Abstract
Progressive renal enlargement due to the growth of innumerable fluid-filled cysts is a central pathophysiological feature of autosomal dominant polycystic kidney disease (ADPKD). These epithelial neoplasms enlarge slowly and damage noncystic parenchyma by mechanisms that have not been clearly defined. In a microarray analysis of cultured human ADPKD cyst epithelial cells, periostin mRNA was overexpressed 15-fold compared with normal human kidney (NHK) cells. Periostin, initially identified in osteoblasts, is not expressed in normal adult kidneys but is expressed transiently during renal development. We found periostin in cyst-lining cells in situ in the extracellular matrix adjacent to the cysts and within cyst fluid. ADPKD cells secreted periostin across luminal and basolateral plasma membranes. Periostin increased proliferation of cyst epithelial cells 27.9 ± 3.1% (P < 0.001) above baseline and augmented in vitro cyst growth but did not affect proliferation of normal renal cells. Expression of αV-integrin, a periostin receptor, was ninefold higher in ADPKD cells compared with NHK cells, and antibodies that block αV-integrin inhibited periostin-induced cell proliferation. We conclude that periostin is a novel autocrine mitogen secreted by mural epithelial cells with the potential to accelerate cyst growth and promote interstitial remodeling in ADPKD.
Keywords: polycystic kidney disease, ADPKD, extracelluar matrix, interstitial filrosis, epithelial
autosomal dominant polycystic kidney disease (ADPKD) is a hyperplastic disorder in which aberrant growth of tubule epithelial cells causes the formation of fluid-filled cysts, massively enlarged kidneys, and progressive loss of renal function. ADPKD is caused by mutations in PKD1 or PKD2 (26, 37, 52). Polycystin-1 (PC-1) and polycystin-2 (PC-2), products of these genes, form a signaling complex implicated in a variety of cell functions. The processes by which mutations in these genes initiate cyst formation remain unclear. In ADPKD, progressive enlargement of renal cysts has features in common with neoplasms, including aberrant cell proliferation, disordered extracellular matrix composition and deposition (53), and increased angiogenesis (3). Although cysts are benign neoplasms, they ultimately cause renal insufficiency through extensive nephron loss and replacement of adjacent parenchyma with fibrosis. The mechanisms by which cysts destroy the kidneys are poorly understood.
To determine potential pathways through which cyst epithelial cells contribute to the loss of renal structure and function, we used differential microarray analysis to compare autonomous gene expression in cultured ADPKD and normal human kidney (NHK) cells. Mural epithelial cells cultured from cyst walls closely reflect the functions of cystic cells in situ (2, 10, 25, 27, 28, 45, 46, 50, 54, 56–59). As noted in earlier studies (21, 41, 53), mRNAs of several genes involved in tissue remodeling were elevated in cystic cells compared with normal cells. Here, we show that periostin, formerly named osteoblast specific factor-2 (OSF-2 Genbank D13665), was one of the most highly overexpressed genes in cyst epithelial cells compared with normal tubule cells. Periostin is a 90-kDa extracellular protein that was initially identified in osteoblasts and shares structural homology to βig-H3, a 68-kDa transforming growth factor (TGF)-β1 inducible protein, and fascilin, a neural adhesion protein in insects (19, 23, 44, 48). These proteins have highly conserved fascilin-1 (fas-1)-like repeats that are essential for their interaction with cell surface integrins (22). Although the explicit function of periostin is unclear, a variety of tissues including bone, heart, and kidney express periostin during development and tissue remodeling (38, 43). High periostin levels were found in the nephrogenic zone of the embryonic kidney, suggesting a role in tubulogenesis and vasculogenesis (20); however, periostin does not appear to be essential for normal renal function (47).
Periostin binds αV-integrin and activates integrin-linked kinase (ILK), a component of the focal adhesion plaques (7), and has been associated with the regulation of a diverse array of cell functions, including cell adhesion, proliferation, migration, survival, and differentiation (1, 13, 19, 23, 39, 40). Periostin is highly expressed in epithelial neoplasms, including ovary (13), breast (42), and colon (1) cancers, and it is thought that periostin enhances tumor growth by stimulating cell proliferation and cell survival and by promoting angiogenesis.
In the current study, we explored the expression and functional action of periostin in tissues and cultured cells derived from cystic kidneys of ADPKD patients. The results lead us to conclude that periostin is an autocrine mitogen that is synthesized by cyst epithelial cells and is involved in the pathogenesis and progression of ADPKD.
METHODS
Primary cultures of human kidney epithelial cells.
Primary cultures of ADPKD and NHK cells were generated by the PKD Biomaterials Research Core Laboratory at the Kansas University Medical Center (KUMC). ADPKD kidneys were obtained from the surgery department at KUMC or hospitals participating in the Polycystic Kidney Research Retrieval Program. Normal regions of human kidneys, confirmed by histological examination, were collected from nephrectomy specimens. The protocol for the use of discarded human tissues complied with federal regulations and was approved by the Institutional Review Board at KUMC.
The collection of tissue and cyst fluid was performed, while the kidneys were on ice. Primary cultures were prepared as described previously (50). Cells were propagated in DMEM-F12 supplemented with 5% FBS, 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml sodium selenite (ITS) and penicillin G and streptomycin (P/S). ADPKD and NHK cells were epithelial (31, 50) and stained Arachis hypogaea and Dolichos biflorus (55) and antibodies to aquaporin-2 (32), markers for collecting ducts and distal tubules.
Cyst fluid was collected at the time the tissues were harvested. Cyst fluids were cleared of cellular debris by centrifugation (3,200 g), and aliquots were frozen at −20°C.
RNA extraction and microarray analysis.
NHK and ADPKD cells were grown in media containing 0.002% FBS for 24 h, and total RNA was isolated in a buffer containing β-mercaptoethanol and precipitated in EtOH. Total RNA was loaded onto the column of an RNeasy Mini Kit (Qiagen) and eluted with RNase free water. RNA quality was verified using an Agilent Bioanalyzer (Santa Clara, CA), and cDNA was synthesized by the Affymetrix Gene Core facility at KUMC. Human genome U133 Plus 2.0 Affymetrix chips (Santa Clara, CA) was used to compare relative expression of 38,500 known human genes between ADPKD and NHK cells.
