Abstract
In eukaryotic cells, the apparent maintenance of 1:1 stoicheometry between the Na-K-ATPase α- and β-subunits led us to question whether this was alterable and thus if some form of regulation was involved. We have examined the consequences of overexpressing Na-K-ATPase β1-subunits using Madin-Darby canine kidney (MDCK) cells expressing flag-tagged β1-subunits (β1flag) or Myc-tagged β1-subunits (β1myc) under the control of a tetracycline-dependent promoter. The induction of β1flag subunit synthesis in MDCK cells, which increases β1-subunit expression at the plasma membrane by more than twofold, while maintaining stable α1 expression levels, revealed that all mature β1-subunits associate with α1-subunits, and no evidence of “free” β1-subunits was obtained. Consequently, the ratio of assembled β1- to α1-subunits is significantly increased when “extra” β-subunits are expressed. An increased β1/α1 stoicheometry is also observed in cells treated with tunicamycin, suggesting that the protein-protein interactions involved in these complexes are not dependent on glycosylation. Confocal images of cocultured β1myc-expressing and β1flag-expressing MDCK cells show colocalization of β1myc and β1flag subunits at the lateral membranes of neighboring cells, suggesting the occurrence of intercellular interactions between the β-subunits. Immunoprecipitation using MDCK cells constitutively expressing β1myc and tetracycline-regulated β1flag subunits confirmed β-β-subunit interactions. These results demonstrate that the equimolar ratio of assembled β1/α1-subunits of the Na-K-ATPase in kidney cells is not fixed by the inherent properties of the interacting subunits. It is likely that cellular mechanisms are present that regulate the individual Na-K-ATPase subunit abundance.
Keywords: sodium pump, beta, expression, epithelial cell, alpha, ratio
the sodium-potassium-adenosinetriphosphatase (Na+-K+-ATPase, or sodium pump) is an integral membrane protein complex that mediates the active transport of three Na+ out of, and two K+ ions into, the cell across the plasma membrane (PM) for each molecule of hydrolyzed ATP. This maintains low internal Na+, resulting in an inwardly directed chemical gradient necessary for secondary transport of nutrients and fluid homeostasis (19, 24). The sodium pump is a heterodimeric complex consisting of one α- and one β-subunit (7). Both α and β have four distinct isoforms that have been identified, all of which are tissue specific in their expression (3, 35). In kidney epithelial cells, α1- and β1-subunits are the predominantly expressed isoforms (37). The α1-subunit is a 113-kDa protein containing 10 transmembrane segments (18) and contains the residues and sequences associated with ATP hydrolysis and ion transport (24). The β1-subunit contains a single transmembrane spanning segment. It has three N-linked glycosylation sites on the extracellular segment, which increases the molecular mass of unglycosylated β1 from ∼33 kDa to a glycosylated β1 mass of 40–65 kDa, depending on the extent of glycosylation. The β1-subunit is necessary for the intracellular trafficking of the α1/β1 complex to the PM (5, 28). Both α1- and β1-subunits are synthesized in the endoplasmic reticulum (ER), where they rapidly assemble. The heterodimer then traffics to the Golgi (G) for posttranslational modifications of β1-subunit glycans. Finally, the α1β1 complex is integrated into the PM and the Na-K-ATPase enzyme functions in ion transport (19). A great deal is known about the reaction mechanism of the Na-K-ATPase and the associated structure-function relations, culminating in the recent high-resolution structure of the protein (27).
Although much is known about the catalytic α1-subunit, a complete description of the functions of the β1-glycoprotein remains elusive. It is clearly involved in the transport cycle, as the reductive loss of its intramolecular Cys-Cys bonds results in the loss of the ability to occlude K+ ions and the associated loss of Na-K-ATPase activity (29), and it undergoes conformational transitions in concert with the α-subunit during the reaction cycle (8, 25). Studies in Madin-Darby canine kidney (MDCK) cells (17) confirmed the 1:1 β-to-α ratio previously reported for purified enzyme (7). Recent studies suggest that, in addition to enabling transport and stabilization of the Na-K-ATPase complex in the PM, β1 may be involved in the structural organization of epithelial cell monolayers. In MDCK-Moloney sarcoma virus (MSV) cells, a renal carcinoma cell line, it has been reported that the expression level of β1-subunit is greatly reduced compared with normal MDCK cells. Upon additional expression of both E-cadherin and Na-K-ATPase β1-subunit in MDCK-MSV cells, cell motility and invasiveness decrease, and polarization and tight junction formation increase (32). The β1-subunits may aid in increased polarization by the interactions formed between the N-linked glycans of β1-subunits in neighboring cells, resulting in strengthened cell-cell contacts (1). The extent to which β1-subunit N-linked glycans are processed, compared with glycans of known adhesion molecules, as well as the recognition that β2 is identical to the adhesion molecule of glia, suggests an additional role for β1-subunit as an adhesion molecule (15, 38). Although β1-glycosylation is not necessary for sodium pump activity or transport to the PM, it is implicated as an important feature of sodium pump stability (2, 22). When MDCK cells exogenously express the unglycosylated form of β1-subunit, a reduction in adherens junctional resistance to detergent extraction has been reported (41). Additional studies conclude that, although some β1-glycosylation is necessary for enhanced cell adhesion, the complexity of N-glycan branching is inversely related to tightness of cell junctions, paracellular permeability, and cell-cell contact formation in MDCK cells (39–41). Whatever the precise roles of the β-subunit, the apparently invariable equimolar stoicheometry of the α- and β-subunits in sodium pump complexes and in PMs suggests that either this αβ stoicheometry is fixed by the nature of intersubunit protein-protein interactions in the heterodimer, or else the cellular abundance of the subunits is regulated in some other way.
Our present studies utilize the overexpression of “extra” β-subunits in MDCK cells and provide evidence that β1-subunits associate with each other in stable αβ complexes that contain the extra β1-subunits. When the β1-subunit is overexpressed in MDCK cells, the ratio of assembled β1/α1-subunits is increased. The increase in β1-subunits associated per α1-subunit is achieved by interactions between the subunits, which results in α1-β1n complexes. Furthermore, no “free” β1-subunits unassociated with α1 are present at significant levels in either normal MDCK cells or cells overexpressing the β1-subunit. Our results suggest that cellular mechanisms are present that regulate the abundance of one or both Na-K-ATPase subunits.
MATERIALS AND METHODS
Cell line and culture.
The MDCK/FlpIn cell line was a kind gift of the late Dr. Robert B. Gunn and modified by us to create a MDCK/FRT/T-Rex cell line. The MDCK/FRT/T-Rex cell line has a Flp recombination target (FRT) site incorporated into the genome. The FRT site is the exact location of insertion of the gene of interest into the genome for every transfected cell. Cells are transfected via the Flp-In expression vector, pcDNA5/FRT/TO, in addition to the Flp recombinase vector, pOG44. The MDCK/FRT/T-Rex host cell line, which will be referred to as MDCK cell line in this study, also includes a tetracycline (Tet) regulatable promoter introduced via the T-Rex kit (Invitrogen). In this kit, a pcDNA6/TR vector incorporates a Tet repressor under control of the cytomegalovirus promoter. Tet present in the growth media binds to the operon and allows the cytomegalovirus promoter to induce expression of the gene of interest. In this study, we inserted into the FRT site either Na-K-ATPase sheep β1- or rat β2-subunit with either a flag or Myc epitope tag on the carboxyl terminus. β1 with a flag tag (β1flag), β1 with a Myc tag (β1myc), or β2 with a Myc tag (β2myc) were transfected into cells according to manufacturer's protocols using Lipofectamine 2000 (Invitrogen), as previously described (21). Another cell line containing both a Tet-regulatable β1flag subunit and a stable β1myc subunit was created by transfecting MDCK/β1flag cells with pcDNA3.1 vector (Invitrogen) containing sheep β1-subunit with a carboxyl terminal Myc epitope tag (MDCK/β1flag/β1myc). In this cell line, β1myc is constantly expressed, whereas β1flag expression is regulated via Tet addition to the growth medium.
