ, Sheu JR, Chen HH, Liao HF, Yang YC, Yang S, Chen YJ. Modulation of monocyte-derived dendritic cell differentiation is associated with ischemic acute renal failure. J Surg Res 132: 104–111, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Yoo JK, Kwon H, Khil LY, Zhang L, Jun HS, Yoon JW. IL-18 induces monocyte chemotactic protein-1 production in macrophages through the phosphatidylinositol 3-kinase/Akt and MEK/ERK1/2 pathways. J Immunol 175: 8280–8286, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Yoshino J, Monkawa T, Tsuji M, Hayashi M, Saruta T. Leukemia inhibitory factor is involved in tubular regeneration after experimental acute renal failure. J Am Soc Nephrol 14: 3090–3101, 2003. [DOI] [PubMed] [Google Scholar]