Quantitative real-time PCR.
cDNA was synthesized from total RNA using the Invitrogen SuperScript III first-strand synthesis system for RT-PCR. Relative expression levels were determined by SYBR green real-time PCR using a SmartCycler (Cepheid, Sunnyvale, CA). The threshold cycle (Ct), the cycle number at which amplification crossed a fixed threshold, was determined for periostin, αV-integrin, and GAPDH, a housekeeping gene. The amplification efficiencies were 1.88, 1.75, and 1.85, respectively. Relative quantification was calculated using a comparative CT method. Periostin, αV-intergrin, and GAPDH primers were obtained from SuperArray (Frederick, MD).
Production of anti-periostin IgY antibody.
IgY antibodies offer several advantages over conventional antibodies raised in mammals (5). Due to phylogenetic differences, mammalian proteins are more immunogenic in birds than in mammals causing a better antibody response and long-lasting IgY titers in the egg yolk from immunized hens. Anti-human periostin IgY was generated by Affinity BioReagents (Golden, CO) using a peptide homologous to a hydrophilic region located at the C terminus of periostin (KVQGSRRRLREGRSQ) as the immunogen and affinity purified.
Immunohistochemistry.
ADPKD and NHK tissues were fixed in 4% paraformaldehyde and embedded in paraffin wax. Sections were deparaffinized and rehydrated in Reveal (Biocare Medical, Concord, CA). Endogenous peroxidase activity was destroyed by incubating sections in 3% H2O2/methanol for 10 min, and then the sections were incubated with a rabbit anti-human periostin antibody (1:1,000; BioVendor, Candler, NC) overnight at 4°C. Sections were rinsed three times with PBS, and the antigen was detected using the Zymed SuperPicTure polymer detection kit (San Francisco, CA). Sections were counter-stained with a hematoxylin solution.
Immunoblot analysis.
Soluble proteins were resolved by SDS-PAGE using the BioRad Mini-Protean III cell system (55) and then transferred from the gels to nitrocellulose membranes. Membranes were blocked overnight at 4°C, incubated with rabbit anti-human periostin primary antibody (AbCam, Cambridge, MA) for 2 h at room temperature, rinsed, and incubated in anti-rabbit IgG secondary antibody (Santa Cruz). Proteins were visualized using the ECL system and images were captured for analysis.
Immunoprecipitation.
Periostin was immunoprecipitated from growth medium conditioned by ADPKD cell monolayers grown on permeable supports (Transwell Cat No. 3491; CoStar, Cambridge, MA) using a hen anti-human periostin IgY antibody conjugated to agarose beads (Santa Cruz). Imunoprecipitated periostin was detected by immunoblot as described in Immunoblot analysis. A clear advantage of using IgY primary antibody for immunoprecipitating periostin is that the secondary anti-rabbit IgG antibody does not recognize IgY on the immunoblot. This method was also used to detect periostin in pooled cyst fluids from ADPKD kidneys.
Cell proliferation measurements.
NHK and ADPKD cells were incubated for 24 h in DMEM-F12 with 1% FBS, ITS, and P/S. The FBS concentration was reduced to 0.002% and ITS was removed for 24 h before the addition of 8-bromo-cAMP, epidermal growth factor (EGF), with or without recombinant periostin (6 wells/condition). After 72 h, cell proliferation was determined by counting cells with a hemocytometer or by the Promega Cell Titer 96 MTT assay method (56).
Flow cytometry analysis.
ADPKD cells were incubated in medium with or without periostin or EGF. Cells were detached from the culture plate with trypsin and treated with 50 μg/ml propidium iodide to stain DNA. Cell cycle profiles were analyzed with a Becton Dickinson LSRII flow cytometer and FACS Diva software (Becton Dickinson, Franklin Lakes, NJ). The percentage of cells in S and G2/M phases was determined from the total number of cells (single events ≥10,000).
Measurement of cultured ADPKD cysts.
Primary cultures of ADPKD cells (4 ×103 cells/well) were dispersed within ice-cold type I collagen in wells of a 96-well culture plate (50, 54). Warming the plate to 37° C caused polymerization of the collagen, trapping the cells within the collagen gel. Defined medium (DME-F12 with ITS, 5 × 10−8 M hydrocortisone, and 5 × 10−5 M triiodothyronine), supplemented with 5 μM forskolin and 5 ng/ml EGF, was added for 3 days to initiate cyst growth. The diameter of cysts after 3 days was <100 μm. EGF was removed, and forskolin with or without periostin was added for an additional 9 days. Outer diameters of cross-sectional images of spherical cysts with distinct lumens were measured using an inverted microscope with a digital camera connected to a video analysis system. Total surface area (SA) of the cysts within each well was calculated from individual cyst diameters (≥100 μm). Experiments were repeated for a total of three cell preparations derived from different ADPKD kidneys.
Human periostin.
We expressed full-length (90 kDa) human periostin protein in Sf-9 insect cells using the baculovirus expression system. Cells were infected with baclovirus containing human periostin cDNA with a hexa-histidine tag located on the C terminus (gift from Dr. Xiao-Fan Wang), and cultures were maintained for 72 h. Cells were scraped from the flasks, centrifuged at 2,000 g for 5 min, and resuspended in lysis buffer. Cell lysates were cleared by centrifugation, and full-length periostin was purified using Ni-NTA agarose resin (Qiagen). In addition, we purchased a 75-kDa periostin (BioVendor, LLC), which has an amino acid sequence that is 100% homologous to the 22–669 amino acid sequence of human periostin and contains the four fas-1 domains. A polyhistidine tag (HisTag) replaces the signal sequence, and the truncated protein lacks 142 amino acids of the C terminus.
Statistics.
Data are means ± SE. Statistical significance was determined by ANOVA and Student-Newman-Keuls posttest for multiple comparisons or unpaired t-test for comparison between control and treated groups.
RESULTS
Periostin expression in human ADPKD cells.
Several structural and soluble extracellular matrix components were overexpressed by ADPKD cyst cells compared with NHK cells (Table 1). Surprisingly, periostin was one of the most highly differentially expressed genes in the data set. To confirm this observation, we compared periostin mRNA levels in cells derived from ADPKD and normal kidneys by quantitative real-time RT-PCR. Periostin expression, normalized to GAPDH, was 13.6-fold higher in ADPKD cells compared with normal kidney cells (Table 2), confirming the microarray data.
Table 1.