All cell lines were grown in Dulbecco's minimal essential medium (DMEM), supplemented with 25 mM HEPES buffer, 10% Tet-free FBS, 10 μg/ml streptomycin, 10 U/ml penicillin, 2.5 μg/ml fungizone, and 5 μg/ml plasmocin. For cells not yet transfected with the gene of interest, 6 μg/ml blasticidin and 150 μg/ml zeocin were added to media. Posttransfection, cells containing the gene of interest in the FRT site were selected and grown with 400 μg/ml hygromycin B and 6 μg/ml blasticidin. Genticin (500 μg/ml) was also added to posttransfection media to select for MDCK/β1flag/β1myc cells. To express the β-subunits, 1 μg/ml Tet was added to the growth media for desired times, usually 3–5 days. In tunicamycin experiments, 1 μg/ml tunicamycin was added to growth media for 24 h. All cell lines were split every 2–4 days or when cells reached confluency by washing two times with PBS and were incubated with 0.05% Trypsin-EDTA until they detach from plates. Cells were maintained in a humidified incubator with 5% CO2 at 37°C.
Membrane isolation.
Cell monolayers were grown to confluence, washed twice with PBS, harvested, and pelleted at 1,000 g for 5 min, and supernatant was removed. The cell pellets were either stored at −20°C or resuspended in cold homogenizing buffer (10 mM Tris·HCl, 2 mM EDTA, 250 mM sucrose, pH 7.4), supplemented with Complete-Mini Protease Inhibitor Cocktail Tablets (Roche). To collect total membranes (TM), cells were sonicated and centrifuged at 1,000 g for 5 min to remove the whole cells and debris. The supernatant was centrifuged at 55,000 rpm for 30 min using a TLA 55 rotor (BioRad), and TM was collected and used for subsequent experiments. To collect enriched membrane fractions, sonicated cells were placed in a five-step sucrose gradient. Gradients were spun at 45,000 rpm in a SW55 rotor for 1.5 h. Then, ER-, G-, or PM-enriched layers were collected and spun at 55,000 rpm for 1 h (18). Membrane protein concentrations were determined by the Lowry assay.
ATPase activity assay.
The ATPase activity assay of TM isolated from either MDCK or MDCK/β1flag cells grown in the presence of Tet for 96 h was performed as previously described (18). TM protein (10 or 20 μg) was incubated at 37°C for 30 min with ATPase Assay Solution (0.6 mM EGTA, 156 mM NaCl, 24 mM KCl, 3.6 mM MgCl2, 3.6 mM NaATP, 10 mM sodium-azide, 50 mM imidazole, pH 7.2) in the presence or absence of 20 μM ouabain. The amount of phosphate liberated in the absence of ouabain less the amount liberated in the presence of ouabain was the determined Na-K-ATPase activity, expressed in micromoles of Pi per milligram of protein per hour.
SDS-PAGE and Western blot analysis.
TM or membrane fractions were subjected to SDS-PAGE and Western blot analysis, as previously described (22). Protein (3–10 μg) was incubated with 2× sample buffer containing 1% 2-mercaptoethanol for 1 h at room temperature. Proteins were resolved on 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes. After proteins were transferred, 5% milk in PBS was used to block polyvinylidene difluoride membranes for at least 1 h. Primary antibodies were diluted in PBS + 0.1% Tween 20 containing 0.5% dried milk and used to probe for 1 h or overnight at 37 or 4°C, respectively. Following immunoprobing with primary antibody, blots were washed with PBS + 0.1% Tween 20 three times for 10 min each. Secondary antibodies were then added to the probing solution as for primary antibodies, and membranes were washed. Horseradish peroxidase-conjugated secondary antibody was detected by chemilumenescent solutions (Pierce), and protein expression was detected and quantified using a BioRad Chemidoc XRS with Quantity One version 4.6.2 software. The histograms shown as quantification of β overexpression in Figs. 1, 5, and 6 do not contain error bars. This is because overexpression was variable in each experiment. However, the data shown are representative of at least three experiments of each type.
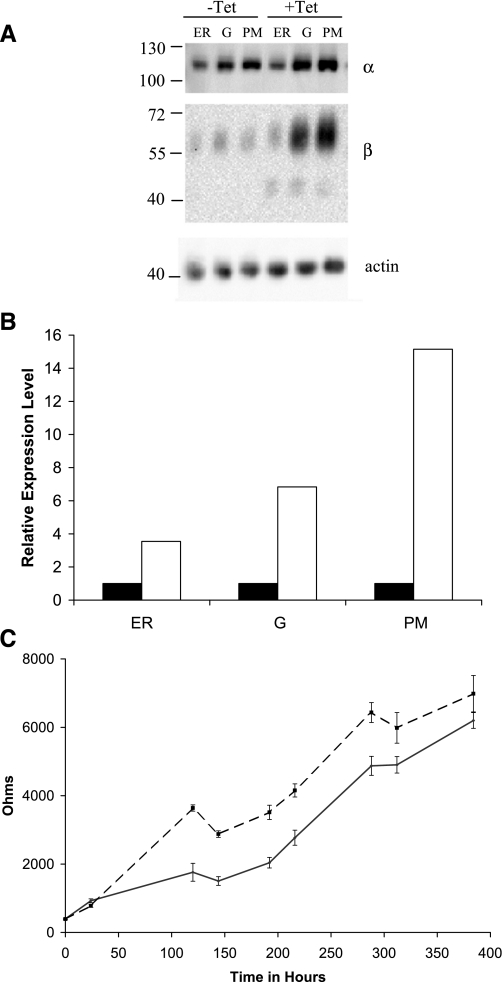
Fig. 1.
Subunit expression and distribution in Madin-Darby canine kidney (MDCK)/β1 with flag tag (β1flag) cells. In MDCK/β1flag cells, β1flag expression was induced with tetracycline (Tet) for 5 days (+Tet) or uninduced (−Tet). Endoplasmic reticulum (ER), Golgi (G), and plasma membrane (PM) enriched fractions were separated via a five-step sucrose gradient. Protein (3 μg) from each fraction was resolved by Western blot analysis detecting with anti-actin, anti-α, or anti-β antibodies. A: relative β signal intensities from Western blot were quantified, normalized using actin, and graphically displayed in B. B: solid bars represent β signal from −Tet samples, and open bars represent β signal from +Tet samples. C: the transepithelial resistance (RTE) of MDCK/β1flag cell monolayers grown in the presence (dashed line) or absence (solid line) of Tet was measured at various times during their growth in transwells. Data points show the means ± SE of 3 independent experiments.
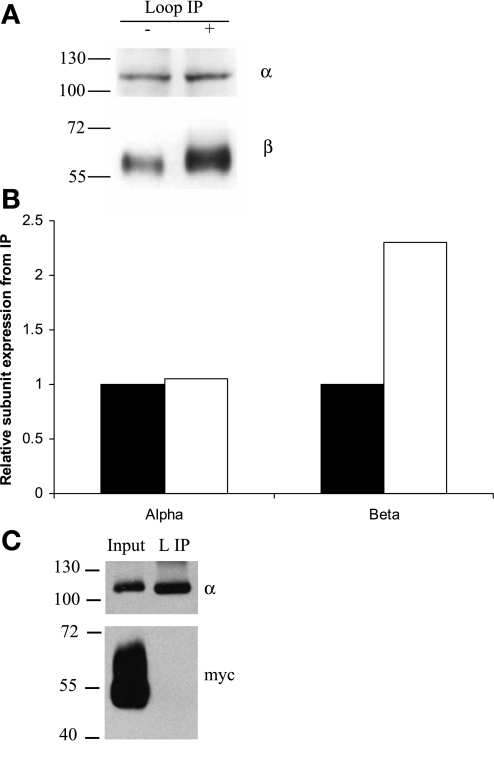
Fig. 5.
The β-to-α ratio is increased in MDCK/β1flag cells inducing β1flag. TM from MDCK/β1flag grown with Tet for 5 days (+) or grown in the absence of Tet (−) were collected, and protein concentrations determined. TM (30 μg) was solubilized in IPB containing 2% DDM. The soluble fraction was subjected to IP using anti-α-loop antibody optimized to bind all α and associated proteins. A: IP eluates were subjected to Western blot analysis using anti-α- and anti-β-antibodies. B: α and β signal intensities from −Tet (solid bars) or +Tet (open bars) loop IP were quantified using densitometry. C: MDCK/β2 with Myc tag (β2myc) cells were grown in the presence of Tet for 5 days, PM collected, and 30 μg PM were solubilized in IPB. 10% of the soluble protein was used as input, and the rest was subjected to L IP followed by SDS-PAGE and Western blot analysis using anti-α- or anti-Myc antibodies.