Microarray analysis of selected ECM components in ADPKD versus NHK cells
| Gene Name | GenBank ID | Average Fold-Increase |
|---|---|---|
| Collagen I | K01228 | 15.7 |
| Periostin (probe 1) | D13665 | 15.3 |
| Periostin (probe 2) | AY140646 | 14.3 |
| Collagen III | AU144167 | 12.4 |
| Lumican | NM_002345 | 4.6 |
| Laminin | NM_018897 | 3.2 |
| Matrix metalloproteinase-2 | NM_004530 | 2.7 |
| TGF-β | AU145950 | 2.6 |
| big-H3 | NM_000358 | 2.4 |
| Heparan sulfate proteoglycan 2 | AI991033 | 2.2 |
| SPARC | NM_003118 | 2.0 |
| Thrombospondin | AI812030 | 2.0 |
Autosomal dominant polycystic kidney disease (ADPKD) and normal human kidney (NHK) cells (n = 2 kidney preparations each) were grown to 70–80% confluency in 0.002% FBS, and total RNA was isolated for whole genome expression using the Affymetrix GeneChip Human Genome U133 Plus 2.0 Array. SPARC, secreted protein; acidic, cysteine-rich (osteonectin); βig-H3, transforming growth factor (TGF), β-induced.
Table 2.
Quantitative real-time RT-PCR determination of periostin gene expression in normal NHK and ADPKD cells
| Group | n | CT Periostin | CT GAPDH | Fold-Change |
|---|---|---|---|---|
| NHK | 6 | 28.5±0.9 | 16.5±0.04 | 1.0 |
| ADPKD | 6 | 24.8±1.6 | 16.5±0.3 | 13.6 |
Total RNA was isolated from primary cultures of NHK and ADPKD cells. RNA was reverse transcribed into cDNA, and equal amounts of cDNA were amplified with a Cepheid Smart Cycler. Relative fold change in periostin expression was calculated by ΔΔCT method, where CT is the threshold cycle.
Periostin is displayed in cyst epithelial cells and adjacent extracellular matrix of human ADPKD kidneys.
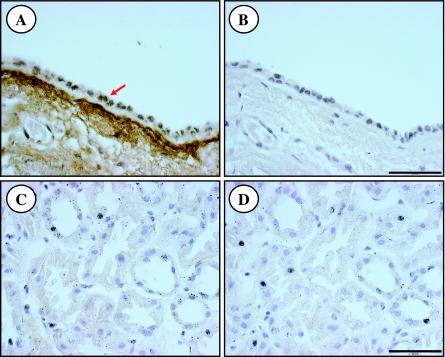
Immunohistochemistry of human ADPKD kidneys revealed periostin-specific punctuate staining within cyst epithelial cells and intense staining in the extracellular matrix lining the basal surface of most cysts (Fig. 1A). The intensity of the staining was variable among cysts even within the same kidney, and some cysts did not stain for periostin. By contrast, periostin was not detected in sections of NHK (Fig. 1C). Immunostaining for periostin was eliminated by incubation of the antibodies with excess competing peptides (Fig. 1, B and D), demonstrating antigen specificity. A similar staining pattern was observed in two additional ADPKD kidneys.
Fig. 1.
Periostin immunostaining of sections of autosomal dominant polycystic kidney disease (ADPKD) and normal human kidneys. A: representative section of a human ADPKD cyst displaying punctuate staining for periostin in mural epithelial cells (arrow) and robust staining in the extracellular matrix lining the basal surface of the cyst. Staining for periostin was variable among cysts even within a single kidney and some cysts did not stain for periostin. By contrast, periostin was not detected in normal human kidney (NHK; C). Immunostaining for perisotin was eliminated by preincubation of the antibody with excess competing peptide (B, D). A similar pattern of staining was detected in 2 additional ADPKD kidneys. Scale bar = 50 μm.
Periostin accumulates in cyst fluid of ADPKD kidneys.
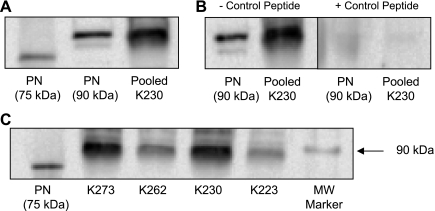
To determine if periostin was present within cyst fluid, we used an anti-periostin IgY antibody to immunoprecipitate periostin from cyst fluid pooled from multiple cysts within ADPKD kidneys and an IgG antibody for detection by immunoblot. Figure 2A displays bands for recombinant full-length (90 kDa) and truncated (75 kDa) periostin that were loaded directly on the gel for immunoblot. A 90-kDa protein was immunoprecipitated from 0.5 ml of cyst fluid, consistent with periostin. Bands for endogenous periostin and recombinant periostin were eliminated by incubation with excess competing (control) peptide (Fig. 2B). Periostin was detected in cyst fluids of male (n = 2) and female (n = 2) ADPKD patients, ages ranging from 37 to 61 yr (Fig. 2C). To quantify the periostin concentration in ADPKD cyst fluid, a standard curve using 75-kDa recombinant periostin (concentrations ranging from 10 to 500 ng) was also loaded in the gel; the respective band intensities were used to determine the amount of periostin in 500 μl of cyst fluid. The concentrations of periostin in pooled cyst fluids from ADPKD kidneys K223 and K230 were 112 and 328 ng/ml, respectively.
Fig. 2.
Periostin (PN) accumulates within cyst fluid of human ADPKD kidneys. To detect periostin in cyst fluid, an anti-human periostin IgY antibody was used for immunoprecipitation and an anti-periostin IgG antibody (AbCam, Cambridge, MA) was used for detection. A: full-length (90 kDa) and truncated (75 kDa) recombinant periostin and native periostin immunoprecipitated from 0.5 ml human ADPKD cyst fluid (K230) were detected using an antibody to human periostin. B: detection of recombinant and native periostin from cyst fluid was eliminated by incubation of the antibody with excess competing (control) peptide, demonstrating antigen specificity. C: PN was detected in cyst fluid from four ADPKD kidneys (K233 = 61-yr-old female, K230 = 49-yr-old male, K262 = 52-yr-old male, and K273 = 37-yr-old female). MW, molecular weight.
Periostin is secreted by ADPKD cyst epithelial cells.
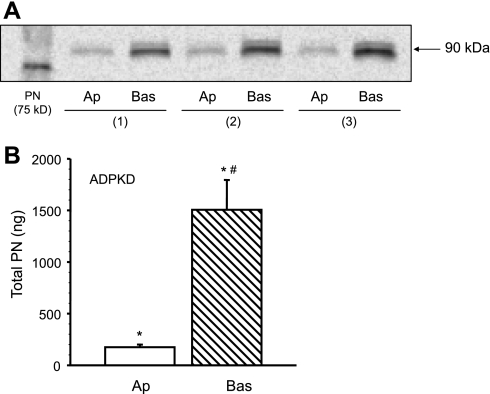
To determine if ADPKD cells secrete periostin, epithelial cell monolayers were grown on collagen-coated Transwell supports and incubated in DME-F12 supplemented with 0.002% FBS (50). In the absence of cells, the supports are freely permeable to proteins, including periostin. After cells form a polarized monolayer, secreted proteins accumulate in the media within the upper and lower reservoirs. After 5 days, conditioned media were collected individually from upper (apical, 1.18 ml) and lower (basolateral, 2.25 ml) chambers of the Transwells. Periostin was immunoprecipitated from the conditioned media and detected by immunoblot analysis. A major band was detected at 90 kDa in the apical and basolateral media (Fig. 3A) in accordance with the calculated molecular mass of periostin (48). Quantification of periostin in conditioned media obtained from 12 ADPKD cell monolayers (3 kidneys) revealed that the amount of periostin secreted across the apical and basolateral membranes was 174.7 ± 25.3 and 1505.3 ± 289.4 ng, respectively (Fig. 3B).
Fig. 3.
Periostin was detected in apical and basolateral media conditioned by ADPKD cells. Cyst epithelial cells were grown as confluent monolayers on collagen-coated permeable supports (Transwell; CoStar). Serum was reduced to 0.002% and after 5 days conditioned media were collected individually from the upper (apical, 1.18 ml) and lower (basolateral, 2.25 ml) chambers of the Transwell. A: periostin was immunoprecipitated from the basolateral (Bas) and apical (Ap) media using an anti-human periostin antibody and detected by immunoblot. B. Quantification of periostin in conditioned media bathing cell monolayers (n = 12) cultured from three ADPKD kidneys revealed that periostin was secreted into both apical and basolateral compartments (*P < 0.001, compared with zero); however, the amount of periostin in the basolateral fluid was significantly greater than that in apical fluid. #P < 0.001, compared with apical PN.
Periostin stimulates ADPKD cell proliferation.
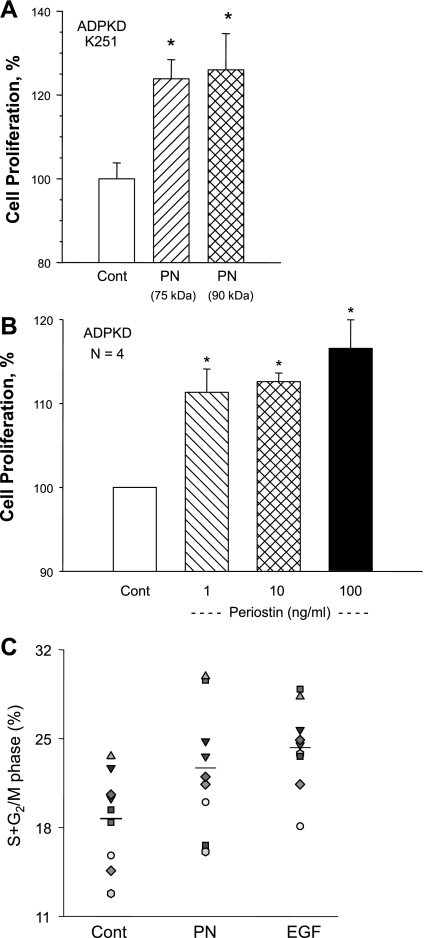
To determine if periostin accelerates cell proliferation as it does in cancer (1, 47), we incubated ADPKD cells for 72 h in minimal growth medium containing periostin and determined the relative rate of cell proliferation. Incubation of ADPKD cells with 90- and 75-kDa recombinant periostin (100 ng/ml) increased the number of cells 26.0 ± 8.6 and 23.9 ± 4.6%, respectively, compared with control (Fig. 4A). These data demonstrate that full-length and truncated periostin increase ADPKD cell proliferation to the same magnitude, consistent with the idea that the conserved fas-1 domains located in the N terminus are required for functional activity of the protein. Concentrations as low as 1 ng/ml periostin (75 kDa) caused a significant increase in the rate of ADPKD cell proliferation (Fig. 4B).
Fig. 4.
Effect of periostin on ADPKD cell proliferation. A: ADPKD cells were incubated in control media (Cont) or media containing 100 ng/ml 90-kDa full-length or 75-kDa truncated (BioVendor) human periostin. Relative rates of cell proliferation were determined by cell count using a hemocytometer and normalized to control. B: ADPKD cells were incubated in the absence and presence of varying concentrations of 75-kDa periostin. Cell proliferation (means ± SE) was determined by a Promega MTT proliferation assay and normalized to control. C: ADPKD cells were incubated with 75-kDa periostin (250 ng/ml) or EGF (25 ng/ml); treated for 12, 24, or 48 h; and stained with propidium iodide to label DNA for cell cycle analysis by flow cytometry. Data represent percentage of cells in the cell cycle (S and G2/M phases) determined from the total number of cells counted (single events ≥10,000); n = 9 experiments using 6 ADPKD kidney cell preparations. Data from 12, 24, and 48 h incubation times were combined since the effect was similar at these time points. Average for each group is indicated by a line. *P < 0.05, compared with control.
To determine if periostin promoted cell cycle progression, ADPKD cells were incubated with 75-kDa periostin (250 ng/ml) or EGF (25 ng/ml) for 12 to 48 h. DNA was labeled with propidium iodide for cell cycle analysis. The percentage of cells in S and G2/M phases was determined from the total number of cells sorted (Fig. 4C). Periostin increased the number of cells in S and G2/M phases from 18.7 ± 0.01 to 22.7 ± 0.02% (P < 0.05), and EGF increased the percentage to 24.4 ± 0.01% (P < 0.05). These data confirm that periostin and EGF individually increased ADPKD cell cycle progression.