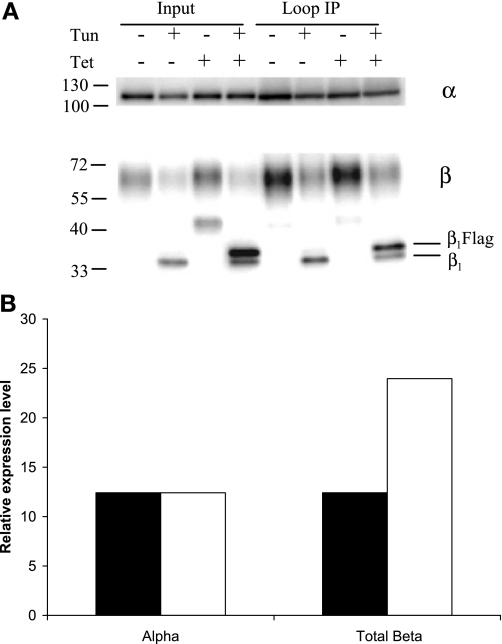
Fig. 6.
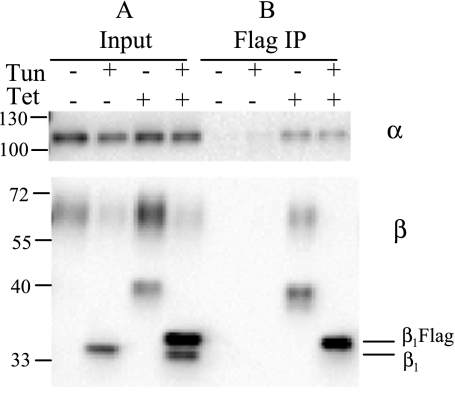
β1 glycosylation is not necessary for increased associations with α in MDCK/β1flag expressing cells. MDCK/β1flag cells were grown in the presence (+) or absence (−) of Tet or tunicamycin (Tun) for 24 h. TM (30 μg) was solubilized, and 10% was used as input. The remaining soluble protein was subjected to IP with anti-α-loop antibody followed by Western blot analysis. Anti-α- and anti-β-antibodies were used to probe Western blots. A: relative levels of β-subunits from Tun-treated samples bound by L IP (lanes 6 and 8) were quantified, normalized to α, and displayed in B. B: quantification shows more β associated per α when β1flag is overexpressed (open bars) than when β1flag is not expressed (solid bars).
In some experiments, endoglycosidases were used to determine the molecular mass of deglycosylated β1-subunit. Before SDS-PAGE and Western blot analysis, 10 μg of membrane protein were subjected to endoglycosidase H (Endo H) or peptide N-glycosidase F (PNGase F) treatment, according to the manufacturer's instructions (New England Biolaboratories).
Antibodies.
Dilutions of primary antibodies used for Western blots were as follows: 1:200,000 of anti-α1 (Affinity Bioreagents MA3-929); 1:200,000 of anti-β1 (Affinity Bioreagents MA3-930), 1:1,000 of anti-Myc (Cell Signaling 2276), 1:1,000 of anti-flag M2 (Sigma F3165), and 1:2,000 of anti-actin (abCam ab20272). Affinipure goat anti-mouse IgG (Jackson Immunoresearch 115-035-146), a horseradish peroxidase-conjugated secondary antibody, was diluted 1:5,000. The antibody dilutions optimized for immunoprecipitation (IP) were 1:50 rabbit polyclonal raised against the α1-cytoplasmic 4/5 loop (13) or 1:50 polyclonal rabbit anti-flag (Sigma F7425).
IP.
To assess Na-K-ATPase subunit interactions in membrane fractions, IP experiments were performed. Protein (5–100 μg) from TM-, ER-, or PM-enriched fractions were solubilized for 1 h at 4°C with 300–500 μl IP buffer (IPB) (150 mM NaCl, 10 mM Tris·HCl, 2 mM EDTA, pH 7.5) (4) containing 2% n-dodecyl β-d-maltoside (DDM) and protease inhibitor cocktail tablets. To remove insoluble material, the samples were spun in a TLA 55 rotor for 30 min at 55,000 rpm. The soluble supernatant was collected, and a 10% aliquot was saved for the input lane on a Western blot. The remaining supernatant was transferred to a new tube containing the IP antibody. The antibody/protein solution was incubated for 1 or 24 h at 4°C with end-over-end rotation. Immobilized protein G-agarose beads (100 μl) (Pierce 20399) were equilibrated in IPB and added to samples for a 1- or 24-h incubation at 4°C with rotation. Antibody-bound proteins were isolated from the IPB solution by spinning the antibody/bead complex at 500 g for 5 min. Of the remaining proteins in the supernatant, either 10% were kept for SDS-PAGE or 100% were used in a second round of IP. The beads were washed three times for 5 min each with 1 ml IPB + 2% DDM, one time with 0.5 M NaCl in IPB, and one time with HPLC H2O to remove unassociated proteins. Followed each wash, samples were spun by centrifugation at 500 g for 1 min, and supernatant was removed by aspiration. Proteins were eluted from the beads with 2× sample buffer + 2% 2-mercaptoethanol for 30 min at room temperature. The eluted proteins were resolved on a 10% SDS-PAGE and detected by Western blot analysis.
Confocal imaging.
MDCK/β1myc and MDCK/β1flag cells were grown separately in 1 μg/ml Tet for 72 h. The confluent cells were then trypsinized, combined at equal proportions on transwell filters, and induced with Tet for 24 h. The cocultured cells were fixed onto the filters with cold acetone for 20 s, blocked with blocking buffer (1% bovine albumin, 1% gelatin in PBS), and immunoprobed. Cells were probed consecutively with 1:500 anti-flag (Sigma F3165) and then 1:500 anti-Myc (Cell Signaling 2276) antibodies, followed by washing with PBS three times for 10 min each. Alexa 488 goat anti-mouse (Molecular Probes) and Cy3 goat anti-rabbit (Jackson Laboratories) secondary antibodies were used at 1:1,000 dilutions in blocking buffer followed by three PBS washes. Transwell filters were placed on slides, covered with Vectashield hard set mounting medium with DAPI (Vector Laboratories), and sealed with a glass coverslip. Images were detected and analyzed using Zeiss LSM 5 Pascal microscope and software.
Transepithelial resistance.
MDCK/β1flag cells were plated at 50% confluency on 0.4-μm pore-size transwells (Costar #3450) in the presence or absence of Tet. Every 24 h, the electrode (STX2 Electrode) on the EVOM epithelial voltohmeter (World Precisions Instruments) was washed with PBS and then DMEM before measurement of the transepithelial resistance (RTE) of the cell monolayer (Rtotal). A transwell containing medium but no cells was used as control (Rblank). The measurements were converted to resistance in Ohms using the equation RTE = (Rtotal − Rblank)(πd2/4), where d is the diameter of the transwell in centimeters.
RESULTS
Protein expression and membrane distribution in MDCK/β1flag cells.
To investigate the cellular response to overexpression of the β1flag subunit, we grew MDCK/β1flag cells with or without Tet for 5 days. ER-, G-, and PM-enriched fractions were then isolated. Equal amounts of membrane protein were separated by SDS-PAGE, and Western blots were probed with anti-α1, anti-β1, or anti-actin antibodies. The α1- and β1-protein expression levels and membrane distributions were analyzed and are shown in Fig. 1. All fractions from uninduced (−Tet) cells display β1- and α1-subunit bands at ∼60 and 113 kDa, respectively. In cells expressing β1flag (+Tet), α1 expression was similar to its expression level in uninduced fractions. However, the total β1 expression was increased significantly in all fractions, with the majority in the PM. Additionally, a smaller β1-band at ∼43 kDa was detected in +Tet samples and is most prominent in ER and G fractions. β1-Subunit expression levels in Fig. 1A were quantified, normalized using an actin loading control, and are displayed in Fig. 1B. Similar results were observed in MDCK cells overexpressing β1myc subunit and in human embryonic kidney cells (HEK-293) overexpressing β1flag (unpublished observations). The extent of overexpression of the β1flag varied among different cell harvests, ranging from ∼2- to 10-fold over native levels, depending on the number of times the cell colonies had been split, Tet induction time, and cell confluence at the time of harvest. In all cases, α1 expression was unaffected by increased β1 expression.