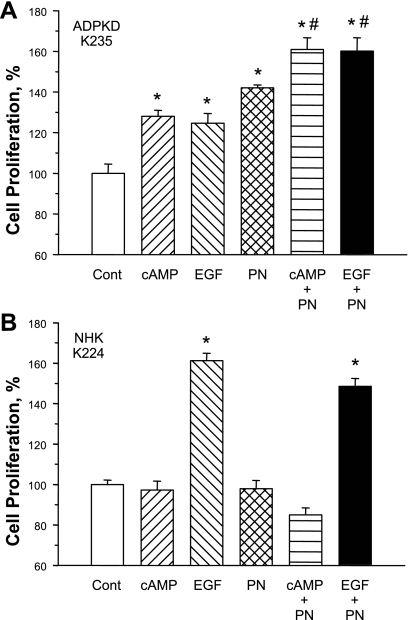
To examine further the mitogenic effect of periostin, NHK and ADPKD cells were incubated for 72 h with 100 ng/ml periostin alone or in combination with 100 μM 8-bromo-cAMP or 25 ng/ml EGF. EGF increased ADPKD and NHK cell proliferation rates; however, cAMP only stimulated the proliferation of ADPKD cells (Fig. 5A), consistent with previous observations (18, 54, 56). Periostin increased ADPKD cell proliferation 40% above control and enhanced cell proliferation induced by either EGF or cAMP. The mitogenic effect of periostin in ADPKD cells was independent of cAMP, since periostin had no effect on intracellular cAMP and periostin-induced proliferation was insensitive to H-89, a protein kinase A inhibitor (data not shown). These data suggested that periostin stimulated a pathway unique from the MEK/ERK pathway (55). In 20 experiments using cells derived from 10 different ADPKD kidneys, periostin increased cell proliferation 27.9 ± 3.1% (P < 0.001) above control. By contrast, periostin had no effect on the growth rate of NHK cells (Fig. 5B). In a series of experiments using cultured cells from six normal kidneys, periostin had no effect on cell proliferation (99.3 ± 2.4 vs. 100 ± 0% for the control group).
Fig. 5.
Effect of periostin, cAMP and epidermal growth factor on ADPKD and normal kidney cell proliferation. A: 8-bromo-cAMP (100 μM) and epidermal growth factor (EGF; 25 ng/ml) stimulated ADPKD cell proliferation, consistent with previous results (56). Recombinant periostin (PN; 100 ng/ml) increased the rate of ADPKD cell proliferation, an effect that was additive to the response of cAMP or EGF. B: in NHK cells EGF, but not cAMP, stimulated cell proliferation (56). PN had no effect on NHK cell proliferation in the absence or presence of cAMP or EGF. *P < 0.001, compared with control. #P < 0.001, compared with cAMP or EGF alone.
Effect of periostin on the proliferation of cells from phenotypically normal tubules and cysts of an early stage ADPKD kidney.
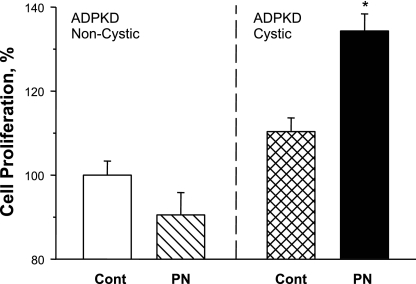
In ADPKD, the explicit cyst initiation mechanism remains unclear. Although all cells carry a germline mutation in either PKD1 or PKD2, cysts form in a minority of nephrons. Consequently, in early and intermediate stages of the disease when cysts are relatively small, substantial portions of the kidney may not be cystic. Initiation of cyst formation is thought to require somatic loss or insufficient expression of the normal allele (52). To determine if periostin stimulates the proliferation of cells from phenotypically normal tubules (heterozygous for the mutated polycystin), epithelial cells were cultured from noncystic and cystic regions of an early stage ADPKD kidney. The serum creatinine level was normal in this patient; however, one kidney was removed for the treatment of severe pain. Periostin had no effect on cells from noncystic regions but increased proliferation 34% in cells derived from the cysts (Fig. 6). Thus periostin appears to be an autocrine mitogen only in established cysts.
Fig. 6.
Effect of periostin on noncystic and cystic cells cultured from an early-stage ADPKD kidney. Primary cultures were generated from tubule epithelial cells cultured from noncystic regions, confirmed by histology examination, and cysts of an early-stage ADPKD kidney (K233). There was no effect of 100 ng/ml periostin on the noncystic cells, whereas PN increased the proliferation rate of the cystic cells. Cell proliferation was determined with the Promega MTT cell proliferation assay (n = 6 wells per condition), and data (means ± SE) were normalized to the control-treated noncystic ADPKD cells. *P < 0.001, compared with control.
Roles of αV-integrin receptor and TGF-β in periostin signaling.
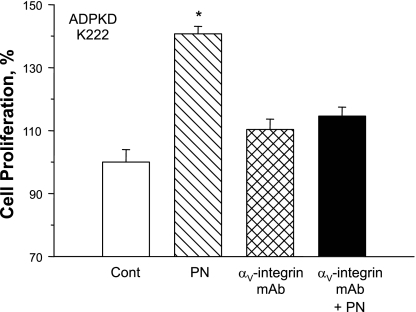
Periostin stimulates cell proliferation by binding αV-integrin and subsequent activation of ILK (7). Using quantitative RT-PCR, we found a ninefold higher expression of αV-integrin in ADPKD cells compared with NHK cells (n= 3). To determine if the effect of periostin was mediated by αV-integrin, ADPKD cells were treated with an antibody that inhibits αV-integrin signaling (7). We found that αV-integrin blockade eliminated the effect of periostin on ADPKD cell proliferation (Fig. 7).
Fig. 7.
Effect of αV-integrin blockade on periostin-induced ADPKD cell proliferation. Cultured ADPKD cells were incubated in 0.002% FBS control media or media containing 100 ng/ml periostin in the absence or presence of 0.5 μg/ml αV-integrin “function blocking” antibody. Cell proliferation was determined by Promega MTT cell proliferation assay. αV-Integrin blockade prevented PN-induced ADPKD cell proliferation. *P < 0.01, compared with control.
TGF-β is a multifunctional cytokine that regulates cell proliferation, differentiation, and ECM remodeling. Recent evidence indicates that there is cross-talk between αV-integrin and TGF-β signaling (17). αV-Integrin binds and activates latent TGF-β, which upregulates the expression of ECM, including periostin (9, 19, 48). We found that TGF-β expression was elevated in ADPKD cells compared with normal cells (Table 1), consistent with a previous study (41). To determine if TGF-β increases periostin expression, we treated NHK and ADPKD cells with 25 ng/ml TGF-β for 48 h. TGF-β increased periostin mRNA expression 15.8-fold in NHK cells and caused a further 2.2-fold increase in ADPKD cells in which periostin expression was elevated de novo.