The observations in Fig. 1 showing that MDCK/β1flag cells display a significant increase in β1-subunit abundance at the PM, in conjunction with studies finding that expression and activity of Na-K-ATPase are necessary for tight junction formation (26, 30, 31), made it of interest to determine the effects of “extra” β-subunit on cell monolayer RTE. MDCK/β1flag cells grown in the absence or presence of Tet were plated onto transwells in triplicate and allowed to grow for 16 days. RTE measurements were taken every 1–4 days before changing growth medium. Figure 1C demonstrates that the RTE of cells grown in the presence of Tet (dotted lines) was higher between 24 and 120 h compared with cells grown in the absence of Tet (solid lines). The difference in RTE between MDCK/β1flag cells grown in the presence or absence of Tet was maintained up to 16 days. After 16 days, the development of domes and cell overgrowth rendered RTE measurements less meaningful. The cell polarity was not altered by β overexpression, as previously reported biotinylation studies of normal MDCK and β1-overexpressing cells display similar targeting of apical and basolateral protein markers (21).
ATPase activity of β1flag expressing cells.
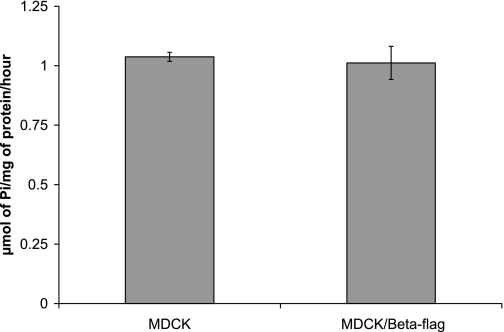
Expression of both α- and β-subunits is necessary to have a catalytically active enzyme (9, 12). When α-subunit is overexpressed in A549 human lung adenocarcinoma (11) or opossum kidney (16) cells, ATPase activity is increased. To test whether the overexpression of β-subunit also alters the level of sodium pump activity, even though α-subunit expression is unaffected, ATPase assays were performed using either MDCK cells or MDCK cells overexpressing β1flag. The Na-K-ATPase activity in TM of MDCK cells is comparable to the activity in MDCK cells expressing β1flag, 1.04 ± 0.02 and 1.01 ± 0.07 μmol Pi·mg protein−1·h−1, respectively (Fig. 2).
Fig. 2.
ATPase assay of β1flag expressing or nonexpressing cells. MDCK cells or MDCK/β1flag cells were grown in the presence of Tet for 4 days before total membrane (TM) harvesting and subjected to ATPase assays. The values displayed represent the micromoles of phosphate liberated by 1 mg of TM protein per hour of three independent experiments.
Characterization of β1-glycosylation in overexpressing cells.
Since the induction of β1flag results in an additional β1-band at 43 kDa, we sought to examine the oligosaccharides present in the 43- and 60-kDa β1-bands. In Fig. 3A, we treated ER (panel A) or PM (panel B) enriched fractions from MDCK/β1flag cells with either Endo H or PNGase F. Endo H cleaves only high mannose and hybrid N-linked glycans from glycoproteins, leaving intact complex oligosaccharides. The immunoblot of ER samples probed with anti-β1 or anti-flag (Fig. 3A, panel A) shows that the glycans attached to the 43-kDa β1-subunits are removed when they are treated with Endo H and are thus core glycosylated. Probing with the anti-flag antibody shows that the 43-kDa core glycosylated β1-subunits in the ER are β1flag subunit (Fig. 3A, bottom left). The PM fraction (Fig. 3A, panel B) contains only 60-kDa β1 (and/or β1flag) subunits that are not cleaved by Endo H. Therefore, it is apparent that all β1-subunits in the PM are complex glycoproteins and that the core β-glycoproteins are retained in the ER. Treatment of ER and PM β-subunits with PNGase F (F), which hydrolyzes all N-linked oligosaccharides, resulted in a band near the estimated mass for unglycosylated endogenous β1-subunit and β1flag subunit at 33.6 and 34.9 kDa, respectively (Fig. 3A, panels A and B).
Fig. 3.
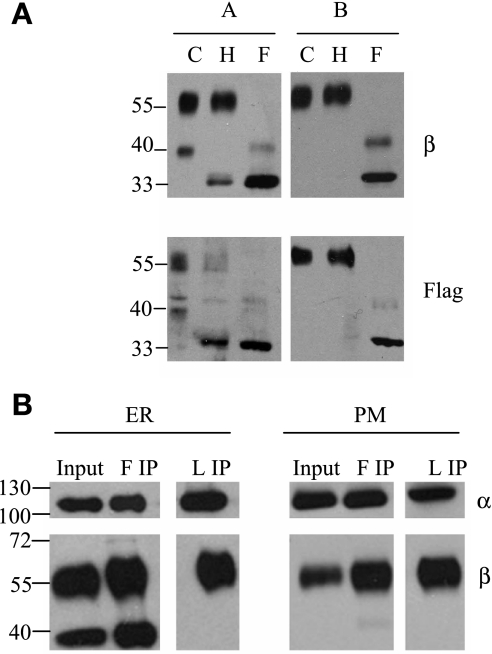
Core glycosylated β-subunits do not associate with α-subunits. MDCK/β1flag cells expressing β1flag were harvested, and membrane fractions collected. A: 10 μg ER (panel A) or PM (panel B) was left untreated [control (C)] or treated with either endoglycosidase H (Endo H; H) or peptide N-glycosidase F (PNGase F; F) and subject to SDS-PAGE followed by Western blotting procedures. Polyvinylidene difluoride membranes were probed with either anti-β or anti-flag antibodies. B: 30 μg ER or PM were solubilized; 10% soluble protein (Input) was used for Western blot analysis. The remaining soluble protein was subject to immunoprecipitation (IP) with flag (F IP) or loop (L IP) antibody. Western blot detection was performed using either anti-α- or anti-β-antibody.
In epithelial cells, the α- and β-subunits are cotranslationally inserted into the ER membrane, where they rapidly assemble, and β-subunit is core glycosylated (14). Within 1 h, the αβ heterodimer is transported to the G, and β-glycans are further modified with complex oligosaccharide additions before PM transport (36). The three N-linked glycans on β-subunit are not necessary for αβ assembly, enzymatic activity, or αβ complex sorting to the PM (42), but their removal reduces the stability of the αβ complex at the adherens junction (40). Previous reports using glycosylation inhibitors to halt β-subunit glycan extension at the core glycosylation stage revealed that core glycosylated β-subunits assemble with α-subunit, and normal enzymatic activity is maintained at the PM (43). To test whether core glycosylated β1flag associates with the α1-subunit in MDCK cells, we employed IP. The β1flag expression was induced with Tet in MDCK/β1flag cells for 3 days. Then, ER and PM fractions were collected and used for IP using either anti-flag or anti-α-loop antibody. IP proteins were eluted and resolved on SDS-PAGE. The resulting immunoblots were probed with anti-α1- or anti-β1-antibody and are shown in Fig. 3B. Figure 3B shows that the input from the ER contains both 60-kDa (complex) and 43-kDa (core) glycosylated β-subunits. When the ER is subjected to IP by anti-flag antibody (F IP), a portion of β1flag associates with α-subunit (Fig. 3B, left). However, it is apparent from anti-α-loop (an anti-α-subunit antibody) IP (L IP, see Fig. 3B, left), that the α-subunit only associates with complex glycosylated β-subunit and fails to associate with core glycosylated β-subunit in the ER. Thus the core glycosylated β-subunits in ER, according to anti-flag IP results, do not assemble with protein complexes containing the α-subunit. This may represent a fraction of the core-glycosylated β-subunit that is retained in the ER as a consequence of misfolding and is then unable to assemble with α. The β-subunits present in the PM are only the 60-kDa glycoproteins, which associate with α-subunits as determined by IP using either anti-flag (F IP) or anti-α-loop (L IP) antibody (Fig. 3B, right).
More than 95% of total β1-subunits associate with the α1-subunit in MDCK cells overexpressing β1flag.
Since the induction of β1flag expression increases total β expression levels by more than twofold and α-subunit levels are unchanged, either there are “free” β-subunits in the membrane, or the stoicheometry of the Na-K-ATPase αβ complex is altered. To examine this question, we employed a double round of IP using anti-α-loop antibody (Fig. 4, L IP1 and L IP2), followed by a third round of IP using anti-flag antibody (Fig. 4, F IP3) to see whether, after thorough precipitation of α-subunits, any additional β-subunits remained. It can readily be seen that, after the second round of anti-α-loop IP (Fig. 4, L IP2), essentially all (>95%) of the α1- and mature β1-subunits were removed. The remaining β1flag subunits consist only of high-mannose β1flag. The remaining protein in the supernatant after the third round IP was analyzed, and no β1- or α1-subunits were detected (data not shown).
Fig. 4.
Nearly all complex β-subunits associate with α in TM from induced MDCK/β1flag cells. MDCK/β1flag cells were induced for 5 days with Tet, and the TM was collected. TM (20 μg) was suspended in IP buffer (IPB) containing 2% n-dodecyl β-d-maltoside (DDM). Soluble protein was subject to first round IP using loop antibody (L IP1) to bind α and associated proteins. Following pull down with immobilized protein G-Sepharose beads, the unassociated proteins in the supernatant were collected and subject to another round of IP by loop antibody (L IP2). The remaining protein in the supernatant after the second pull down was collected and subject to a third round of IP using anti-flag antibody (F IP3) to bind any remaining β1flag subunits that were not assembled with α-subunits. Detection of associated α- and β-subunits was determined by Western blot analysis using anti-α- and anti-β-antibodies.
Ratios of associated Na-K-ATPase α1- to β1-subunits.
The functional sodium pump is composed of an α1-subunit noncovalently linked to a β1-subunit (or perhaps some higher oligomer of this composition). Previous studies indicate that, in MDCK cells, the ratio of α1 to β1 is 1:1 (7, 17, 20). Evidence in Fig. 1 and a previous report (21) show that α1-subunit abundance is unaltered by β overexpression in MDCK cells. This observation, together with evidence from Fig. 4, in which no free β-subunits are present in normal MDCK cells or cells overexpressing the β-subunit, suggests that the ratio of assembled α/β-subunits is changed when cells overexpress the β-subunit. To determine the ratio of associated α1/β1-subunits in cells when β1flag is overexpressed, TM protein from MDCK/β1flag cells induced with Tet for 5 days to overexpress the β-subunit or from uninduced cells was subjected to IP with anti-α-loop antibody (Fig. 5). When β1flag was overexpressed, the amount of α1-subunit pulled down by the loop IP did not change, but the amount of β1-subunit associated per α1-subunit increased (Fig. 5A). Quantification of these α1- and β1-subunit Western blots was performed (Fig. 5B). Clearly, the amount of β1-subunits in the sodium pump complexes has increased with increased β-subunit expression. Similar results were obtained in MDCK/β1myc cells and in HEK-293 cells overexpressing β1flag (data not shown). If normal MDCK cells have an equimolar ratio of assembled α1- and β1-subunits (7, 17, 20), the densitometric analysis of β-subunits associated with α-subunit, represented in Fig. 5, A and B, suggests that the induced MDCK/β1flag cells have approximately a 1:2 to 1:5 ratio of interacting α1- to β1-subunits, depending on the extent of β1flag overexpression. Negative controls, IP omitting either antibody or protein revealed no artifactual precipitation (data not shown). To ensure that the anti-α-loop antibody procedure we employ does not precipitate proteins present in membrane patches, but not in association with the α-subunit, we used PM-enriched fractions from MDCK cells overexpressing Myc-tagged β2-subunit. We have previously shown that the β2-subunit colocalizes with α1 at the basolateral membrane but does not associate with α1-subunit in MDCK cells (21). Our results confirmed that IP using anti-α-loop antibody does not co-immunoprecipitate β2-subunits (Fig. 5C).
Interactions between β1-subunits of adjoining cells via their N-linked glycans has been implicated in increasing cellular adhesion and Na+ pump stability at the PM (1, 39). It was interesting to determine whether the β-subunit N-linked glycans are important in the complexes that have increased β1/α1 ratio. MDCK/β1flag cells were grown in the presence or absence of Tet or tunicamycin for 24 h before membrane fractionation. PM-enriched fractions were solubilized, and the soluble protein was subject to IP with anti-α-loop antibody. Input samples in Fig. 6A (lanes 2 and 4) show β1-subunits lacking N-linked glycans due to tunicamycin treatment have molecular masses comparable to those seen following deglycosylation in Fig. 3A. The molecular mass differences between β1flag (34.9 kDa) and the endogenous β1-subunits (33.6 kDa) in the absence of glycans are distinguishable. Additionally, unglycosylated β-subunits were about twofold more abundant in cells expressing β1flag than in cells not treated with Tet, and α1-subunit expression was unchanged by unglycosylated β overexpression. IP with the loop antibody (Fig. 6, lanes 6 and 8) reveals that the amount of β1-subunit that associates with α1-subunit is increased twofold, even when β1-subunit is unglycosylated. Quantification of α1- and β1-subunits co-immunoprecipitated by anti-α-loop antibody from tunicamycin-treated cells is displayed in Fig. 6B. These results show that β1 glycosylation is not necessary for an increased ratio of associated β1/α1-subunits in cells overexpressing β1-subunit.
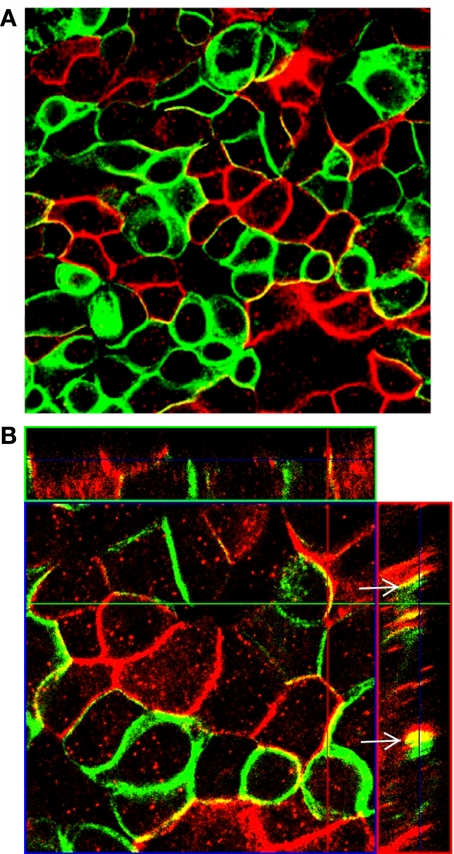
β1-β1-subunits colocalize at intercellular lateral membranes.
Na-K-ATPase α1- and β1-subunits are basolateral marker proteins primarily located at the lateral membranes in MDCK cells (10, 33). It has been suggested that lateral interactions between β1-subunits on neighboring cells play a role in cellular adhesion and protein stability in MDCK cells (40). To identify distinct β1-subunits on adjoining cells, we employed two MDCK lines expressing differently tagged β-subunits: MDCK/β1flag and MDCK/β1myc. MDCK/β1flag cells and MDCK/β1myc cells were mixed and grown together at high density with Tet for 24 h. Confocal images of the XY-plane show colocalization of β1myc and β1flag subunits at nearly all points of contact between the different cell types, as detected with the anti-Myc and anti-flag antibodies (Fig. 7A). Z-stacks of these monolayers show that β-tagged subunits are colocalized primarily at the lateral membranes (Fig. 7B, arrows). Intracellular staining in cells expressing β1flag or β1myc is likely due to the core glycosylated β-subunit, which is found primarily in the ER and G compartments, as shown in Figs. 1A and 3A.
Fig. 7.