Effect of periostin on ADPKD cyst growth in vitro.
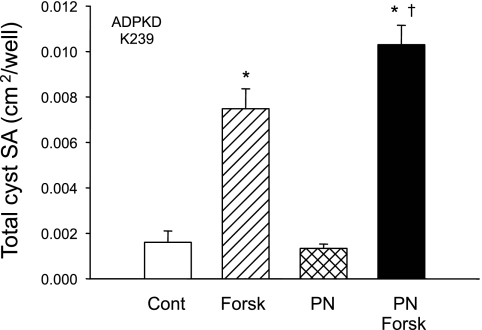
ADPKD cell microcysts were generated in polymerized collagen gels as reported previously from this laboratory (50, 54). After cysts formed, agonists were removed, and forskolin, a cAMP agonist, and periostin were added to culture media individually or together. Forskolin stimulated ADPKD cell proliferation and fluid secretion, and it increased the number and size of the cysts (Fig. 8). Periostin alone had no effect on cyst enlargement even though the compound increased ADPKD cell proliferation (Figs. 4–7). On the other hand, periostin enhanced the effect of forskolin on cyst growth. The total number of cysts (≥100 μm) increased from 55 to 68, and the total cyst surface area per well increased by 39%, in keeping with an action to complement cAMP-induced cell proliferation (Fig. 5). The lack of an effect of periostin in the absence of forskolin reinforces the view that cAMP-dependent transepithelial fluid secretion is required for cyst expansion in vitro (45, 50).
Fig. 8.
Effect of periostin on in vitro cyst growth of ADPKD cells cultured within collagen gel. ADPKD cells were seeded within a collagen gel and incubated in forskolin plus EGF for 3 days to initiate cyst formation. Agonists were removed, and gels were incubated with control media or media containing 5 μM forskolin (Forsk) ± 100 ng/ml periostin for 9 days. Total surface area (SA) of cysts (≥ 100 μm) per well was determined with a video analysis system attached to an inverted microscope. Forskolin, a direct activator of adenylyl cyclase, stimulated cAMP-dependent cell proliferation and fluid secretion (36, 50). PN alone had no effect of cyst expansion; however, PN amplified the effect of forskolin. The result was confirmed in a second ADPKD cell preparation (K235). The average fold increase in total cyst SA induced by forskolin in the absence and presence of PN was 3.7 and 6.8, respectively. *P < 0.001, compared with control. †P < 0.01 compared with forskolin alone.
DISCUSSION
During kidney development, nephron formation involves a coordinated orchestration of cell proliferation, differentiation, apoptosis, and angiogenesis, processes that are regulated by diverse proteins expressed by tubule epithelial cells. Matrix molecules and autocrine factors play important roles in the growth and elongation of these structures to form tubules with a relatively uniform diameter of ∼40 μm (16). In ADPKD, reduction in PC-1 or PC-2 expression below a critical threshold is thought to cause incomplete cellular differentiation and aberrant expression of proteins, including ECM molecules. Abnormal proliferation, loss of planar cell polarity and transepithelial fluid secretion (4, 11, 15, 35) result in the formation and progressive expansion of fluid-filled cysts that can increase to several centimeters in diameter.
There is a tremendous accumulation of ECM proteins around renal cysts (8, 53); basement membranes that line the cysts become laminated and fragmented; and mononuclear cells infiltrate the interstitial space adjacent to the cysts (29). These changes are forerunners to functional loss of nephrons and chronic kidney disease. We show here that aberrant expression of ECM molecules is remembered by ADPKD cystic cells in culture (Table 1). There was overexpression of structural ECM proteins, including type I and III collagens and laminin, and soluble ECM-associated proteins, including matrix metalloproteinases, TGF-β, and βig-H3. Surprisingly, mRNA for periostin, a protein originally considered a bone factor, was highly overexpressed in ADPKD cells (Tables 1 and 2). We found that periostin was secreted into luminal and interstitial compartments (Fig. 3), localized within cyst epithelial cells and the subjacent interstitial space of ADPKD cysts in situ (Fig. 1), and accumulated in cyst fluid (Fig. 2) to concentrations that are biologically active.
Periostin is structurally similar to βig-H3, a TGF-β inducible factor, and both proteins are composed of highly conserved fas-1-like repeats, indicating that the proteins share functional overlap (22). βig-H3 was shown to be involved in the regeneration of proximal tubule cells after injury (33), and periostin expression was found to be upregulated after vascular injury (23). The current study, in light of previous work (6, 60), leads us to think that as cysts expand, they elaborate chemokine, cytokine, and other autocrine/paracrine factors. It is very likely that tissue remodeling, perhaps led by periostin, in association with collagens, laminin, matrix metalloproteinases, and TGF-β, contributes importantly to renal fibrosis and the decline in renal function in ADPKD.
In ADPKD, mutations in PKD1 or PKD2 confer a cellular phenotype in the proliferation response to cAMP that is not present in normal renal cells. cAMP agonists stimulate the MEK/ERK pathway and proliferation of ADPKD cyst cells (12, 18, 49, 51, 54–57), and cAMP is equally potent and complimentary to growth factor stimulation (14, 30, 34, 55). In this study, we found that periostin increased ADPKD cell proliferation (Fig. 4) and in vitro cyst expansion (Fig. 8) but had no effect on the proliferation of normal cells (Fig. 5B). This mitogenic effect of periostin was additive to cAMP or EGF (Fig. 5A), suggesting that integrin signaling independently contributes to the overall proliferation rate. Normal appearing tubule cells cultured from noncystic regions of ADPKD kidneys were insensitive to periostin (Fig. 6) and cAMP (54); thus cyst epithelial cells have a unique functional phenotype in response to periostin and cAMP that is absent in normal cells.
Periostin binds αV-integrins (αVβ3- and αVβ5-integrins), leading to activation of ILK, a serine/threonine protein kinase. ILK inhibits GSK-3β, leading to β-catenin stabilization, increased levels of nuclear β-catenin, and activation of T cell factor/lymphoid enhancer binding factor (TCF/LEF), a transcription factor involved in cell proliferation (13, 47). ILK has also been shown to activate Akt causing a decrease in expression of p27Kip1, a cell cycle kinase inhibitor (1). Thus periostin promotes cell proliferation through its action on αV-integrin receptors. In ADPKD cells, αV-integrin was highly overexpressed compared with NHK cells and inhibition of αV-integrin with a blocking antibody inhibited the effect of periostin on cell proliferation (Fig. 7). Elevated expression of αV-integrin may cause ADPKD cystic cells to become sensitive to the mitogenic effects of periostin; thus periostin acts as an autocrine factor to promote the growth of only established cysts.