Colocalization of β1flag and β1myc subunits occurs in cocultured cells. MDCK/β1flag and MDCK/β1myc cells were grown separately in growth medium containing Tet for 4 days and then combined for 24 h with Tet present. Cells were fixed, immunostained with anti-Myc (green) and anti-flag (red) antibodies, and analyzed by confocal fluorescence imaging. A: the XY-plane revealing colocalization at the basolateral membrane; B: Z-stacks show colocalization of β-subunits at the lateral membranes (arrows).
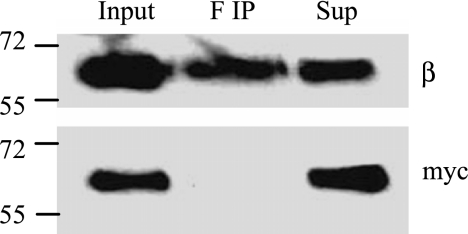
To determine whether β1-β1 interactions accompany the colocalization between neighboring cells, we collected PM from MDCK/β1flag and MDCK/β1myc cocultured cells and IP proteins associated with β1flag using anti-flag antibody. Western blots of the IP proteins were probed with anti-Myc or anti-β1 antibodies (Fig. 8). The Western blot probed with anti-Myc antibody shows β1myc was not precipitated by anti-flag (Fig. 8, bottom). The top panel probed with anti-β1-antibody was used as a positive control to show that the IP was successful in pulling down β1flag. These results suggest that strong intercellular associations were not detected between β1-tagged subunits. However, in Fig. 7A, the amount of β1flag in colocalized regions is only ∼5% compared with the total β1flag in the PM. Therefore, intercellular β1-β1 interactions cannot be excluded by our data because of the limited potential associations between β1flag and β1myc subunits in these cocultures. This limitation is a consequence of the radial nature of cell proliferation, resulting in most of the neighbors of a cell being of the same type.
Fig. 8.
Interactions between β1flag and β1myc subunits are not detected in cocultured MDCK cells. MDCK cells expressing β1flag or β1myc subunits were grown separately in growth medium with Tet for 4 days. Then, equal number of cells from each culture were mixed and plated together for 24 h in medium containing Tet. Cells were harvested, and PM collected. PM (20 μg) from cocultured cells was solublized in IPB containing 2% DDM. Ten percent of the solublized protein were used for input, the rest was immunoprecipitated with anti-flag antibody (F IP), and 10% of the remaining protein in the supernatant (Sup) were collected. Samples were subject to SDS-PAGE and Western blot analysis. Blots were probed with either anti-β or anti-Myc antibodies.
Intracellular β1flag and β1 interactions.
To ascertain if endogenous β1 associates with β1flag, either within the same cell or between cells, we used a procedure that incorporates tunicamycin, the N-glycosylation blocking reagent, in cell culture media. This enables us to distinguish the two β-subunit forms based on their slightly differing mobilities (because of the flag addition) that are not discernable in glycosylated protein. It has been shown that expression of β-subunits lacking glycosylation (caused by DNA point mutations or tunicamycin treatment) does not hinder the α/β associations seen in normal MDCK cells (22, 36). MDCK/β1flag cells were grown with or without Tet or tunicamycin for 24 h. Membranes were then solubilized with DDM. The soluble proteins were subjected to IP with anti-flag antibody. In Fig. 9, panel A, the input sample not treated with tunicamycin shows β expressed as a 60-kDa complex glycoprotein in uninduced membranes (lane 1). In the Tet-induced input sample, β1flag is expressed as both a 60-kDa complex glycosylated and a 43-kDa core glycoprotein (lane 3). In tunicamycin-treated input samples, it is possible to distinguish between the unglycosylated 33.6-kDa endogenous β1-subunit from uninduced samples (lane 2) and the unglycosylated 34.9-kDa β1flag subunit from Tet-induced samples (lane 4). Presumably, the small amount of the 60-kDa complex β1-subunit present in tunicamycin-treated samples (lanes 2 and 4) was synthesized before tunicamycin addition to the cells and is endogenous β1. In uninduced samples, the anti-flag antibody does not IP any β1-subunits (panel B, lanes 1 and 2). IP of membranes from Tet-induced and tunicamycin-treated cells with anti-flag antibody resulted in pull down of the unglycosylated 34.9-kDa β1flag subunit but not the unglycosylated 33.6-kDa endogenous β1-subunit (panel B, lane 4). These results suggest that unglycosylated β1flag and endogenous β1-subunits do not associate at a detectable level. Previous studies show that cell-cell adhesion only occurs when the neighboring cells express at the PM β1-subunits originating from the same species (33), thus β homo-oligomerization may be species specific. Based on this hypothesis, interactions between endogenous β1 and β1flag subunits are unlikely because they are from different species, dog and sheep, respectively. Alternatively, the overexpressed β1flag subunits immediately associate into β1flag complexes in the ER at their site of synthesis.
Fig. 9.
Unglycosylated β1flag and endogenous β1-subunits do not associate intercellularly at detectable levels. MDCK/β1flag cells grown in media with (+) or without (−) Tun and with (+) or without (−) Tet were harvested, and TM collected. TM (20 μg) was solublized in IPB containing 2% DDM. Ten percent of the soluble protein was used for input and shown in panel A; the rest was immunoprecipitated with anti-flag antibody, shown in panel B. Samples were run on SDS-PAGE and subject to Western blot analysis using either anti-α- or anti-β-antibody.
To detect whether β1-β1 interactions can occur on the same species, we used the MDCK/β1flag/β1myc cell line. In this cell line, synthesis and expression of β1myc is stable, whereas β1flag subunit is only expressed when cells are grown with Tet. TM from +Tet- or −Tet-treated cells were collected and used for IP with anti-flag antibody. Precipitated proteins were probed with anti-Myc antibody to recognize β1myc subunits associated with β1flag subunits. The data in Fig. 10 show that a small percentage of β1myc interacts with β1flag in TM from cells grown with Tet (lane 4). Cells grown without Tet did not synthesize β1flag, and, therefore, the anti-flag antibody does not IP any β-subunits. This demonstrates that interactions exist between β1-subunits of the same species, either directly, or via interaction with the α-subunit.
Fig. 10.
β1Flag and β1myc subunits associate at detectable levels in MDCK/β1flag/β1myc cells. MDCK/β1flag/β1myc cells grown in media with (+) or without (−) Tet were harvested, and TM collected. TM (100 μg) was solublized in IPB containing 2% DDM. Two percent of the soluble protein were used for input, and the rest was immunoprecipitated with anti-flag antibody. Samples were treated with PNGase F, run on SDS-PAGE, and subject to Western blot analysis using anti-Myc antibody.
DISCUSSION
In the present work, we have shown that, in MDCK cells overexpressing β1, the Na-K-ATPase α1- and β1-subunits are not restricted to their usual 1:1 stoicheometry. IP reveals that the α1/β1 complex assembles with multiple β1-subunits when β1 is overexpressed, resulting in an increased ratio of associated β1/α1-subunits. This increased ratio is not dependent on the presence of N-linked glycans on β-subunit. The overexpression of β-subunits causes a more rapid development of RTE, and it does not alter either cellular α-subunit levels (Fig. 1A) or MDCK cell Na-K-ATPase activity (Fig. 2).
Characterization of overexpressed β1-subunit's N-linked glycans and its interaction with α-subunit.
We have shown that inducing β1flag subunit overexpression in MDCK cells increases overall β1 abundance by greater than twofold over endogenous β1 levels, and that the majority of this protein is present in the PM. Furthermore, >90% of the β1-subunits are present in the PM in their fully glycosylated state. The ER of both β1flag-expressing cells and normal MDCK cells contain complex glycosylated β-subunits. The core glycosylated form of β-subunit is present in the ER of only β1flag-expressing cells.
Results from α-subunit IP (Fig. 3B) show that α1-subunit interacts with the 60-kDa complex glycosylated β1-subunits but not the 43-kDa core glycosylated β1-subunits. Previous reports using oligosaccharide inhibitors demonstrate that core glycosylated β-subunits can assemble with α-subunits (43). However, the time it takes for β-subunit synthesis and complex glycosylation modifications to begin is <1 h, suggesting that core glycosylated β-subunits do not occur at significant levels at any one time in normal epithelial cells (36). In our studies, the core glycosylated β1-subunits are retained in the ER, possibly due to protein misfolding, which may account for their inability to associate with the α1-subunit. Since the core β1-subunits do not localize to the PM, interact with α1-subunit, or express in native cells at sufficient levels, they were not included in the subsequent studies of α1/β1 ratios, which focus primarily on unglycosylated or fully glycosylated β1 proteins.