Expression of αV-integrin increases the level of activated TGF-β (17, 24). In ADPKD, overexpression of αV-integrin and its stimulation by periostin may activate latent TGF-β and subsequently increase the production of ECM molecules, including periostin. Thus periostin may be an important factor involved in interstitial fibrosis.
In summary, our study supports the hypothesis that aberrant expression of ECM molecules, including periostin, accelerates cyst growth and contributes to structural changes in the kidney, including interstitial fibrosis. Periostin was highly overexpressed in human ADPKD cyst-lining cells compared with normal cells and accumulated within the interstium and cyst fluid of ADPKD kidneys in situ. Periostin stimulated ADPKD cell proliferation via αV-integrin signaling and increased in vitro cyst growth. We conclude that periostin is a novel autocrine mitogen with the potential to accelerate cyst growth and promote interstitial remodeling in ADPKD.
GRANTS
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (P50 DK-057301 to D. P. Wallace) and the PKD Foundation (D. P. Wallace).
Acknowledgments
We thank Dr. Anh-Nguyet Nguyen for technical help.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, Shao R, Anderson RM, Rich JN, Wang XF. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell 5: 329–339, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Belibi FA, Reif G, Wallace DP, Yamaguchi T, Olsen L, Li H, Helmkamp GM Jr, Rantham JJ. Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells.Kidney Int 66: 964–973, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bello-Reuss E, Holubec K, Rajaraman S. Angiogenesis in autosomal-dominant polycystic kidney disease. Kidney Int 60: 37–45, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Calvet JP Molecular genetics of polycystic kidney disease. J Nephrol 11: 24–34, 1998. [PubMed] [Google Scholar]
- 5.Camenisch G, Tini M, Chilov D, Kvietikova I, Srinivas V, Caro J, Spielmann P, Wenger RH, Gassmann M. General applicability of chicken egg yolk antibodies: the performance of IgY immunoglobulins raised against the hypoxia-inducible factor 1alpha. FASEB J 13: 81–88, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Cowley BD, Ricardo SD, Nagao S, iamond JR. Increased renal expression of monocyte chemoattractant protein-1 and osteopontin in ADPKD in rats. Kidney Int 60: 2087–2096, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Cruet-Hennequart S, Maubant S, Luis J, Gauduchon P, Staedel C, Dedhar S. Alpha(v) integrins regulate cell proliferation through integrin-linked kinase (ILK) in ovarian cancer cells. Oncogene 22: 1688–1702, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Cuppage FE, Huseman RA, Chapman A, Grantham JJ. Ultrastructure and function of cysts from human adult polycystic kidneys. Kidney Int 17: 372–381, 1980. [DOI] [PubMed] [Google Scholar]
- 9.D'Angelo M, Chen JM, Ugen K, Greene RM. TGF beta 1 regulation of collagen metabolism by embryonic palate mesenchymal cells. J Exp Zool 270: 189–201, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Davidow CJ, Maser RL, Rome LA, Calvet JP, Grantham JJ. The cystic fibrosis transmembrane conductance regulator mediates transepithelial fluid secretion by human autosomal dominant polycystic kidney disease epithelium in vitro. Kidney Int 50: 208–218, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Edelstein CL What is the role of tubular epithelial cell apoptosis in polycystic kidney disease (PKD)? Cell Cycle 4: 1550–1554, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Gattone VH, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323–1326, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res 62: 5358–5364, 2002. [PubMed] [Google Scholar]
- 14.Grantham JJ 1992 Homer Smith Award. Fluid secretion, cellular proliferation, and the pathogenesis of renal epithelial cysts. J Am Soc Nephrol 3: 1841–1857, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Grantham JJ Lillian Jean Kaplan International Prize for advancement in the understanding of polycystic kidney disease. Understanding polycystic kidney disease: a systems biology approach. Kidney Int 64: 1157–1162, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Grantham JJ Polycystic kidney disease: a predominance of giant nephrons. Am J Physiol Renal Fluid Electrolyte Physiol 244: F3–F10, 1983. [DOI] [PubMed] [Google Scholar]
- 17.Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, Chun Wang L, Leone DR, Lobb RR, McCrann DJ, Allaire NE, Horan GS, Fogo A, Kalluri R, Shield CF, Sheppard D 3rd, Gardner HA, Violette SM. Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol 170: 110–125, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanaoka K, Guggino WB. cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J Am Soc Nephrol 11: 1179–1187, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 14: 1239–1249, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Ito T, Suzuki A, Imai E, Horimoto N, Ohnishi T, Daikuhara Y, Hori M. Tornado extraction: a method to enrich and purify RNA from the nephrogenic zone of the neonatal rat kidney. Kidney Int 62: 763–769, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Joly D, Morel V, Hummel A, Ruello A, Nusbaum P, Patey N, Noel LH, Rousselle P, Knebelmann B. Beta4 integrin and laminin 5 are aberrantly expressed in polycystic kidney disease: role in increased cell adhesion and migration. Am J Pathol 163: 1791–1800, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JE, Jeong HW, Nam JO, Lee BH, Choi JY, Park RW, Park JY, Kim IS. Identification of motifs in the fasciclin domains of the transforming growth factor-beta-induced matrix protein betaig-h3 that interact with the alphavbeta5 integrin. J Biol Chem 277: 46159–46165, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Lindner V, Wang Q, Conley BA, Friesel RE, Vary CP. Vascular injury induces expression of periostin: implications for vascular cell differentiation and migration. Arterioscler Thromb Vasc Biol 25: 77–83, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(−/−) mice. Am J Pathol 163: 1261–1273, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magenheimer BS, St John PL, Isom KS, Abrahamson DR, De Lisle RC, Wallace DP, Maser RL, Grantham JJ, Calvet JP. Early embryonic renal tubules of wild-type and polycystic kidney disease kidneys respond to cAMP stimulation with cystic fibrosis transmembrane conductance regulator/Na(+),K(+),2Cl(−) co-transporter-dependent cystic dilation. J Am Soc Nephrol 17: 3424–3437, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272: 1339–1342, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Nagao S, Nishii K, Katsuyama M, Kurahashi H, Marunouchi T, Takahashi H, Wallace DP. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol 17: 2220–2227, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Nagao S, Nishii K, Yoshihara D, Kurahashi H, Nagaoka K, Yamashita T, Takahashi H, Yamaguchi T, Calvet JP, Wallace DP. Calcium channel inhibition accelerates polycystic kidney disease progression in the Cy/+ rat. Kidney Int 73: 269–277, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Nagao S, Yamaguchi T, Kusaka M, Maser RL, Takahashi H, Cowley BD, Grantham JJ. Renal activation of extracellular signal-regulated kinase in rats with autosomal-dominant polycystic kidney disease. Kidney Int 63: 427–437, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T, Ebihara I, Nagaoka I, Tomino Y, Nagao S, Takahashi H, Koide H. Growth factor gene expression in kidney of murine polycystic kidney disease. J Am Soc Nephrol 3: 1378–1386, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Neufeld TK, Douglass D, Grant M, Ye M, Silva F, Nadasdy T, Grantham JJ. In vitro formation and expansion of cysts derived from human renal cortex epithelial cells. Kidney Int 41: 1222–1236, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen AN, Wallace DP, Blanco G. Ouabain binds with high affinity to the Na,K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation. J Am Soc Nephrol 18: 46–57, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Park SW, Bae JS, Kim KS, Park SH, Lee BH, Choi JY, Park JY, Ha SW, Kim YL, Kwon TH, Kim IS, Park RW. Beta ig-h3 promotes renal proximal tubular epithelial cell adhesion, migration and proliferation through the interaction with alpha3beta1 integrin. Exp Mol Med 36: 211–219, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Parker E, Newby LJ, Sharpe CC, Rossetti S, Streets AJ, Harris PC, O'Hare MJ, Ong AC. Hyperproliferation of PKD1 cystic cells is induced by insulin-like growth factor-1 activation of the Ras/Raf signalling system. Kidney Int 72: 157–165, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet 17: 1578–1590, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Putnam WC, Swenson SM, Reif GA, Wallace DP, Helmkamp GM Jr, rantham JJ. Identification of a forskolin-like molecule in human renal cysts. Am Soc Nephrol 18: 934–943, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Rossetti S, Strmecki L, Gamble V, Burton S, Sneddon V, Peral B, Roy S, Bakkaloglu A, Komel R, Winearls CG, Harris PC. Mutation analysis of the entire PKD1 gene: genetic and diagnostic implications. Am J Hum Genet 68: 46–63, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy S, Patel D, Khanna S, Gordillo GM, Biswas S, Friedman A, Sen CK. Transcriptome-wide analysis of blood vessels laser captured from human skin and chronic wound-edge tissue. Proc Natl Acad Sci USA 104: 14472–14477, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sasaki H, Dai M, Auclair D, Fukai I, Kiriyama M, Yamakawa Y, Fujii Y, Chen LB. Serum level of the periostin, a homologue of an insect cell adhesion molecule, as a prognostic marker in nonsmall cell lung carcinomas. Cancer 92: 843–848, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki H, Yu CY, Dai M, Tam C, Loda M, Auclair D, Chen LB, Elias A. Elevated serum periostin levels in patients with bone metastases from breast but not lung cancer. Breast Cancer Res Treat 77: 245–252, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Schieren G, Rumberger B, Klein M, Kreutz C, Wilpert J, Geyer M, Faller D, Timmer J, Quack I, Rump LC, Walz G, Donauer J. Gene profiling of polycystic kidneys. Nephrol Dial Transplant 21: 1816–1824, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Shao R, Bao S, Bai X, Blanchette C, Anderson RM, Dang T, Gishizky ML, Marks JR, Wang XF. Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol 24: 3992–4003, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shapiro IM Discovery: Osf2/Cbfa1, a master gene of bone formation. Clin Orthod Res 2: 42–46, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Skonier J, Neubauer M, Madisen L, Bennett K, Plowman GD, Purchio AF. cDNA cloning and sequence analysis of beta ig-h3, a novel gene induced in a human adenocarcinoma cell line after treatment with transforming growth factor-beta. DNA Cell Biol 11: 511–522, 1992. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan LP, Wallace DP, Grantham JJ. Chloride and fluid secretion in polycystic kidney disease. J Am Soc Nephrol 9: 903–916, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan LP, Wallace DP, Grantham JJ. Epithelial transport in polycystic kidney disease. Physiol Rev 78: 1165–1191, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Tai IT, Dai M, Chen LB. Periostin induction in tumor cell line explants and inhibition of in vitro cell growth by anti-periostin antibodies. Carcinogenesis 26: 908–915, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J 294: 271–278, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH 2nd. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 10: 363–364, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Wallace DP, Grantham JJ, Sullivan LP. Chloride and fluid secretion by cultured human polycystic kidney cells. Kidney Int 50: 1327–1336, 1996. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 19: 102–108, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watnick T, Germino GG. Molecular basis of autosomal dominant polycystic kidney disease. Semin Nephrol 19: 327–343, 1999. [PubMed] [Google Scholar]
- 53.Wilson PD, Hreniuk D, Gabow PA. Abnormal extracellular matrix and excessive growth of human adult polycystic kidney disease epithelia. J Cell Physiol 150: 360–369, 1992. [DOI] [PubMed] [Google Scholar]
- 54.Yamaguchi T, Hempson SJ, Reif GA, Hedge AM, Wallace DP. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol 17: 178–187, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, Pelling JC, Grantham JJ. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int 63: 1983–1994, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Yamaguchi T, Pelling JC, Ramaswamy NT, Eppler JW, Wallace DP, Nagao S, Rome LA, Sullivan LP, Grantham JJ. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int 57: 1460–1471, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 279: 40419–40430, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Ye M, Grant M, Sharma M, Elzinga L, Swan S, Torres VE, Grantham JJ. Cyst fluid from human autosomal dominant polycystic kidneys promotes cyst formation and expansion by renal epithelial cells in vitro. J Am Soc Nephrol 3: 984–994, 1992. [DOI] [PubMed] [Google Scholar]
- 59.Ye M, Grantham JJ. The secretion of fluid by renal cysts from patients with autosomal dominant polycystic kidney disease. N Engl J Med 329: 310–313, 1993. [DOI] [PubMed] [Google Scholar]
- 60.Zheng D, Wolfe M, Cowley BD Jr, Wallace DP, Yamaguchi T, Grantham JJ. Urinary excretion of monocyte chemoattractant protein-1 in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 14: 2588–2595, 2003. [DOI] [PubMed] [Google Scholar]