Effects of β1 induction and glycosylation on the associated α/β ratio.
Using the MDCK/β1flag cell line, we tested whether increasing β1-subunit expression would change α1 expression levels and/or the ratio of associated α1/β1-subunits. Our results demonstrate that, although expression of complex glycosylated β-subunit increases 2- to 10-fold over native levels, the expression level of the α-subunit does not change. IP, using an anti-α-subunit antibody demonstrates that essentially all of the β-subunits present are associated with α-subunits. Accordingly, the ratio of assembled β1/α1-subunits in the PM of MDCK cells overexpressing β1-subunit is increased over twofold compared with the native ratio of 1:1 (20, 23). The extent to which the ratio of associated β/α-subunits is increased in β1flag-expressing cells vs. noninduced cells depends on the level of exogenous β1flag overexpression. Since it is unlikely that the α-subunit has an unlimited or even large capacity for specific interactions with β-subunits, it seems most likely that the increased stoicheometry arises from β-β-subunit interactions. This may not be surprising, bearing in mind the apparent propensity of this type of protein to engage in protein-protein interactions as an adhesion molecule.
The Na-K-ATPase β1-subunit displays a significant homology to the known adhesion molecule on glia, which is identical to the β2-subunit isoform (15, 38), suggesting that a new physiological role for the β1-subunit in cell-cell adhesion may exist. Since β1-subunits are important for maintaining cell-cell contacts and initiating tight junction formation (34), and it has been shown that MSV-MDCK cells lacking sufficient amounts of β1-subunit display higher motility than MSV-MDCK cells overexpressing β1-subunit (32), it seems likely that the RTE of MDCK monolayers would increase with β1-subunit overexpression. Our results in Fig. 1C demonstrate that the RTE from cells overexpressing β-subunits is increased more rapidly compared with cells with native β-subunit levels during the first 5 days after plating.
In MDCK cells overexpressing the β-subunit, the increased β/α ratio may be caused by multiple β-subunits in associations made possible by either direct or indirect protein interactions. Previous reports demonstrate that the oligosaccharides on β-subunits are important for cell-cell adhesion and protein stability at the PM (39–41); they may also facilitate β-β interactions. To determine whether αβn complexes in β-subunit overexpressing MDCK cells occur via linkages between β1-subunit N-linked glycans, we used tunicamycin to prevent N-linked glycosylation of newly synthesized β1-subunits. The ratio of unglycosylated β1-subunits assembled with α1-subunit was similar to the ratio in untreated cells. Although cell-cell linkages via β-subunit glycans may exist in MDCK cell monolayers, they do not apparently account for an increased β/α ratio in the Na-K-ATPase complexes.
β-β-subunit interactions.
Confocal images of cocultured MDCK/β1flag and MDCK/β1myc cell monolayers show that β-subunits on adjoining cells colocalize at the lateral membrane of nearly all heterotypic cell borders. While β-β interactions may occur between adjoining MDCK cells overexpressing β-subunit, the contact sites between β1flag- and β1myc-expressing cells are few, making detection of β1flag-β1myc intercellular interactions difficult. Our observation of colocalized intercellular β-subunits is consistent with a previous report by Shoshani et al. (33), which shows colocalization of β1-subunits occurring at the lateral membranes of adjoining cells. An earlier study claimed that the β-β-subunit interactions were species specific and occurred only at the PMs of adjoining homotypic cells and not at heterotypic cell borders (6). CHO cells cocultured with MDCK cells exhibit β-subunit colocalization between the two different cell types only when the CHO cells exogenously express dog β1-subunit (33).
While intercellular β-β interactions may exist, we explored the presence of intracellular interactions in the increased β/α ratio. If same-species β-subunits are a requirement for intracellular β-β interactions as they are for intercellular ones, β1flag-endogenous β1 interactions would not occur in β1flag-overexpressing cells, since the native β1-subunit is from dog and the β1flag subunit is derived from sheep. Such interactions do not exist at detectable levels, as revealed by anti-flag IP in Fig. 9. Interestingly, while examining the presence of β-subunit interactions, we observed that, when the β1flag subunit is overexpressed, the level of endogenous β1-subunits is reduced (see Fig. 6). We are currently investigating the mechanism of this apparent regulation.
To determine whether β-subunits from the same species interact, β1myc and β1flag subunits, both derived from sheep, were expressed in MDCK cells, MDCK/β1flag/β1myc. We observe that ∼2% of the Na-K-ATPase protein complexes contain both β1flag and β1myc subunits (Fig. 10). This low level of interaction may be due to the depression of β1myc synthesis. Direct interactions between β1myc and β1flag subunits are most likely, although the α1-subunit may be involved in linking multiple β-subunits. Previous cross-linking studies of purified Na-K-ATPase from canine or pig kidney membranes provided evidence for β-β-subunit interactions; such interactions may also be present in our αβn complexes. Interactions between the β-subunits may be accomplished via the glycine zipper motif, GxxxGxxxG, which is found in the highly conserved transmembrane domain of both dog and sheep β1-subunit. The glycine zipper is important for homooligomerization of β-subunits, and the removal of the glycine zipper motif hinders cell-cell adhesion (1).
In summary, we have shown that α1- and β1-subunits are not restricted to equimolar heterodimer assembly, through an inherent limitation in subunit interactions. Rather, α1-β1n-subunit complexes can occur in β1-overexpressing MDCK cells, suggesting that other mechanisms may function to limit the individual subunit expression levels. Glycosylation of the β-subunit is not a necessary feature for assembly of the familiar 1:1 α1/β1 complexes or of the novel αβ assemblies containing “extra” copies of the β-subunit that we describe here. Our studies suggest that the formation of the normal bimolecular assemblies of Na-K-ATPase subunits is not limited by inherent subunit-subunit interactions, but that regulatory mechanisms exist that affect subunit abundance, and the availability of the Tet-regulated expression system may provide a valuable tool for the investigation of these processes.
GRANTS
This work was supported by National Institute of General Medical Sciences Grant GM-39500.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barwe SP, Kim S, Rajasekaran SA, Bowie JU, Rajasekaran AK. Janus model of the Na,K-ATPase β-subunit transmembrane domain: distinct faces mediate α/β assembly and β-β homo-oligomerization. J Mol Biol 365: 706–714, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beggah AT, Jaunin P, Geering K. Role of glycosylation and disulfide bond formation in the beta subunit in the folding and functional expression of Na,K-ATPase. J Biol Chem 272: 10318–10326, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol Renal Physiol 275: F633–F650, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Caplan MJ, Palade GE, Jamieson JD. Newly synthesized Na,K-ATPase alpha-subunit has no cytosolic intermediate in MDCK cells. J Biol Chem 261: 2860–2865, 1986. [PubMed] [Google Scholar]
- 5.Chow DC, Forte JG. Functional significance of the beta-subunit for heterodimeric P-type ATPases. J Exp Biol 198: 1–17, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Contreras R, Lázaro A, Bolivar J, Flores-Maldonado C, Sánchez S, González-Mariscal L, García-Villegas M, Valdés J, Cereijido M. A novel type of cell-cell cooperation between epithelial cells. J Membr Biol 145: 305–310, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Craig WS, Kyte J. Stoichiometry and molecular weight of the minimum asymmetric unit of canine renal sodium and potassium ion-activated adenosine triphosphatase. J Biol Chem 255: 6262–6269, 1980. [PubMed] [Google Scholar]
- 8.Dempski RE, Friedrich T, Bamberg E. The β subunit of the Na+/K+-ATPase follows the conformational state of the holoenzyme. J Gen Physiol 125: 505–520, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeTomaso AW, Xie ZJ, Liu G, Mercer RW. Expression, targeting, and assembly of functional Na,K-ATPase polypeptides in baculovirus-infected insect cells. J Biol Chem 268: 1470–1478, 1993. [PubMed] [Google Scholar]
- 10.Ernst SA, Mills JW. Autoradiographic localization of tritiated ouabain-sensitive sodium pump sites in ion transporting epithelia. J Histochem Cytochem 28: 72–77, 1980. [DOI] [PubMed] [Google Scholar]
- 11.Factor P, Senne C, Dumasius V, Ridge K, Ari Jaffe H, Uhal B, Gao Z, Iasha Sznajder J. Overexpression of the Na+,K+-ATPase alpha 1 subunit increases Na+,K+-ATPase function in A549 cells. Am J Respir Cell Mol Biol 18: 741–749, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Gatto C, McLoud SM, Kaplan JH. Heterologous expression of Na+-K+-ATPase in insect cells: intracellular distribution of pump subunits. Am J Physiol Cell Physiol 281: C982–C992, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Gatto C, Wang AX, Kaplan JH. The M4M5 cytoplasmic loop of the Na,K-ATPase, overexpressed in Escherichia coli, binds nucleoside triphosphates with the same selectivity as the intact native protein. J Biol Chem 273: 10578–10585, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Geering K, Meyer DI, Paccolat MP, Kraehenbuhl JP, Rossier BC. Membrane insertion of alpha- and beta-subunits of Na+,K+-ATPase. J Biol Chem 260: 5154–5160, 1985. [PubMed] [Google Scholar]
- 15.Gloor S, Antonicek H, Sweadner KJ, Pagliusi S, Frank R, Moos M, Schachner M. The adhesion molecule on glia (AMOG) is a homologue of the beta subunit of the Na,K-ATPase. J Cell Biol 110: 165–174, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomes P, Soares-da-Silva P. Upregulation of apical NHE3 in renal OK cells overexpressing the rodent α1-subunit of the Na+ pump. Am J Physiol Regul Integr Comp Physiol 290: R1142–R1150, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi Y, Takagi T, Maezawa S, Matsui H. Molecular weights of αβ-protomeric and oligomeric units of soluble (Na+,K+)-ATPase determined by low-angle laser light scattering after high-performance gel chromatography. Biochim Biophys Acta 748: 153–167, 1983. [DOI] [PubMed] [Google Scholar]
- 18.Hu YK, Kaplan JH. Site-directed chemical labeling of extracellular loops in a membrane protein. The topology of the Na,K-ATPase alpha-subunit. J Biol Chem 275: 19185–19191, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan JH Biochemistry of Na,K-ATPase. Annu Rev Biochem 71: 511–535, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Kyte J Purification of the sodium- and potassium-dependent adenosine triphosphatase from canine renal medulla. J Biol Chem 246: 4157–4165, 1971. [PubMed] [Google Scholar]
- 21.Laughery MD, Clifford RJ, Chi Y, Kaplan JH. Selective basolateral localization of overexpressed Na-K-ATPase β1- and β2-subunits is disrupted by butryate treatment of MDCK cells. Am J Physiol Renal Physiol 292: F1718–F1725, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Laughery MD, Todd ML, Kaplan JH. Mutational analysis of α-β subunit interactions in the delivery of Na,K-ATPase heterodimers to the plasma membrane. J Biol Chem 278: 34794–34803, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Lescale-Matys L, Putnam DS, McDonough AA. Na(+)-K(+)-ATPase alpha 1- and beta 1-subunit degradation: evidence for multiple subunit specific rates. Am J Physiol Cell Physiol 264: C583–C590, 1993. [DOI] [PubMed] [Google Scholar]
- 24.Lingrel JB, Kuntzweiler T. Na+,K+-ATPase. J Biol Chem 269: 19659–19662, 1994. [PubMed] [Google Scholar]
- 25.Lutsenko S, Kaplan JH. Molecular events in close proximity to the membrane associated with the binding of ligands to the Na,K-ATPase. J Biol Chem 269: 4555–4564, 1994. [PubMed] [Google Scholar]
- 26.Madan P, Rose K, Watson AJ. Na/K-ATPase beta1 subunit expression is required for blastocyst formation and normal assembly of trophectoderm tight junction-associated proteins. J Biol Chem 282: 12127–12134, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Morth JP, Pedersen BP, Toustrup-Jensen MS, Sorensen TLM, Petersen J, Andersen JP, Vilsen B, Nissen P. Crystal structure of the sodium-potassium pump. Nature 450: 1043–1049, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Muth TR, Gottardi CJ, Roush DL, Caplan MJ. A basolateral sorting signal is encoded in the alpha-subunit of Na-K-ATPase. Am J Physiol Cell Physiol 274: C688–C696, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Noguchi S, Mutoh Y, Kawamura M. The functional roles of disulfide bonds in the [beta]-subunit of (Na,K)ATPase as studied by site-directed mutagenesis. FEBS Lett 341: 233–238, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Rajasekaran SA, Hu J, Gopal J, Gallemore R, Ryazantsev S, Bok D, Rajasekaran AK. Na,K-ATPase inhibition alters tight junction structure and permeability in human retinal pigment epithelial cells. Am J Physiol Cell Physiol 284: C1497–C1507, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Rajasekaran SA, Palmer LG, Moon SY, Peralta Soler A, Apodaca GL, Harper JF, Zheng Y, Rajasekaran AK. Na,K-ATPase activity is required for formation of tight junctions, desmosomes, and induction of polarity in epithelial cells. Mol Biol Cell 12: 3717–3732, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajasekaran SA, Palmer LG, Quan K, Harper JF, Ball WJ Jr, Bander NH, Soler AP, Rajasekaran AK. Na,K-ATPase β-subunit is required for epithelial polarization, suppression of invasion, and cell motility. Mol Biol Cell 12: 279–295, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shoshani L, Contreras RG, Roldan ML, Moreno J, Lazaro A, Balda MS, Matter K, Cereijido M. The polarized expression of Na+,K+-ATPase in epithelia depends on the association between β-subunits located in neighboring cells. Mol Biol Cell 16: 1071–1081, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sigrid AR, Sonali PB, Ayyappan KR. Multiple functions of Na,K-ATPase in epithelial cells. Semin Nephrol 25: 328–334, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Sweadner KJ Enzymatic properties of separated isozymes of the Na,K-ATPase. Substrate affinities, kinetic cooperativity, and ion transport stoichiometry. J Biol Chem 260: 11508–11513, 1985. [PubMed] [Google Scholar]
- 36.Tamkun MM, Fambrough DM. The (Na+ + K+)-ATPase of chick sensory neurons. Studies on biosynthesis and intracellular transport. J Biol Chem 261: 1009–1019, 1986. [PubMed] [Google Scholar]
- 37.Therien AG, Nestor NB, Ball WJ, Blostein R. Tissue-specific versus isoform-specific differences in cation activation kinetics of the Na,K-ATPase. J Biol Chem 271: 7104–7112, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Treuheit MJ, Costello CE, Kirley TL. Structures of the complex glycans found on the beta-subunit of (Na,K)-ATPase. J Biol Chem 268: 13914–13919, 1993. [PubMed] [Google Scholar]
- 39.Vagin O, Sachs G, Tokhtaeva E. The roles of the Na,K-ATPase beta 1 subunit in pump sorting and epithelial integrity. J Bioenerg Biomembr 39: 367–372, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Vagin O, Tokhtaeva E, Sachs G. The role of the beta1 subunit of the Na,K-ATPase and its glycosylation in cell-cell adhesion. J Biol Chem 281: 39573–39587, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Vagin O, Tokhtaeva E, Yakubov I, Shevchenko E, Sachs G. Inverse correlation between the extent of N-glycan branching and intercellular adhesion in epithelia: contribution of the Na,K-ATPase beta 1 subunit. J Biol Chem: 2192–2202, 2008. [DOI] [PMC free article] [PubMed]
- 42.Zamofing D, Rossier BC, Geering K. Inhibition of N-glycosylation affects transepithelial Na+ but not Na+-K+-ATPase transport. Am J Physiol Cell Physiol 256: C958–C966, 1989. [DOI] [PubMed] [Google Scholar]
- 43.Zamofing D, Rossier BC, Geering K. Role of the Na,K-ATPase beta-subunit in the cellular accumulation and maturation of the enzyme as assessed by glycosylation inhibitors. J Membr Biol 104: 69–79, 1988. [DOI] [PubMed] [Google Scholar]